Abstract
Our aim was to study the protective effect of quercitin on liver cirrhosis induced by carbon tetrachloride (CCl4) in rats and its relationship with liver morphology. Thirty male Wistar rats weighing 200–250 g were randomly divided into three groups: control, CCl4, and CCl4+ quercetin. Rats in the experimental groups were given CCl4 (0.5 ml/kg i.p.), diluted 1:6 in vegetable oil (5 mmol/kg body wt), at 10:00 p.m. every 4 days for 17 weeks. Quercetin (500 μl/kg i.p.; 150 μmol/kg body wt) or vehicle was administered at 6:00 p.m. for the last 3 weeks of the study. Control group rats were given only olive oil for the same period. At the end of the 17 weeks, all rats were sacrificed. Blood samples were taken for determination of serum indicators (ALT, AST, total bilirubin, conjugated bilirubin, factor V) and the livers were dissected out and divided into two parts: one was homogenized and the supernatant was used for measurement of superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) activities, as well as lipid peroxidation. The other part was used for the histopathological study. CCl4 caused a marked rise in serum levels of ALT, AST, total bilirubin, and conjugated bilirubin, as well as a decrease in factor V (P<0.05). Lipid peroxidation levels were significantly increased, whereas GSH, SOD, catalase, GPx, and GST levels were decreased in the liver of CCl4-treated rats. Quercetin (50 mg/kg/day) successfully attenuated these effects of CCl4. We conclude that quercetin has beneficial effects on liver fibrosis in rats by enhancing antioxidant enzyme activity and decreasing the pro-oxidant effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic injury leading to liver fibrosis occurs in response to a variety of insults, including alcohol abuse, drugs, metabolic diseases due to iron or copper overload, and autoimmune attack on hepatocytes or bile duct epithelium. Carbon tetrachloride (CCl4) administration is a well-known model for the production of chemical hepatic injury [1]. Evidence of CCl3 • formation from CCl4 was provided in the 1960s by the identification of CHCl3 [2] and C2Cl6 [3] in tissues of animals that were given CCl4. More recently, spin trapping experiments have directly verified the production of CCl3 • from CCl4 by the hepatic microsomal cytochrome P450 system [4]. The major reactions of CCl3 • with liver microsomes appear to be covalent binding and H• abstraction from polyunsaturated fatty acids. The H• abstraction results in CHCl3 and PUFA• formation. PUFA• can enter into cross-linking reactions or lipid peroxidation, depending on the O2 tension. In 1978, Packer et al. [5] reported that O2 reacts very rapidly with CCl3 • to yield CCl3OO•. This radical is many orders of magnitude more reactive with PUFA than is CCl3 • and therefore is a potent initiator of lipid peroxidation. Therefore, both CCl3 • and CCl3OO• can cause membrane injury.
The sufficient time to develop cirrhosis by CCl4 administration is variable. According to Perez-Tamayo [6], the interval between CCl4 administrations must not be so long that the damaged cells cannot recover; generally, two doses per week are recommended, but this may vary depending on the model. Human dense cirrhosis, with nodule formation and portal hypertension, is usually considered irreversible, but the reversibility of cirrhosis in a number of diseases is well documented [7].
Although no successful therapeutic approach to this pathogenic mechanism in liver disease has been developed, antioxidant therapies have been shown to achieve some positive effects [8–11]. Use of antioxidants may therefore have therapeutic implications for the management of liver cirrhosis. Flavonoids are phenolic phytochemicals that represent substantial constituents of the nonenergetic part of the human diet and are thought to promote optimal health, partly via their antioxidant effects in protecting cellular components against reactive oxygen species (ROS) [12]. Quercetin (3,5,7,3,4-pentahydroxy flavon) is one of the most distributed flavonoids, semiessential food components, in certain species of plants [13]. Previous studies have shown that quercetin and other flavonoids have a broad range of pharmacological properties, including carcinostatic and antiviral activities, suppression of cell proliferation, modification of eicosanoid synthesis, protection of LDL from oxidation, prevention of platelet aggregation, stabilization of immune cells, and relaxation of cardiovascular smooth muscle [8, 14].
The present study was designed to investigate the ability of quercetin to prevent CCl4-induced oxidative stress and liver injury in rats.
Materials and methods
Experimental protocol
All experiments were performed in accordance with the Guiding Principles for Research Involving Animals (NAS) [15]. Male Wistar rats were obtained from the CREAL (Centro de Reprodução Animal de Laboratório) of the UFRGS, Porto Alegre, Brazil. They were kept on standard rat chow with free access to tap water, in rooms with controlled temperature and humidity, under a 12-hr light-dark cycle. Cirrhosis was induced by intraperitoneal injection of CCl4 (0.5 ml, diluted 1:6 in vegetable oil) at 10:00 p.m. every 4 days for 14 weeks [11].
Quercetin (Sigma, St. Louis, MO, USA) was suspended immediately before administration in a 0.2% Tween aqueous solution. Control and cirrhotic animals received daily a 500-μl intraperitoneal injection of quercetin (150 μmol/kg body wt at 6:00 p.m.) or vehicle for the last 3 weeks of the study.
To eliminate diurnal effects, all rats were killed at the same time of day. After the animals were decapitated and exsanguinated, the livers were immediately removed and blood samples were centrifuged at 1800 g for 15 min at 4°C to obtain plasma.
Analytical procedures
Blood was removed from the vena cava to determine the serum activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Bilirubin concentration was estimated using commercially available kits (Boehringer, Mannheim, Germany). Activity level of clotting factor V was measured using STA compact diagnostic STAGO.
Tissue homogenate preparation
Immediately following blood collection, rats were killed by cervical dislocation and their livers were removed. The livers were cooled in ice and homogenized in 1.15% (w/v) KCl containing 1 mM PMSF. The homogenates were centrifuged at 700 g for 10 min to discard nuclei and cell debris, and the supernatant fraction obtained was frozen at −70°C for further measurements [16].
Antioxidant enzyme activities
Total superoxide dismutase (CuZnSOD + MnSOD) activity was determined in liver as the inhibition rate of autocatalytic adenocrome generation at 480 nm in a reaction medium containing 1 mM epinephrine and 50 mM glycine-NaOH (pH 10.5). Enzyme activity is expressed as units per milligram of protein [17].
Glutathione peroxidase (GPx) activity in liver was measured by monitoring NADPH oxidation at 340 nm. The reaction medium consisted of 30 nM sodium phosphate buffer, pH 7.0, 0.17 mM reduced glutathione, 0.2 U/ml glutathione reductase, and 0.5 mM tert-butyl-hydroperoxide. GPx activity is reported as nanomoles per minute per milligram of protein [18].
Catalase (CAT) activity was determined essentially using the method described by Beers and Sizer [19], in which the disappearance of H2O2 is followed spectrophotometrically at 240 nm. The reaction medium consisted of 50 mM sodium phosphate buffer, pH 7.2, and 10 mM H2O2 [20]. The results are reported as nanomoles per milligram of protein.
Glutathione S-transferase (GST) activity toward CDNB was determined spectrophotometrically at 340 nm by the method of Habig et al. [21] with slight modifications. Briefly, the assay was performed at 25°C using 100 mM potassium phosphate buffer, pH 6.5, with GSH and CDNB (dissolved in ethanol) at a final concentration of 1 mM each. The reaction was followed for 4 min and the activity was calculated from the changes in absorbance at 340 nm using the extinction coefficient of 9.6 mmol−1 cm−1 for the reaction product, dinitrophenyl-S-glutathione (DNP-SG). Nonenzymatic conjugation was subtracted using a blank containing buffer and the substrate, but no enzyme. One unit of GST activity was defined as the amount of enzyme catalyzing the conjugation of 1 μmol of CDNB with GSH per minute at 25°C.
Lipid peroxidation measurement
Lipid peroxidation (LPO) was measured by thiobarbituric acid reactive substances (TBARS). In the TBARS method, absorbance measurements at 535 nm were used to measure the reaction between thiobarbituric acid and the LPO products, resulting in the formation of a chromogen (Schiff’s base). The homogenate was diluted to 1 mg protein/ml in 140 mM KCl and 20 mM potassium phosphate buffer, pH 7.3, and the same volume of 10% trichloroacetic acid was added (1:1). The precipitate was removed by centrifugation, and the supernatant incubated with 0.67% thiobarbituric acid for 15 min at 100°C. The results are reported as nanomoles per milligram of protein. Commercially available malonaldehyde was used as a standard [22].
Protein measurement
The protein content of the homogenate was measured by the method of Lowry et al. [23] using bovine serum albumin as a standard.
Histology
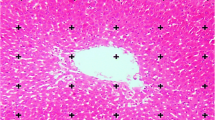
For histological studies, a piece of the liver was trimmed and fixed by immersion in Bouin’s fluid. The tissues were processed according to standard histological methods. Fibrosis was semiquantitatively assessed in 3-μm sections stained with hematoxylin/eosin or Sirius red, which mainly stains collagen fibers. The histopathological fibrosis scores were as follows: 0, no fibrosis; 1, perivenular and/or pericellular fibrosis; 2, septal fibrosis; 3, incomplete cirrhosis; and 4, complete cirrhosis [24]. The tissue slices were blindly scored by two expert pathologists. The degree of fibrosis was expressed as the mean of eight fields in each slide.
Statistical analysis
The data were compared by one-way ANOVA followed by the Student-Newman-Keuls multiple-comparison test. Results are reported as means ± SE and differences were considered to be significant at P < 0.05.
Results
CCl4 significantly decreased body weight, by 10%, and increased liver weight, by 59%, compared with those in the control group. However, quercetin reversed body weight loss and liver swelling induced by CCl4 to the levels of the control group (Table 1).
The results of the biochemical tests showed that the CCl4 group had higher plasma levels of ALT and AST activities, total bilirubin, and conjugated bilirubin concentration and significantly lower factor V activity than the control group. Quercetin prevented the rise in bilirubin concentration and the fall in factor V, and significantly reduced the increase in transaminase activities observed in the CCl4 group (Table 2).
The effects of quercetin on CCl4-induced lipid peroxidation products, measured as TBARS in the liver, are reported shown in Table 3. CCl4 significantly increased the TBARS concentration, by 98%, but quercetin inhibited it by 26%, compared with that in the CCl4 group.
CCl4 significantly decreased the hepatic GSH concentration, by 10%, compared to that in the control group, but when quercetin was also administered, the GSH concentration was maintained at the level detected in the control group (Table 3).
Antioxidant enzyme (SOD, CAT, GPx, GST) activities were significantly different between the CCl4 and the CCl4 + quercetin groups (Table 3). Compared to the control group, CCl4 administration significantly decreased SOD activity in the liver, by 63%, while quercetin administration increased it to 70% compared with that in the CCl4 group (Table 3). Likewise, CCl4 significantly decreased CAT (67%), GPx (68%), and GST (58%) activities in the liver, which were restored by quercetin administration to 77%, 54%, and 52%, respectively, compared to those in the CCl4 group.
Specimens from the normal control group presented a normal structure. In specimens from the experimental group there was an apparent formation of fibrotic septa, encompassing regenerated hepatocytes into pseudo-lobules. Regenerated hepatocytes underwent severe lipoid degeneration. Specimens from the CCl4 + quercetin group showed only mild fibrogenesis without pseudo-lobule formation. Statistical analysis showed that fibrogenesis in the CCl4 + quercetin group was less severe than in the CCl4 group (Table 4).
Discussion
Liver injuries caused by CCl4 are the best-characterized system of xenobiotic-induced hepatotoxicity and commonly used models for the screening of antihepatotoxic/hepatoprotective activity of drugs [25–28]. The results of the present study demonstrate that quercetin treatment effectively protected rats against CCL4-induced hepatotoxicity.
By considering the hepatosomatic indexes (HIS), note that the increase in liver weight during induction in the CCl4 model was reduced by quercetin. Such data are in agreement with those reported by Jeon et al., who observed increased HSIs in animals receiving CCl4 and reduced HSIs in those receiving chitosan (an oligosaccharide derived from alkaline deacetylation of chitin, superoxide, and hydroxyl radical scavenger) [28]. Other authors showed that, in animals receiving CCl4 [24, 36] and in animals with biliary duct obstruction, there was an increase in liver weight [34, 37] as measured by the HSI in these models. However, Pastor et al. [37] administered N-acetylcysteine and observed liver weight reduction in animals with biliary duct obstruction.
LPO determines alterations in structures and cell membranes by two mechanisms: (1) covalent binding with cell macromolecules and (2) action on lipids [38]. The animals receiving CCl4 presented elevated LPO (70%). This is a result of CCl4 metabolization by cytochrome P-450, which yields two such highly toxic radicals as CCl3 • and CCl3O2 • [39]. Administration of quercetin to the animals determined a 26% reduction in LPO. Quercetin’s possible mechanism of action is through the improvement of damage caused by CCl4, preventing injuries by the generated radicals. Quercetin can act as a scavenger of CCl3 •.
GSH is a major antioxidant and its levels can be altered by various chemical agents such as CCl4. GSH is produced in the liver and is kept at high levels in most tissues. Normally there is more GSH and GSSG within the cell than in the plasma, something which does not occur during liver injury processes [41]. In our work, GSH levels were found to be decreased in cirrhotic animals, similar to reports by other authors [34, 42–45]. The administration of quercetin was able to restore GSH levels to those in the control group. These results are close to those reported by Lee et al. [46], who administered quercetin orally in order to reduce dimethylnitrosamine-induced liver injury. Those authors noted that quercetin produced an increase in serum albumin and liver GSH levels and a decrease in lipid peroxidation, data similar to those found in our work.
In our study, SOD and CAT were significantly decreased [63%] compared to those in controls and were elevated in animals receiving quercetin, an increase of 70% for SOD and of 77% for CAT. These results match those of previous work [47–49] showing a significant increase in the activity of these enzymes when CCl4-intoxicated animals were treated with antioxidants. Other authors have observed an increase in these enzymes also when PMC (2,2,5,7,8-pentametyl-6-hydroxychrome), a derivative of α-tocopherol, was administered [47]. Other antioxidant enzymes were evaluated in this work, such as GPx and GST. Under oxidative stress conditions, the GSH consumed by these enzymes tries to eliminate CCl4 toxicity. In this case, GPx and GST activities are decreased. In animals treated with quercetin, a reduction in oxidative damage was observed, with regeneration of GSH, and an increase in the activity of antioxidant enzymes. GPx activity is significantly reduced in animals with CCl4-induced cirrhosis compared with the control group, possibly because of the increase in superoxide and hydrogen peroxide anions resulting from the action of xenobiotics.
GST favors protection against lipid peroxides, promoting the conjugation of toxic radicals with electrophilic characteristics with glutathione [51].
CCl4-induced cirrhosis is considered a classic, replicable model used to study the different morphophysiological and histological aspects present in the complex structure of the human liver. A normal lobular structure was observed in the liver of control animals in the present study. The main structural modifications observed in the cirrhotic liver are those related to nodular disorganization, whose structure is characterized by fibrotic septa that cross the parenchyma or form variously sized nodules [47, 52]. In this work, when the animals received quercetin, little portal and periportal fibrosis was observed, with occasional portal-portal bridges, nevertheless without clearly outlining the formation of nodules, without the presence of steatosis, results which suggest an improvement of liver histology.
In conclusion, chronic liver diseases, no matter the etiology, frequently determine similar cellular alterations as observed in this experimental model—cellular damage, inflammation, and necrosis—often with fibrosis and cirrhosis, leading to a gradual loss of the hepatic function despite the use of immunosuppressive agents and antiviral and anti-inflammatory drugs. The finding of a substance with well-tolerated antifibrotic therapeutic action is urgently needed to block this progression and to maintain the hepatocyte capacity of synthesis. The greatest obstacle to the development of drugs is the slow progression of significant fibrosis, which takes years or even decades in humans [53].
References
Recknagel RO, Glende EA, Hruszkewycz AM (1977) Chemical mechanisms in carbon tetrachloride toxicity. In: Free radicals in biology. Pryor WA (ed). Academic Press, New York, pp 97–132
Butler TC (1961) Reduction of carbon tetrachloride in vivo and reduction of carbon tetrachloride and chloroform in vitro by tissues and tissue constituents. J Pharmacol Exp Ther 134:311–319
Fowler JSL (1969) Carbon tetrachloride metabolism in the rabbit. Br J Pharmacol 37:733–737
Poyer JL, McCay PB, Lai EK, Jenzen EG, Davis ER (1980) Confirmation of assignment of the trichloromethyl radical spin adduct detected by spin trapping during 13C-carbon tetrachloride metabolism in vitro and in vivo. Biochem Biophys Res Commun 94:1154–1160
Packer JE, Slater TF, Willson RL (1978) Reactions of the carbon tetrachloride-related peroxy free radical with amino acids: pulse radiolysis evidence. Life Sci 23:2617–2620
Bonis P, Friedman SL, Kaplan MM (2001) Is liver fibrosis reversible. N Engl J Med 344(6):452–454
Perez-Tamayo R (1983) Is cirrhosis of the liver experimentally produced by CCl4 an adequate model of human cirrhosis? Hepatology 3:112–120
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080
Iwao K, Tsukamoto I (1999) Quercetin inhibited DNA synthesis and induced apoptosis associated with increase in c-fos mRNA level and the upreglutation of p21WAF1C1P1 mRNA and protein expression during liver regeneration after partial hepatectomy. Biochim Biophys Acta 1427:112–120
Sanders RA, Rauscher FM, Watkins JB (2001) Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol 15:143–149
Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, Marroni N (2003) Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci 4:824–829
Poli G, Parola M (1997) Oxidative damage and fibrogenesis. Free Rad Biol Med 22:287–305
Manach C, Texier O, Morand C, Crespy V, Regerat V, Demigne C, Remesy C (1999) Comparison of the bioavailability of quercetin and catechin in rats. Free Radic Biol Med 27:1259–1266
Park C, So H, Shin C, Baek S, Moon B, Shin S, Lee HS, Lee DW, Park R (2003) Quercetin protects the hydrogen peroxide-induced apoptosis via inhibition of mitochondrial dysfuntion in H9c2 cardiomyoblast cells. Biochem Pharmacol 66(7):1287–1295
National Academy of Sciences (1991) The guiding principles for research involving animals. National Institutes of Health, Bethesada, MD
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4’-epiadriamycin in mice. Tumori 71:241–249
Fridovich I (1974) Superoxide and evolution. Horizons Biochem Biophys 1:1–18
Flohé L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:114–121
Beers RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–625
Habig WH, Pabst MJ, Jakoby WB (1974) The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Buege JÁ, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309
Lowry OH, Rosebrough AL, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Aleynik SI, Leo MA, Ma X, Aleynik MK, Lieber CS (1997) Polyenylphosphatidylcholine prevents carbon tetrachloride-induce lipid peroxidation while it attenuates. J Hepatol 27(3):554–561
Brattin WJ, Glende EA Jr, Recknagel RO (1985) Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med 1(1):27–38
Glende EA Jr, Recknagel RO (1991) An indirect method demonstrating that CCl4-dependent hepatocyte injury is linked to a rise in intracellular calcium ion concentration. Res Commun Chem Pathol Pharmacol 73(1):41–52
Williams AT, Burk RF (1990) Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Semin Liver Dis 10(4):279–284
Jeon TI, Hwang SG, Park NG, Jung YR, Shin SI, Choi SD, Park DK (2003) Antioxidative effect of chitosan on chronic carbon tetrachloride induced hepatic injury in rats. Toxicology 187:67–73
Fort J, Oberti F, Pilette C, Veal N, Gallois Y, Douay O, Rousselet MC, Rosenbaum CP (1998) Antifibrotic and hemodynamic effects of the early and chronic administration of octreotide models of liver fibrosis in rats. Hepatology 28(6):1525–1531
Castilla-Cortazar I, Garcia M, Muguereza B, Quiroga J, Perez R, Santidrian S, Prieto J (1997) Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology 13:1682–1691
Muriel P (1998) Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol 56:773–779
Hernández-Muñhoz R, Diaz-Muñhoz M, Suárez-Cuena JÁ, Trejo-Solís C, López V, Sánchez-Sevilla Y, De Sánchez VC (2001) Adenosine reverses a preestablished CCl4-induced micronudular cirrhosis trhough enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology 34(4):677–687
García L, Hernádez I, Sandoval A, Salazar A, Garcia J, Vera J, Grijalva G, Muriel P, Margolin S, Armendariz-Borunda J (2002) Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol 37:797–805
Peres W, Tuñón MJ, Collado PS, Matos SH, Marroni N, Gonzalez-Gallego J (2000) The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol 33:742–750
Brandão ABL, Marroni CA (2001) Testes de função hepática. In: Compêndio de Hepatologia., Mattos AA, Dantas W (eds). Fundação BYK, São Paulo
Yokogawa K, Watanabe M, Takeshita H, Nomura M, Mano Y, Myamoto KI (2004) Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm 269:479–489
Pastor A, Sánchez Collado P, Almar M, Barrientos C, González-Gallego J (1997) Antioxidant enzyme status in biliary obstructed rats: effects of S-adenosylmethionine. J Hepatol 27:363–367
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, New York
Tappel AC (1973) Lipid peroxidation damage to cell components. Fed Proc 32:1870–1874
Lin CC, Yen MH, Lo TS, Lin JM (1998) Evaluation of the hepatoprotective and antioxidant activity of Boehmeria nivea var. nivea and B. nivea var. tenacissima. J Ethnopharmacol 60:9–17
Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal R S, Hatch GE, Jones DP, Mason RP, Barrett JC (2000) Biomarkes of oxidative stress study: are plasma antioxidants markers of CCl4 poisoning? Free Rad Biol Med 28(6):838–845
Corrales F, Giménez A, Alvarez L, Caballería J, Pajares M A, Andreu H, Parés A, Mato JM, Rodés J (1992) S-Adenosylmethionine treatment prevents carbon tetrachloride-induced S-adenosylmethionine synthetase inactivationand attenuates liver injury. Hepatology 16:1022–1027
Gassó M, Rubio M, Varela G, Cabré M, Caballería J, Alonso E, Deulofem R, Camps, J, Giménez A, Pajares M, Parés A, Mato JM, Rodés J (1996) Effects of S-adenosylmethionine on lipid peroxidation and liver fibrogenesis in carbon tetrachloride-induced cirrhosis. J Hepatol 25:200–205
Hernández-Muñhoz R, Díaz-Muñhoz M, López V, López-Barrera F, Yánez L, Vidrio S, Aranda-Fraustro A, Sánchez VC (1997) Balance between oxidative damage and proliferative potential in an experimental rat model of CCl4-induced cirrhosis: protective role of adenosine administration. Hepatology 26:1100–1110
Cabre M, Camps J, Paternain JL, Ferre N, Joven J (2000) Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin Exp Pharmacol Physiol 27(9):694–699
Lee MH, Yoon S, Moon JO (2004) The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull 27(1):72–76
Hsiao G, Lin YH, Lin CH, Chou DS, Lin WC, Sheu JR (2001) The protective effects of pmc against chronic carbon tetrachloride induced hepatotoxicity in vivo. Biol Pharm Bull 24(11):1271–1276
Murthy KNC, Jayaprakasha GK, Singh RP (2002) Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agr Food Chem 50:4791–4795
Lee TY, Mai LM, Wang GJ, Chiu JH, Lin YL, Lin HC (2003) Protective mechanism of salvia miltiorrhiza on carbon tetrachloride-induced acute hepatotoxicity in rats. J Pharmacol Sci 91:202–210
Flohé L, Gunzler Wa, Schock HH (1973) Glutathione peroxidase: a seleno-enzyme. Febs Lett 32:132–134
Jakoby WB (1988) Detoxification, conjugation and hydrolysis in liver biology and pathology. Raven Press, New York
Gaudio E, Onori P, Franchitto A, Sferra R, Riggio O (1997) Liver metabolic zonation and hepatic microcirculation in carbon tetrachloride-induced experimental cirrhosis. Dig Dis Sci 42(1):167–177
Schuppan D, Bauer M, Krebs A, Hahn EG (2001) Antifibrogenic treatment- present status and future directions. In: Therapy in hepatology. Arroyo V, Bosch J, Bruix J, Ginés P, Navasa M, Rodés J (eds). Ars Medica, Barcelona, pp 395–405
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amália, P.M., Possa, M.N., Augusto, M.C. et al. Quercetin Prevents Oxidative Stress in Cirrhotic Rats. Dig Dis Sci 52, 2616–2621 (2007). https://doi.org/10.1007/s10620-007-9748-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9748-x