Abstract
In the present study, Nile tilapia (Oreochromis niloticus) were used to assess the endocrine disruption potential of Microcytis aeruginosa. Male Nile tilapia were exposed to lyophilized M. aeruginosa or purified microcystin-LR (8.3 μg/L) for 28 days. The levels of serum hormones (17β-estradiol and testosterone) and transcripts of selected genes in the hypothalamus-pituitary-gonadal-liver axis were analyzed. The results showed that serum hormones were significantly up-regulated, and transcripts of 13 genes (GHRH, PACAP, GH, GHR1, GHR2, IGF1, IGF2, CYP19a, CYP19b, 3β-HSD1, 20β-HSD, 17β-HSD1 and 17β-HSD8) were significantly altered after Microcytis exposure. These results indicate that fish reproduction can be altered in a Microcystis bloom-contaminated aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Toxic cyanobacterial blooms occur in worldwide freshwaters and represent a human health and ecological concern. There are many species of toxin-producing cyanobacteria which are harmful to aquatic animals (Malbrouck and Kestemont 2006). Microcystins (MCs) belong to a family of cyanotoxins that are mainly produced by the genus Microcytis. Up to now, more than 100 variants of MCs have been isolated from Microcytis and cultures, with microcystin-LR (MC-LR), microcystin-RR (MC-RR) and microcystin-YR (MC-YR) being the most common (Puddick et al. 2014).
In recent decades, the toxicity of MCs has been well documented in aquatic organisms. For example, a number of studies have demonstrated that MCs cause a range of effects including oxidative stress, developmental toxicity, neurotoxicity, immunotoxicity, as well as hepatotoxic, cancerogenic, mutagenic and cytotoxic effects (Malbrouck and Kestemont 2006; Chen et al. 2016). However, microcystins are only one kind of compound isolated from cyanobacteria. There are also other biologically active compounds in cyanobacteria. Some previous studies have shown that crude extracts from cyanobacterial biomass or Microcystis cells may have greater effects than those of purified cyanotoxins (Palíková et al. 2007; Rogers et al. 2011). In fact, aquatic organisms, such as fish, are not only exposed to MCs but rather to Microcystis cells and lysates that contain other bioactive substances in Microcystis blooms in the real aquatic environment. For example, Microcystis also can produce multitudinous peptides classified as aeruginosins (Ishida et al. 1999), micropeptins (Yamaki et al. 2005), and microoviridins (Rohrlack et al. 2003) that have diverse types of biological function (Smith et al. 2008). However, the toxic effects of the complex mixtures of chemicals in Microcystis blooms have not been studied in detail. Recently, several studies have revealed insight into the potential for cyanobacterial extracts to exhibit estrogenic effects (Sychrová et al. 2012), and for Microcytis to represent a natural source of environmental estrogens that can up-regulate vitellogenin genes (vtg) of embryonic zebrafish (Rogers et al. 2011). Therefore, it is important to consider the toxicity of Microcystis in fish.
The Nile tilapia (Oreochromis niloticus) is an important economic species and a widely farmed freshwater food fish in southern China. In recent years, this species has been considered as a bioindicator for aquatic environmental contaminants, because of its significant tolerance to environmental stress as well as its potential to be used as an intensive aquaculture species. Due to the tropical growth environment, Nile tilapia may be exposed to Microcystis blooms which frequently occur in aquaculture ponds. Although there are several studies demonstrating the estrogenic activities of Microcytis in fish, the effects on the hypothalamic-pituitary-gonadal-liver (HPGL) axis remain to be elucidated. In the present study, we investigated serum hormones and several important steroidogenic enzymes of the HPGL axis in Nile tilapia following exposure to Microcystis cells and purified MC-LR. Our results suggest that Microcystis and MC-LR have the potential to affect the endocrine system in male Nile tilapia.
Materials and Methods
Male Nile tilapia weighing between 40 and 50 g at the beginning of the experiment were obtained from the fish farm of Freshwater Fisheries Research Center, Chinese Academy of Fishery Science (Wuxi, CN). Fish were acclimated under laboratory culture conditions for two weeks and maintained at 28 ± 0.5° C in a 12 h light: 12 h dark cycle in aquaria with recirculation and dechlorinated tap water. The adults were fed with commercial floating pellets at 10% of their body weight twice daily.
The Microcystis treatment was prepared from lyophilized cells of Microcystis aeruginosa, which was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (Wuhan, CN). The total dry weight mass of algal cells was 10 g. For exposure of Nile tilapia, lyophilized Microcystis was reconstituted back to its original nominal concentration of 100 mg of lyophilized cells/L. The solution for the MC-LR treatment was prepared by dissolving 100 μg of purified microcystin-LR (CAS#: 101043-37-2, Beijing Lianlixin BioTech, Beijing, CN) and dilution to a series of concentrations in culture water.
Six Nile tilapia were randomly distributed into an aquarium and exposed to lyophilized Microcystis, purified MC-LR and control (culture water) for 28 days, in triplicate, for each treatment group. The exposure solutions were renewed daily. Water samples for quality measurements and microcystin analysis were taken during the experiment. After 28 days of exposure, all fish were euthanized in ice water. Body weight was measured at the start and end of the 28-day exposure period, and the specific growth rate was determined. Blood was collected from the caudal vein using a heparinized syringe, and then centrifuged at 1200×g for 15 min. The separated serum samples were stored at −80°C for analysis of sex hormones. The brain, gonads and liver were removed from each fish per treatment group, rinsed with cold phosphate buffered saline (PBS, pH 7.0), and snap frozen in liquid nitrogen for later gene expression analysis.
Water quality parameters were measured daily, with dissolved oxygen = 7.3 ± 0.3 mg/L, pH 7.4 ± 0.1, and total hardness = 28.3 ± 0.4 mg CaCO3/L. The concentrations of MC-LR were measured using the Beacon Microcystin Tube Kit (Beacon Analytical Systems Inc., Saco, ME, USA) following the manufacturer’s instructions. In the lyophilized Microcystis treatment, measured MC-LR concentration was 8.3 ± 0.5 μg of MC-LR equiv/L. Therefore, as a positive control, Nile tilapia were also exposed to a nominal concentration of 8.3 μg of purified MC-LR/L under the same conditions. The actual concentration of MC-LR was 9.6 ± 1.2 μg/L in the purified MC-LR-treated groups. There were no fish deaths in the treatment groups or control group during the exposure period.
Serum hormone 17β-estradiol (E2) and testosterone (T) were measured using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s protocol.
For gene transcription analysis, six of the same tissues from one aquarium were set as one sample (n = 1), and there were triplicate samples from each treatment group. Total RNA was extracted from tissue samples using TRIzol reagent (Sangon, Shanghai, CN) following the manufacturer’s protocol. The quality and quantity of RNA were determined by UV spectrophotometry and by 1% agarose gel electrophoresis. First-strand cDNA synthesis was performed using the AMV First Strand cDNA Synthesis Kit (Sangon, Shanghai, CN) according to the manufacturer’s instructions. Real-time PCR with SYBR green detection was performed on the ABI StepOnePlus™ Real-Time PCR Systems (ABI, Foster, CA, USA) according to protocols established by the manufacturer (ABI SYBR Green PCR Master Mix, ABI). A total of 11 functionally relevant genes associated with the pathways of the HPGL axis of Nile tilapia were selected for the present study based on previous literature (Table S1, Supplementary Information). In addition, reference genes, actin and RPL8 were selected as internal quantitative controls.
Experimental data were checked for normality and homogeneity of variance using the Kolmogorov–Smirnov one-sample test and Levene’s test. Intergroup differences were assessed using one-way analysis of variance (ANOVA) followed by Duncan’s test, using SPSS Statistics 18 (SPSS Inc., Chicago, IL, USA). The level for statistical significance was set at p < 0.05. All data are shown as mean ± standard error (S.E.M.).
Results and Discussion
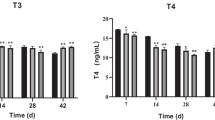
We investigated the potential of Microcystis and purified MC-LR to directly disrupt the levels of serum hormones and influence the expression of genes in HPGL axis of male Nile tilapia. Serum E2 and T levels increased in both treatments (p < 0.01) (Fig. 1). No significant differences of the E2/T ratio were observed. Sex steroid hormones play crucial roles at all stages of the reproductive cycle in vertebrates. Measurement of sex steroid hormones could be suggested as one of the most integrative and functional endpoints for understanding the effects of a chemical on reproduction in fish (Ma et al. 2012; Jo et al. 2014). In the present study, serum E2 and T levels increased in Microcystis and MC-LR exposed Nile tilapia. These results are consistent with a previous study which demonstrated increases in those hormones in male zebrafish upon exposure to purified MC-LR (Liu et al. 2016). Therefore, our results suggest that both Microcystis and microcystin have the potential to alter sex hormone balance.
The transcription of genes in the growth hormone (GH)-insulin-like growth factor (IGF) pathway of the HPLG axis was affected in Nile tilapia after Microcystis and MC-LR exposure (Fig. 2; Table S2, Supplementary Information). In brain, the levels of GH mRNA were down-regulated and no significant differences were observed for growth hormone–releasing hormone (GHRH) and pituitary adenylate cyclase-activating polypeptide (PACAP) expression in both Microcystis and MC-LR group. Expressions of growth hormone receptor 1 and 2 (GHR1 and GHR2) in liver were not significantly different, while those of IGF1 and IGF2 were down-regulated in the Microcystis group. However, transcripts of GHR1, GHR2, IGF1 and IGF2 in liver did not exhibit significant changes in MC-LR treatment relative to the control. In gonad, the transcripts of GHR1 and IGF1 were down-regulated in the Microcystis-treated group, while only IGF1 was down-regulated in the MC-LR-treated group. Our observations showed differential expressions of these genes between the Microcystis and purified MC-LR treatments. Rogers et al. (2011) evaluated gene expression in zebrafish larvae after exposure to Microcystis and purified MC-LR treatment, and their results suggested that there were biological effects beyond just the toxin MC-LR.
The GH-IGF axis plays a crucial role in the regulation of growth. In previous studies, the growth rate of fish had been reported to be associated with the expression levels of GH-IGF axis related genes (Reinecke et al. 2005). In aquaculture, cyanobacteria, including Microcystis, were suggested as feasible fish diets due to their nutritive protein (Dong et al. 2012; Ziková et al. 2010). However, most fish growth was inhibited after ingesting cyanobacteria-containing food (Kamjunke et al. 2002; Liang et al. 2015). Furthermore, microcystins from Microcystis were accumulated in fish tissue (Zhao et al. 2006; Palikova et al. 2011). In the present study, although GH-IGF axis related gene expressions exhibited different patterns between Microcystis and purified MC-LR treatments, the specific growth rate of Nile tilapia was slightly inhibited in the treatment groups compared with the control group (Table S3, Supplementary Information). These results indicated that Microcystis and the low concentration of MC-LR may have influenced growth in the present study.
The transcription of cytochrome P450 aromatase gene (CYP19a), 3β-hydroxysteroid dehydrogenase type 1 (3β-HSD1), 20β-hydroxysteroid dehydrogenase (20β-HSD), and 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1) exhibited similar expressed patterns in Nile tilapia between Microcystis- and MC-LR-treated groups (Fig. 2; Table S2, Supplementary Information). In detail, the mRNA levels CYP19a and 3β-HSD1 decreased in both exposed groups, while expression of 17β-HSD1 was significantly increased by exposure to Microcystis and MC-LR. In teleosts, CYP19a is one isoform of aromatase involved in the synthesis of estrogens from androgens (Chang et al. 2005). 17β-HSD1 catalyzes the conversion of androstenedione (A) into T (Zhou et al. 2005). Liu et al. (2016) reported that the increase in 17β-HSD mRNAs and decrease in CYP19a mRNA agreed with the greater levels of T and 11-kekotestosterone (11-KT) in male zebrafish exposed to MC-LR. In the present study, we observed similar results in Nile tilapia exposed to Microcystis and MC-LR. The gonadal 3β-HSD is linked to the conversion of pregnenolone to progesterone and of dehydroepiandrosterone (DHEA) to A (Senthilkumaran et al. 2009). In the present study, 3β-HSD was down-regulated after exposure to Microcystis and MC-LR for 28 days. Adult male zebrafish showed similar decreases in 3β-HSD after 30 days of exposure to purified MC-LR (Liu et al. 2016). Generally, 3β-HSD has the potential to decrease testosterone in males. However, the reduction of 3βHSD level detected in both treatments did not decrease the serum levels of T in male Nile tilapia. We speculated that 17β-HSD1 induced the levels of T in male Nile tilapia. Fish 20β-HSD, expressed in various tissues, including testis and ovary, is known to be involved in the production of 17α, 20β-dihydroxy-4-pregnen-3-one (17α,20β-DP), the maturation inducing hormone (Senthilkumaran et al. 2002). In females, the alternation of this gene potentially suggests reproductive dysfunction or disruption. However, the levels of 20β-HSD were not changed significantly after exposure to Microcystis and MC-LR in the present study.
In conclusion, our findings showed that exposure to Microcystis may interfere with the expression of genes involved in the HPGL axis and balance of sex hormones of male Nile tilapia. Therefore, the possibility that Microcystis may release endocrine disrupting substances is of considerable environmental interest. The results also indicated that Microcystis may pose a potential threat to fish growth and reproduction in a Microcystis bloom-contaminated aquatic environment.
References
Chang X, Kobayashi T, Senthilkumaran B, Kobayashi-Kajura H, Sudhakumari CC, Nagahama Y (2005) Two types of aromatase with different encoding genes, tissue distribution and developmental expression in Nile tilapia (Oreochromis niloticus). Gen Comp Endocrinol 141:101–115
Chen L, Chen J, Zhang X, Xie P (2016) A review of reproductive toxicity of microcystins. J Hazard Mater 301:381–399
Dong GF, Xie SQ, Zhu XM, Han D, Yang YX (2012) Nutri-toxicological effects of cyanobacteria on fish. Acta Ecol Sin 32:6233–6241
Ishida K, Okita Y, Matsuda H, Okino T, Murakami M (1999) Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron 55:10971–10988
Jo A, Ji K, Choi K (2014) Endocrine disruption effects of long-term exposure to perfluorodecanoic acid (PFDA) and perfluorotridecanoic acid (PFTrDA) in zebrafish (Danio rerio) and related mechanisms. Chemosphere 108:360–366
Kamjunke N, Schmidt K, Pflugmacher S, Mehner T (2002) Consumption of cyanobacteria by roach (Rutilus rutilus): useful or harmful to the fish? Fresh Biol 47:243–250
Liang H, Zhou W, Zhang Y, Qiao Q, Zhang X (2015) Are fish fed with cyanobacteria safe, nutritious and delicious? A laboratory study. Sci Rep 5:15166 doi:10.1038/srep15166
Liu W, Chen C, Chen L, Wang L, Li J, Chen Y, Jin J, Kawan A, Zhang X (2016) Sex-dependent effects of microcystin-LR on hypothalamic-pituitary-gonad axis and gametogenesis of adult zebrafish. Sci Rep 6:22819 doi:10.1038/srep22819
Ma Y, Han J, Guo Y, Lam PKS, Wu RSS, Giesy JP, Zhang X, Zhou B (2012) Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol. Aquat Toxicol 106–107:173–181
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Environ Toxicol Chem 25:72–86
Palikova M, Mares J, Kopp R, Hlavkova J, Navratil S, Adamovsky O, Chmelar L, Blaha L (2011) Accumulation of microcystins in Nile tilapia, Oreochromis niloticus L., and effects of a complex cyanobacterial bloom on the dietetic quality of muscles. Bull Environ Contam Toxicol 87:26–30
Palíková M, Krejčí R, Hilscherová R, Babica P, Navrátil S, Kopp R, Bláha L (2007) Effect of different cyanobacterial biomasses and their fractions with variable microcystin content on embryonal development of carp (Cyprinus carpio L.). Aquat Toxicol 81:312–318
Puddick J, Prinsep MR, Wood SA, Kaufononga SAF, Cary SC, Hamilton DP (2014) High levels of structural diversity observed in microcystins from microcystis CAWBG11 and characterization of six new microcystin congeners. Mar Drugs 12:5372–5395
Reinecke M, Björnsson BT, Dickhoff WW, McCormick SD, Navarro I, Power DM, Gutiérrez J (2005) Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen Comp Endocrinol 142:20–24
Rogers ED, Henry TB, Twiner MJ, Gouffon JS, McPherson JT, Boyer GL, Sayler GS, Wilhelm SW (2011) Global gene expression profiling in larval zebrafish exposed to microcystin-LR and Microcystis reveals endocrine disrupting effects of cyanobacteria. Environ Sci Technol 45:1962–1969
Rohrlack T, Christoffersen K, Hansen PE, Zhang W, Czarnecki O, Henning M, Fastner J, Erhard M, Neilan BA, Kaebernick M (2003) Isolation, characterization, and quantitative analysis of microviridin J, a new Microcystis metabolite toxic to Daphnia. J Chem Ecol 29:1757–1770
Senthilkumaran B, Sudhakumari CC, Chang XT, Kobayashi T, Oba Y, Guan G, Yoshiura Y, Yoshikuni M, Nagahama Y (2002) Ovarian carbonyl reductase-like 20beta-hydroxysteroid dehydrogenase shows distinct surge in messenger RNA expression during natural and gonadotropin-induced meiotic maturation in Nile tilapia. Biol Reprod 67:1080–1086
Senthilkumaran B, Sudhakumari CC, Wang DS, Sreenivasulu G, Kobayashi T, Kobayashi HK, Yoshikuni M, Nagahama Y (2009) Novel 3beta-hydroxysteroid dehydrogenases from gonads of the Nile tilapia: phylogenetic significance and expression during reproductive cycle. Mol Cell Endocrinol 299:146–152
Smith JL, Boyer GL, Zimba PV (2008) A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 280:5–20
Sychrová E, Štěpánková T, Nováková K, Bláha L, Giesy JP, Hilscherová K (2012) Estrogenic activity in extracts and exudates of cyanobacteria and green algae. Environ Int 39:134–140
Yamaki H, Sitachitta N, Sano T, Kaya K (2005) Two new chymotrypsin inhibitors isolated from the cyanobacterium Microcystis aeruginosa NIES-88. J Nat Prod 68:14–18
Zhao M, Xie S, Zhu X, Yang Y, Gan N, Song L (2006) Effect of dietary cyanobacteria on growth and accumulation of microcystins in Nile tilapia (Oreochromis niloticus). Aquaculture 261:960–966
Zhou LY, Wang DS, Senthilkumaran B, Yoshikuni M, Shibata Y, Kobayashi T, Sudhakumari CC, Nagahama Y (2005) Cloning, expression and characterization of three types of 17beta-hydroxysteroid dehydrogenases from the Nile tilapia, Oreochromis niloticus. J Mol Endocrinol 35:103–116
Ziková A, Trubiroha A, Wiegand C, Wuertz S, Rennert B, Pflugmacher S, Kopp R, Mareš J, Kloas W (2010) Impact of microcystin containing diets on physiological performance of Nile tilapia (Oreochromis niloticus) concerning stress and growth. Environ Toxicol Chem 29:561–568
Acknowledgements
This work was supported financially by the National Major Project on Quality & Safety Risk Assessment for Agricultural Products (Grants GJFP2014009), Natural Science Foundation of Jiangsu Province of China (Grants BK20130488).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiazhang Chen and Shunlong Meng have contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Meng, S., Xu, H. et al. Effects of Microcystis on Hypothalamic-Pituitary-Gonadal-Liver Axis in Nile Tilapia (Oreochromis niloticus). Bull Environ Contam Toxicol 98, 562–566 (2017). https://doi.org/10.1007/s00128-017-2051-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2051-4