Abstract
Limited evidence exists on the latent effects of toxicant exposure on the seawater adaptability of anadromous salmon and steelhead. It is unclear whether such an effect exists for the widely used and relatively non-toxic herbicide endothall. Coho salmon, Oncorhynchus kisutch (coho), Chinook salmon, O. tshawytscha (Chinook), and anadromous rainbow trout, O. mykiss (steelhead) were subjected to a 10-day seawater challenge following freshwater treatments [0–12 mg acid equivalent (a.e)./L at 96 h]. Mean survival resulted in 82 % (n = 225), 84 % (n = 133), 90 % (n = 73) and 59 % (n = 147) survival for 0, 3–5, 6–8, and 9–12 mg a.e./L, respectively. Our results indicate a lower toxicity threshold compared with previously reported acute toxicity results, but higher compared with previous seawater challenge studies. We demonstrate the utility of the seawater challenge assay to accurately define toxic effects of pesticides on salmonids with complex life-histories.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Reasonable concern exists regarding potential deleterious effects of herbicides on non-target species, particularly species listed as threatened or endangered. Herbicide applications in the Pacific Northwest often occur within anadromous salmonid-supporting watersheds where currently, 28 of 52 distinct populations of anadromous Pacific salmonids are listed as threatened or endangered under the U.S. Endangered Species Act (ESA) (Gustafson et al. 2007). For this reason, chemical registration and permitting for the chemical application in salmonid-supporting watersheds is complicated, as there is an apparent need for baseline toxicity data in order to accurately assess risk to culturally and economically important anadromous fish (NRC 2013). Currently, standardized tests do not require the testing of ESA-listed salmonid species, or assay for latent or sublethal endpoints. Therefore, it is plausible that available data underestimates a toxicant’s effect to salmonids.
The dipotassium endothall (Fig. 1) formulation is a fast-acting contact herbicide used to control aquatic and terrestrial plant species. Due to its high efficacy at low concentrations (Westerdahl and Getsinger 1988), and its relatively low acute and chronic toxic effects on fish compared with concentrations needed for aquatic weed control, endothall has been a commonly used herbicide for over 50 years. For salmonids, the LC50 is significantly greater than the maximum application rate of 5 mg acid equivalent (a.e)./L; >100 mg a.e./L for coho (Johnson and Finley 1980; Mayer and Ellersieck 1986) and steelhead (Johnson and Finley 1980), and 23 mg a.e./L for Chinook [as reported by WDOE (2000)]. The no observable effect concentration (NOEC) for rainbow trout was found to be approximately 41 mg a.e./L (Bettencourt 1993), approximately ten times more than the application limit. These data have historically suggested that endothall is relatively safe within salmon-bearing watersheds.
Given that anadromous salmonids rely on fresh and saltwater habitats, and successful transition is critical to their fitness, latent toxic effects by herbicides and other toxicants should be evaluated. Early marine survival rate is low due to predation, starvation, and disease (Mathews and Buckley 1976; Parker 1971; Walters et al. 1978), and it is dependent on the successful physiological adaptation to maintain homeostasis during the transition from a hyper- to hypo-osmoregulatory strategy (Quinn 2005). Lack of physiological readiness, or stress – such as toxicant exposure – can reduce survival during seawater transition (Bjornsson et al. 2011).
Few studies have attempted to capture the potential lethality of endothall during seawater transition by employing a seawater challenge assay. It has been shown that latent lethality occurs in salmon smolts following exposure to low endothall concentrations (Bouck and Johnson 1979; Liguori et al. 1983), but confounding results (Serdar and Johnson 1996) has created uncertainties in understanding endothall’s toxicological profile. To resolve these data uncertainties, we designed a seawater challenge assay ideal for Pacific salmon and steelhead. The aim of this study was twofold: (1) to determine if latent lethality during seawater transition occurs following endothall exposure in three unique ESA-listed salmonid species; and, (2) to refine the seawater challenge assay to generate a protocol that can accurately generate toxicity information for anadromous salmonids.
Materials and Methods
Steelhead and coho and fall Chinook smolts (11–15 cm in length) were obtained from Washington Department of Fish and Wildlife (WDFW) lower Columbia River Basin hatchery programs, and transported to the Fisheries Technology laboratory facility at Mt. Hood Community College in Gresham, Oregon. We acclimated fish with optimal health in flow-through 350-gallon holding tanks [coho, steelhead: 8 gallons per minute (gpm); Chinook: 15 gpm] for 2 weeks. Well water was UV-treated and passed through ammonia-fixing bacteria (Aquabac-T, Argent Labs, Redmond, WA) and polypropylene biomedia to minimize ammonia and nitrite accumulation. A natural light photoperiod (16 h light, 8 h dark) and a 14–15°C temperature were maintained. Oregon Moist Pellets (Moore Clark Co., LaConner, WA) were administered once daily (coho, steelhead: 0.7 % body weight/day; Chinook: 2.4 % body weight/day), and feeding ended 24 h prior to transfer to exposure aquaria. Aquaria and tanks were sanitized (Wescodyne; West Chemical Co, NY) and thoroughly rinsed between experiments. Fish were monitored daily for mortalities and behavioral and anatomical abnormalities. Prior to the experiment, we confirmed that the well water supply did not exhibit the presence of heavy metals and total suspended solids. Specifically, no detection [below method reporting level (MRL)] was reported for mercury (EPA method 245.1; MRL = 0.0001 mg/L), cadmium (MRL = 0.002 mg/L), copper (MRL = 0.01 mg/L), molybdenum (MRL = 0.01 mg/L), selenium (MRL = 0.05 mg/L), silver (MRL = 0.005 mg/L) and zinc (MRL = 0.05 mg/L) (EPA method 200.8), and no detection for total suspended solids (Standard Method 2540D; MRL = 2 mg/L) (Pyxis Labs, Portland OR). We also confirmed that plumbing did not leach detectable levels of vinyl chloride, tetrahydrofuran, methyl ethyl ketone, and cyclohexanone into the rearing and testing systems (no detection, where MRLs = 0.005 mg/L; EPA method 8260C) (Anatek Labs, Moscow ID).
Optimum water quality parameters were maintained daily; total dissolved gas (approximately 100 %), dissolved oxygen (>8 mg/L), pH (6.7–8.5), temperature (14–16°C), total ammonia (NH4 +, <0.5 mg/L), un-ionized ammonia (NH3, ≤0.03 mg/L), nitrate (NO3N, ≤0.55 mg/L), water flow (13.25 L/min; acute/96 h exposure in freshwater), and salinity (30 parts per thousand or 30 g/L; seawater challenge). Chemical test reagents used were compatible for both fresh and saltwater use; pH (PrimaLine, ELOS, Verona, Italy), ammonia (API, Chalfont PA), and nitrogen (API, Chalfont PA; Hach, Loveland CO). Within the seawater challenge system, ammonia and nitrogen levels were controlled with Proline ® ammonia remover (Aquatic Ecosystems, Inc, Apopka, FL) and carbon filtration.
The Cascade ® formulation (United Phosphorus, Inc) of endothall was used throughout the study. It contains 40.3 % (w/w) of the active ingredient (a.i.; Fig. 1a) dipotassium salt of endothall, and 28.6 % (w/w) or 36 % (w/v) dicarboxylic (acid equivalent; a.e.). Treatment concentrations were calculated from the molecular weight of the dicarboxylic acid, the parent acid responsible for endothall’s herbicidal activity (Fig. 1b). Endothall was diluted with carbon-filtered well water in a 2 L Mariotte bottle positioned above the test aquaria, providing adequate head pressure to the chemical dispensing pumps (Fluid Metering, Inc., Syosset, NY). The pumps allowed for controlled release of endothall into downstream mixing chambers before dispensing into test aquaria at 1 mL/13.25 L/min. Time-lapse photography and application of 1 mg/L sodium fluorescein (Sigma, St. Louis MO) ensured adequate mixing and consistent laminar flow throughout the aquaria. Fish were not placed in tanks with sodium fluorescein, and tanks were flushed at 13.25 L/min for 48 h prior to placement. The chemical delivery system was recalibrated prior to each experiment to ensure appropriate concentrations of endothall were dispensed.
Fish were transferred from holding tanks to test aquaria and allowed to acclimate for 24 h, during which the temperature increased 2°C per hour from 14°C until 20°C. The three aquaria were partitioned into three compartments by 1 cm3 screens to divide fish by species. In total, the experimental control aquarium (Tank 1) and both of the treatment aquaria (Tanks 2, 3) contained 30 smolts of each species (see Table 1). Fish were exposed to endothall in the aquaria with a flow-through system (13.25 L/min), where flow was monitored by in-line digital rotameters (Aquatic Ecosystems, Inc, Apopka FL). Temperature was maintained at 20°C (±0.5°C) using an in-line circulation heating system (Condex Wattco Inc., Lachine, Quebec), and continually monitored with HOBO electronic data logging system and software (Onset, Pocasset, MA). In order to achieve relevancy to extreme temperatures during field application and because higher temperatures exacerbate herbicide toxicity (e.g. Cairns et al. 1975; Folmar et al. 1979), 20°C was maintained during acute exposure to endothall. Water samples were collected and stabilized with HCl (pH 2.0) at 24 and 72 h to monitor actual endothall concentrations (U.S. EPA Method 548.1; Anatek Labs). Actual concentrations (Table 1) are the concentration means at both sample collection times.
Chemical delivery concluded at 96 h and water temperature incrementally decreased 2°C per hour and maintained at 14–15°C for 24 h. Fish were transferred and separated by species to 40-gallon circular tanks with a recirculating flow-through system (10 gpm) supplied with 14–15°C well water. Water temperature was maintained with a Teco SeaChill chiller (Aquatic Ecosystems, Inc.). Following 24 h acclimation, water salinity was brought to 8 g/L, increased to 20 g/L at 8 h and 30 g/L at 24 h using H2Ocean Magnesium Pro Plus (D–D The Aquarium Solution, Ltd., Scottsdale, AZ). The 10-day seawater challenge commenced following a sustained water salinity of 30 g/L for 24 h, as previously described (Clarke and Blackburn 1977; Serdar and Johnson 1996). The methodology for seawater introduction for steelhead was amended due to lower survival (but not significant) in the control fish (70 %), compared with Chinook (82 %) and coho (82 %). We suspected this difference was due to physiological differences in steelhead smoltification compared with the other species. Therefore, for steelhead, salinity increased to 6 g/L at 17 h, 12 g/L at 32 h, 18 g/L at 2.5 days, held at 24 g/L for days 4–6, 28 g/L at 7 days, and 30 g/L for days 8–10.
Surviving fish from the seawater challenge were given a lethal dose of tricaine methanesulfonate or euthanized by stunning (blood sample collection). Blood was collected from the caudal artery (Chinook) or by cardiac puncture (steelhead, coho) using lithium-heparin coated needles and collection tubes (MP Biomedicals, Solon, OH). Blood was pooled from 2–3 fish (steelhead), 4–5 fish (coho), and 7–10 fish (fall Chinook) per treatment, in duplicate or triplicate and immediately placed on ice. Plasma sodium levels were determined by indirect potentiometry (SYNCHRON®, Beckman Coulter, Brea, CA).
A Linear Mixed Effects Model (LMM) was used to compare the mean plasma sodium level for “low” (3–8 mg a.e./L) and “high” (9–12 mg a.e./L) treatments relative to control for each species. Potential variation in the tanks from the two different experiments were modeled as crossed random effects and an additional random effect term for blood pooling size was included to account for potential increased variation introduced by pooling blood from more fish to generate a sample. Species and treatment group were included as multiplicative fixed effects so that statistical comparisons between treatments and within species could be made. LMM was fit using the R Statistical Platform (RDC Team 2011) with lme4 package (Bates et al. 2011).
Generalized Linear Mixed Models (GLMM) (Bates et al. 2011) were fit to the data to evaluate how different levels of endothall affected overall survival of each species. Potential variation in tanks from different experiments in the study was modeled as nested random effects. Potential variables include subtle variation in chemical exposure levels and flow rates, and changes in fish size between experiments. Incorporating random effects allowed us to account for such small differences. Species and endothall exposure are included as additive fixed effects. Endothall exposure was treated as a categorical variable defined by four groups: control (0 mg a.e./L), 3–5, 6–8 and 9–12 mg a.e./L. This approach facilitated testing whether there were significantly different rates of survival between different levels of endothall exposure. The four groups chosen for this study ensured that each species was represented within each category of endothall exposure level.
Results and Discussion
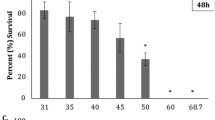
Consistent with previous reports, we found no acute lethality by endothall on coho, Chinook and steelhead following freshwater exposure at all treatment concentrations. However, we observed ≥70 % lethality at 12 mg a.e/L (coho, Chinook), and ≥50 % lethality at 9 mg a.e./L (steelhead) following the 10-day seawater challenge (Fig. 2) and decreased survival probability for steelhead at the highest endothall concentrations of 9–12 mg a.e./L (Fig. 3). Across all treatment concentrations, mean survival for steelhead decreased significantly compared with coho and Chinook (p < 0.01; Table 2). In addition, we found that across all species, mean survival significantly decreased at 9–12 mg a.e./L treatments compared with lower concentrations (p < 0.01; Table 2). This significantly lower toxicity threshold conflicts with accepted extrapolations of lethality (LC50 = 100–320 mg a.e./L) (Johnson and Finley 1980; Mayer and Ellersieck 1986).
Predicted survival probabilities for each species – Chinook (circles), coho (triangles), and steelhead (squares) – across each treatment category following a 10-day seawater challenge. The 95 % confidence intervals (bars) of survival probabilities are derived from the main (additive species and treatment effects) and random effects portions of the GLMM model
The seawater challenge is an underutilized assay for determining toxic thresholds of anadromous fish. As a result, many experimental design parameters for this assay are not standardized and results are difficult to compare. One major parameter discrepancy is assay duration; for example, 24 h (Serdar and Johnson 1996) to 48 h (Liguori et al. 1983) to 10 days (Bouck and Johnson 1979). In a 24 h study, no effects were observed at ≤10 mg a.e./L for coho (Serdar and Johnson 1996). It is plausible that the seawater challenge assay duration did provide adequate time for effects to manifest. This assertion is supported by our observations that lethality occurred on day three post-saltwater entry (all species), and survival gradually declined over time, and plateaued by day six. Our findings demonstrate that seawater challenge durations shorter than 6–10 days may not adequately capture latent lethality. Any lethal effect by endothall could be expected to occur within a 10 days assay duration. Such lethal effects could be caused by: (1) physical damage, such as altered gill epithelia structure; or, (2) altered physiological processes, including endocrine and metabolic disruption – any or all of which may take more than 24 h to cause a lethal effect.
Other study design parameter differences likely explain the disparities in previously reported findings and from what we report here. Physiological immaturity for smoltification may explain the 100 % mortality observed at 48 h post-seawater introduction for Chinook Salmon following ≤5 mg a.e./L endothall (Liguori et al. 1983). Smoltification timing varies between species (Quinn 2005); however, the 4-g Chinook Salmon used in the Liguori et al. study (1983) were fry and physiologically immature for smoltification. Fish size (i.e. weight, length) can identify physiological maturity for seawater tolerance (Berggren and Filardo 1993; Giorgi et al. 1997); therefore, to ensure age maturity for this experiment, we used adequately sized fish. For example, in our study Chinook Salmon smolts averaged 13 g and at least 11 cm in length (McCormick and Saunders 1987). A static exposure system is another explanation for the high mortality observed by Liguori et al., where they noted approximately 0.07 mg/L unionized ammonia [NH3]. Such NH3 concentrations are undesirable husbandry conditions for salmonids (Bond et al. 1960) and could cause or potentiate lethality. To negate possible effects by poor water quality, we designed a flow-through recirculating system complete with carbon filtration and administered ammonia-blocking reagents. Furthermore, we monitored and maintained water temperature, photoperiod, and water flow since smoltification can be negatively impacted by suboptimal environmental conditions, as previously reviewed (Bjornsson et al. 2011).
Steelhead were most sensitive and experienced higher mortality, compared with coho and Chinook salmon. Modeled predictions of survival for each species revealed significantly lower survival for steelhead compared with coho and Chinook salmon for all endothall treatment levels, and significantly lower survival of all species exposed to concentrations between 9 and 12 mg a.e./L (9 mg a.e./L for steelhead and 12 mg a.e./L for coho and Chinook salmon; Fig. 3). It is plausible that the diversity of steelhead life history pathways explain our observations. Steelhead can adopt both freshwater resident (rainbow trout) and anadromous (steelhead) life histories, and although our study fish were hatchery-origin steelhead with anadromous parents, both resident and anadromous O. mykiss are known to produce substantial numbers of offspring of the alternate life-history type (Courter et al. 2013). Therefore, it is possible that a portion of fish in our study may have chosen a freshwater life history and these fish would not have been physiologically prepared for saltwater. It is also reasonable to expect that a steelhead’s life-history plasticity increases vulnerability to toxicants by reduced adaptability to hypo-osmotic conditions, compared with salmon (Morgan and Iwama 1991).
The demand for physiological adaptation to successfully enter full-strength seawater requires precise changes to kidney and gill Na+, K+-ATPase enzyme activity levels and changes to endocrine and metabolic systems (Bjornsson et al. 2011; Quinn 2005). Plasma sodium measurements provide a basic index of osmoregulatory function; therefore, we collected blood of surviving fish at the conclusion of the seawater challenge assay. We found no significant difference in plasma sodium levels for coho and Chinook salmon in “low” and “high” treatment groups, relative to control (Table 3). The higher plasma sodium levels in steelhead [196 and 184 mmol/L for control and treatment (p = 0.02), respectively; Table 3] could be attributed to methodology differences employed (see “Materials and Methods” section) or conflicting physiological differences between species (Morgan and Iwama 1991). Higher plasma sodium levels in surviving steelhead may also suggest the need for longer seawater challenge duration to fully capture potential lethal effects in steelhead.
We present evidence that the seawater challenge assay provides a more accurate determination of a toxicant’s lethal thresholds for anadromous salmonids. This assay determines whether a toxicant disturbs the physiological ability to maintain internal equilibria of osmotic and ionic exchange necessary for seawater transition. We carefully considered the physiological differences between anadromous salmonid smolts and other freshwater fish species and incorporated suitable parameters to ensure that lethality was solely a response to toxicant exposure. Our design was attentive to: (1) optimal water quality; (2) physiological maturity; (3) sufficient sample sizes and replicates; and, (4) a seawater challenge with a duration adequate to capture delayed lethality following seawater entry. A seawater challenge can deliver relevant and quantifiable data on a sensitive toxicological endpoint needed to make accurate ecological risk assessments for anadromous salmonids. These data would reduce the need to extrapolate toxicity levels exclusively from acute 96-h exposures, and/or from non-relevant or surrogate species. Our results affirm the assertion that traditional lethality-based metric standards may underestimate toxicity for anadromous salmonids and there is a need to consider their unique life-cycle when designing toxicity investigations (NRC 2013) for chemicals applied within, or adjacent to, anadromous salmon-bearing watersheds.
References
Bates D, Maechler M, Bolker B (2011) lme4: linear mixed-effects models using S4 classes, version 0.999375-40. R Foundation for Statistical Computing, Vienna
Berggren TJ, Filardo MJ (1993) An analysis of variables influencing the migration of juvenile salmonids in the Columbia River basin. N Am J Fish Manag 13(1):48–63
Bettencourt M (1993) Aquathol K (dipotassium salt of endothall)—acute toxicity to rainbow trout (Oncorhynchus mykiss) under flow-through conditions; Project Number: 92-3-4163: 12442.0591.6132.10; MRID 42695402. Final Technical Report. Springborn Labs, Inc
Bjornsson BT, Stefansson SO, McCormick SD (2011) Environmental endocrinology of salmon smoltification. Gen Comp Endocrinol 170(2):290–298
Bond EE, Lewis RH, Fryer JL (1960) Toxicity of various herbicides and herbicidal materials to fish. W-60-3 Final Technical Report. Robert A. Taft Sanitary Engineering Center, Cincinatti
Bouck GR, Johnson DA (1979) Medication inhibits tolerance to seawater in coho salmon smolts. Trans Am Fish Soc 108:63–66
Cairns J Jr, Heath AG, Parker BC (1975) Temperature influence on chemical toxicity to aquatic organisms. J Water Pollut Control Fed 47(2):267–280
Clarke WC, Blackburn J (1977) A seawater challenge test to measure smolting of juvenile salmon. Research and Development Directorate, Pacific Biological Station, B.C., Canada
Courter II, Child DB, Hobbs JA, Garrison TM, Glessner JJ, Duery S, Fraser D (2013) Resident rainbow trout produce anadromous offspring in a large interior watershed. Can J Fish Aquat Sci 70(5):701–710
Folmar LC, Sanders HO, Julin AM (1979) Toxicity of the herbicide glyphosphate and several of its formulations to fish and aquatic invertebrates. Arch Environ Contam Toxicol 8(3):269–278
Giorgi A, Hillman T, Stevenson J, Hays S, Peven C (1997) Factors that influence the downstream migration rates of juvenile salmon and steelhead through the hydroelectric system in the mid-Columbia River basin. N Am J Fish Manag 17(2):268–282
Gustafson RG, Waples RS, Myers JM, Weitkamp LA, Bryant GJ, Johnson OW, Hard JJ (2007) Pacific salmon extinctions: quantifying lost and remaining diversity. Conserv Biol 21(4):1009–1020
Johnson JW, Finley MT (1980) Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. U.S. Department of the Interior, Fish and Wildlife Service, Washington
Liguori VM, Zakour HR, Landolt ML, Felton SP (1983) Toxicity of the herbicide endothall to juvenile chinook salmon (Oncorhynchus tshawytscha). In: Bishop WE, Cardwell RD, Heidolph BB (eds) Aquatic toxicology and hazard assessment: sixth symposium. Special technical publication 802. American Society for Testing and Materials, Philadelphia, pp 530–544
Mathews SB, Buckley R (1976) Marine mortality of Puget Sound coho salmon (Oncorhynchus kisutch). J Fish Res Board Can 33:1677–1684
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. U.S. Department of the Interior, U.S. Fish and Wildlife Service, Washington D.C.
McCormick SD, Saunders RL (1987) Preparatory physiological adaptations for marine life of salmonids: osmoregulation, growth, and metabolism. Proc Am Fish Soc Symp 1:211–244
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall Chinook Salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 48(11):2083–2094
NRC (2013) assessing risks to endangered and threatened species from pesticides. National Academy Press, Washington
Parker RR (1971) Size selective predation among juvenile salmonid fishes in a British Columbia inlet. J Fish Res Board Can 28:1503–1510
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout. University of Washington Press, Seattle
RDC Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Serdar DM, Johnson AF (1996) Seawater challenge of coho salmon smolts following exposure to the herbicide endothall. Prog Fish Cult 58:131–134
Walters CJ, Hilborn R, Peterman RM, Staley MJ (1978) Model for examining early ocean limitation of Pacific salmon production. J Fish Res Board Can 35:1303–1315
WDOE (2000) Herbicide risk assessment for the aquatic plant management final supplemental environmental impact statement; endothall, section 4. Final Report. Washington Department of Ecology, Olympia
Westerdahl HE, Getsinger KD (1988) Aquatic plant identification and herbicide use guide. Final report. U.S. Army Corps of Engineers, Vicksburg
Acknowledgments
The Washington Water Resources Association funded this research, and United Phosphorus, Inc. provided publication funding. We thank the Washington Department of Ecology and WDFW for contributions toward the experimental design. D. Child, S. Duery, D. Cramer, E. Buckner, T. Hanna, and M. Chaney assisted with fish delivery, husbandry, and daily data collection. Our technical reviewers included A. Kolosseus, P. Harvester, and R. Marshall.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Courter, L.A., Garrison, T.M. & Courter, I.I. Latent Toxicity of Endothall to Anadromous Salmonids During Seawater Challenge. Bull Environ Contam Toxicol 96, 573–579 (2016). https://doi.org/10.1007/s00128-016-1781-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1781-z