Abstract
In this study, bioleaching was coupled with electrokinetics (BE) to remove heavy metals (Cu, Zn, Cr and Pb) from contaminated soil. For comparison, bioleaching (BL), electrokinetics (EK), and the chemical extraction method were also applied alone to remove the metals. The results showed that the BE method removed more heavy metals from the contaminated soil than the BL method or the EK method alone. The BE method was able to achieve metal solubilization rates of more than 70 % for Cu, Zn and Cr and of more than 40 % for Pb. Within the range of low current densities (<1 mA cm−2), higher current density led to more metal removal. However, the metal solubilization rates did not increase with increasing current density when the current density was higher than 1 mA cm−2. Therefore, it is suggested that bioleaching coupled with electrokinetics can effectively remediate heavy metal-contaminated soils and that preliminary tests should be conducted before field operation to detect the lowest current density for the greatest metal removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anthropogenic activities in agriculture and industry have caused serious environmental problems, including soil contamination by toxic heavy metals such as Cu, Zn, Cr, and Pb (Lee et al. 2011). Unlike most organic pollutants, heavy metals persist in the environment for very long periods as they are generally non-biodegradable and are mainly distributed in the soil as either exchangeable, carbonate-bound, Fe/Mn oxide-bound, organic matter/sulfide-bound or residual (Tessier et al. 1979) components. The exchangeable, carbonate-bound, and Fe/Mn oxide-bound components are more mobile and bioavailable and consequently more dangerous, whereas the organic matter/sulfide-bound and residual fractions are more stable and less bioavailable (Liu et al. 2008). Heavy metals accumulate in the pedosphere (Han et al. 2001), are transported to the groundwater, and enter the food chain, which poses a threat to plants, animals, and human beings (Maksymiec 2007; Nahmani et al. 2005). Therefore, effective remediation of heavy metal-contaminated soils is of great importance for ecosystems and human health.
Many in situ and ex situ remediation technologies have been developed for remediating heavy metal-contaminated soils. Generally, these technologies can be classified into two categories: the physio-chemical and biological treatments (Mulligan et al. 2001). Physio-chemical technologies include solidification/immobilization, extraction, and electrokinetics, whereas biological methods involve phytoremediation, microbial precipitation, and bioleaching. Traditional physio-chemical methods usually have high operational cost and require high energy with correspondingly low efficiency (Martin and Ruby 2004). Biological approaches are considered cost-effective, efficient and environmentally friendly (Seidel et al. 2004). As one of the possible bioremediation techniques, phytoremediation has gained tremendous attention. However, its disadvantages are that it is time-consuming and requires the disposal of the harvested plants (Cunningham et al. 1995). Bioleaching based on the ability of microorganisms to transform solid compounds into soluble and extractable elements that can be recovered has been rapidly developed in recent decades (Olson et al. 2003). During bioleaching, iron-oxidizing bacteria (Wong et al. 2004) or sulfur-oxidizing bacteria (Villar and Garcia 2006) oxidize the reduced sulfur or ferrous ions and create acidic conditions favorable to metal removal. Bioleaching technology has the advantages of a mild reaction condition without the need for additives (e.g., acids, chelators) that are needed in physio-chemical processes, low energy consumption, simplicity compared with excavation and soil washing, and low environmental damage. Yet, because of the time it takes for the bacteria to be stimulated, the method requires a long operational period, which has prevented its practical application on a large scale (Nareshkumar et al. 2008).
Electrokinetic remediation technology can simultaneously recover multiple metal pollutants (Wang et al. 2005). A combination of electrokinetics and bioremediation has been reported to enhance the biodegradation of organic pollutants (Wick et al. 2007), but few attempts have been made to apply the integrated method to remove heavy metals. This is likely because of the non-degradable nature of heavy metals. Maini et al. (2000) demonstrated that bioleaching can be combined with electrokinetics to enhance Cu removal from contaminated soil by amending sulfur.
In this study, bioleaching was coupled with electrokinetics to remove Cu, Zn, Cr, and Pb from the polluted soils. In addition, FeSO4 was amended to the soil to activate the indigenous iron oxidizing bacteria.
Materials and Methods
Soil used in this study was taken from a demolished electroplating plant site located in Jingzhou City, China. After stones and plastic debris were removed from the soil surface, a soil composite sample from the top 20 cm layer was taken using a plastic scoop, immediately transported to the laboratory in airtight polythene bags using a cooler at 4°C, and stored at 4°C until use. The soil had an organic matter content of 8.3 % and a cation exchange capacity (CEC) of 19.5 cmol kg−1. The soil consisted of 12.3 % clay, 42.6 % silt, and 45.1 % sand. The soil pH was determined with a water/dry soil ratio of 2.5:1 using a pH meter. To determine the total heavy metal contents, the soil sample was digested using the method of Ure (1995) and the metal concentrations were measured using an atomic absorption spectrophotometer (Vario-6, Analytik Jena, Germany). Fractionation of the heavy metals present in the soil was carried out by selective sequential extraction following the methods of Tessier et al. (1979).
The concentrations of Cu, Zn, and Cr exceed the Environmental Quality Standards for Soils (GB15618-1995) in China, whereas the total content of Pb was close to the standard (Table 1). Only a minor fraction of the four metals was exchangeable (Fig. 1). The predominant fraction of Cu, Zn and Cr was organic matter/sulfide-bound, followed by the component in the residual form. For Pb, nearly 50 % was in the residual form; the second largest fraction was the Fe/Mn oxide-bound metals.
All chemicals used were of analytical grade and were used as received. Deionized water was used for all analyses.
The collected fresh soil sample was incubated for iron-oxidizing bacteria enrichment. A 10 g soil sample was mixed with 250 mL deionized water in a 500-mL Erlenmeyer flask, and FeSO4·7H2O was added at a final concentration of 20 g L−1 as an energy source for the iron-oxidizing bacteria. The Erlenmeyer flask was agitated on a gyratory shaker (HZQ-X300C, China) at 200 rpm and 30°C until the pH dropped to 2.0. Then, 25 mL of the soil suspension was taken and transferred to a 500-mL Erlenmeyer flask containing 250 mL of deionized water and 20 g L−1 of FeSO4·7H2O. The above enrichment procedure was repeated three times, and the soil slurry obtained was used as an inoculum.
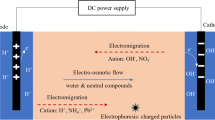
A self-designed experimental device, as shown in Fig. 2, was used in the bioleaching experiment. The main body of the device is an open PVC cell of 26 cm × 10 cm × 15 cm (length × width × height). Two clapboards of 10 cm × 15 cm (length × height) with fiberglass filter papers (0.45 μm) on both sides were used to separate the cell into three subcells with sizes of 3 cm × 10 cm × 15 cm (length × width × height), 20 cm × 10 cm × 15 cm (length × width × height), and 3 cm × 10 cm × 15 cm (length × width × height). Two graphite electrodes with a diameter of 1 cm and a length of 15 cm were inserted into the two smaller subcells, and the electrodes were connected with a direct current power supply (WYK301B4, EKSI). An aerator pipe with a diameter of 10 mm and a length of 16 cm was placed so that it was approximately 1 cm away from the bottom of the middle soil chamber. The PVC cell was placed in a constant temperature water bath (GDH-0530, China) at 30°C for the entire experiment.
Many studies have noted the importance of the solid content in bioleaching efficiency. For example, high solid content may result in a decrease in the metal solubilization (Kim et al. 2005) as collision and friction between solid particles could cause a decrease in the oxidation activity of bacterial cells (Liu et al. 2007). In this study, a solid content of 4 % was employed when preparing the soil slurry. Considering that the fluid shear rate had little adverse effect on the oxidation activity of the bacterial cells in the bioleaching process (Liu et al. 2007), an agitation speed of 200 rpm was used.
To evaluate the advantages of a combination of bioleaching and electrokinetics over chemical extraction, bioleaching or electrokinetics alone for the removal of heavy metals from contaminated soils, four treatments were set up: bioleaching combined with electrokinetics (BE), electrokinetics (EK), bioleaching (BL), and chemical extraction (CE). For the BE treatment, the soil suspension was prepared by mixing a 40 g soil sample with 1000 mL of deionized water and FeSO4·7H2O was added at a final concentration of 10 g L−1. Inoculum was added at 10 % (v/v). The mixture was added to the middle soil chamber of the PVC cell. The aerator pipe was pumped with air to ensure an effective supply of oxygen and to ensure homogeneity of the soil suspension. Using peristaltic pumps, tap water was pumped into the anode and cathode compartments to be used as electrolyte solutions. The EK treatment was identical to the BE treatment except that no FeSO4·7H2O was added and the soil suspension was not inoculated. Samples were taken every day for 6 days for the BE and EK treatments. For the BL treatment, only 220 mL of soil slurry of the same solid content (4 %) was used and FeSO4·7H2O was added at a final concentration of 10 g L−1. The slurry was incubated at 30°C and 200 rpm for 18 days. Samples were taken every day for the first 6 days and every other day for another 12 days. For the CE treatment, 200 mL of soil slurry of the same solid content (4 %) was prepared, acidified to a pH of 2.0 using 0.1 mol L−1 H2SO4 and incubated at 30°C and 200 rpm for 24 h. For the BE treatment, the effects of current density on the heavy metal solubilization rate were also evaluated by applying direct current densities of 0, 0.25, 0.5, 0.75, 1, 1.25 and 1.5 mA cm−2.
All the experiments were conducted in triplicate, and the results were reported as the mean ± SD (standard deviation).
Results and Discussion
The pH is the most important operating parameter influencing the metal solubilization during bioleaching (Du et al. 1995). The soil had an initial pH of 6.8. As shown in Fig. 3, in all three treatments (BL, EK and BE), the pH dropped rapidly with time and the pH in the BE treatment decreased most rapidly, followed by those in the BL and EK treatments. At day 6, the pH in the BE treatment dropped to 2 and the pH in the EK treatment was just slightly lower than 4. In addition, it took as long as 18 days for the pH to drop to 2 in the BL treatment. A rapid decrease in the pH led to a rapid dissolution of the metals, which is favorable for efficient metal removal and has been commonly documented in the literature (Zagury et al. 1994). The acidification can be attributed to the precipitation of ferric ions in the form of jarosite because the net reactions resulting in jarosite formation are acid yielding (Blais et al. 1993). The protons released into the liquid phase can replace heavy metals adsorbed on the soil particles (Chen and Lin 2001) and can also help dissolve the carbonate-bound fraction. The reason for the rapid pH drop in the BE treatment was that the growth and metabolic activities of iron oxidizing bacteria created a favorable acidic condition for electrokinetics, which in turn stimulated the growth of the bacteria. Consequently, the pH continuously decreased as a result of this feedback.
After the soil was treated via bioleaching, electrokinetics or a combination of the two, the four metals began to dissolve into the soil solution due to the decreasing pH in the systems (Fig. 4). Metal solubilization rates increased with time in all the treatments. It is expected that the soluble fraction mainly came from the exchangeable and carbonate-bound portions of the heavy metals. Previous studies have shown that copper bound to the exchangeable and carbonate states were completely solubilized by bioleaching (Naresh Kumar and Nagendran 2009). The Fe/Mn oxide-bound fraction might be partly solubilized, whereas solubilization of the residual fraction might be minimum. Among the three treatments, BE displayed the highest efficiency in metal solubilization, achieving the highest metal solubilization rates within the same time period. For 6 days, the solubilization rates of Cu, Zn and Cr in BE treatments achieved approximately 70 %, followed by 60 % in BL and 40 % in EK. In other words, the coupling of electrokinetics with bioleaching can effectively lower the metal contents in the soil to environmentally safe levels, improve remediation efficiency and shorten the operation time of bioleaching. All three remediation methods appeared to be more effective on Cu, Zn and Cr solubilization than Pb solubilization. Low Pb solubilization had also been observed by other scientists (Nareshkumar et al. 2008), which may be related to the high proportion of the residual fraction of Pb. Another causal factor may be the presence of high amounts of sulfate (as FeSO4 was amended), which led to the formation of lead sulfate, a compound with very low solubility (K sp = 1.62 × 10−8) (Mercier et al. 1996).
As observed in Fig. 5, the current density influenced the metal solubilization when the heavy metal-contaminated soil was treated with a combination of bioleaching and electrokinetics. Generally, when a higher current density was applied, a higher metal solubilization rate was achieved. This was more prominent when the current density was <1 mA cm−2. When the current density was higher than 1 mA cm−2, a further increase in the current density did not produce a better metal solubilization rate than the value at 1 mA cm−2. That is to say, a higher current density does not necessarily produce greater metal solubilization. In real operations, preliminary studies should be performed to determine the optimum current density value at which the highest metal removal rate can be achieved with minimal energy input.
It is clearly shown in Fig. 6 that of the five remediation methods, BE achieved the highest metal solubilization rates. The five methods can be ordered by decreasing solubilization rates as BE, 18-day BL, 6-day BL, CE, and EK for Cu, Zn and Cr, or as BE, 18-day BL, EK, 6-day BL, and CE for Pb. In other words, the combination of bioleaching and electrokinetics can remove more metal than bioleaching or electrokinetics alone in an equivalent or even shorter amount of time. After remediation with the integrated technology of bioleaching coupled with electrokinetics, the contents of the four heavy metals decreased to below their corresponding critical values based on the Environmental Quality Standards for Soils in China (GB15618-1995) (Table 2). In addition, the advantage of this method over the other four methods was most clearly exhibited for Cr as none of the other four methods was able to decrease the Cr content to an environmentally safe level. For BL, after the reduced sulfur or ferrous ion is oxidized by the bacteria, heavy metals coexisting with the primary sulfide minerals lose their stability. In addition, the final product of the oxidation process, H2SO4, increases the metal mobility by decreasing the soil pH. However, the mobilized heavy metals are not effectively separated from the soil. Applying an electric field, as is done for the electrokinetics method, can enhance metal migration by reducing the electrical resistance of the soil (Lee et al. 2009). This explains the synergistic effect of the BE method in metal removal from contaminated soils.
Lee et al. (2011) have also reported that the BE approach improves the metal removal performance compared to that of EK. However, lower metal removal efficiencies (20 %–50 % for Cu, Zn, Cr and Pb) were achieved in their study, which can be attributed to the fact that the metals existed in relatively strongly bound chemical forms. The findings in this study show that BE is a promising technology for the cleanup of heavy metal-contaminated soils as it is less time-consuming compared with phytoremediation and has been proven to work well for soils with a high content of fines (silt/clay) (Lee and Kim 2010), for which soil washing fails to yield good results. When implemented in practice, EDTA can be employed to enhance the performance of BE in terms of Pb removal (Lee and Kim 2010).
References
Blais J, Tyagi R, Auclair J (1993) Metals removal from sewage sludge by indigenous iron-oxidizing bacteria. J Environ Sci Health Part A 28:443–467
Chen SY, Lin JG (2001) Bioleaching of heavy metals from sediment: significance of pH. Chemosphere 44:1093–1102
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Du YG, Tyagi RD, Sreekrishnan TR (1995) Operational strategy for metal bioleaching based on pH measurements. J Environ Eng 121:527–535
Han FX, Kingery WL, Selim HM (2001) Accumulation, redistribution, transport and bioavailability of heavy metals in waste-amended soils. In: Iskandar IK, Kirkham MB (eds) Trace elements in soil: bioavailability, flux, and transfer. CRC Press, Boca Raton, FL, pp 141–168
Kim IS, Lee JU, Jang A (2005) Bioleaching of heavy metals from dewatered sludge by Acidithiobacillus ferrooxidans. J Chem Technol Biotechnol 80:1339–1348
Lee KY, Kim KW (2010) Heavy metal removal from shooting range soil by hybrid electrokinetics with bacteria and enhancing agents. Environ Sci Technol 44:9482–9487
Lee KY, Yoon IH, Lee BT, Kim SO, Kim KW (2009) A novel combination of anaerobic bioleaching and electrokinetics for arsenic removal from mine tailing soil. Environ Sci Technol 43:9354–9360
Lee KY, Kim HA, Lee BT, Kim SO, Kwon YH, Kim KW (2011) A feasibility study on bioelectrokinetics for the removal of heavy metals from tailing soil. Environ Geochem Health 33:3–11
Liu G, Yin J, Cong W (2007) Effect of fluid shear and particles collision on the oxidation of ferrous iron by Acidithiobacillus ferrooxidans. Miner Eng 20:1227–1231
Liu YG, Zhou M, Zeng GM, Wang X, Li X, Fan T, Xu WH (2008) Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: effects of substrate concentration. Bioresour Technol 99:4124–4129
Maini G, Sharman AK, Sunderland G, Knowles CJ, Jackman SA (2000) An integrated method incorporating sulfur-oxidizing bacteria and electrokinetics to enhance removal of copper from contaminated soil. Environ Sci Technol 34:1081–1087
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Martin TA, Ruby MV (2004) Review of in situ remediation technologies for lead, zinc, and cadmium in soil. Remediation J 14:35–53
Mercier G, Chartier M, Couillard D (1996) Strategies to maximize the microbial leaching of lead from metal-contaminated aquatic sediments. Water Res 30:2452–2464
Mulligan C, Yong R, Gibbs B (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Nahmani J, Capowiez Y, Lavelle P (2005) Effects of metal pollution on soil macroinvertebrate burrow systems. Biol Fertil Soils 42:31–39
Naresh Kumar R, Nagendran R (2009) Fractionation behavior of heavy metals in soil during bioleaching with Acidithiobacillus thiooxidans. J Hazard Mater 169:1119–1126
Nareshkumar R, Nagendran R, Parvathi K (2008) Bioleaching of heavy metals from contaminated soil using Acidithiobacillus thiooxidans: effect of sulfur/soil ratio. World J Microbiol Biotechnol 24:1539–1546
Olson G, Brierley J, Brierley C (2003) Bioleaching review part B. Appl Microbiol Biotechnol 63:249–257
Seidel H, Löser C, Zehnsdorf A, Hoffmann P, Schmerold R (2004) Bioremediation process for sediments contaminated by heavy metals: feasibility study on a pilot scale. Environ Sci Technol 38:1582–1588
Tessier A, Campbell PG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Ure AM (1995) Methods of analysis for heavy metals in soils. In: Alloway BJ (ed) Heavy Metals in the Environment. Blackie Academic and Professional, London, pp 58–102
Villar LD, Garcia O Jr (2006) Effect of anaerobic digestion and initial pH on metal bioleaching from sewage sludge. J Environ Sci Health Part A 41:211–222
Wang JY, Zhang DS, Stabnikova O, Tay JH (2005) Evaluation of electrokinetic removal of heavy metals from sewage sludge. J Hazard Mater 124:139–146
Wick LY, Shi L, Harms H (2007) Electro-bioremediation of hydrophobic organic soil-contaminants: a review of fundamental interactions. Electrochim Acta 52:3441–3448
Wong J, Xiang L, Gu X, Zhou L (2004) Bioleaching of heavy metals from anaerobically digested sewage sludge using FeS2 as an energy source. Chemosphere 55:101–107
Zagury GJ, Narasiah KS, Tyagi RD (1994) Adaptation of indigenous iron-oxidizing bacteria for bioleaching of heavy metals in contaminated soils. Environ Technol 15:517–530
Acknowledgments
This work was financially supported by the Foundation for Excellent Young Scientist in Guangdong Academy of Sciences (qnjj201401), the Science and Technology Planning Project of Guangzhou City, China (2014Y2-00194) and the Scientific Research Fund of Hunan Provincial Education Department (13C1082).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Q., Yu, Z., Pang, Y. et al. Coupling Bioleaching and Electrokinetics to Remediate Heavy Metal Contaminated Soils. Bull Environ Contam Toxicol 94, 519–524 (2015). https://doi.org/10.1007/s00128-015-1500-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1500-1