Abstract
Electrobioremediation (EBR, electrokinetic-enhanced in situ biodegradation of soil pollutants) is a technology whose objective is to improve in situ bioremediation by the enhancement of different transport processes by means of the application of electrokinetics. Some of the potential benefits are enhancement of contaminant bioavailability, the increase of bacteria mobility and the electrokinetic-induced transport of nutrients and electron acceptors. However, electrokinetics should be used under controlled conditions as they also could produce disadvantages. It is very important to avoid extreme temperature or pH values, or the lack of nutrients and electron acceptors. It is currently assumed that electrokinetics will improve the transport and contact between the different species involved in the biological mechanism into the soil although it is necessary to maintain environmental conditions in values adequate for microbial life. The first EBR fundamental studies were reported in the 1990s and subsequently numerous works have expanded and deepened the study of this technology. The present chapter focus on the main factors that influence EBR, including some relevant findings by many authors. Some strategies such as electrokinetic biostimulation (improvement of environmental conditions into the soil to enhance the in situ bioremediation rate by means of the positive influence of electrokinetic phenomena) and electrokinetic bioaugmentation (the delivery of microorganisms to the soil by using EK transport mechanisms) have been described. Finally, research needs and future developments are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Bioremediation of Polluted Soil
Bioremediation is usually known as the technology used for restoration of polluted sites through the biodegradation of the soil organic pollutants. Its fundamental concept does not differ from the fundamentals or principles of conventional water-suspended biodegradation technology using selected microbes or mixed microbial cultures [1]. Because of the usual occurrence of soil pollution problems, bioremediation is mainly applied to eliminate chemicals such as petroleum hydrocarbons, polyaromatic hydrocarbons, organochlorinated compounds (solvents, pesticides, or herbicides), polychlorinated biphenyls (PCBs), and heavy metals [2]. The pollutant biodegradability, by means of adapted microorganisms, is the key factor affecting the performance of such technology, which in turn depends mainly on the pollutant chemical structure. However, the fact that the pollutant is included in a heterogeneous matrix that could involve up to four different phases (solid, water, organic, and gas phases) supposedly causes the pollutant to be transported and distributed between such phases, making bioremediation a more complex process than a conventional biodegradation process in water [3].
Bioremediation can be achieved by in situ or ex situ options. Ex situ bioremediation is the most commonly used technology: the polluted soil is excavated and removed from its original contaminated site and subsequently soil is transported to be treated using an external bioreactor [4]. In situ bioremediation is not so common and it consists on remediating the soil in its original site, and thus soil excavation and transport is not necessary. The in situ treatments have several advantages among which it can be highlighted the minimal disruption to activities on site or on adjacent land. It usually involves the movement of air or water through the polluted soil, which is favored by more permeable media and by lower heterogeneity of physical conditions and pollution distribution. However, one of the main disadvantages of in situ treatments is the reduced mobility of microorganisms, pollutants and nutrients into the soil, especially in low permeable soil, causing reduced bioavailability for biodegradation, while ex situ approaches generally offer greater scope for managing conditions to optimize treatment efficiency and for controlling potential spread of pollutants [5].
Bioavailability of nonsoluble pollutants to microorganisms is also one of the main factors that influences soil bioremediation performance. The low water solubility and the adsorption to particulate matter in soil and sediments are important factors that can reduce in situ biodegradation of organic pollutants such as hydrocarbons or nonpolar organochlorines. The rates of desorption and dissolution of such pollutants in interstitial water in soil can be improved by adding surfactants (either biosurfactants or synthetic detergents) to the contaminated zone [6].
There are additional environmental factors such as pH, temperature, salinity, inorganic nutrients and electron acceptors availability that influence bioremediation [7]. Most natural environments have pH values between 5.0 and 9.0 because of the natural buffering capacity of soil that contains carbonates and other minerals. pH values out of this interval can inhibit microbial growth. Regarding temperature, bioremediation is generally carried out under mesophilic conditions (20–40 °C). Low temperatures can kill or inactivate microbes. The biodegradation rate increases with temperature up to a maximum above which the rate declines as enzyme denaturation occurs. Requirements of inorganic nutrients (N and P) depend of the nutrient availability in soil (N and P are usually present in agricultural soils), the nature of pollutants and the type of metabolism (aerobic, anoxic, anaerobic). Thus, electron acceptors availability is also important. The amount of available oxygen will determine whether bioremediation is carried out under aerobic or anaerobic conditions. Hydrocarbons are readily degraded under aerobic conditions while organochlorines can be degraded under anaerobic ones [7]. The depth of pollution in soil is an additional factor that conditions the oxygen availability. Other electron acceptors such as nitrate could be used. Megharaj and Naidu [2] deepen the effect of different conditions and physicochemical characteristics in soil bioremediation technology.
There are different bioremediation approaches that can be selected and applied either in situ or ex situ depending on the characteristics of pollutants and site conditions [1]. Additionally, three possible general strategies can be considered in order to enhance the biological process: natural attenuation, biostimulation, and bioaugmentation [1, 4]. Natural attenuation involves slow microbial degradation only if adapted microorganisms are present into the soil, together with processes such as volatilization, sorption and immobilization, but no actions to stimulate the biological process are considered. Biostimulation consists of providing favourable conditions for the enhancement of the biological process (through homogenization, addition of nutrients, electron acceptors, or pH buffering) as the polluted soil already contains a native population of microorganisms adapted to biodegradation of the soil pollutants (usually because the pollution episode happened long time ago). Finally, if soil microbial populations to efficiently degrade the pollutants do not exist (what happens in recent spills), inoculation of enriched/acclimated consortia or strains can be provided, and this operation is called bioaugmentation . Mixed cultures with a large variety of microorganisms are utilized in bioaugmentation practice [8]. Additional updated information about species and experimental conditions used in bioremediation is available in a recent reported review [9].
Taking into account the influence of the abovementioned factors, in situ bioremediation can be considered as an adequate and cost-effective treatment to eliminate organic pollutants in soil although the transport and contact between microorganisms, water, pollutants, nutrients and electron acceptors in order to stimulate biodegradation is still a challenge, especially in low permeable soil.
2 Electrobioremediation of Polluted Soil. Concepts and Objectives
Electrobioremediation (EBR) is a generic name that can be used for different technological approaches focused on biodegradation of pollutants combined with electrochemical methods. The electrobioremediation concept in the present chapter refers to the electrokinetic-enhanced in situ biodegradation of soil pollutants. Its objective is to improve in situ bioremediation by the enhancement of different transport processes by means of the application of low-voltage direct electric currents through the soil. This method would previously assume that the limiting step in the bioremediation process would be the transport processes rather than the pollutants biodegradability.
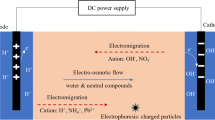
The basic method to achieve EBR is schemed in Fig. 1, and it consists of the insertion of appropriately distributed electrodes into the soil (or inside electrolyte wells) so that the polluted zone is located between them. The most widely studied arrangement of electrodes is facing two linear rows of them with different polarities because this configuration has been related to better electric current distribution lines, which would produce a well-distributed pattern of electrokinetic flows. However, this is not the only possibility of electrodes arrangement in soil, and configurations in which various electrodes surround a central electrode of the opposite polarity have also been checked [10, 11]. The results reported in these studies suggest that the most effective electrode configuration (from a pollutant transport viewpoint) is a hexagonal arrangement, that is, a ring of six cathodes with a central anode, or vice versa. When DC electric current is applied, many transport processes previously described in previous chapters may occur (such as electroosmosis, electromigration, and electrophoresis). These transport phenomena could help (or disturb, if not correctly applied) biological processes by allowing contact between pollution, nutrients and microorganisms. Electric fields (between 1 and 5 V cm−1 approximately) induce microscale dispersion rather than macroscale movement, which was reported to be favourable to the stimulation of the microbial activity [9]. In general, the potential benefits of such technology may include (a) the enhancement of contaminant bioavailability; (b) increase of bacteria mobility; (c) electrokinetic-induced transport of nutrients and electron acceptors and (d) in situ electrochemical generation of electron donors and acceptors [12]. EBR is specially recommended for low permeable soil, as clay soil, where the hydraulic conductivity is very low and hydrodynamic transport would not be suitable. Regarding pollutants, EBR is used mainly for hydrocarbons and organochlorines removal, but also metal remediation has been reported [13]. The first EBR fundamental studies were reported in the 1990s by Marks et al. [14], Ho et al. [15] and Chillingar et al. [16], and subsequently numerous works have expanded and deepened the study of this technology. Wick [17] reported an interesting review about the first decade of EBR works and offered a conceptualization of the “electrobioremediation tetrahedron” which described the critical factors influencing the biotransformation of hydrophobic organic compounds during subsurface electrokinetic treatment. The following sections in the present chapter focus on the main factors that influence EBR, including some relevant findings by many authors.
3 Microbial Transport and Activity Under Electrokinetics
One important aspect that must be considered in EBR is the feasibility of microbial mobility and transport into the soil under electrokinetic treatment. This point is important as it opens the possibility of microbial inoculation in bioaugmentation processes (Sect. 5), or just because it enables the possibility of moving microorganisms into the soil to contact pollutants and nutrients in biostimulation processes (Sect. 4). Microorganisms in the soil under electrokinetic treatment can move by electrophoresis (EF) and electroosmosis (EO) , and they have a strong tendency to adhere onto the surface of soil particles [18]. Research works about microbial electrokinetic mobility in soil are usually carried out at bench-scale setups (laboratory model aquifers or soil columns) and batch mode, using electric fields between 0.5 and 4.0 V cm−1 approximately. Figure 2 shows an example of a bench-scale installation used to study microbial transport through a soil sample under electrokinetics using different voltages and different soil textures [19].
Bench-scale installation used to study microbial transport through a soil sample under electrokinetics. (Adapted from Mena et al. [19])
It has been observed in most of the published works that the negative electric charge on the microbial surface causes the movement of microorganisms towards the anode by EF whereas EO simultaneously moves them towards the cathode. Different works tried to study which is the predominant mechanism. Lee and Lee [20] supplied Pseudomonas to a diesel-contaminated soil bed of 15 cm under 40 mA. They observed the transport of diesel-degrading microorganisms towards the anode mainly by EF. The cells acted as negatively charged particles at neutral pH, and they concluded that pH and ionic concentration played an important role. Da Rocha et al. [21] performed electrokinesis on a low hydraulic reconstituted clayey soil column subjected to a 5 mA electrical current for 24 h. They studied the efficacy of EF against the electroosmotic flow to transport endospores of Bacillus subtilis LBBMA 155 and nitrogen-starved cells of Pseudomonas sp. LBBMA 81. They observed EF to be the predominant mechanism, and they observed that the negative charge on the cells surface played an important influence.
On the contrary, Suni and Romantschuk [22] reported that the microbial mobility of phenol-degrading bacteria in three types of soil (garden soil, fine sand, and clay) was mainly produced by EO , and the transport velocities ranged between 0.1 cm h−1 (clay soil) and 1 cm h−1 (fine sand). Wick et al. [23] reported the mobility of different strains of hydrocarbon-degrading bacteria that presented different adhesion potential to soil particles, and they found that EO transport mechanism was more important than EF if bacteria were not strongly adhered to soil. Shi et al. [24] observed the important role of EO. They reported microbial velocity of 0.6 cm min−1 using hydrocarbon-degrading bacteria under 1 V cm−1, and they proposed that the different electrokinetic behaviour of individual cells could be solely attributed to intra-population heterogeneity of the cell surface charge.
There is also an important aspect to be studied such as the possible influence of the electric current on the soil microbial activity, physiology and the microbial diversity. Wick et al. [25] did not observe harmful effects on the soil microbial population when using 1.4 V cm−1 and 1 mA cm−2 except in areas near the electrodes because of the extreme pH values (<1.5 pH units) caused by water electrolysis.
Kim et al. [26] also observed a decrease in microbial concentration and microbial diversity under 0.6 mA cm−2 in zones at extreme pH values, but on the contrary, they observed positive effects on microbial activity and soil enzyme activity in other areas. Mena et al. [27] found that voltages higher than 2 V cm−1 caused an important increase in the endogenous cell decay rate because of harmful pH effects using graphite electrodes. Finally, Li et al. [28] isolated hydrocarbon-degrading bacteria capable of growing under electrokinetic conditions after an acclimation and enrichment procedure. They found that strains PB4 (Pseudomonas fluorescens) and FB6 (Kocuria sp.) were the most efficient hydrocarbon degraders under electrokinetic conditions, and that their degradation capabilities were enhanced compared to experiments without application of electric fields. They observed that the electric field acted as a selective pressure for isolating those bacteria capable of growing under such conditions. However, to understand the ability of these particular species, authors suggested future research focused on the particular biological functions that set these species apart from others.
4 Electrokinetic Biostimulation
EK-biostimulation consists on the improvement of environmental conditions into the soil to enhance the in situ bioremediation rate by means of the positive influence of electrokinetic phenomena. However, electrokinetics should be used under controlled conditions as they also could produce disadvantages. It has been previously indicated (Sect. 3) that soil microorganisms can move by EF and EO and that low-voltage DC does not cause negative effects to microorganisms (i.e., no considerable increase in the endogenous cell decay rate is observed), but it is very important to avoid extreme temperatures or pH values, or lack of nutrients and electron acceptors. It is currently assumed that EK will improve the transport and contact between the different species involved in the biological mechanism into the soil although it is necessary to maintain environmental conditions in values adequate for microbial life. Lohner et al. [12] reported data about transport rates between 0.4 and 5 cm h−1 approximately for microbial nutrients and electron acceptors (nitrate, sulfate, phosphate, and ammonium) but also limitations because of undesirable side reactions, electroporation, irreversible permeabilization of cell membranes, oxidative stress, and cell death due to electrochemically generated oxidants and electrochemical oxidation of vital cellular constituents.
Most of the scientific works that study EK-biostimulation use bench-scale installations and, because of the experimental conditions in microcosms should be perfectly controlled (approximately: temperatures between 10 and 30 °C, pH close to neutrality, electrical conductivity between 500 and 3000 μS cm−1, constant values of moisture and porosity), many of these works use artificially polluted soil in order to simulate real pollution, and they also inoculate acclimated microorganisms into the soil before the experiments in order to simulate the presence of a native microbial population. Then, the objective of batch experimental studies is to know the influence of variables such as pH, temperature, and nutrient concentrations. A scheme of a typical bench-scale setup for EK-biostimulation studies is shown in Fig. 3.
Some works just study the feasibility of the combination of bioremediation and electrokinetics. Yuan et al. [29] studied EBR in soil microcosms that were spiked with n-hexadecane at 1.0% (v/w) and inoculated with a mixture of petroleum-degrading bacteria (107–108 CFU g−1) before being subjected to a constant voltage gradient of 1.3 V cm−1 for 42 days. They observed that the degradation rate of n-hexadecane by electrobioremediation was up to 53.7%, representing an increase of 20.3% compared to conventional in situ bioremediation without an electric field. Guo et al. [30] observed the positive effect of the biological–electrochemical combination in EBR experiments (100 days duration, 1 V cm−1 and using polarity changes every 5 min) for petroleum hydrocarbons removal in soil. Most of the experimental studies carried out also use simultaneous reference tests (only biological test or only electrokinetic tests) in order to evaluate the feasibility of the combination of both technologies. The following subsections describe specific reported works regarding the influence of some factors such as pH, nutrients, and pollutant bioavailability.
4.1 pH Control into the Soil
Previous chapters in the present issue have described the formation of extreme pH fronts into the soil (low pH near the anode and high pH near the cathode) because of water electrolysis. These extreme pH values cause inhibition of the biological mechanisms and the microbial activity is drastically reduced in a few days [31]. Different strategies have been proposed to maintain a suitable pH in the soil during EBR processes [32]. One of the most interesting methods is the periodic change of the polarity of the electric field (the so-called periodic polarity reversal).
Different works have been reported in the last decade regarding the application of polarity reversal in order to control pH during EBR treatment, and some of them also showed beneficial effects in temperature and moisture control. Kim and Han [33] used EK (12.5 V cm−1) for clay soil remediation and proposed circulation of anolyte and catholyte between electrode wells. pH was maintained continuously only by circulation of electrolytes (H2SO4 and NH4OH) in each chamber without any buffering solutions, and pH in soil showed a difference not greater than 0.2 of initial pH. Alternatively, they used periodic polarity reversal and they found that electrode polarity reversal prevented the development of pH gradient, and it was inferred that electrode polarity reversal enabled an effective ion injection, and ions were distributed more uniformly in soil. Luo et al. [34] studied EBR of sandy loam spiked with phenol. They found that nonuniform electrokinetics could accelerate the movement and in situ phenol biodegradation. Low polarity-reversing intervals (every 3 h) induced a higher and more uniform removal of phenol (a maximum removal efficiency of 58% was achieved in 10 days and the bioremediation rate was increased about five times as compared to that with no electric field applied), and it was also observed a better moisture control in the soil. The same authors [35] used 2D nonuniform electrokinetic operation modes (bidirectional and rotational) to test EBR at bench-scale with a sandy loam as the model soil and 2,4-dichlorophenol (2,4-DCP) as the model organic pollutant. At the bidirectional mode, an average 2,4-DCP removal of 73.4% was achieved in 15 days, whereas 34.8% of 2,4-DCP was removed on average in the same time period at the rotational mode. Harbottle et al. [36] studied EBR of pentachlorophenol-polluted soil (approximately 100 mg kg−1) and inoculated with a specific pentachlorophenol-degrading bacterium (Sphingobium sp. UG30) and subjected to constant and regularly reversed electric currents (10 mA) during different experimental periods (between 36 and 95 days). When both pH and moisture content were controlled using a regularly reversed electric field, instead of unidirectional field, it was found a positive effect on biodegradation of PCP.
Li et al. [37] studied the influence of polarity reversal and electrical intensity on oil removal from soil by EBR. Soil pH remained at around 6.6 obtaining nearly 30% removal rate after 6 weeks when using polarity reversal (1 h−1) and 1 V cm−1. These authors also studied the biodegradation of Pyrene in contaminated soil under electrokinetic treatment. Three strategies were conducted: In situ conventional bioremediation (Bio), electrobioremediation without polarity-reversal (EK-Bio), and electrobioremediation with polarity-reversal every 2 h (EK-Bio-PR). Pyrene degradation efficiency was 55.9% after 6 weeks under EK-Bio-PR at the end of experiment.
Despite polarity reversal has been proved to be an efficient method for pH control during EBR, no studies were reported regarding the optimization of the reversal frequency. Barba et al. [38] studied EBR of pesticide (oxyfluorfen) polluted clay. Two-weeks duration batch experiments were carried out and used different reversal frequencies between 1 and 6 day−1, and they found 2 day−1 as optimal value that would produce the optimum pH control and mixture effect in soil.
4.2 Availability and Supply of Inorganic Nutrients and Electron Acceptors
The availability of electron acceptors and nutrients is often a key factor influencing the success of microbiological remediation at contaminated sites. Despite nutrients requirements may be very low (because of the low organic pollutant concentration or because of the anaerobic mechanism) a lack of nutrients can occur in EBR. Moreover, adsorption in soil, chemical precipitation or ion exchange can reduce nutrients bioavailability, and thus nutrient replacement is needed.
Nutrient injection into low permeable soil could be done using electrokinetics as the most important inorganic nutrients (ammonium, nitrate, and phosphate) are ionic substances that could be transported by electromigration, and also by water electroosmosis. However, electrokinetics also could produce nutrients depletion into the soil in long-time EBR processes because of their transport to the electrode wells. Barba et al. [39] found that the biological process could be inactivated in a clay soil EBR study after 11 weeks because of nutrients depletion, despite they were partially supplemented at intermediate operation times.
Some works have been reported regarding electrokinetic injection of nutrients in order to avoid possible inactivation of the bioremediation mechanisms. Schmidt et al. [40] studied the feasibility of injecting inorganic nutrients (ammonium, nitrate, and phosphate) to low permeable clay soil by the addition of prepared solutions into the soil in an electrokinetic cell. Ammonium moved but decreased in the system during the tests because of chemical reactions. Nitrate showed greater mobility and a behaviour less reactive, remaining in the system and only moving towards the anode. Phosphate was not successfully transported, probably because of reactions with calcium carbonate and precipitation; thus, the injection of phosphorous did not prove to be successful. Suni et al. [41] studied enhanced bioremediation of creosote-contaminated soil with a combination of electric heating and infiltration-electrokinetic introduction of oxygenated, nutrient-rich liquid. Nutrient and oxygen levels in the soils were elevated by hydraulic and electrokinetic pumping of urea and phosphate amended, aerated water into the soil. Total hydrocarbon concentrations decreased by 50–80% during 12 weeks of treatment. Xu et al. [42] investigated an EK injection method, which combined electrolyte circulation between electrodes and electrode polarity reversal in bioremediation of phenanthrene-polluted low permeable soil. As expected, soil pH was successfully controlled, but additionally it was also possible supply and distribution of nutrients and electron acceptors (ammonium and nitrate) uniformly in soil. Over 80% of phenanthrene was removed in 20 days.
Regarding the availability of electron acceptors, it is important to notice that oxygen (the most usual acceptor necessary for the most efficient aerobic biodegradation mechanisms) will not be easily available in the low permeable soil. Mena Ramírez et al. [43] studied the feasibility of supply oxygen to a soil by electrokinetics. Oxygen was generated by water electrolysis in the anode and transported to the soil in a bench-scale electrokinetic cell. It was found that oxygen transport was only available in silty and sandy soils by oxygen diffusion, while transport was not possible in clay soil. Moreover, electroosmotic flow in clay soil did not contribute to the transport of oxygen, and only a minimum fraction of the electrolytically generated oxygen was efficiently used.
Nitrate could be alternatively used instead of oxygen if denitrifying microorganisms were able to be used in EBR of organics-polluted soil, and it would be an important advantage as nitrate is easily transported by EK. Additionally, nitrate-polluted soil remediation could be also achieved. Choi et al. [44] studied nitrate removal in soil by electrobioremediation using reducing bacteria (Bacillus spp.) and humic substances as electron donor. Thiem et al. [45] studied electrokinetic nitrate transport and toluene biodegradation under denitrifying conditions and different voltage gradients. A denitrifying microbial mixed culture was inoculated. Application of the electric field allowed nitrate migration into toluene-polluted areas and resulted in toluene biodegradation.
Finally, anaerobic treatment has also been reported. Wu et al. [46] studied in situ EBR of tetrachloroethylene low permeable soil by electrokinetic injection of lactate, a common electron donor for anaerobic biodegradation. The soil was inoculated with KB-1® dechlorinators. They concluded that ionic migration delivered organic additives and induced biological activity and complete tetrachloroethylene transformation in soil.
4.3 Bioavailability of Nonpolar Pollutants. Surfactant Applications
Previous chapters in the present issue discussed the role of surfactants in electrokinetic soil washing to improve the nonpolar pollutant transport and it is known that surfactant addition is a common strategy in electroremediation. However, there are few studies regarding the use of surfactants in electrobioremediation. From the biological perspective, surfactants are also necessary to allow accessibility of microorganisms to nonsoluble organic substrates. Mena et al. [47] used sodium dodecyl sulfate (SDS) to improve diesel hydrocarbons removal by EBR. The same authors [48] used again this surfactant to improve oxyfluorfen removal: SDS was introduced in the electrode compartments and transported into the soil. An optimum amount (2.5 g L−1) was found and it increased the oxyfluorfen removal efficiency by 52%.
5 Electrokinetic Bioaugmentation
Biodegradation of soil pollutants is usually carried out by soil indigenous microorganisms as they developed the ability to consume the organic pollutants after a long period of time since the spill or pollution episode happened. However, if the spill was recent, and thus the soil was recently polluted, it is possible that no indigenous acclimated microbial population able to degrade the pollutants was present. In these occasions, it is necessary to develop a microbial culture adapted to the biodegradation of such pollutants in external bioreactors, and then introduce it into the soil.
Another situation could be that, although the soil indigenous population is adapted to the soil pollutants biodegradation, the microbial concentration in soil is very low. Bioremediation could be accelerated under high microbial concentration. Thus, it is possible to obtain and isolate indigenous microbial seeds from the soil, in order to develop again external growth processes in bioreactors, and then introduce high amounts of such cultures into the soil.
Both situations correspond to the bioaugmentation option [8]. Electrokinetic bioaugmentation consists on the delivery of microorganisms to the soil by using the EK transport mechanisms, and it is again recommended in low permeable soils where hydraulic advection is not feasible. On one hand, it is necessary to know the performance of the microbial culture under low DC electric fields (which has been previously discussed in Sect. 3). On the other hand, it is necessary to study what are the options to deliver external microorganisms into the soil. EK injection of inorganic nutrients and electron acceptors has been successfully studied (Sect. 4.2) but EK injection of microorganisms is not so common.
Only some previous works has attempted to use EK as the sole delivery mechanism to inoculate nonnative bacteria into soils for bioremediation purposes, and they tried to do this through the injection in the electrode wells or in soil positions far from the electrodes, and they always tried to avoid extreme pH values in the delivery point.
Mao et al. [49] studied EK-enhanced bioaugmentation for remediation of clays contaminated with chlorinated solvents in laboratory experiments under 5 A m−2. Dehalococcoides (Dhc) bacterial strain and lactate ions were uniformly injected in contaminated clay. To bioaugment the soil, the power was turned off and the microbial culture solution was added to the electrode compartments and to a central injection well. After 2 days of acclimation following bioaugmentation, the power supply was turned on to resume the EK operation. The distribution of Dhc within the clay suggested that electrokinetic microbial transport was primarily driven by electroosmosis. The injected bacteria were able to survive and grow, and complete effective dechlorination of chlorinated ethene was observed after 94 days.
Secord et al. [50] evaluated the possibility of using EK as a delivery mechanism to introduce organic pollutant degrading bacteria, Sphingomonas paucimobilis EPA505 and Mycobacterium vanbaalenii PYR-1 , into hydrocarbons-polluted low permeability soils. Bacterial cultures were previously grown to exponential phase using hydrocarbons as carbon source, and then transferred to EK reservoirs (one or both bacterial strains were inoculated into either the anolyte or catholyte). It was demonstrated that in situ inoculation of nonnative bacterial species using EK is possible. Although hydrocarbon degradation was not monitored in this study, it was hypothesized that the presence of carbon sources enabled bacteria to thrive in the polluted soil.
One alternative option for EK-bioaugmentation is the application of biological PRBs (permeable reactive barriers), also called “biobarriers.” Generally, a PRB is a reactive material that is placed into the soil in the direction of the groundwater flow to help intercept a pollution plume that is carried within an aquifer by degrading or retaining the pollutants [51]. The subsurface pollution plume can be moved through the PRB using the natural hydraulic gradient, or forced by a pump-and-treat method. However, for low permeability soils, the EK mobilization of water is recommended (in that case, EK-PRB is used).
PRBs can be built using different materials that are based on different mechanisms (adsorption with porous high-surface materials, ion exchange with resin-based materials, reduction using elemental metals, biological degradation, etc.). In particular, a barrier based on biological degradation (BioPRB , or biobarrier) is a fixed culture bioreactor that includes a porous supporting material and a microbial biofilm attached on its surface. The working principle of a biobarrier is the same as that of a conventional biofilm reactor. In fact, the only difference is that it is inserted in the soil during EK treatment. Figure 4 shows a conceptual scheme of an EK-BioPRB process and a typical experimental setup used in bench-scale studies.
Although numerous works have been previously reported regarding the use of EK-PRB technology, that is, different types of PRBs coupled to electrokinetics [52], only a limited number of publications regard biological barriers. Fonseca et al. [53] developed EK-BioPRB to treat soils contaminated with hexavalent chromium. The electric field promoted the electromigration of chromium oxyanions towards the anode while the biobarriers, placed before the anode electrode, promoted the reduction and retention of the chromium migrating in its direction. The reactive biobarriers were composed by Arthrobacter viscosus bacteria, supported either in activated carbon or zeolite. They reported removal values of 60% and 79% under 10 V and after 18 days when electrokinetic treatment was coupled with zeolite and activated carbon biobarriers, respectively.
Mena et al. [54] studied electrobioremediation of diesel hydrocarbons–polluted clay soil by means of coupled electrokinetic soil flushing and biobarriers, using bench scale setups under 0.5 and 1.0 V cm−1 and 14 day-long tests. Biobarriers were introduced in a central position of the soil to be treated in order to prevent extreme pH values near the electrodes. They evaluated two types of biobarriers: one of them (BB1) was a fixed-bed biofilm reactor previously developed in the laboratory, with a culture of diesel-degrading microorganisms supported on gravel particles; the other one (BB2) was obtained by mixing clean clay soil with activated sludge obtained from a wastewater treatment plant. Results showed diesel removal rates of 19.36% and 27.36% (using BB1 under 0.5 and 1.0 V cm−1, respectively) and of 23.33% and 29.10% (when using BB2) indicating that despite the nonspecific barrier BB2 did not contain an acclimated culture, it reached similar results than BB1. The same authors [55] studied the application of EK-BioPRB for the elimination of organochlorinated pesticides in clay soil. Two compounds were used as model pollutants: oxyfluorfen (a nonpolar pollutant) and 2,4-dichlorophenoxyacetic acid (2,4-D, polar pollutant). The two types of biobarriers previously mentioned were tested again (BB1 and BB2). Compared to the EK-Biostimulation option, it was found that the inclusion of a central biobarrier slightly decreased the pollutant removal rates probably because a decrease in EO flow. However, the EK-BioPRB technology was checked to be a feasible option when no native microbial population is available and there is no option to apply EK-biostimulation.
However, in general, bioaugmentation studies (not only EK-bioaugmentation) have not been successful. It has been repeatedly reported that it is often difficult to maintain the survival of exogenous microbes introduced to a foreign environment [56]. Often the inoculum shows a dramatic decrease in colony forming units (CFU) upon soil inoculation but this behaviour is not well-understood [57]. The lack of success has been related to the formation of antibiotics by native bacteria, or predation and adaptability of external bacteria to the contaminated soil [58]. A possible solution could be the addition of endospores instead of active bacteria because endospores are more robust and migrate faster than bacteria under EK due to a high associated surface charge [21].
6 Research Needs
There are some challenges regarding EBR that should be addressed in the near future. One of them is scaling up of the EBR technology. Studies regarding scale-up are scarce. Mena et al. [59] studied EBR of diesel hydrocarbons polluted soil in a prototype (650 kg, 0.25 m3) using BioPRBs, and the bioremediation performance was strongly affected by the high temperatures reached because of ohmic heating. Similar conclusions were obtained by Barba et al. [60]. They used a large prototype (32 m3) and they studied EBR of organochlorinated pollutants. Most of the pollutant removal was caused by volatilization because of the high temperatures reached. It is clear that maintenance of adequate conditions for microbial life should be one of the future research efforts.
Regarding the lack of success of bioaugmentation, successive periodic inoculations after several days of treatment could be applied to compensate microbial mortality in the soil. The use of enzymes instead of microorganisms has also been proposed [58]. The use of enzymes in bioaugmentation can result in avoiding the competition between indigenous bacteria and the new strains. Using enzymes in bioaugmentation could offer additional advantages such as the simplification of the process (they do not generate by-products), it is easier to work with enzymes than with the whole microorganism, and enzyme capabilities can be improved at the production stage. However, the cost of enzyme production is high. Enzyme delivery via electrokinetics transport mechanisms has not been investigated to date.
In conclusion, it is important to note that once the viability of the soil microbial metabolism was assured, electrobioremediation does not increase costs and energy consumption compared to conventional electrokinetic remediation; moreover, the pollutant is not transferred to another phase or matrix but eliminated in situ.
References
A.A. Juwarkar, S.K. Singh, A. Mudhoo, A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Biotechnol. 9, 215–288 (2010)
M. Megharaj, R. Naidu, Soil and brownfield bioremediation. Microbial Biotechnol. 10, 1244–1249 (2017)
E.L. Fernández, E.M. Merlo, L.R. Mayor, J.V. Camacho, Kinetic modelling of a diesel-polluted clayey soil bioremediation process. Sci. Total Environ. 557–558, 276–284 (2016)
M.C. Tomei, A.J. Daugulis, Ex situ bioremediation of contaminated soils: an overview of conventional and innovative technologies. Crit. Rev. Environ. Sci. Technol. 43, 2107–2139 (2013)
J. Scullion, Remediating polluted soils. Naturwissenschaften 93, 51–65 (2006)
C.N. Mulligan, Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Interface Sci. 14, 372–378 (2009)
M. Vidali, Bioremediation. An overview. Pure Appl. Chem. 73, 1163–1172 (2001)
S. Di Toro, G. Zanaroli, F. Fava, Intensification of the aerobic bioremediation of an actual site soil historically contaminated by polychlorinated biphenyls (PCBs) through bioaugmentation with a nonacclimated, complex source of microorganisms. Microbial. Cell Fact. 5, 11–20 (2006)
C.M. Quintella, A.M.T. Mata, L.C.P. Lima, Overview of bioremediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. J. Environ. Manage. 241, 156–166 (2019)
C. Risco, H. Rubí-Juárez, S. Rodrigo, R. López-Vizcaíno, C. Saez, P. Cañizares, C. Barrera-Díaz, V. Navarro, M.A. Rodrigo, Removal of oxyfluorfen from spiked soils using electrokinetic soil flushing with the surrounding arrangements of electrodes. Sci. Total Environ. 559, 94–102 (2016)
G.L. Grundmann, Spatial scales of soil bacterial diversity—the size of a clone. FEMS Microbiol. Ecol. 48, 119–127 (2004)
S.T. Lohner, A. Tiehm, S.A. Jackman, P. Carter, Coupled Electrokinetic-bioremediation: applied aspects, in Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater, ed. by K. R. Reddy, C. Cameselle, (Wiley, Hoboken, 2009), pp. 389–416
K.Y. Lee, H.A. Kim, B.T. Lee, S.O. Kim, Y.H. Kwon, K.W. Kim, A feasibility study on bioelectrokinetics for the removal of heavy metals from tailing soil. Environ. Geochem. Health 33, 3–11 (2011)
R.E. Marks, Y.B. Acar, R.J. Gale, In situ remediation of contaminated soils containing hazardous mixed wastes by bioelectric remediation and other competitive technologies, in Remediation of Hazardous Waste Contaminated Soils, ed. by D. L. Wise, D. J. Trantolo, (Marcel Dekker, New York, 1994), pp. 405–436
S.V. Ho, P.W. Sheridan, C.J. Athmer, M.A. Heitkamp, J.M. Brackin, D. Weber, P.H. Brodsky, Integrated in situ soil remediation technology: the Lasagna process. Environ. Sci. Technol. 29, 2528–2534 (1995)
G.V. Chillingar, W.W. Loo, L.F. Khilyuk, S.A. Katz, Electrobioremediation of soils contaminated with hydrocarbons and metals: progress report. Energy Source. 19, 129–146 (1997)
L.Y. Wick, L. Shi, H. Harms, Electro-bioremediation of hydrophobic organic soil-contaminants: a review of fundamental interactions. Electrochim. Acta 52, 3441–3448 (2007)
M.F. Deflaun, C.W. Condee, Electrokinetic transport of bacteria. J. Hazard. Mater. 55, 263–277 (1997)
E. Mena, P. Rubio, P. Cañizares, J. Villaseñor, M.A. Rodrigo, Electrokinetic transport of diesel-degrading microorganisms through soils of different textures using electric fields. J. Environ. Sci. Health A 47, 274–279 (2012)
H.S. Lee, K. Lee, Bioremediation of diesel-contaminated soil by bacterial cells transported by electrokinetics. J. Microbiol. Biotechnol. 11, 1038–1045 (2001)
U.N. Da Rocha, M.R. Tótola, D.M.M. Pessoa, J.T.A. Júnior, J.C.L. Neves, A.C. Borges, Mobilisation of bacteria in a fine-grained residual soil by electrophoresis. J. Hazard. Mater. 161, 485–491 (2009)
S. Suni, M. Romantschuk, Mobilisation of bacteria in soils by electro-osmosis. FEMS Microbiol. Ecol. 49, 51–57 (2004)
L.Y. Wick, P.A. Mattle, P. Wattiau, H. Harms, Electrokinetic transport of PAH-degrading bacteria in model aquifers and soil. Environ. Sci. Technol. 38, 4596–4602 (2004)
L. Shi, S. Müller, H. Harms, L.Y. Wick, Factors influencing the electrokinetic dispersion of PAH-degrading bacteria in a laboratory model aquifer. Appl. Microbiol. Biotechnol. 80, 507–515 (2008)
L.Y. Wick, F. Buchholz, I. Fetzer, S. Kleinsteuber, C. Härtig, L. Shi, A. Miltner, H. Harms, G.N. Pucci, Responses of soil microbial communities to weak electric fields. Sci. Total Environ. 408, 4886–4893 (2010)
S.H. Kim, H.Y. Han, Y.J. Lee, C.W. Kim, J.W. Yang, Effect of electrokinetic remediation on indigenous microbial activity and community within diesel contaminated soil. Sci. Total Environ. 408, 3162–3168 (2010)
E. Mena, J. Villaseñor, P. Cañizares, M.A. Rodrigo, Effect of a direct electric current on the activity of a hydrocarbon-degrading microorganism culture used as the flushing liquid in soil remediation processes. Sep. Purif. Technol. 124, 217–223 (2014)
F. Li, S. Guo, N. Hartog, Y. Yuan, X. Yang, Isolation and characterization of heavy polycyclic aromatic hydrocarbon-degrading bacteria adapted to electrokinetic conditions. Biodegradation 27, 1–13 (2016)
Y. Yuan, S.H. Guo, F.M. Li, T.T. Li, Effect of an electric field on n-hexadecane microbial degradation in contaminated soil. Int. Biodeter. Biodegr. 77, 78–84 (2013)
S. Guo, R. Fan, T. Li, N. Hartog, F. Li, X. Yang, Synergistic effects of bioremediation and electrokinetics in the remediation of petroleum-contaminated soil. Chemosphere 109, 226–233 (2014)
E. Mena Ramírez, J. Villaseñor Camacho, M.A. Rodrigo Rodrigo, P. Cañizares Cañizares, Combination of bioremediation and electrokinetics for the in-situ treatment of diesel polluted soil: a comparison of strategies. Sci. Total Environ. 533, 307–316 (2015)
A.T. Yeung, Y.Y. Gu, A review on techniques to enhance electrochemical remediation of contaminated soils. J. Hazard. Mater. 195, 11–29 (2011)
S.S. Kim, S.J. Han, Application of an enhanced electrokinetic ion injection system to bioremediation. Water Air Soil Pollut. 146, 365–377 (2003)
Q. Luo, H. Wang, X. Zhang, X. Fan, Y. Qian, In situ bioelectrokinetic remediation of phenol-contaminated soil by use of an electrode matrix and a rotational operation mode. Chemosphere 64, 415–422 (2006)
X. Fan, H. Wang, Q. Luo, J. Ma, X. Zhang, The use of 2D non-uniform electric field to enhance in situ bioremediation of 2,4-dichlorophenol-contaminated soil. J. Hazard. Mater. 148, 29–37 (2007)
M.J. Harbottle, G. Lear, G.C. Sills, I.P. Thompson, Enhanced biodegradation of pentachlorophenol in unsaturated soil using reversed field electrokinetics. J. Environ. Manage. 90, 1893–1900 (2009)
T. Li, S. Guo, B. Wu, L. Zhang, Y. Gao, Effect of polarity-reversal and electrical intensity on the oil removal from soil. J. Chem. Technol. Biotechnol. 90, 441–448 (2015)
S. Barba, J. Villaseñor, M.A. Rodrigo, P. Cañizares, Effect of the polarity reversal frequency in the electrokinetic-biological remediation of oxyfluorfen polluted soil. Chemosphere 177, 120–127 (2017)
S. Barba, J. Villaseñor, M.A. Rodrigo, P. Cañizares, Can electro-bioremediation of polluted soils perform as a self-sustainable process? J. Appl. Electrochem. 48, 579–588 (2018)
C.A.B. Schmidt, M.C. Barbosa, M.D.S.S. de Almeida, A laboratory feasibility study on electrokinetic injection of nutrients on an organic, tropical, clayey soil. J. Hazard. Mater. 143, 655–661 (2007)
S. Suni, E. Malinen, J. Kosonen, H. Silvennoinen, M. Romantschuk, Electrokinetically enhanced bioremediation of creosote-contaminated soil: laboratory and field studies. J. Environ. Sci. Health A 42, 277–287 (2007)
W. Xu, C. Wang, H. Liu, Z. Zhang, H. Sun, A laboratory feasibility study on a new electrokinetic nutrient injection pattern and bioremediation of phenanthrene in a clayey soil. J. Hazard. Mater. 184, 798–804 (2010)
E. Mena Ramírez, J. Villaseñor Camacho, M.A. Rodrigo Rodrigo, P. Cañizares Cañizares, Feasibility of electrokinetic oxygen supply for soil bioremediation purposes. Chemosphere 117, 382–387 (2014)
J.H. Choi, S. Maruthamuthu, H.G. Lee, T.H. Ha, J.H. Bae, Nitrate removal by electro-bioremediation technology in Korean soil. J. Hazard. Mater. 168, 1208–1216 (2009)
A. Tiehm, T. Augenstein, D. Ilieva, H. Schell, C. Weidlich, K.M. Mangold, Bio-electro-remediation: electrokinetic transport of nitrate in a flow-through system for enhanced toluene biodegradation. J. Appl. Electrochem. 40, 1263–1268 (2010)
X. Wu, D.B. Gent, J.L. Davis, A.N. Alshawabkeh, Lactate injection by electric currents for bioremediation of tetrachloroethylene in clay. Electrochim. Acta 86, 157–163 (2012)
E. Mena, C. Ruiz, J. Villaseñor, M.A. Rodrigo, P. Cañizares, Biological permeable reactive barriers coupled with electrokinetic soil flushing for the treatment of diesel polluted clay soil. J. Hazard. Mater. 283, 131–139 (2015)
S. Barba, M. Carvela, J. Villaseñor, M.A. Rodrigo, P. Cañizares, Improvement of the electro-bioremediation process of a non-polar herbicide polluted soil by means of surfactant addition. Sci. Total Environ. 650, 1961–1968 (2019)
X. Mao, J. Wang, A. Ciblak, E.E. Cox, C. Riis, M. Terkelsen, D.B. Gent, A.N. Alshawabkeh, Electrokinetic-enhanced bioaugmentation for remediation of chlorinated solvents contaminated clay. J. Hazard. Mater. 213–214, 311–317 (2012)
E.L. Secord, A. Kottara, P. Van Cappellen, A.T. Lima, Inoculating bacteria into polycyclic aromatic hydrocarbon-contaminated oil sands soil by means of electrokinetics. Water Air Soil Pollut. 227, 288–301 (2016)
E.K. Nyer, In situ treatment technology (Lewis Publishers, Boca Raton, 2001)
H.I. Chung, M. Lee, A new method for remedial treatment of contaminated clayey soils by electrokinetics coupled with permeable reactive barriers. Electrochim. Acta 52, 3427–3431 (2007)
B. Fonseca, M. Pazos, T. Tavares, M.A. Sanromán, Removal of hexavalent chromium of contaminated soil by coupling electrokinetic remediation and permeable reactive biobarriers. Environ. Sci. Pollut. Res. 19, 1800–1808 (2012)
E. Mena, J. Villaseñor, P. Cañizares, M.A. Rodrigo, Influence of electric field on the remediation of polluted soil using a biobarrier assisted electro-bioremediation process. Electrochim. Acta 190, 294–304 (2016)
S. Barba, M. Carvela, J. Villaseñor, M.A. Rodrigo, P. Cañizares, In-situ electro-bioremediation of soil polluted with organochlorinated compounds, in: 7th European Bioremediation Conference & 11th ISEB Conference, Chania, 25–28 June 2018
M. Megharaj, B. Ramakrishnan, K. Venkateswarlu, N. Sethunathan, R. Naidu, Bioremediation approaches for organic pollutants: a critical perspective. Environ. Int. 37, 1362–1375 (2011)
T.T. Fida, S.K. Moreno-Forero, P. Breugelmans, H.J. Heipieper, W.F.M. Röling, D. Springael, Physiological and transcriptome response of the polycyclic aromatic hydrocarbon degrading Novosphingobium sp. LH128 after inoculation in soil. Environ. Sci. Technol. 51, 1570–1579 (2017)
I. Hassan, E. Mohamedelhassan, E.K. Yanful, Z.C. Yuan, A review article: electrokinetic bioremediation current knowledge and new prospects. Adv. Microbiol. 6, 57–72 (2016)
E. Mena, S. Barba, C. Sáez, V. Navarro, J. Villaseñor, M.A. Rodrigo, P. Cañizares, Pre scale-up of electrobioremediation processes, in Geo-Chicago 2016. Sustainable Waste Management and Remediation, Geotechnical Special Publication, 273, (American Society of Civil Engineers, Reston, 2016), pp. 264–273
S. Barba, R. López-Vizcaíno, C. Saez, J. Villaseñor, P. Cañizares, V. Navarro, M. Rodrigo, Electro-bioremediation at the prototype scale: what it should be learned for the scale-up. Chem. Eng. J. 334, 2030–2038 (2018)
Acknowledgments
Financial support from the Spanish Government and European Union through projects CTM2016-76197-R (AEI/FEDER, UE) from Ministry of Economy, Industry and Competitiveness, and EQC2018-004240-P from Ministry of Science, Innovation and Universities is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Villaseñor Camacho, J. (2021). Electrobioremediation of Polluted Soils. In: Rodrigo, M.A., Dos Santos, E.V. (eds) Electrochemically Assisted Remediation of Contaminated Soils. Environmental Pollution, vol 30. Springer, Cham. https://doi.org/10.1007/978-3-030-68140-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-68140-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68139-5
Online ISBN: 978-3-030-68140-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)