Abstract
Different grasses and trees were tested for their growth in a crude oil contaminated soil. Three grasses, Lolium perenne, Leptochloa fusca, Brachiaria mutica, and two trees, Lecucaena leucocephala and Acacia ampliceps, were selected to investigate the diversity of hydrocarbon-degrading rhizospheric and endophytic bacteria. We found a higher number of hydrocarbon degrading bacteria associated with grasses than trees and that the endophytic bacteria were taxonomically different from rhizosphere associated bacteria showing their spatial distribution with reference to plant compartment as well as genotype. The rhizospheric soil yielded 22 (59.45 %), root interior yielded 9 (24.32 %) and shoot interior yielded 6 (16.21 %) hydrocarbon-degrading bacteria. These bacteria possessed genes encoding alkane hydroxylase and showed multiple plant growth-promoting activities. Bacillus (48.64 %) and Acinetobacter (18.91 %) were dominant genera found in this study. At 2 % crude oil concentration, all bacterial isolates exhibited 25 %–78 % oil degradation and Acinetobacter sp. strain BRSI56 degraded maximum. Our study suggests that for practical application, support of potential bacteria combined with the grasses is more effective approach than trees to remediate oil contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The release of petroleum oil in soil and water, due to various human activities, is posing serious threats to our environment. Petroleum hydrocarbons are considered very hazardous to living organisms due to their toxicity, mutagenicity and carcinogenicity (Anderson et al. 2014; Cohen et al. 2014). The combined use of plants and hydrocarbon-degrading microorganisms is a promising strategy for the cleanup of environment polluted with petroleum hydrocarbons (Lin et al. 2008; Afzal et al. 2013a; Khan et al. 2013a). During phytoremediation of soil polluted with hydrocarbons, plant-associated rhizobacteria largely participate in the mineralization of these contaminants. The proliferation and activity of pollutant-degrading rhizobacteria are maintained through the release of root exudates. As plants can take up and accumulate organic pollutants in their roots, shoots and leaves, endophytic bacteria seem to be the best candidate for their degradation in planta. Endophytic bacteria colonize plant tissues without causing any apparent symptoms of disease (Sessitsch et al. 2002).
Both grasses and trees have been found to be suitable for the cleanup of crude oil polluted soil (Tesar et al. 2002; Yousaf et al. 2010). Grasses have an extensive root system which provides a high root surface area for the colonization of pollutant-degrading bacteria and nutrient uptake (Frank and Dugas 2001). Trees show fast growth and high biomass production and also enhance microbial mineralization of organic pollutants (Tesar et al. 2002).
The microbial capability to mineralize hydrocarbons is mainly attributed to enzymes such as the alkane monooxygenase encoded by alkB and cytochrome P450 alkane hydroxylase encoded by CYP153 (van Beilen and Funhoff 2007). In addition to degrading organic pollutants, bacteria can also improve plant growth due to their plant growth-promoting activities, such as 1-amino-cyclopropane-1-carboxylic acid (ACC) deaminase, siderophore production and phosphorous solubilization (Naveed et al. 2014).
Regarding bacterial-assisted phytoremediation of hydrocarbon polluted soil, less knowledge exists on the diversity and distribution of rhizospheric and endophytic bacteria associated with grasses and trees and their hydrocarbon-degrading and plant growth-stimulating activities. Therefore, the objective of the present study was to assess whether grasses and trees growing in crude oil contaminated soil were hosting distinct hydrocarbon-degrading and/or plant growth-stimulating bacteria, which might affect the phytoremediation efficacy. Based on the capability to grow in crude oil polluted soil, we selected three grass species, Lolium perenne, Leptochloa fusca, Brachiaria mutica, and two tree species Lecucaena leucocephala and Acacia ampliceps, and determined the diversity and distribution of rhizospheric and endophytic bacteria associated with these plant species. Moreover, hydrocarbon degrading and plant growth promoting activities of isolated bacteria were determined.
Materials and Methods
Crude oil contaminated soil (25.6 g oil kg−1 soil, pH 7.4, electrical conductivity 3.7 ds m−1, clay 26.5 %, silt 19.7 %, sand 53.8 %, total bacterial population 2.7 × 105 cfu g−1 soil, N 0.02 % and p 0.02 %) was collected from an oil pumping site located in Chakwal, Pakistan. Soil was homogenized manually by thorough mixing and sieved with a 2 mm sieve and subsequently transferred into pots. One hundred seeds of grass/one seedling of tree of 27 different plant species were sown/planted in these pots in triplicates. Seeds/seedlings were also planted in uncontaminated agricultural soil (pH 7.2, electrical conductivity 3.9 ds m−1, clay 28.6 %, silt 19.3 %, sand 52.1 %, total bacterial population 6.7 × 105 cfu g−1 soil, total N 0.033 %, p 0.08 % and organic matter 0.34 %). The biomass of each plant species vegetated in the crude oil contaminated soil was determined and compared with that produced in the uncontaminated soil. The growth and biomass of L. perenne, L. fusca, B. mutica, L. leucocephala and A. ampliceps were least affected by the crude oil-contamination (Table 1) and were chosen for the isolation of rhizospheric and endophytic bacteria.
Grasses and trees were grown for about 3 and 6 months, respectively. The grasses have shorter life span than trees, therefore, the growth period of grasses was shorter than trees. The plants were uprooted carefully, the soil closely attached to roots was collected and the shoots were cut 2 cm above the soil surface. The isolation of hydrocarbon degrading rhizospheric and endophytic bacteria was performed on minimal medium (having 1 % filtered sterilized diesel as sole carbon source) as explained previously (Yousaf et al. 2010). All the isolates were differentiated by restriction fragment length polymorphism (RFLP) analysis as described earlier (Afzal et al. 2013b). On the basis of RFLP analysis, 37 isolates were distinguished and identified by 16S rRNA gene sequencing. Sequences were subjected to BLAST analysis with NCBI database and submitted to GenBank (accession numbers KF478211-KF478226, KF478228-KF478231, KF478235-KF478236, KF478238-KF478241, KF318035-KF318040, KJ620868-KJ620869, KJ620860, KJ620863 and KF312211).
Strains were tested for their ability to utilize alkanes and crude oil as sole carbon source by growing them in flasks containing liquid minimal medium amended with either 2 % (v/v or w/v) of crude oil and n-alkanes (C8, C10, C12 and C16). The flasks were incubated for 7 days at 37 ± 2°C. The amount of residual hydrocarbons/oil was analyzed as described earlier (Das and Mukherjee 2007). The presence of two different alk genes (alkB and CYP153) in hydrocarbon-degrading bacterial strains was determined as demonstrated previously (Yousaf et al. 2010). Different plant growth-promoting activities were determined using the protocols as described earlier (Naveed et al. 2014). Phosphate solubilization activity was determined by development of clear zone around bacterial growth on Pikovskaya’s agar medium. Bacterial isolates were assayed for siderophore production on the Chrome azurol S (CAS) agar medium. ACC deaminase activity of the isolates was tested on minimal medium containing 0.7 g ACC L−1 as sole nitrogen source. The IAA production activity was determined using Salkowski reagent.
Results and Discussion
All plant species tested in the present study exhibited reduced growth and less biomass production as compared to plants vegetated in uncontaminated soil. Among others, Sorghum bicolor, Terminalia bellirica, Camelina sativa, Trifolium alexandrium and Conocarpus erectus plant species showed reduced growth (83.15 %, 67.69 %, 62.60 %, 62.18 % and 59.13 %, respectively) in hydrocarbon contaminated soil as compared to plants vegetated in uncontaminated soil, hence were considered as more hydrocarbon-sensitive plants. Crude oil inhibits plant growth and biomass production due to the toxic nature of its low molecular weight components. Often, contaminants such as petroleum hydrocarbons alter the physical and chemical properties of the soil. Hydrophobic contaminants can change the water/soil interactions that would normally occur, thereby potentially affecting oxygen transfer, available water uptake, and nutrient mobility (Nwaichi et al. 2010; Khan et al. 2013b). Biomass production of L. perenne, L. fusca, B. mutica, L. leucocephala, and A. ampliceps was least affected by the crude oil-contamination as compared to the respective plants vegetated in uncontaminated soil. These five plants (three grasses and two tree plants) were considered as hydrocarbon-tolerant species and chosen for analysis of plant-associated rhizospheric and endophytic bacterial communities.
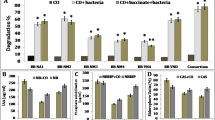
By a cultivation-dependent approach, 37 hydrocarbon-degrading rhizospheric and endophytic bacteria were obtained that can utilize crude oil as sole carbon source. On the whole, the rhizosphere soil yielded 22 (59.45 %), root interior yielded 9 (24.32 %) and shoot interior yielded 6 (16.21 %) hydrocarbon-degrading bacterial isolates (Table 2). The maximum numbers (29.72 %) of the diesel-utilizing bacteria were found to be associated with L. perenne plant (both rhizo- and endophytes), of which Bacillus species were dominant (54.54 %). It has been reported that this grass can host a large number of microorganisms because of its fibrous root system providing a large surface area for microbial colonization (Yousaf et al. 2010). The fact that Bacillus sp. strains are frequent colonizers of the rhizosphere of grasses and trees further shows that the members of this genus might be dominant among hydrocarbon degrading bacterial community (Peng et al. 2013). Besides Bacillus sp., some Staphylococus and Oceanimonas sp. strains were also detected in the rhizosphere and root/shoot interior of L. perenne. Bacterial population associated with the other plants was more limited in terms of diversity as compared to L. perenne. Higher numbers of bacteria were isolated from the rhizosphere, root and shoot of both B. mutica (24.32 %) and L. leucocephala (21.62 %) as compared to A. ampliceps and L. fusca. Similarly, Yousaf et al. (2010) and Siciliano et al. (2001) observed that the colonization of diesel-utilizing bacteria in the rhizosphere and endosphere of a host depends on plant species.
Root exudates and other plant metabolites determine the potential, density and diversity of microorganisms in the rhizosphere or endosphere (Costa et al. 2006). Grasses are known to release alkanes in soil (Marseille et al. 1999), which might favor the population of hydrocarbon-degrading bacteria in the vicinity. Only Bacillus cereus was found in the rhizospheric soil and root and shoot of three different plants, showing that mostly distinct plant species host different hydrocarbon degraders.
In the present study, all cultured rhizospheric and endophytic bacterial strains showed 99 % sequence similarity to known 16S rRNA genes when subjected to BLAST analysis. Generally, endophytic bacteria were different from rhizospheric bacteria and also shoot and root possessed different hydrocarbon-degrading bacteria. The fact that a higher number of bacterial endophytes were isolated from root as compared shoot supports the theory that the density of the endophytic population decreases with the distance from their entry points such as root tip and/or the site of the emergence of lateral roots (Khan et al. 2013a). These endophytes may play a great role in reducing the toxicity of the hydrocarbons that are taken up by plants (Afzal et al. 2013b).
On the whole, two bacterial genera i.e., Bacillus (48.64 %) and Acinetobacter (18.91) were found dominant both in the rhizosphere as well as endosphere. Both of these genera have been frequently reported to be involved in the degradation of hydrocarbons (Tesar et al. 2002; Yousaf et al. 2010). It was important to note that in the rhizosphere and shoot interior of A. ampliceps, four out of five strains were Acinetobacter spp., showing that strains of this genus form tight endophytic association with A. ampliceps. A large number of bacteria were unable to proliferate in contaminated soil, and possibly were outcompeted by the pollutant-degrading bacteria. Moreover, it might be due to the toxicity of low molecular weight alkanes and aromatic compounds present in crude oil (Tesar et al. 2002).
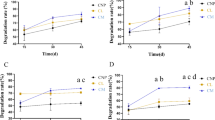
Many rhizospheric and endophytic bacteria have been reported to be tolerant of hydrocarbons, and some of these can even utilize them as sole carbon source (Tesar et al. 2002). In this study, all isolated bacterial strains were tested for their ability to degrade different hydrocarbons (C8, C10, C12 and C16) and crude oil. Among all isolated bacterial strains, only Acinetobacter sp. strain LCRH81, isolated from the rhizosphere of L. leucocephala, could utilize all tested alkanes (Table 3) and also possessed alkB gene. However, eight strains could not utilize any of the tested alkanes although they showed growth on crude oil (Table 4). They were possibly involved in the utilization of other crude oil components such as low molecular weight alkanes and/or aromatic hydrocarbons. The highest proportion of crude oil was degraded by Acinetobacter sp. strain BRSI56 followed by Acinetobacter sp. strain ACRH77, Acinetobacter sp. strain LCRH81 and Pseudomonas aeruginosa strain BRRI54 with 78 %, 77 %, 72 % and 71 %, respectively.
Although most of the bacterial strains showed potential to degrade hydrocarbons, only 54.05 % of them showed the amplification of alkB and CYP153 genes with the primers used in this study. Most of the strains isolated from the grasses possessed alkB and CYP153 genes, as revealed by PCR. This suggests that the genes or the mechanism involved in hydrocarbon-degradation in bacteria associated with trees might be different so different set of degenerate primers may be used for the amplification of genes involved in the phenomenon.
The beneficial effects of rhizospheric and endophytic bacteria on their host plant appear to occur through mechanisms widely described for plant growth promoting (PGP) bacteria. In this study, many of the isolated rhizo/endophytic bacteria showed the in vitro PGP activities or mechanisms that are thought to be responsible for plant growth promotion (Table 3). It could be one of the possible reasons of the survival and growth of the 5 selected plants in the crude oil contaminated soil. Most of the strains showed the ability to produce IAA and ACC deaminase showing that these two mechanisms are more common among the hydrocarbon-degrading bacteria. It has been reported that ACC deaminase activity plays a key role in plant–microbe partnerships especially under stress conditions including contaminant stress. This bacterial ACC deaminase enzyme regulates ethylene levels in plants and consequently contributes to the production of longer roots (Khan et al. 2013a). Phosphorus solubilization activity was found to be relatively less common. One strain, Klebsiella sp. strain LCRI87, isolated from the root of L. leucocephala exhibited multiple plant growth promoting activities.
Grasses and trees vegetated in the crude oil contaminated soil hosted versatile, heterogeneously distributed rhizospheric and endophytic bacteria, which may influence the efficiency of plants to stimulate the mineralization of organic pollutants. Regarding the application of plants for phytoremediation of hydrocarbon contaminated soil, where a large number of hydrocarbon degraders is essentially needed for the maximum degradation of pollutants, two grass species, L. perenne and B. mutica and one tree species, L. leucocephala, seem to be more appropriate than other plants investigated in this study. Further studies are in progress to evaluate and quantify the potential of selected rhizo/endophytic bacteria for hydrocarbon degradation and plant growth promotion during phytoremediation of crude oil-contaminated soil.
References
Afzal M, Yousaf S, Reichenauer TG, Sessitsch A (2013a) Ecology of alkane-degrading bacteria and their interaction with the plant. In: Bruijn FJD (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken, pp 975–989
Afzal M, Khan S, Iqbal S, Mirza MS, Khan QM (2013b) Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int Biodeterior Biodegrad 85:331–336
Anderson JA, Kuhl AJ, Anderson AN (2014) Toxicity of oil and dispersed oil on Juvenile Mud Crabs, Rhithropanopeus harrisii. Bull Environ Contam Toxicol 92:375–380
Cohen JH, Mccormick LR, Burkhardt SM (2014) Effects of dispersant and oil on survival and swimming activity in a marine copepod. Bull Environ Contam Toxicol 92:381–387
Costa R, Gotz M, Mrotzek N, Lottmann J, Berg G, Smalla K (2006) Effects of site and plant species on rhizosphere community structure as revealed by molecular analysis of microbial guilds. FEMS Microbiol Ecol 56:236–249
Das K, Mukherjee AK (2007) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol 98:1339–1345
Frank AB, Dugas WA (2001) Carbon dioxide fluxes over a northern, semiarid, mixed-grass prairie. Agric For Meteorol 108:317–326
Khan S, Afzal M, Iqbal S, Khan QM (2013a) Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90:1317–1332
Khan S, Afzal M, Iqbal S, Mirza MS, Khan QM (2013b) Inoculum pretreatment affects bacterial survival, activity and catabolic gene expression during phytoremediation of diesel contaminated soil. Chemosphere 91:663–668
Lin X, Li X, Li P, Li F, Zhang L, Zhou Q (2008) Evaluation of plant-microorganism synergy for the remediation of diesel fuel contaminated soil. Bull Environ Contam Toxicol 81:19–24
Marseille F, Disnar J, Guillet B, Noack Y (1999) n-Alkanes and free fatty acids in humus and A1 horizons of soils under beech, spruce and grass in the Massif-Central (Mont-Lozère), France. Eur J Soil Sci 50:433–441
Naveed M, Mitter B, Yousaf S, Pastar M, Afzal M, Sessitsch A (2014) The endophyte Enterobacter sp. FD17: a maize growth enhancer selected based on rigorous testing of plant beneficial traits and colonization characteristics. Biol Fertil Soils 50:249–262
Nwaichi EO, Onyeike EN, Wegwu MO (2010) Characterization and safety evaluation of the impact of hydrocarbon contaminants on ecological receptors. Bull Environ Contam Toxicol 85:199–204
Peng A, Liu J, Gao Y, Chen Z (2013) Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from soils contaminated by polycyclic aromatic hydrocarbons. PLoS ONE 8:e83054
Sessitsch A, Reiter B, Pfeifer U, Wilhelm E (2002) Cultivation-independent population analysis of bacterial endophytes in three potato varieties based on eubacterial and Actinomycetes-specific PCR of 16S rRNA genes. FEMS Microbiol Ecol 39:23–32
Siciliano SD, Fortin N, Mihoc A, Wisse G, Labelle S, Beaumier D, Ouellette D, Roy R, Whyte LG, Banks MK, Schwab P, Lee K, Greer CW (2001) Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol 67:2469–2475
Tesar M, Reichenauer TG, Sessitsch A (2002) Bacterial rhizosphere populations of black poplar and herbal plants to be used for phytoremediation of diesel fuel. Soil Biol Biochem 34:1883–1892
Van Beilen JB, Funhoff EG (2007) Alkane hydroxylases involved in microbial alkane degradation. Appl Microbiol Biotechnol 74:13–21
Yousaf S, Andria V, Reichenauer TG, Smalla K, Sessitsch A (2010) Phylogenetic and functional diversity of alkane degrading bacteria associated with Italian ryegrass (Lolium multiflorum) and birdsfoot trefoil (Lotus corniculatus) in a petroleum oil-contaminated environment. J Hazard Mater 184:523–532
Acknowledgments
The authors would like to thank Higher Education Commission (HEC), Government of Pakistan, for providing the financial support (Grant Number 1997) for this study. We also thank Mr. Sajad Ali for his help with the pot experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatima, K., Afzal, M., Imran, A. et al. Bacterial Rhizosphere and Endosphere Populations Associated with Grasses and Trees to be Used for Phytoremediation of Crude Oil Contaminated Soil. Bull Environ Contam Toxicol 94, 314–320 (2015). https://doi.org/10.1007/s00128-015-1489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1489-5