Abstract
The link between metal enrichment and the addition of a magmatic volatile phase in volcanogenic massive sulfide deposits and actively forming seafloor massive sulfide deposits remains poorly characterized. This is especially true when considering how metal, sulfur and fluid flux change with time. In this study, we combine in situ sulfur isotope (δ34S; n = 31) measurements with trace metal chemistry of pyrite (n = 143) from the Mala VMS deposit, Troodos, Cyprus. The aim of our study is to assess the links between volatile influx and metal enrichment and establish how, or indeed if, this is preserved at the scale of individual mineral grains. We classify pyrite based on texture into colloform, granular, disseminated and massive varieties. The trace metal content of different pyrite textures is highly variable and relates to fluid temperature and secondary reworking that are influenced by the location of the sample within the mound. The sulfur isotope composition of pyrite at Mala ranges from − 17.1 to 7.5‰ (n = 31), with a range of − 10.9 to 2.5‰ within a single pyrite crystal. This variation is attributed to changes in the relative proportion of sulfur sourced from (i) SO2 disproportionation, (ii) thermochemical sulfate reduction, (iii) the leaching of igneous sulfur/sulfide and (iv) bacterial sulfate reduction. Our data shows that there is no correlation between δ34S values and the concentration of volatile elements (Te, Se) and Au in pyrite at Mala indicating that remobilization of trace metals occurred within the mound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The source of metals in volcanogenic massive sulfide (VMS) and actively forming seafloor massive sulfide (SMS) deposits remains poorly constrained and actively debated (Sillitoe et al. 1996; Yang and Scott 1996; Jowitt et al. 2012; Keith et al. 2018; Martin et al. 2020; Patten et al. 2020). Principally, two metal sources can contribute to the metal budget of VMS deposits: leaching of metals from underlying host rocks during hydrothermal alteration (Richardson et al. 1987; Jowitt et al. 2012; Banerjee et al. 2000; Patten et al. 2017) and the addition of a magmatic volatile phase to the overlying hydrothermal system (Yang and Scott 1996, 2002; de Ronde et al. 2011; Martin et al. 2020, 2021; Patten et al. 2020). The relative contribution of metals from these two sources and how they change with system maturity (i.e. time) and the subsequent preservation of trace metal signatures to distinguish these sources in sulfide minerals in VMS deposits remains enigmatic. Here, we apply in situ geochemical and sulfur isotope measurements on pyrite from the 92-million-year-old Mala VMS deposit of the Troodos ophiolite (Cyprus) to assess co-variations in trace metal content and sulfur isotope ratios (δ34S) in an ancient mafic VMS deposit. These data will be used to investigate the variability in metal and sulfur sources during the growth of individual pyrite crystals and to understand if the influx of certain volatile metals can be linked to variations in sulfur isotopic composition.
To investigate the source(s) of metals in VMS deposits, previous studies utilized either trace metal enrichment profiles (Halbach et al. 1998; Butler and Nesbitt 1999; Maslennikov et al. 2009; Wohlgemuth-Ueberwasser et al. 2015; Keith et al. 2016a; Grant et al. 2018; Wang et al. 2018), the ratio of Se to S (Yamamoto 1976; Huston et al. 1995; Hannington et al. 1999; Layton-Matthews et al. 2008, 2013; Martin et al. 2019) or the sulfur isotopic composition of sulfide minerals (Herzig et al. 1998; Gemmel et al. 2004; Huston et al. 2011; Yeats et al. 2014; Brueckner et al. 2015; Lode et al. 2017; Zeng et al. 2017). However, very few studies utilize a combined approach (e.g. Rouxel et al. 2004; Sharman et al. 2015; Meng et al. 2020; Martin et al. 2021) that takes into consideration any systematic relationship between trace element enrichment profiles and the sulfur isotopic composition of sulfide minerals; this is especially true when considering the complex nature of different pyrite textures, generations (i.e. overgrowths) and variation that occurs at the scale of individual mineral grains.
In volatile-rich, subduction influenced VMS deposits, the systematic enrichment of certain metals such as Pb, As, Sb, Bi, Hg and Te (Wohlgemuth-Ueberwasser et al. 2015); Se, Cu and Te (Keith et al. 2016a); Bi and Te (Mathieu 2019); or Se, Cu, Te and Au (Martin et al. 2021) have been proposed to indicate the addition of a metal-rich magmatic volatile phase to the hydrothermal system from degassing of shallow magma reservoirs (e.g. Huston et al. 2011). Additionally, elevated Se/S (expressed as Se/S*106) that are > 500 in pyrite are also interpreted as representing an increased magmatic volatile influx in VMS deposits (Yamamoto 1976; Huston et al. 1995; Layton-Matthews et al. 2008, 2013). The sulfur isotopic composition of sulfide minerals provides further evidence of a magmatic volatile contribution to VMS deposits with the addition of degassing magmatic SO2 that undergoes disproportionation upon mixing with hydrothermal fluid and results in a characteristic sulfur isotope composition defined by δ34S values that are less than the magmatic mean (< 0‰, MORB; Herzig et al. 1998; Kusakabe et al. 2000; de Ronde et al. 2005). These low δ34S values are generally limited to felsic or subduction influenced environments (e.g. Herzig et al. 1998; Huston et al. 2011), where magmas are more volatile-rich relative to mafic-hosted mid-ocean ridge (MOR) environments (Wallace 2005). However, this signature was also recorded in an ancient mafic VMS deposit (Martin et al. 2021). This sulfur isotope composition contrasts signatures typical of MOR environments, where δ34S values in sulfide minerals are positive, indicating a combination of sulfur from thermochemical sulfate reduction (TSR) of seawater at high temperatures (> 160 °C) in the presence of iron-bearing minerals and leaching of sulfur and primary igneous sulfide minerals from igneous host rocks (Ono et al. 2007).
Previous studies utilising laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) of sulfide minerals from Troodos VMS deposits indicate that deposits associated with the Solea graben, one of three fossil spreading axes in the Troodos ophiolite are enriched in Se, Cu and Au (Keith et al. 2016a; Fig. 1). The enrichment of these elements is attributed to the contribution of a magmatic volatile phase to the hydrothermal systems of the Solea graben (Martin et al. 2019, 2020).
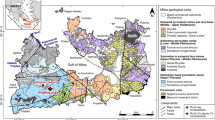
Simplified geological map of the Troodos ophiolite, Cyprus. Graben axes are indicated by the dashed lines. Mala is located in the SW of the ophiolite at the LPL/BG transitional horizon (after Martin et al. 2018)
Variation in the sulfur isotope composition of pyrite has largely been constrained in Troodos VMS deposits using whole-rock analytical methods of sulfide minerals with the median composition for all Troodos VMS sulfide minerals of 4.6 ± 2.7‰ (1σ, n = 220) indicating that sulfur is primarily sourced from both the leaching of igneous sulfur/sulfide and TSR of seawater (Hannington et al. 1998; Parvaz 2014; Keith et al. 2016a; Pedersen et al. 2017; Martin et al. 2020). However, significant variation is noted in the sulfur isotope composition of pyrite across Troodos VMS deposits with the δ34S values of pyrite ranging from − 7.6 to 13.2‰ (Martin et al. 2020, 2021). Samples of disseminated pyrite that occur within the volcanic stratigraphy exhibit significantly lower δ34S values than those typical for Troodos VMS deposits; pyrite is depleted in 34S, with δ34S values ranging from − 22.2 to − 6.9‰. These values indicate that bacterial sulfate reduction (BSR) occurred at low temperatures (< 120 °C) in the shallow subsurface, away from areas of high-temperature fluid discharge (Pedersen et al. 2017).
Sulfide mineral chemistry and the sulfur isotopic composition of sulfide minerals analysed using bulk analytical techniques leads to the homogenization of any variation in the chemical and isotopic composition of the sample. Thus, there is a need to apply in situ analytical techniques to better understand the spatial zonation in isotopic and chemical composition within individual mineral grains. Utilizing a combined approach of in situ LA-ICP-MS analysis to measure trace metal concentrations and secondary ion mass spectrometry (SIMS) to measure sulfur isotope ratios, this study aims to establish if links exist between trace metal enrichment and variations in the source of sulfur during VMS deposit formation. We focus on the Mala VMS deposit located in the Troodos ophiolite of Cyprus, as Mala represents an immature magmatic volatile-influenced deposit where links between magmatic volatile influx, sulfur isotope ratios and trace metal enrichment signatures in pyrite have not been extensively modified during fluid overprinting associated with the maturation of the VMS mound (Martin et al. 2021). We present in situ sulfur isotope data and trace element geochemistry of different pyrite textures that highlight variable trace element enrichment profiles that can be related to mound-scale fluid flow. We then combine trace metal geochemistry and sulfur isotope ratios to test if any systematic relationship exists between magmatic volatile influx and the enrichment of Te, Se and Au in pyrite at Mala.
Geological setting
The 92-Ma Troodos ophiolite in Cyprus comprises a complete, un-deformed oceanic pseudo-stratigraphy (Gass 1968; Mukasa and Ludden 1987). Mantle peridotites are surrounded radially by gabbro and plagiogranite of the plutonic sequence, a sheeted dyke complex (SDC), and the basal group (BG), which consists of a transitional horizon between the SDC and overlying upper and lower pillow lavas (UPL-LPL; Fig. 1) (Gass 1968). It has long been recognized that the Troodos ophiolite formed in a sediment-free subduction influenced environment due to the occurrence of boninite (Pearce and Robinson 2010; Woelki et al. 2018), the trace element and isotopic composition of volcanic glass (Rautenschlein et al. 1985), elevated H2O contents of parent melts (> 2 wt% H2O; Muenow et al. 1990) and an enrichment of magmatic volatile-derived elements (e.g. Te, Se) in some VMS deposits (Keith et al. 2016b; Martin et al. 2019, 2021).
Observations from actively forming intermediate to slow seafloor spreading centres are akin to processes and structures preserved in the Troodos ophiolite (Varga and Moores 1985). On the northern flank of the ophiolite, three structural grabens are delineated by inversely dipping sheeted dykes along a series of ~ north–south normal faults (Varga and Moores 1985). The grabens from east to west are Larnaca, Mitsero and Solea (Fig. 1). The grabens are widely accepted as representing fossil axial spreading ridges (Varga and Moores 1985). Associated with these graben bounding faults are VMS deposits that formed within or at the contact between the BG, LPL and UPL units at the periphery of the ophiolite that once represented the Cretaceous seafloor (Hannington et al. 1998; Adamides 2010; Fig. 1).
Hydrothermal alteration of the Troodos crust is well-characterized and the leaching of metals from the SDC during spilitisation and epidosite formation is interpreted as a possible source of metal and sulfur in overlying VMS deposits (Richardson et al. 1987; Jowitt et al. 2012; Patten et al. 2017). More recently, a pulsed magmatic volatile component has been suggested as an additional source of some metals (Cu, Au, Te and Se) in Troodos VMS deposits, especially in magmatic volatile-influenced VMS deposits such as Mala and deposits associated with the Solea graben in particular (Keith et al. 2016a; Martin et al. 2020; Fox et al. 2020).
The Mala VMS deposit
The Mala VMS deposit is located in the southwest Troodos ophiolite, northwest of the town of Pano Pangia within the Pafos Forest region (Fig. 1). Mala is located deep in the lava stratigraphy at the BG-LPL transition. Previous studies have identified an enrichment in Te, Se, Cu and Au in pyrite at Mala relative to other Troodos VMS deposits (Martin et al. 2021). Mala has been historically mined for Cu and Zn (0.8 Mt total; Brazilian Metals Group, 2013) leading to the exposure of a massive sulfide mound at the northern end of the open pit (Fig. 2).
Field observations from the Mala VMS deposit. A The exposed portion of the Mala mound. The VMS mound contains pyrite and crudely layered gypsum capped by a veneer of leached volcanic rocks. The margin of the VMS mound is denoted by the white dashed line. B Coarse-grained euhedral pyrite with infilling gypsum (white). C Massive pyrite. D Massive gypsum horizon located in the upper mound region containing coarse (1–2 cm) euhedral pyrite grains. E Gypsum containing finely disseminated euhedral pyrite. F Euhedral pyrite in a gypsum matrix with minor Fe oxide staining. G Aggregates of euhedral pyrite in gypsum
The exposed pyrite mound measures approximately 12 × 8 m (width × height) and contains pyrite and gypsum (Fig. 2A). Gypsum occurs as three distinct morphologies: massive-bedded, breccia infill and mesh textured varieties (Fig. 2B–G). Within the gypsum are euhedral pyrite crystals which vary in size from < 1 mm up to 2 cm in width (Fig. 2D–G). In the VMS mound pyrite forms as five distinct textures: massive euhedral grains (Fig. 3A), dendritic growths (Fig. 3B, C), colloform bands (Fig. 3D), granular pyrite (Fig. 3E) and as disseminated grains within gypsum and the surrounding wall rock (Fig. 3F). A previous study by Martin et al. (2021) demonstrated that the pyrite–gypsum relationships preserved at Mala are analogous to those observed in actively forming SMS deposits and reflect primary hydrothermal processes and are not the result of supergene weathering during uplift and exposure.
Photomicrographs in reflected light of common pyrite textures at Mala. A Massive pyrite. Note porous inclusion trails delineating crystal growth zones. B The relationship between massive and dendritic pyrite textures. C Dendritic pyrite. D Colloform pyrite in a matrix of porous pyrite. E Granular pyrite consisting of anhedral rounded pyrite in a matrix of Fe oxides and pyrite. F Finely disseminated euhedral to subhedral pyrite in surrounding altered volcanic rocks
Methods
Mineral chemistry
Laser ablation ICP-MS was used to determine in situ trace element concentrations in pyrite. Spot analyses (n = 143) were performed on 10 representative polished blocks. Analyte masses used were 57Fe, 65Cu, 59Co, 66Zn, 75As, 77Se, 109Ag, 111Cd, 121Sb, 125Te, 185Re, 189Os, 193Ir, 195Pt, 197Au 206Pb and 209Bi (Table S1, ESM). Data were collected using a New Wave Research UP213 laser coupled to a Thermo iCAP RQ-ICP-MS at Cardiff University, UK. Samples were analysed in time-resolved analysis mode with a nominal spot diameter of 55 μm at a frequency of 10 Hz. Each analysis lasted 40 s and a gas blank was measured for 20 s prior to each analysis. Data correction and the subtraction of gas blanks was performed using the Thermo Qtegra software. External calibration was performed on a series of synthetic NiFeS standards (see Prichard et al. 2013; Smith et al. 2016) and the reproducibility of analyses was monitored through the repeat analysis of UQAC FeS-1. The repeat analysis of UQAC FeS-1 yielded a relative standard deviation (RSD) of < 10% for Fe, Co, Ni, As, Ru, Rh, Pd, Ag, Re, Os, Ir, Pt, Au and Bi; < 15% for Cu, Se, Sb and Te; and < 20% for Zn and Pb. The RSD for Cd was 24% (Table S2, ESM). Detection limits for all elements analysed are available in ESM Table S2. Sulfur-33 was used as an internal standard for all analyses and a stoichiometric concentration of 53.5 wt% sulfur was used that is within error of the measured average sulfur concentration for pyrite from Troodos VMS deposits (Martin et al. 2019).
Sulfur isotope analysis
Secondary ion mass spectrometry microanalysis was used to determine the sulfur isotope composition (δ34S) of pyrite. Analysis was undertaken on the same samples analysed via LA-ICP-MS. Epoxy mounted polished blocks (n = 6) were first coated with 300 Å of Au and were analysed using a Cameca IMS 4f SIMS at the MAF-IIC Microanalysis Facility at Memorial University of Newfoundland following the procedures detailed in Brueckner et al. (2015) and Lode et al. (2017). Systematic analysis of points across pyrite grains was performed proximal to LA-ICP-MS ablation pits to determine variation and correlation between δ34S values and trace element concentrations. The sample was bombarded with a primary ion beam of 350–750 pA of Cs+, accelerated through a potential of 10 keV and focused into a 5–1-μm-diameter spot. The duration of each analyses was 16.3 min including 2 min of pre-sputtering. To discriminate between 33SH− (and 32SH2−) from 34S−, the instrument was operated with a medium contrast aperture (150 μm), with entrance and exit slits paired to give flat topped peaks at a mass resolving power of 2975 (10% peak height definition; Brueckner et al. 2015). Negatively charged sputtered secondary ions were accelerated into the mass spectrometer using a potential of + 4.5 keV. To exclude sulfur contamination from the sample surface, the spot was pre-sputtered for 120 s with a 15-μm square rastered beam prior to analysis. Reproducibility based on the repeat analysis of standard reference material pyrite-UL9 (δ34S = 16.3‰) and KH87 (δ34S = 0.4‰), is typically better than ± 0.4‰ (1σ) (Table S3, ESM). All analyses are reported in standard notation (‰) relative to Vienna-Canyon Diablo Troilite (V-CDT).
Results
Sample characterization
Samples in this study were collected from the exposed Mala VMS mound (Fig. 2A and Fig. S1, ESM). We subdivide pyrite samples based on texture into massive, dendritic, colloform, granular and disseminated varieties (Fig. 3). Massive pyrite occurs in discrete meter-scale pods consisting of coarse-grained (mm to cm) aggregates of euhedral grains (Figs. 2C and 3A and B). Multiple pyrite generations occur as overgrowths within massive pyrite samples and can be delineated by inclusion-rich zones at the grain margin (Fig. 3A). Within massive pyrite, dendritic horizons were common but were not analysed in this study (Fig. 3B, C). Colloform pyrite is rare, occurring spatially associated with granular horizons (Fig. 3D). Granular massive pyrite contains anhedral aggregates of highly resorbed and often spherical pyrite in a pyrite matrix (Fig. 3E). Disseminated pyrite grains are commonly euhedral and vary in size from < 1 mm to > 2 cm and occur within gypsum horizons as well as within the volcanic rocks that surround the massive sulfide mound (Figs. 2D and 3F).
Trace element geochemistry
To assess trace metal enrichment profiles, results are grouped based on pyrite texture, and for disseminated grains, the location of the analytical point in relation to the core or rim of the individual mineral grain that was analysed. The division between core and rim is arbitrary and depends on the number of analyses performed within an individual pyrite grain, which in turn reflects the size of the pyrite grain (Table S1, ESM).
Colloform pyrite (n = 15) contains the highest median concentrations of Au, Pb and Re at 0.13, 9.8 and 0.17 ppm, respectively. All Ni and Cd analyses are below the detection limit, as are the majority of Cu, Zn and Co (Table 1; Fig. 4A). The concentration of Se is relatively homogenous ranging from 190 to 302 ppm with a median concentration of 245 ppm (n = 15) (Fig. 4B, Table 1). The total measured trace metal content for colloform pyrite is 371 ppm (Fig. 4D). A strong positive correlation is noted between Au and Ag (R2 = 0.95) and a moderate positive correlation between Pb and Ag (R2 = 0.72), Te and Au (R2 = 0.56) and Te and Bi (R2 = 0.52).
Pyrite chemistry analysed via LA-ICP-MS. Pyrite analyses are divided based on grain morphology into disseminated (n = 78), massive (n = 40), granular (n = 10) and colloform (n = 15). Analyses that are below detection limit are excluded (Table S1, ESM). A Te vs. Co, B Te vs. Se, C Co vs. Se. D Total measured trace metal by pyrite texture (Co, Ni, Cu, Zn, As, Se, Ag, Cd, Sb, Te, Re, Au, Pb and Bi). Data in ESM, Table S1
Granular pyrite (n = 10) is depleted in most trace metals relative to other pyrite textures considered here. All analyses for Ni, Ag, Zn, Cd, Re and Pb are below detection limit as are the majority of analyses for Au, Bi, Cu and Sb (Table 1). Cobalt and Te are notably enriched relative to colloform pyrite with median concentrations of 17.9 and 5.9 ppm, respectively (n = 10; Fig. 4A; Table 1). As observed in colloform pyrite, the concentration of Se is relatively homogenous, ranging from 5 to 31 ppm, with a median concentration of 6.4 ppm (n = 10) (Fig. 4B, Table 1). With the exception of a moderate correlation between As and Se (R2 = 0.63), no notable correlations exist. Granular pyrite contains the lowest measured total trace metal content at 198 ppm (Fig. 4D).
Trace metal enrichment profiles in massive pyrite (n = 40) are highly variable between samples. For example, sample MAL 17 is enriched in Cu with a median concentration of 0.04 wt% with a range of between 0.01 and 0.11 wt% (n = 10) but is relatively depleted in Se with a median concentration of 61 ppm (n = 10) (Fig. 4A, Table 1). Across all massive samples, Se exhibits the most variation, ranging from 30 to 1253 ppm with a median concentration of 178 ppm (n = 40) (Fig. 4B). With the exception of one point, all analyses for Ni and Cd were below the detection limit (Table 1). Massive pyrite contains the second highest total measured metal content at 566 ppm (Fig. 4D). A weak correlation is noted between Te and Se (R2 = 0.38; Fig. 4B).
Grains of disseminated pyrite within gypsum (n = 78) contain the highest concentration of Se, Te, As and Bi relative to all other pyrite textures (Fig. 4A–C, Table 1). Trace metal concentrations are highly variable within and between individual samples relative to other pyrite textures analysed in this study. For example, Se concentrations range from 18 to 3261 ppm with a median concentration of 645 ppm (n = 78). Similar variability is also noted for Co, As and Te (Table 1; Fig. 4B, C). Sample MAL 05 is notably enriched in Se and Te relative to all other samples with median concentrations of 1668 and 16.4 ppm, respectively (n = 14). Disseminated grains have the highest total measured metal content at 981 ppm (Fig. 4D), largely consisting of Se and Cu. Disseminated pyrite grains are sub-divided further based on the location of the analytical point relative to the margin of the pyrite grain to assess metal enrichment trends across individual grains (Fig. 5). With the exception of Co and Cu that are enriched at the margin of grains (n = 43), pyrite cores exhibit a minor enrichment in As, Se, Te and Au relative the grain margins (n = 35; Fig. 5).
Pyrite chemistry analysed via LA-ICP-MS from the core (n = 35) and rim (n = 43) of individual disseminated pyrite grains. The rim of pyrite grains is enriched in Co and Cu and relative to the margin. Data in ESM, Table S1
Sulfur isotopes
Secondary ion mass spectrometry analysis was performed on representative samples of colloform (n = 6), granular (n = 2), massive (n = 9) and disseminated (n = 14) textured pyrite (Fig. 6, Table S3, ESM). The median δ34S value for all samples is − 1.2‰ (n = 31) with a range of 24.6‰. Granular pyrite exhibits the lightest median sulfur isotopic composition with two analyses yielding δ34S values of − 10.5‰ and − 4.7‰. Colloform pyrite consistently exhibits δ34S values < 0‰ ranging from − 3.8 to − 0.1‰ with a median of − 2.2‰ (n = 6) (Fig. 6). The sulfur isotopic composition of massive pyrite is highly variable, ranging from − 17.1 to 7.5‰ with a median composition of − 1.8‰ (n = 9).
Summary of sulfur isotope analyses (δ34S) analysed by SIMS (n = 31) and classified based on pyrite texture. Massive pyrite exhibits the largest range in its δ34S composition whilst colloform pyrite clusters between 0 and − 5‰. Data and standard information in ESM, Table S3
To assess intra-grain variation in sulfur isotopic composition of disseminated pyrite, transects of analytical points were analysed across different pyrite grains (Fig. 7). The same grains were then etched with NaClO for 90 s to reveal any chemical zonation. A significant amount of variability is recorded in a single pyrite grain, for example MAL 05 where δ34S values range from 2.5 to − 10.9‰ (sub-grain SG1) with a median of 2.0‰ (n = 4, Fig. 7A). There is no systematic relationship between δ34S values and the location of the analysis with respect to the core or rim of the grain, for example, low values do not only occur at the margin of the grain. This is best illustrated in sample MAL 05, an aggregate of three pyrite grains disseminated within gypsum where opposing edges of the pyrite sub-grain SG1 have δ34S values of − 10.9‰ and 2.5‰, respectively (Fig. 7A). Individual pyrite grains that are located within the same region of the VMS mound (Fig. 2 and Fig. S1, ESM) also exhibit notable variation, for example, two grains from sample MAL 11 (Fig. 7B, C) that have different median δ34S values of − 0.9‰ and 0.9‰, respectively (n = 7). Again, there is no systematic pattern of enrichment from the grain core to rim within these grains.
Discussion
Mound-scale trace element systematics
The trace metal content and ratio of metals in pyrite can be used as a proxy for the past physical and chemical conditions of hydrothermal fluids (e.g. temperature, pH, fO2 and fS2) (Butler and Nesbitt 1999; Hannington et al. 2005; Maslennikov et al. 2009; Genna and Gaboury 2015; Wohlgemuth-Ueberwasser et al. 2015; Keith et al. 2016b; Monecke et al. 2016; Grant et al. 2018; Wang et al. 2018). Thus, variations in trace metal enrichment profiles in pyrite in VMS deposits reflect changes in the physicochemistry of fluids and the flux of metals and fluid entering the VMS deposit during growth of the VMS mound. At the mound scale (~ 10 s of m), steep temperature gradients occur due to variations in the relative amounts of seawater ingress and hydrothermal fluid influx with depth within the mound. Increasing temperatures within the mound lead to the dissolution, recrystallization and remobilization of metals associated with lower temperature sulfide minerals (e.g. Zn, Au, Ag, As, Pb, Sb) from the mound interior to the cooler margin of the mound during zone refining (Eldridge et al. 1983; Hannington et al. 1986; Petersen et al. 2000; Galley et al. 2007). This process is preserved in active SMS deposits as systematic variations in trace metal enrichment profiles between different pyrite textures, such as colloform pyrite that forms near the seawater interface that is enriched in elements that are associated with low-temperature sulfide minerals or those sourced from seawater (e.g. Mo, Tl, Pb; Grant et al. 2018). We note the same systematic variation in the distribution of trace metals between different pyrite textures at the Mala VMS deposit.
Colloform pyrite occurs in active SMS deposits towards the margin of the sulfide mound or during the early stage of chimney growth in environments where seawater ingress is high and fluid temperatures are lower producing non-equilibrium conditions (Maslennikov et al. 2009, 2017; Melekestseva et al. 2014; Wohlgemuth-Ueberwasser et al. 2015; Keith et al. 2016a, b; Grant et al. 2018). Colloform pyrite from Mala is enriched in Au and Pb relative to all other pyrite and contains the lowest median concentration of Co, Cu and Te, with all Ni analyses below detection limit, indicating it formed at lower fluid temperatures. At these low concentrations, both Au and Pb are likely incorporated into the pyrite structure as a lattice bound substitution, whilst at higher concentrations inclusions may occur (Huston et al. 1995; Cook et al. 2009). This trace metal enrichment profile is similar to those reported in colloform pyrite from black smoker chimneys of Urals VMS deposits that are enriched in Au, Ag, Pb and elements derived from seawater such as Mo and Tl that were not analysed in this study (Maslennikov et al. 2009) or colloform pyrite from PACMANUS that is enriched in Au and As but depleted in Te and Se relative to massive pyrite (Wohlgemuth-Ueberwasser et al. 2015). A depletion in Co and Te, which are enriched in high-temperature pyrite varieties (e.g. massive pyrite) in active SMS deposits, further supports a low temperature origin for colloform pyrite at Mala (Wohlgemuth-Ueberwasser et al. 2015; Monecke et al. 2016; Grant et al. 2018). The trace element enrichment profile in colloform pyrite at Mala is comparable to colloform pyrite from the actively forming TAG SMS deposit located at the Mid-Atlantic Ridge (Grant et al. 2018) and from colloform pyrite at the Skouriotissa VMS deposit (Keith et al. 2016a). Primarily, this trace metal enrichment profile reflects the location of colloform pyrite at shallow depths within the VMS mound in close proximity to the seawater interface and its formation under lower fluid temperatures generated by increased amounts of seawater mixing relative to massive pyrite at deeper stratigraphic levels in the VMS mound (Monecke et al. 2016).
Mala colloform pyrite is strongly enriched in Se (Fig. 4) relative to concentrations reported for colloform pyrite at the Skouriotissa VMS deposit (Troodos) that average 12 ± 10 ppm (1σ, n = 52; Keith et al. 2016a), black smoker chimneys from Urals VMS deposits that average 9 ± 5 ppm (1σ, n = 8; Maslennikov et al. 2009), concentrations of < 4.5 ppm at the Semenov vent field (Melekestseva et al. 2014), and the TAG and PACMUS (Roman Ruins) deposits where colloform pyrite did not contain any detectable Se (Wohlgemuth-Ueberwasser et al. 2015; Grant et al. 2018). This concentration is also notably higher than that typically recorded in colloform pyrite in other Troodos VMS deposits where the median concentration is 49 ppm (Martin et al. 2019, 2020). Several studies indicate that Se is preferentially enriched in pyrite that formed in high-temperature regions of the VMS mound or the interior conduit of black smoker chimneys, indicating an affinity for high-temperature fluids (Butler and Nesbitt, 1999; Maslennikov et al. 2009; Revan et al. 2014; Grant et al. 2018). However, at Mala Se is also enriched in pyrite that is interpreted to have formed at lower temperatures, and we suggest that the enrichment of Se in colloform pyrite is a consequence of an increased magmatic volatile influx.
Granular textured massive pyrite occurs towards the margin of the sulfide mound and forms through the collapse and physical reworking of sulfide fragments during the growth of the VMS deposit (Humphris et al. 1995; Petersen et al. 2000). Granular pyrite contains the lowest total measured trace metal content but is relatively enriched in Te compared to other pyrite varieties (Table 1; Fig. 4A). We suggest that metal enrichment profiles in granular pyrite reflect the remobilization and subsequent loss of some metals during zone refining and oxidization of primary massive pyrite at low temperatures (< 100 °C) at or near the seafloor towards the periphery of the VMS mound (Herzig et al. 1991; Hannington et al. 1998; Fallon et al. 2017; Murton et al. 2019). Assuming that massive pyrite samples represent the primary trace metal content of granular samples prior to reworking, then all elements have been depleted during reworking with the exception of Te. The enrichment of Te, which is readily mobilized under oxidizing and low-temperature fluid conditions (e.g. McPhail 1995), possibly indicates that the granular sample analysed was initially enriched in Te prior to reworking.
Massive pyrite at Mala occurs as discrete pods throughout the sulfide mound. The trace metal composition is highly variable depending on the individual sample analysed (Table S1 and Fig. S1, ESM). Fluid flow regimes evolve with time in response to permeability fluctuations related to the collapse and occlusion of permeability pathways, leading to the disruption of high-temperature fluid flow pathways within the VMS mound (e.g. Tivey et al. 1995; You and Bickle 1998; Tivey 2007). These local-scale fluctuations (cm to m’s) in fluid flow led to changes in temperature and the remobilization of metals stable at low temperatures from areas proximal to zones of high-temperature fluid flow (> 350 °C) within the mound, leading to highly heterogeneous trace metal enrichment profiles between different massive pyrite samples. For example, samples located proximal to high-temperature (> 350 °C) fluid pathways (e.g. MAL 14) are relatively enriched in Se, Co and Te compared with samples that have experienced zone refining during fluid overprinting by later cooler fluids (< 350 °C) (e.g. MAL 17). Hence, such large variations in trace metal concentrations between massive pyrite samples reflects the dynamic nature of fluid flow within the VMS mound. Alternatively, pyrite that contains low total trace metal concentrations may have been affected by a higher degree of zone refining leading to the localized leaching and remobilization of most trace metals, even those associated with high-temperature assemblages as suggested for the Kokkinopezula VMS deposit in Troodos (Hannington et al. 1998). However, high degrees of zone refining are considered to occur in mature VMS deposits, whereas the Mala deposit is suggested to represent an immature system based on the elevated concentration of magmatic volatile elements and low-sulfur isotope values (Martin et al. 2021), and therefore, it is unlikely that enough time has elapsed to reach the high degree of zone refining.
Disseminated pyrite occurs as euhedral grains within gypsum (Fig. 2D) and is strongly enriched in Se compared to all other pyrite textures (Fig. 5). This enrichment in Se is most likely a function of the preferential partitioning of Se into disseminated pyrite grains relative to the surrounding gypsum/anhydrite matrix (Yamamoto 1976; Huston et al. 1995). Alternatively, the enrichment of Se could indicate that disseminated pyrite formed under lower fluid temperatures, assuming that Se is transported as H2Se in the hydrothermal fluid, as thermodynamic models for Se in pyrite predict that it is preferentially enriched at lower temperatures (150 °C) and more oxidizing conditions (ΣSO4 > ΣH2S; Huston et al. 1995). However, observations from the active TAG deposit indicate that anhydrite-rich zones, which are analogous to gypsum-rich zones at Mala where disseminated pyrite occurs, typically form at temperatures > 350 °C (Edmond et al. 1995; Grant et al. 2018; Petersen et al. 2000). Furthermore, pyrite grains appear euhedral with no haematite or magnetite that would indicate more oxidized fluids. Thus, there is no evidence supporting the formation of disseminated pyrite from more oxidized or low-temperature (~ 150 °C) fluids at Mala that are favourable for the incorporation of Se in pyrite (Yamamoto 1976; Huston et al. 1995).
As observed in massive pyrite samples, notable variation exists between individual disseminated samples (Fig. 8). We use the Te and Se content of pyrite as proxies for magmatic volatile influx as these elements are enriched in VMS deposits in volatile-rich subduction influenced environments (Keith et al. 2016b) and Co and As as indicators of high- and low-temperature fluids, respectively (Grant et al. 2018) (Fig. 8). Samples MAL 18 and MAL 05 are located in the upper mound region, whilst MAL 04 and MAL 11 are located in the lower mound (Fig. 2A). Based on studies of the active TAG mound, spatial trends in the trace metal content of pyrite exists with, for example, Ni, Co and Se enrichment in high-temperature stockwork zones and As, Pb, Zn and Ag enrichment in lower temperature massive pyrite (Grant et al. 2018). Sample MAL 05 contains notably higher Se and Te concentrations relative to all other grains and is depleted in Co and enriched in As, indicating that it formed at low temperatures. In contrast, sample MAL 18, which also formed in the upper mound area, is relatively depleted in Se, Te and As but is strongly enriched in Co, indicating that it formed from high-temperature fluids (> 350 °C; Metz and Trefry 2000; Keith et al. 2016a; Grant et al. 2018) (Fig. 8). Some samples (e.g. MAL 04) from the lower mound are depleted in As, Co, Te and Se relative to other samples, possibly providing evidence for high degrees of zone refining (Hannington et al. 1998). The trace metal enrichment profile in samples from the lower mound are highly variable with some analyses strongly enriched in Te, Se, Co and As, indicating a change in fluid temperature and/or magmatic volatile influx as the pyrite grain grew. The sporadic variation in trace metal concentrations within individual disseminated pyrite grains indicates the influence of dynamic fluid flow regimes within the VMS mound that leads to strong and transient physicochemical gradients localized over cm to m scales. Hence, our data indicate that the well-established zone refining model that is modelled on the active TAG deposit (Humphris et al. 1995; Grant et al. 2018) is more complex when considering the distribution of trace metals, especially when accounting for the distribution of metals over individual mineral grains. This notwithstanding, there are obvious similarities between actively forming SMS deposits and the zonation of metals in black smoker chimneys with the ancient Mala VMS deposit, suggesting that processes that control the mound scale distribution and cycling of trace metals are broadly analogous between the two and did not change since the Cretaceous.
Sulfur isotope systematics
Sulfur isotope analysis of sulfide minerals, such as pyrite, in VMS deposits can be used to discriminate contributions from isotopically distinct reservoirs of sulfur in hydrothermal fluids, such as sulfur leached from oceanic crust, seawater sulfate and SO2 degassing from shallow magma chambers (Lüders et al. 2001; Hannington et al. 2005; Ono et al. 2007; de Ronde et al. 2011; McDermott et al. 2015; Keith et al. 2016a; Martin et al. 2020; LaFlamme et al. 2021). The two primary sources of sulfur in Troodos VMS deposits are the bulk Troodos crust that has a sulfur isotope composition of 1 to 0‰ (Alt 1994), Cretaceous seawater sulfate at δ34S = 18 to 19‰ (Kampschulte and Strauss 2004) and at the Mala VMS deposit, the disproportionation of SO2 (Martin et al. 2021). The mixing of sulfur sourced from TSR of seawater and the leaching of primary igneous sulfur/sulfide from host rocks (Woodruff and Shanks 1988; Shanks 2001) lead to an average δ34S value in pyrite of 4.6 ± 2.8‰ (1σ, n = 160), indicating that, on average, ~ 34% of sulfur is sourced from TSR during VMS deposit formation in Troodos (Martin et al. 2020). The Skouriotissa and Sha, and most notably the Mala VMS deposits, contain pyrite with a sulfur isotopic composition that is less than < 0‰. Previously, the low δ34S values in pyrite have been attributed to the disproportion of SO2 during the degassing of volatiles from a shallow magma chamber (Keith et al. 2016a; Martin et al. 2020). At Mala, disproportionation generates sulfate with a sulfur isotopic composition of 14.5‰ in gypsum (n = 26), and pyrite that is depleted in 34S on average by − 4.3‰ (n = 28) (Martin et al. 2021). Bacterial sulfate reduction of seawater at low temperatures (< 120 °C) can also form negative δ34S values in pyrite as low as − 22.2‰ in altered oceanic crust in Troodos, such as those found in pyrite veins in the pillow lavas, indicating that BSR occurred distally to high-temperature VMS deposits in the shallow lava stratigraphy (Alt 1994; Pedersen et al. 2017).
The in situ analysis of different pyrite textures indicates that the source of sulfur in the Mala VMS deposit was not constant and evolved with time, leading to variations in δ34S values across individual pyrite grains. Variation in δ34S values between pyrite textures relates to mound-scale fluid flow that is directly influenced by the location of specific pyrite samples within the mound that in turn influences pyrite texture (e.g. colloform vs. massive samples). Similar variations are reported across black smoker chimneys where intense gradients in temperature and fluid mixing occur within the chimney wall (Haymon 1983; Crowe and Valley 1992). For example, δ34S varies from 2.1 to 6.4‰ in pyrite from the interior to exterior chimney wall, respectively, in a black smoker chimney from 1–2° S on the EPR (Meng et al. 2020). Given the large range in δ34S values of pyrite at Mala, from − 17.1 to 7.5‰, we suggest four processes that account for the observed variation in the sulfur isotope composition of pyrite: (i) TSR of seawater (18 to 19‰), (ii) the leaching of igneous sulfide (0 to 1‰), (iii) the disproportionation of degassing SO2 (0 to 1‰) and (iv) BSR.
Firstly, considering variation in sulfur isotope composition with pyrite texture, previous studies note a systematic relationship between stratigraphic depth and δ34S values in pyrite from the Skouriotissa VMS deposit (Keith et al. 2016a), where enrichment of 34S in shallow euhedral and colloform textured pyrite leads to δ34S values of 4.7‰ and 4.9‰, respectively (n = 4, Keith et al. 2016a). This variation is attributed to the increased proportion of sulfur that is incorporated from TSR of Cretaceous seawater in regions of the VMS mound near the seawater interface. In contrast, deep, stockwork pyrite has a δ34S value of − 1.4‰ indicating lesser amounts of TSR and leaching of igneous sulfur/sulfide and an increased amount of sulfur sourced from the disproportionation of degassing SO2, producing a sulfur isotope composition in pyrite that is less than the Troodos magmatic mean (0–1‰; Alt 1994) (Keith et al. 2016a). Data presented in this study for the Mala VMS deposit has a range of 24.6‰ (n = 31), notably more variable than Skouriotissa with a range of 7.8‰ (n = 29; Keith et al. 2016a; Martin et al. 2020), with no systematic variation between δ34S values and pyrite texture at Mala (Figs. 6 and 9). Instead, 18 of the 31 analyses across disseminated, granular, colloform and massive textured pyrite contain δ34S values of less than the Troodos magmatic mean of ~ 0‰ (Fig. 9; Alt 1994). This supports previous interpretations that have been proposed for the Mala VMS deposit that indicates sulfur was sourced from a mixture of SO2 disproportion, TSR and leaching of igneous sulfur/sulfide. However, the low δ34S values measured in massive pyrite (down to − 17.1‰), which are much lower than any values previously reported, could be produced either via the low-temperature disproportionation of SO2 or during BSR of Cretaceous seawater. Based on the expected fractionation factors between SO2 and pyrite (after Sakai 1968), SO2 disproportion would have had to occur at a temperature of ~ 120 °C to account for the measured depletion in 34S to − 17.1‰, assuming a starting SO2 composition of 0‰. Alternatively, at low temperatures (< 120 °C) BSR of seawater sulfate can generate large depletions in 34S up to − 38.9‰ in pyrite (Habicht and Canfield 1997; Wortmann et al. 2001; Farquhar et al. 2003; Rouxel et al. 2008; Alt and Shanks 2011; Nozaki et al. 2021).
A comparison between bulk δ34S analyses of Mala samples (Martin et al. 2021) with the same sub-set of samples analysed by SIMS in this study. Samples analysed by SIMS exhibit notably more variation with extremely low (− 17.1‰) and high (7.5‰) values compared to the samples analysed using bulk methods. Cretaceous seawater 18 to 19‰ (SW) (Kampschulte and Strauss 2004) and Troodos magmatic mean 0 to 1‰ (TO magmatic mean; Alt 1994)
In active seafloor hydrothermal systems evidence for both the low-temperature disproportionation of SO2 and BSR exist. Negative δ34S values attributed to the disproportion of SO2 down to − 17.5‰ are found in active seafloor hydrothermal systems that experience an increased influx of magmatic volatiles, such as Conical seamount, Papa New Guinea (Gemmel et al. 2004); however, typical median values are less extreme, for example, the median δ34S values at Conical seamount are − 1.2‰ (n = 66) (Petersen et al. 2002; Gemmel et al. 2004) at Hine Hina, Valu Fa Ridge, Lau Basin, − 4.5‰ (n = 12) (Herzig et al. 1998), at Brothers volcano, Kermadec arc, − 2.9‰ (n = 87) (Kermadec arc; de Ronde et al. 2005, 2011) and − 5.6‰ (n = 13) at SuSu Knolls, Manus back-arc basin (Kim et al. 2004), indicating that sulfur was sourced from a combination of SO2 disproportionation, TSR and leaching of igneous sulfur/sulfide.
Evidence of BSR has also been suggested as an important processes in the Agrokipia B VMS deposit (Troodos) where multiple sulfur isotopes (Δ33S) indicate reworking of sulfide minerals in the upper part of the VMS mound by microbes was an important but localized process (Pedersen et al. 2017). In lower temperature regions of the Troodos stratigraphy, similar δ34S values of − 17.2‰ and as low as − 22.2‰ (Alt 1994; Pedersen et al. 2017) have been reported for vein pyrite from the upper lava stratigraphy where the occurrence of analcime, natrolite and phillipsite indicates the presence of low-temperature fluids (< 100 °C; Gass and Smewing 1973) where BSR could occur.
The negative δ34S signature, down to − 17.1‰, with a median composition of − 13.7‰ (n = 3) for the massive sulfide sample at Mala, is somewhat enigmatic as both disproportionation of SO2 and BSR require low fluid temperatures < 120 °C to explain the observed δ34S signature. Fractionation factors between SO2 and pyrite (Sakai, 1968), using an initial value of 0‰, would have had to occur at a temperature of 187 °C to explain the median sulfur isotopic composition in pyrite of − 13.7‰; assuming that SO2 disproportionation was the only source of sulfur (i.e. a closed system), which as shown in previous studies is not the case (Ono et al. 2007).
Combining trace element geochemistry and sulfur isotope data, we suggest that the δ34S signature is best explained by BSR of seawater. Disproportionation of SO2 that indicates magmatic volatile influx is, at the mound scale, commonly associated with increased concentrations of Se, Te and Au in the Mala VMS deposit (Martin et al. 2021); however, this is not the case in massive pyrite sample MAL 17 that is depleted in Te, Se and Au compared to all other massive pyrite samples. Furthermore, MAL 17 has a median Co concentration of 8.4 ppm (n = 10), compared to 38.7 ppm for all massive samples (n = 40), further supporting its formation from relatively low-temperature fluids (< 350 °C) (Keith et al. 2016a, b; Monecke et al. 2016; Grant et al. 2018). The relative depletion in Te, Se and Au is not consistent with its formation during magmatic degassing, indicating it most likely formed during the BSR of seawater. This is further supported by the location of the sample on the western margin of the VMS mound (Fig. 2A), and assuming that the mound morphology preserved today is representative of how the Mala VMS deposit formed at the Cretaceous seafloor, the sample is located near the seawater interface where seawater ingress is high and the fluid temperature is expected to be low, possibly < 120 °C (cf. Pedersen et al. 2017).
The sulfur isotopic composition of colloform textured pyrite at Mala has a median δ34S value of − 2.2‰ (n = 6; Fig. 4). This is significantly lower than the median δ34S value recorded in colloform pyrite at the Skouriotissa VMS deposit of 4.9‰ (n = 2; Keith et al. 2016a), the ice VMS deposit (Yukon, Canada) at 5.0‰ (n = 8; McDonald et al. 2018) and the Ezuri VMS deposit (Hokuroko, Japan) at 4.5‰ (n = 16; Barrie et al. 2009). The positive values found in colloform pyrite in most VMS deposits demonstrate that the primary source of sulfur is the leaching of igneous basement and TSR of seawater (Ohmoto 1996; Barrie et al. 2009; Keith et al. 2016a; McDonald et al. 2018). By contrast, δ34S values in colloform pyrite at Mala are less than the Troodos magmatic mean (0–1‰; Alt 1994) indicating that sulfur was not only sourced via TSR of Cretaceous seawater and the leaching of igneous lithologies, instead indicating that colloform pyrite formed during the disproportionation of SO2. Using the fractionation between SO2 and pyrite (Sakai 1968); Ohmoto and Lasaga 1982, the expected fractionation at 350 °C would be 8.6‰. In order to explain the observed fractionation between the median δ34S value in colloform pyrite (2.2‰) and primary igneous sulfur/sulfide (0‰) the fluid temperature would have had to be > 880 °C. As previously established for bulk rock samples, this temperature is unrealistically high for VMS deposit formation; hence, we suggest that the disproportion of SO2 occurred at lower fluid temperatures where fractionation between SO2 and pyrite is greater and that the higher than anticipated δ34S values in colloform pyrite reflects the addition of a 34S enriched source from both TSR and the leaching of igneous sulfur/sulfide providing an additional source of sulfur in colloform pyrite.
Disseminated pyrite grains exhibit the largest variation in δ34S values with a median of 0.7‰ and a range of 16.8‰ (n = 14). To assess if the pyrite analysed are singular grains or aggregates of sub-grains, etching was used to reveal any internal variation (Fig. 7). Sample MAL 05 (Fig. 7A) exhibits internal variation appearing as three different coloured sections, indicating it is formed from three sub-grains. In contrast, the etched surface of MAL 11A and B does not show any notable variation indicating that they are a single continuous grain (Fig. 7B and C).
The sulfur isotopic composition of individual pyrite grains is highly variable across disseminated pyrite grains with δ34S values ranging from − 10.9 to 2.5‰ (within a single sub-grain), suggesting that the source of sulfur changed as the pyrite crystal grew. However, there is no systematic variation in sulfur isotope composition between the core and rim of the grain (Fig. 7A), and the two analyses located at the margin of the grain yield δ34S values of 2.5‰ and − 10.9‰, respectively. Instead, δ34S values appear to gradually decrease across the pyrite grain indicating a change in the source of sulfur as the grain grew (Fig. 7A). In other pyrite grains, a more systematic pattern is discernable where the rim of the grain is enriched in 34S relative to the core (Fig. 7B, C). This variation could be related to either pulsed fluid flow events (Butterfield et al. 1994, 2011; Jamieson et al. 2013) that generate fluctuations in fluid flux, temperature and the chemical and physical (e.g. phase separation) composition of hydrothermal fluids (Lalou et al. 1990, 1998; Butterfield et al. 1994) or local-scale variation in mound-scale fluid flow in response to the collapse and occlusion of permeability pathways during growth of the VMS mound (Kleinrock and Humphris 1996; Humphris et al. 1995; Hannington et al. 1998; Petersen et al. 2000).
Pulsed fluid flow events are linked to magmatic intrusions at depth within the oceanic crust that provide a renewed source of heat that increases the temperature of venting hydrothermal fluid, volatile influx (e.g. H2S and SO2) and the metal content of fluid vented at the seafloor (Von Damm 1990; Von Damm et al. 1995; Butterfield et al. 1997; Andersen et al. 2017). Several studies document temporal variations in total sulfur concentrations and major anions (e.g. Cl−) in hydrothermal vent fluids sampled from active vent sites demonstrating that the flux of sulfur in VMS deposits varies temporally (Butterfield and Massoth, 1994; Von Damm et al. 1995; Butterfield et al. 1997, 2011; Von Damm 1995). It does however remain enigmatic exactly what these variations mean; for example, variations in fluid H2S concentration could also be produced during the precipitation or dissolution of sulfide minerals below the seafloor (Reed and Palandri 2006) or during the segregation of H2S into a vapour phase, thus linking these variations to magmatic intrusion events is challenging (Von Damm et al. 1995; Butterfield et al. 1997). The dating of SMS deposits (210Pb/Pb, 230Th/234U and 14C) also provides evidence of aperiodic fluid flow with apparent pulses of activity recorded at the active TAG mound every 2000 to 6000 years (Lalou et al. 1993; You and Bickle1998).
In Troodos VMS deposits, there are no hydrothermal fluids left to sample nor are the deposits young enough to be dated using radiometric isotope techniques commonly applied to active SMS deposits; however, variation in rare earth element profiles and Sr isotopes (87Sr/86Sr) in epidote from the sheeted dyke complex and plagiogranites that formed during hydrothermal alteration of the oceanic crust indicates the episodic release of volatiles into the Troodos hydrothermal systems (Fox et al. 2020). If this was the case, then evidence of pulsed magmatic influx may have been recorded on the scale of individual pyrite mineral grains as variations in across grain sulfur isotope composition (Fig. 7). Following a volcanic event or the liberation of magmatic volatiles from the roof of an underlying magma chamber during dyking events, as hypothesized for Troodos (Gillis and Roberts 1999; Fox et al. 2020), the disproportionation of SO2 occurs and progressively decreases with time from the event, reducing the flux of SO2 and subsequent disproportionation in the overlying hydrothermal system, and the primary source of sulfur changes from one strongly influenced by disproportionation of SO2 to one dominated by TSR and leaching of igneous sulfur/sulfide. This would produce a pronounced shift in the sulfur isotopic composition across individual pyrite grains from negative δ34S values indicating SO2 disproportionation to values > 0‰ that indicate increasing amounts of sulfur sourced from TSR and the leaching of igneous sulfur/sulfide (e.g. Fig. 7C). A similar model has been proposed by earlier studies to explain the sporadic preservation of δ34S values down to − 5.5‰ in the Sha VMS deposit (Martin et al. 2020). The zonation and aperiodic nature of these fluctuations in sulfur isotope composition across each mineral grain may be linked to the pulsed nature of fluid flow during VMS deposit formation; however, further high-resolution mineral-scale data including mapping of individual mineral grains is required to ascertain the true significance and validity of mineral-scale sulfur isotope data when applied to system scale pulsed ore-forming processes.
A comparison between bulk and in situ sulfur isotope data
The sulfur isotope composition of pyrite from the Mala VMS deposit has previously been assessed using bulk analytical methods on powdered whole-rock samples or pure mineral concentrates (Fig. 9). Using bulk analytical methods, the δ34S values in pyrite range from − 7.6 to 0.1‰ with a median value of − 4.3‰ (n = 28; Martin et al. 2021) (Fig. 9). Using in situ sulfur isotope data collected on the same samples, the sulfur isotope composition of pyrite varies from − 17.1 to 7.5‰ with a median value of − 1.0‰ (n = 31), variation larger than for the same samples using bulk analytical techniques (Fig. 9). When comparing between whole-rock and in situ datasets, on average, the difference between the same sample analysed by SIMS and whole-rock methods was 4.9‰ (n = 6 samples), with some samples, such as MAL 17, exhibiting a difference of 7.3‰ between methods. This difference between analytical scales indicates that a significant amount of variability, and therefore, changes in sulfur source and by implication metal source are being overlooked when using bulk analytical methods compared to in situ mineral-scale data. This trend is comparable to those recognized at Axial Seamount where the bulk analysis of pyrite yields a range of 1.1 to 3.9‰ (Hannington and Scott 1989), whilst in situ analysis exhibits more variability with a range of 1.2 to 6.9‰ (Crowe and Valley 1992) or at 1–2° N on the East Pacific Rise (EPR) where bulk pyrite analyses have a range of 3.0 to 5.8‰ (Zeng et al. 2017) versus in situ analysis of pyrite that ranges from 1.8 to 7.5‰ (Meng et al. 2019).
To illustrate the disparity between analytical scales, we highlight two examples of analytical transects across pyrite grains (n = 23 spots; Fig. 10A). Across a coarse-grained (~ 1 cm) pyrite grain with three sub-grains that occurs within gypsum δ34S values exhibit the largest intra-sample range from − 10.9 to 4.9‰ with a median of 2.0‰ (n = 7) (Fig. 10A). Bulk analysis of the same sample yielded δ34S values of − 0.5 and − 1.2‰ (Martin et al. 2021). The application of in situ analytical methods indicates a variable source of sulfur with values of − 10.9‰ indicating dominantly SO2 disproportionation and values of 4.5‰ signifying a larger contribution of sulfur sourced via TSR of seawater and the leaching of igneous sulfur/sulfide (Fig. 9). A similar difference between analytical methods is evident in all other samples we analysed (Table S3, ESM), demonstrating that data obtained at different analytical scales (i.e. mineral vs. bulk samples) can change the resulting interpretation that is reached.
Coupled trace metal and δ34S analyses across individual disseminated pyrite grains. A Sample MAL 05. B Sample MAL 11 A. C Sample MAL 11 B. P1 on X-axis refers to the specific analytical point in the image above. Selenium, As, Co and Te concentrations are shown by red lines with corresponding concentration in ppm on the secondary axis (right). Note varying Y-axis between graphs
Can magmatic volatile influx be traced at the mineral-scale?
Previous studies have identified an enrichment of Te, Se, Au, Bi, As, Sb, Pb and Hg in SMS deposits that form in subduction influenced environments relative to MOR hosted hydrothermal systems, indicating a link between metal enrichment and magmatic volatile degassing (Hannington et al. 1999; de Ronde et al. 2011; Berkenbosch et al. 2012; Layton-Matthews et al. 2013; Wohlgemuth-Ueberwasser et al. 2015; Fuchs et al. 2019; Martin et al. 2019; Mathieu 2019; Patten et al. 2020). For example, at the Brothers NW Caldera site (Kermadec arc), a positive correlation exists between decreasing δ34S values (to − 4.6‰ in sphalerite) and Au concentration, indicating a link between magmatic volatile influx and Au enrichment (de Ronde et al. 2011). If certain metals were sourced from a magmatic volatile phase in the Mala VMS deposit, then they would be expected to show increased concentrations at low δ34S values that indicate an increased magmatic volatile influx associated with SO2 disproportionation (e.g. Herzig et al. 1998). As previously established, sulfur isotopic ratios exhibit notable variation across individual pyrite grains indicating a variation in sulfur source that we ultimately relate to a variable magmatic volatile influx. If magmatic volatile influx occurred as aperiodic releases from the plagiogranites in Troodos into the overlying hydrothermal systems, a coupled relationship between sulfur isotopes and the enrichment of trace metals that are associated with increased levels of volatile influx (e.g. Te and Se) should exist in pyrite (Fox et al. 2020). However, our data exhibits limited correlation between Te, Se, Au, As and Co and δ34S values indicating that the primary magmatic signature/zonation in trace metals, if initially present, was rapidly modified within the VMS mound.
Selenium has been widely applied as an indicator of magmatic volatile influx with Se/S ratios in pyrite used as evidence of an increased magmatic volatile influx in VMS deposits (Yamamoto 1976; Huston et al. 1995; Hannington et al. 1999; Layton-Matthews et al. 2008, 2013; Martin et al. 2019). The contribution of Se from a magmatic volatile phase at Mala is largely supported at the deposit scale by negative δ34S values in pyrite and a moderate positive correlation between Te and Se (R2 = 0.67), a trend that is not observed in any other Troodos VMS deposit (Martin et al. 2021). However, mineral grain-scale observations show that a more complex relationship exists (Figs. 10 and 11).
Se, Te and Co vs. δ34S for all samples. Twinned points in disseminated pyrite (as in Fig. 10) are represented by multiple analytical points. In samples where points were not twinned, an average value is reported and denoted by an asterisk next to each sample in the legend. A Se vs. δ34S, B Te vs. δ34S, C Co vs. δ34S. Volatile trend in plot B shows expected trend between Te, Se and Co and δ34S. The grey box represents the primary magmatic mean for Troodos magmatic rocks (Alt 1994). Solid line represents the average bulk δ34S for all Troodos VMS (4.6‰; Martin et al. 2020) and the dashed line is the bulk median δ34S for Mala (− 4.3‰; Martin et al. 2021)
If the Se content of pyrite is a reliable proxy for magmatic volatile influx at Mala, then a negative correlation is expected between Se content and δ34S values in pyrite, with high Se contents occurring in the samples with low δ34S values indicating a coupled relationship (Yamamoto 1976). The Se content of sample MAL 05, a disseminated pyrite grain in gypsum (Fig. 10A), ranges from 839 to 1727 ppm with the highest Se concentration corresponding to a δ34S value of 2.5‰. The two lowest Se concentrations of 839 and 1273 ppm correspond to the highest δ34S values measured in MAL 05 of 4.9 and 5.9‰, respectively (Fig. 10A). However, the two lowest δ34S values of − 2.0 and − 10.9‰ do not correspond to the highest Se content. Thus, Se content does not accurately track magmatic volatile influx. If this were the case, then a strong correlation between δ34S values and Se concentration would occur; however, this is not observed (Fig. 11B).
Renewed volatile influx is driven by magmatic intrusions at depth below the seafloor that lead to an increase in fluid temperatures preceding the intrusive event, for example at 9° 46.5′ N on the East Pacific Rise where vent fluid temperature varied from 403 °C directly after the intrusive event to 322 °C 3 years later (Von Damm et al. 1995). High Co concentrations in pyrite have been suggested to indicate elevated fluid temperatures (> 350 °C); therefore, Co should be enriched at low δ34S values that precede the intrusive event (Butterfield et al. 1997; Keith et al. 2016a, b; Grant et al. 2018). Arsenic is expected to exhibit the inverse trend as it is concentrated in low-temperature pyrite (< 350 °C) and is expected to be enriched at high δ34S values representing periods of low magmatic volatile influx and correspondingly cooler fluids (Monecke et al. 2016; Grant et al. 2018). Thus, systematic shifts in Te and Se, which indicate renewed volatile influx, and As and Co that signify changes in fluid temperature should correlate with changes in δ34S values in pyrite if they are reliable tracers of magmatic volatile influx at the mineral scale; we assess this relationship in disseminated pyrite grains at Mala.
In sample MAL 05, Co concentration varies from 12 to 23 ppm (Fig. 10A), exhibiting only a minor correlation (R2 = 0.3) with δ34S values (Fig. 10A). Arsenic concentrations are more variable than Co ranging from 5 to 145 ppm with the highest concentration corresponding to a δ34S value of − 2.0‰, but with no notable correlation between As and δ34S values (R2 = 0.02). A similar trend is evident for Te data with an R2 value of 0.1 (Figs. 10 and 11).
In other disseminated grains (Fig. 10B; MAL 11A) the highest Se and Co contents correspond to the lowest δ34S value of 0.2‰, whilst Te exhibits the inverse trend (Fig. 10B). A similar trend is also discernable in MAL 11B where the sulfur isotope composition of pyrite exhibits a saw tooth pattern ranging from − 2.5 to 0.4‰ (Fig. 10C). Selenium and Te concentrations exhibit a moderate correlation with δ34S values with an R2 value of 0.6 and 0.3, respectively. Thus, no clear relationship exists between trace metals and sulfur isotope composition across disseminated pyrite grains at Mala.
For all other samples considered in this study, our data indicate that there is no notable correlation between Te, Se and Co with δ34S values in individual pyrite grains or within the different textures analysed (Fig. 11). The highest measured Se concentration of 3261 ppm corresponds to a δ34S value of 0‰, whilst lower values of − 2.5‰ within the same mineral grain, which suggest an increased magmatic volatile influx correspond to a Se concentration of 325 ppm (Fig. 11A). We exclude massive pyrite sample MAL 17 from our interpretation that is influenced by BSR of seawater leading to a low δ34S value in pyrite and notably lower concentrations of Se, Te and Co relative to massive and disseminated samples (Fig. 11).
Our investigation indicates that variations in Te, Se, Co and As concentrations at the mineral-scale cannot be linked to variations in magmatic volatile influx at the Mala VMS deposit (Fig. 12). The lack of correlation between δ34S values and Te, Se, Co and As in the Mala VMS deposit probably reflects the localized remobilization of trace elements during zone refining related to fluctuations in mound scale fluid flow related to the collapse and occlusion of fluid pathways during the growth of the mound that leads to localized zone refining of trace metals in pyrite (Fig. 12). Any initial zonation of trace metals in pyrite related to the influx of magmatic volatiles is overprinted by later fluid flow that led to the mobilization of trace metals but does not alter the original sulfur isotope composition of < 0‰ related to magmatic volatile influx and SO2 disproportionation; hence, this explains the lack of correlation between δ34S values and Te, Se, Co and As at the mineral scale at Mala (Fig. 12). To investigate this process, further high-resolution in situ isotopic and trace element geochemistry and information on the diffusion rates of trace metals and sulfur in pyrite (e.g. Cherniak 2010) is needed to understand chemical zonation at the scale of individual mineral grains.
A Summary schematic for mound scale metal enrichment processes at the Mala VMS deposit. Granular pyrite forms from the reworking and collapse of the outer mound and chimney material, metals are remobilized during low-temperature fluid flow. Disseminated pyrite forms in gypsum veins and volcanic rocks (Fig. 2). B Colloform and dendritic textured pyrite (Fig. 3C, D) form at the margin of the sulfide mound where seawater ingress is high creating disequilibrium textures and fluid temperatures are lower (< 350 °C) leading to an enrichment in Ag, Au and Pb. C With increasing time, permeability pathways within the mound change in response to the collapse and reworking of anhydrite, creating a prominent brecciated texture leading to localized zone refining on the cm/m scale in response to new high-temperature fluid pathways. D Pyrite grains undergo zone refining leading to the decoupling of δ34S and magmatic volatile elements in pyrite
Summary and conclusion
The distribution of trace metals between different pyrite textures in the Mala VMS deposit appears broadly analogous to actively forming mafic SMS deposits. Colloform pyrite that formed near the seawater interface is enriched in low-temperature elements such as As and Au relative to massive samples that contain euhedral pyrite that are relatively enriched in high-temperature metals Se, Co and Te. Granular samples that formed during the reworking of massive pyrite are depleted in all metals (except Te) due to the mobilization and leaching of metals during low-temperature fluid flow at the margin of the VMS mound. There is no systematic variation in trace metal enrichment profiles with depth in the VMS mound; instead, variation between individual samples can be attributed to dynamic fluid flow patterns within the VMS mound and the localized (cm to m scale) zone refining of metals around individual fluid conduits that vary spatially and temporally during mound growth (Fig. 12). All pyrite textures appear to be enriched in Se relative to both Troodos VMS deposits and mafic-hosted VMS deposits more widely indicating a Se-rich magmatic volatile dominated source.
The large range in the δ34S composition of pyrite relative to previous studies and mafic VMS deposits more widely can be explained by varying combinations of four main processes: (i) TSR, (ii) leaching of sulfur/sulfide from host rocks, (iii) SO2 disproportionation and (iv) BSR. Thermochemical sulfate reduction of seawater and the leaching of igneous sulfur/sulfide generates δ34S values in pyrite that are higher than the Troodos magmatic mean of 0–1‰, whilst low values that are less than this are produced during the addition of sulfur from the disproportionation of SO2 during the degassing of shallow magma chambers. When combined with trace element geochemistry, we suggest low δ34S values (to − 17.1‰) formed during the BSR of seawater in the upper VMS mound at temperatures of < 120 °C.
Mineral-scale sulfur isotope data exhibits notably more variation than the same samples analysed via bulk rock analytical methods. We observe greater amounts of variation over a single pyrite grain than recorded across all bulk rock analyses with δ34S values ranging from − 10.9 to 2.5‰. Analytical transects across individual mineral grains indicate that the source of sulfur in the Mala VMS deposit was variable and alternated between disproportionation of SO2, TSR and the leaching of igneous sulfur/sulfide as the pyrite grain grew. Such variation is not evident when using bulk analytical techniques for sulfur isotopes and warrants further detailed investigation.
Linking variations in trace metal enrichment profiles and sulfur isotopes is key in understanding the role of magmatic volatiles as a potential metal source in mafic VMS deposits. However, findings of this study indicate no simple correlation between magmatic volatile elements such as Te and Se and δ34S values. We attribute this decoupled relationship to local-scale fluid flow within the mound that leads to the remobilization of trace metals during zone refining. Pyrite retains δ34S values that indicate disproportionation (< 0‰) but can be depleted in magmatic volatile metals.
References
Adamides NG (2010) Mafic-dominated volcanogenic sulphide deposits in the Troodos ophiolite, Cyprus Part 2 – a review of genetic models and guides for exploration. Appl Earth Sci 119:193–204
Alt JC (1994) A sulfur isotopic profile through the Troodos ophiolite, Cyprus: primary composition and the effects of seawater hydrothermal alteration. Geochim Cosmochim Acta 58:1825–1840
Alt JC, Shanks WC (2011) Microbial sulfate reduction and the sulfur budget for a complete section of altered oceanic basalts, IODP Hole 1256D (eastern Pacific). Earth Planet Sci Lett 310:73–83
Andersen C, Theissen-Krah S, Hannington M, Rüpke L, Petersen S (2017) Faulting and off-axis submarine massive sulfide accumulation at slow spreading mid-ocean ridges: a numerical modeling perspective. Geochem Geophys Geosyst 18:2305–2320
Banerjee NR, Gillis KM, Muehlenbachs K (2000) Discovery of epidosites in a modern oceanic setting, the Tonga forearc. Geology 28:151–154
Barrie CD, Boyce AJ, Boyle AP, Williams PJ, Blake K, Ogawara T, Akai J, Prior DJ (2009) Growth controls in colloform pyrite. Am Mineral 94:415–442
Berkenbosch HA, de Ronde CEJ, Gemmell JB, McNeill AW, Goemann K (2012) Mineralogy and Formation of Black Smoker Chimneys from Brothers Submarine Volcano, Kermadec Arc. Econ Geol 107:1613–1633
Brazilian Metals Group (2013) High-grade copper-zinc sulphide mineralisation identified at Mala Prospect – Vrechia. www.bmgl.com.au/investors/annual-reports. Accessed 30 July 2018
Brueckner SM, Piercey SJ, Layne GD, Piercey G, Sylvester PJ (2015) Variations of sulphur isotope signatures in sulphides from the metamorphosed Ming Cu(−Au) volcanogenic massive sulphide deposit, Newfoundland Appalachians, Canada. Miner Deposita 50:619–640
Butler IB, Nesbitt RW (1999) Trace element distributions in the chalcopyrite wall of a black smoker chimney: insights from laser ablation inductively coupled plasma mass spectrometry (LA–ICP–MS). Earth Planet Sci Lett 167:335–345
Butterfield DA, Jonasson IR, Massoth GJ, Feely RA, Roe KK, Embley RE, Holden JF, McDuff RE, Lilley MD, Delaney JR (1997) Seafloor eruptions and evolution of hydrothermal fluid chemistry. Philos Trans Royal Soc A 355(1723):369–386. https://doi.org/10.1098/rsta.1997.0013
Butterfield DA, Massoth GJ (1994) Geochemistry of north Cleft segment vent fluids: temporal changes in chlorinity and their possible relation to recent volcanism. J Geophys Res Solid Earth 99:4951–4968
Butterfield DA, McDuff RE, Mottl MJ, Lilley MD, Lupton JE, Massoth GJ (1994) Gradients in the composition of hydrothermal fluids from the Endeavour segment vent field: phase separation and brine loss. J Geophys Res Solid Earth 99:9561–9583
Butterfield DA, Nakamura K, Takano B, Lilley MD, Lupton JE, Resing JA, Roe KK (2011) High SO2 flux, sulfur accumulation, and gas fractionation at an erupting submarine volcano. Geology 39:803–806
Cherniak DJ (2010) Diffusion in carbonates, fluorite, sulfide minerals, and diamond. Rev Mineral Geochem 72:871–897
Cook NJ, Ciobanu CL, Mao J (2009) Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem Geol 264:101–121
Crowe DE, Valley JW (1992) Laser microprobe study of sulfur isotope variation in a sea-floor hydrothermal spire, Axial Seamount, Juan de Fuca Ridge, eastern Pacific. Chem Geol 101:63–70
de Ronde CEJ, Hannington MD, Stoffers P, Wright IC, Ditchburn RG, Reyes AG, Baker ET, Massoth GJ, Lupton JE, Walker SL, Greene RR, Soong CWR, Ishibashi J, Lebon GT, Bray CJ, Resing JA (2005) Evolution of a submarine magmatic-hydrothermal system: Brothers Volcano, Southern Kermadec Arc, New Zealand. Econ Geol 100:1097–1133
de Ronde CEJ, Massoth GJ, Butterfield DA, Christenson BW, Ishibashi J, Ditchburn RG, Hannington MD, Brathwaite RL, Lupton JE, Kamenetsky VS, Graham IJ, Zellmer GF, Dziak RP, Embley RW, Dekov VM, Munnik F, Lahr J, Evans LJ, Takai K (2011) Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand. Miner Deposita 46:541–584
Edmond JM, Campbell AC, Palmer MR, Klinkhammer GP, German CR, Edmonds HN, Elderfield H, Thompson G, Rona P (1995) Time series studies of vent fluids from the TAG and MARK sites (1986, 1990) Mid-Atlantic Ridge: a new solution chemistry model and a mechanism for Cu/Zn zonation in massive sulphide orebodies. Geol Soc Lond Spec Publ 87:77–86
Eldridge CW, Barton PB, Ohmoto H (1983) Mineral textures and their bearing on formation of the Kuroko orebodies. Econ Geol Mono 5:241–281
Fallon EK, Petersen S, Brooker RA, Scott TB (2017) Oxidative dissolution of hydrothermal mixed-sulphide ore: an assessment of current knowledge in relation to seafloor massive sulphide mining. Ore Geol Rev 86:309–337
Farquhar J, Johnston DT, Wing BA, Habicht KS, Canfield DE, Airieau S, Thiemens MH (2003) Multiple sulphur isotopic interpretations of biosynthetic pathways: implications for biological signatures in the sulphur isotope record. Geobiology 1:27–36
Fox S, Katzir Y, Bach W, Schlicht L, Glessner J (2020) Magmatic volatiles episodically flush oceanic hydrothermal systems as recorded by zoned epidote. Commun Earth Environ 1:52
Fuchs S, Hannington MD, Petersen S (2019) Divining gold in seafloor polymetallic massive sulfide systems. Miner Deposita 54:789–820
Galley AG, Hannington MD, Jonasson IR (2007) Volcanogenic massive sulphide deposits, in: Mineral deposits of Canada: a synthesis of major deposit types. Geological Association of Canada, St. John’s, Newfoundland, pp.141–162
Gass IG (1968) Is the Troodos Massif of Cyprus a fragment of Mesozoic ocean floor? Nature 220:39–42
Gass IG, Smewing JD (1973) Intrusion, extrusion and metamorphism at constructive margins: evidence from the Troodos Massif, Cyprus. Nature 242:26–29
Gemmel JB, Sharpe R, Jonasson IR, Herzig PM (2004) Sulfur isotope evidence for magmatic contributions to submarine and subaerial gold mineralization: Conical Seamount and the Ladolam Gold Deposit, Papua New Guinea. Econ Geol 99:1711–1725
Genna D, Gaboury D (2015) Deciphering the hydrothermal evolution of a VMS system by LA-ICP-MS using trace elements in pyrite: an example from the Bracemac-McLeod deposits, Abitibi, Canada, and implications for exploration. Econ Geol 110:2087–2108
Gillis KM, Roberts MD (1999) Cracking at the magma–hydrothermal transition: evidence from the Troodos Ophiolite, Cyprus. Earth Planet Sci Lett 169:227–244
Grant HLJ, Hannington MD, Petersen S, Frische M, Fuchs SH (2018) Constraints on the behavior of trace elements in the actively-forming TAG deposit, Mid-Atlantic Ridge, based on LA-ICP-MS analyses of pyrite. Chem Geol 498:45–71
Habicht KS, Canfield DE (1997) Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochim Cosmochim Acta 61:5351–5361
Halbach P, Blum N, Münch U, Plüger W, Garbe-Schönberg D, Zimmer M (1998) Formation and decay of a modern massive sulfide deposit in the Indian Ocean. Miner Deposita 33:302–309
Hannington MD, Bleeker W, Kjarsgaard I (1999) Sulfide mineralogy, geochemistry, and ore genesis of the Kidd Creek deposit: Part II. The Bornite Zone*, in: Hannington, MD, Barrie CT (ed), The giant Kidd Creek volcanogenic massive sulfide deposit, Western Abitibi Subprovince, Canada. Society of Economic Geologists, Littleton Colorado
Hannington MD, de Ronde CEJ, Petersen S (2005) Sea-floor tectonics and submarine hydrothermal systems. In: Hedenquist JW, Thompson JFH, Goldfarb RJ, Richards JP (eds) Economic Geology 100th Anniversary Volume. Society of Economic Geologists, Littelton, pp 111–141
Hannington MD, Galley AG, Herzig PM, Petersen S (1998) Comparison of the TAG mound and stockwork complex with Cyprus-type massive sulfide deposits. In: Herzig PM, Humphris SE, Miller DJ, Zierenberg RA (ed), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 158
Hannington MD, Peter JM, Scott SD (1986) Gold in sea-floor polymetallic sulfide deposits. Econ Geol 81(8):1867–1883. https://doi.org/10.2113/gsecongeo.81.8.1867
Hannington MD, Scott SD (1989) Sulfidation equilibria as guides to gold mineralization in volcanogenic massive sulfides; evidence from sulfide mineralogy and the composition of sphalerite. Econ Geol 84:1978–1995. https://doi.org/10.2113/gsecongeo.84.7.1978
Herzig PM, Hannington MD, Scott SD, Maliotis G, Rona PA, Thompson G (1991) Gold-rich sea-floor gossans in the Troodos Ophiolite and on the Mid-Atlantic Ridge. Econ Geol 86:1747–1755
Herzig PM, Hannington MD, Arribas A Jr (1998) Sulfur isotopic composition of hydrothermal precipitates from the Lau back-arc: implications for magmatic contributions to seafloor hydrothermal systems. Miner Deposita 33:226–237
Humphris SE, Herzig PM, Miller DJ, Alt JC, Becker K, Brown D, Brügmann G, Chiba H, Fouquet Y, Gemmell JB, Guerin G, Hannington MD, Holm NG, Honnorez JJ, Iturrino GJ, Knott R, Ludwig R, Nakamura K, Petersen S, Reysenbach A-L, Rona PA, Smith S, Sturz AA, Tivey MK, Zhao X (1995) The internal structure of an active sea-floor massive sulphide deposit. Nature 377:713–716
Huston DL, Sie SH, Suter GF (1995) Selenium and its importance to the study of ore genesis: the theoretical basis and its application to volcanic-hosted massive sulfide deposits using pixeprobe analysis. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At Nucl Microprobe Technol Appl 104:476–480
Huston DL, Relvas JMRS, Gemmell JB, Drieberg S (2011) The role of granites in volcanic-hosted massive sulphide ore-forming systems: an assessment of magmatic–hydrothermal contributions. Miner Deposita 46:473–507
Jamieson JW, Hannington MD, Clague DA, Kelley DS, Delaney JR, Holden JF, Tivey MK, Kimpe LE (2013) Sulfide geochronology along the Endeavour Segment of the Juan de Fuca Ridge. Geochem Geophys Geosyst 14:2084–2099
Jowitt SM, Jenkin GRT, Coogan LA, Naden J (2012) Quantifying the release of base metals from source rocks for volcanogenic massive sulfide deposits: effects of protolith composition and alteration mineralogy. J Geochem Explor 118:47–59
Kampschulte A, Strauss H (2004) The sulfur isotopic evolution of Phanerozoic seawater based on the analysis of structurally substituted sulfate in carbonates. Chem Geol 204:255–286
Keith M, Haase KM, Klemd R, Krumm S, Strauss H (2016a) Systematic variations of trace element and sulfur isotope compositions in pyrite with stratigraphic depth in the Skouriotissa volcanic-hosted massive sulfide deposit, Troodos ophiolite, Cyprus. Chem Geol 423:7–18
Keith M, Häckel F, Haase KM, Schwarz-Schampera U, Klemd R (2016b) Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol Rev 72:728–745
Keith M, Haase KM, Klemd R, Smith DJ, Schwarz-Schampera U, Bach W (2018) Constraints on the source of Cu in a submarine magmatic-hydrothermal system, Brothers volcano. Kermadec Island Arc Contrib Mineral Petrol 173:40
Kim J, Lee I, Lee K-Y (2004) S, Sr, and Pb isotopic systematics of hydrothermal chimney precipitates from the Eastern Manus Basin, western Pacific: evaluation of magmatic contribution to hydrothermal system. J Geophys Res Solid Earth 109:B12210
Kleinrock MC, Humphris SE (1996) Structural control on sea-floor hydrothermal activity at the TAG active mound. Nature 382:149–153
Kusakabe M, Komoda Y, Takano B, Abiko T (2000) Sulfur isotopic effects in the disproportionation reaction of sulfur dioxide in hydrothermal fluids: implications for the δ34S variations of dissolved bisulfate and elemental sulfur from active crater lakes. J Volcanol Geotherm Res 97:287–307
LaFlamme C, Barré G, Fiorentini ML, Beaudoin G, Occhipinti S, Bell J (2021) A significant seawater sulfate reservoir at 2.0 Ga determined from multiple sulfur isotope analyses of the Paleoproterozoic Degrussa Cu-Au volcanogenic massive sulfide deposit, Western Australia. Geochim Cosmochim Acta 295:178–193
Lalou C, Thompson G, Arnold M, Brichet E, Druffel E, Rona PA (1990) Geochronology of TAG and Snakepit hydrothermal fields, Mid-Atlantic Ridge: witness to a long, complex hydrothermal history. Earth Planet Sci Lett 97:113–128
Lalou C, Reyss J-L, Brichet E, Arnold M, Thompson G, Fouquet Y, Rona PA (1993) New age data for Mid-Atlantic Ridge hydrothermal sites: TAG and Snakepit chronology revisited. J Geophys Res 98:9705–9713
Lalou C, Münch U, Halbach P, Reyss J-L (1998) Radiochronological investigation of hydrothermal deposits from the MESO zone, Central Indian Ridge. Mar Geol 149:243–254
Layton-Matthews D, Peter JM, Scott SD, Leybourne MI (2008) Distribution, mineralogy, and geochemistry of selenium in felsic volcanic-hosted massive sulfide deposits of the Finlayson Lake District, Yukon Territory, Canada. Econ Geol 103:61–88
Layton-Matthews D, Leybourne MI, Peter JM, Scott SD, Cousens B, Eglington BM (2013) Multiple sources of selenium in ancient seafloor hydrothermal systems: compositional and Se, S, and Pb isotopic evidence from volcanic-hosted and volcanic-sediment-hosted massive sulfide deposits of the Finlayson Lake District, Yukon, Canada. Geochim Cosmochim Acta 117:313–331
Lode S, Piercey SJ, Layne GD, Piercey G, Cloutier J (2017) Multiple sulphur and lead sources recorded in hydrothermal exhalites associated with the Lemarchant volcanogenic massive sulphide deposit, central Newfoundland, Canada. Miner Deposita 52:105–128
Lüders V, Pracejus B, Halbach P (2001) Fluid inclusion and sulfur isotope studies in probable modern analogue Kuroko-type ores from the JADE hydrothermal field (Central Okinawa Trough, Japan). Chem Geol 173:45–58
Martin AJ, McDonald I, MacLeod CJ, Prichard HM, McFall K (2018) Extreme enrichment of selenium in the Apliki Cyprus-type VMS deposit, Troodos, Cyprus. Min Mag 82:697–724
Martin AJ, Keith M, McDonald I, Haase KM, McFall KA, Klemd R, MacLeod CJ (2019) Trace element systematics and ore-forming processes in mafic VMS deposits: evidence from the Troodos ophiolite, Cyprus. Ore Geol Rev 106:205–225
Martin AJ, Keith M, Parvaz DB, McDonald I, Boyce AJ, McFall KA, Jenkin GRT, Strauss H, MacLeod CJ (2020) Effects of magmatic volatile influx in mafic VMS hydrothermal systems: evidence from the Troodos ophiolite, Cyprus. Chem Geol 531:119325
Martin AJ, McDonald I, Jenkin GRT, McFall KA, Boyce AJ, Jamieson JW, MacLeod CJ (2021) A missing link between ancient and active mafic-hosted seafloor hydrothermal systems – magmatic volatile influx in the exceptionally preserved Mala VMS deposit, Troodos, Cyprus. Chem Geol 567:120127
Maslennikov VV, Maslennikova SP, Large RR, Danyushevsky LV (2009) Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanic-hosted massive sulfide deposit (Southern Urals, Russia) using laser ablation-inductively coupled plasma mass spectrometry (LA-ICPMS). Econ Geol 104:1111–1141
Maslennikov VV, Maslennikova SP, Large RR, Danyushevsky LV, Herrington RJ, Ayupova NR, Zaykov VV, Lein AYu, Tseluyko AS, Melekestseva IYu, Tessalina SG (2017) Chimneys in Paleozoic massive sulfide mounds of the Urals VMS deposits: Mineral and trace element comparison with modern black, grey, white and clear smokers. Ore Geol Rev 85:64–106. https://doi.org/10.1016/j.oregeorev.2016.09.012
Mathieu L (2019) Detecting magmatic-derived fluids using pyrite chemistry: example of the Chibougamau area, Abitibi Subprovince, Québec. Ore Geol Rev 114:103–127
McDermott JM, Ono S, Tivey MK, Seewald JS, Shanks WC, Solow AR (2015) Identification of sulfur sources and isotopic equilibria in submarine hot-springs using multiple sulfur isotopes. Geochim Cosmochim Acta 160:169–187
McDonald MJ, Piercey SJ, Layne GD, Pigage LC, Piercey G (2018) Mineral assemblages, textures and in situ sulphur isotope geochemistry of sulphide mineralization from the Cyprus-type ice volcanogenic massive sulphide (VMS) deposit, ,on Canada. Minerals 8:501
McPhail DC (1995) Thermodynamic properties of aqueous tellurium species between 25 and 350°. Geochim Cosmochim Acta 59:851–866
Melekestseva IY, Tret’yakov GA, Nimis P, Yuminov AM, Maslennikov VV, Maslennikova SP, Kotlyarov VA, Beltenev VE, Danyushevsky LV, Large R (2014) Barite-rich massive sulfides from the Semenov-1 hydrothermal field (Mid-Atlantic Ridge, 13°30.87′ N): evidence for phase separation and magmatic input. Mar Geol 349:37–54
Meng X, Li X, Chu F, Fu B, Lei J, Li Z, Wang H, Chen L (2019) Multi-stage growth and fluid evolution of a hydrothermal sulphide chimney in the East Pacific Ridge 1–2° S hydrothermal field: constraints from in situ sulphur isotopes. Geol Mag 156:989–1002
Meng X, Li X, Chu F, Zhu J, Lei J, Li Z, Wang H, Chen L, Zhu Z (2020) Trace element and sulfur isotope compositions for pyrite across the mineralization zones of a sulfide chimney from the East Pacific Rise (1–2°S). Ore Geol Rev 116:103209
Metz S, Trefry JH (2000) Chemical and mineralogical influences on concentrations of trace metals in hydrothermal fluids. Geochim Cosmochim Acta 64(13):2267–2279. https://doi.org/10.1016/S0016-7037(00)00354-9
Monecke T, Petersen S, Hannington MD, Grant H, Samson I (2016) The minor element endowment of modern sea-floor massive sulfides and comparison with deposits hosted in ancient volcanic successions. Rev Econ Geol 18:245–306
Muenow DW, Garciat MO, Aggrey KE, Bednarz U, Schmincke HU (1990) Volatiles in submarine glasses as a discriminant of tectonic origin: application to the Troodos ophiolite. Nature 343:159–161
Mukasa SB, Ludden JN (1987) Uranium-lead isotopic ages of plagiogranites from the Troodos ophiolite, Cyprus, and their tectonic significance. Geology 15:825–828
Murton BJ, Lehrmann B, Dutrieux AM, Martins S, de la Iglesia AG, Stobbs IJ, Barriga FJAS, Bialas J, Dannowski A, Vardy ME, North LJ, Yeo IALM, Lusty PAJ, Petersen S (2019) Geological fate of seafloor massive sulphides at the TAG hydrothermal field (Mid-Atlantic Ridge). Ore Geol Rev 107:903–925
Nozaki T, Nagase T, Ushikubo T, Shimizu K, Ishibashi J-I (2021) Microbial sulfate reduction plays an important role at the initial stage of subseafloor sulfide mineralization. Geology 49(2):222–227. https://doi.org/10.1130/G47943.1
Ohmoto H (1996) Formation of volcanogenic massive sulfide deposits: the Kuroko perspective. Ore Geol Rev 10:135–177
Ohmoto H, Lasaga AC (1982) Kinetics of reactions between aqueous sulfates and sulfides in hydrothermal systems. Geochim Cosmochim Acta 46:1727–1745
Ono S, Shanks WC, Rouxel OJ, Rumble D (2007) S-33 constraints on the seawater sulfate contribution in modern seafloor hydrothermal vent sulfides. Geochim Cosmochim Acta 71:1170–1182
Parvaz DB (2014) Oxidation zones of volcanogenic massive sulphide deposits in the Troodos Ophiolite, Cyprus: targeting secondary copper deposits. Doctoral Thesis, University of Exeter
Patten CGC, Pitcairn IK, Teagle DAH (2017) Hydrothermal mobilisation of Au and other metals in supra-subduction oceanic crust: insights from the Troodos ophiolite. Ore Geol Rev 86:487–508
Patten CGC, Pitcairn IK, Alt JC, Zack T, Lahaye Y, Teagle DAH, Markdahl K (2020) Metal fluxes during magmatic degassing in the oceanic crust: sulfide mineralisation at ODP site 786B, Izu-Bonin forearc. Miner Deposita 55:469–489
Pearce JA, Robinson PT (2010) The Troodos ophiolitic complex probably formed in a subduction initiation, slab edge setting. Gondwana Res A Tribute Miyashiro 18:60–81
Pedersen L-ER, Staudigel H, McLoughlin N, Whitehouse MJ, Strauss H (2017) A multiple sulfur isotope study through the volcanic section of the Troodos ophiolite. Chem Geol 46:849–862
Petersen S, Herzig PM, Hannington MD (2000) Third dimension of a presently forming VMS deposit: TAG hydrothermal mound, Mid-Atlantic Ridge, 26°N. Miner Deposita 35:233–259
Petersen S, Herzig PM, Hannington MD, Jonasson IR, Arribas A (2002) Submarine gold mineralization near Lihir Island, New Ireland Fore-Arc, Papua New Guinea. Econ Geol 97:1795–1813
Prichard HM, Knight RD, Fisher PC, McDonald I, Zhou M-F, Wang CY (2013) Distribution of platinum-group elements in magmatic and altered ores in the Jinchuan intrusion, China: an example of selenium remobilization by postmagmatic fluids. Miner Deposita 48:767–786
Rautenschlein M, Jenner GA, Hertogen J, Hofmann AW, Kerrich R, Schmincke H-U, White WM (1985) Isotopic and trace element composition of volcanic glasses from the Akaki Canyon, Cyprus: implications for the origin of the Troodos ophiolite. Earth Planet Sci Lett 75:369–383
Reed MH, Palandri J (2006) Sulfide mineral precipitation from hydrothermal fluids. Rev Mineral Geochem 61:609–631
Revan MK, Genç Y, Maslennikov VV, Maslennikova SP, Large RR, Danyushevsky LV (2014) Mineralogy and trace-element geochemistry of sulfide minerals in hydrothermal chimneys from the Upper-Cretaceous VMS deposits of the eastern Pontide orogenic belt (NE Turkey). Ore Geol Rev 63:129–149
Richardson CJ, Cann JR, Richards HG, Cowan JG (1987) Metal-depleted root zones of the Troodos ore-forming hydrothermal systems, Cyprus. Earth Planet Sci Lett 84:243–253
Rouxel O, Fouquet Y, Ludden JN (2004) Subsurface processes at the lucky strike hydrothermal field, Mid-Atlantic ridge: evidence from sulfur, selenium, and iron isotopes. Geochim Cosmochim Acta 68:2295–2311
Rouxel O, Ono S, Alt J, Rumble D, Ludden J (2008) Sulfur isotope evidence for microbial sulfate reduction in altered oceanic basalts at ODP Site 801. Earth Planet Sci Lett 268:110–123
Sakai H (1968) Isotopic properties of sulfur compounds in hydrothermal processes. Geochem J 2:29–49
Shanks WC (2001) Stable isotopes in seafloor hydrothermal systems: vent fluids, hydrothermal deposits, hydrothermal alteration, and microbial processes. Rev Mineral Geochem 43:469–525
Sharman ER, Taylor BE, Minarik WG, Dubé B, Wing BA (2015) Sulfur isotope and trace element data from ore sulfides in the Noranda district (Abitibi, Canada): implications for volcanogenic massive sulfide deposit genesis. Miner Deposita 50:591–606
Sillitoe RH, Hannington MD, Thompson JFH (1996) High sulfidation deposits in the volcanogenic massive sulfide environment. Econ Geol 91:204–212
Smith JW, Holwell DA, McDonald I, Boyce AJ (2016) The application of S isotopes and S/Se ratios in determining ore-forming processes of magmatic Ni–Cu–PGE sulfide deposits: A cautionary case study from the northern Bushveld Complex. Ore Geol Rev 73:148–174. https://doi.org/10.1016/j.oregeorev.2015.10.022
Tivey MK (2007) Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20:50–65
Tivey MK, Humphris SE, Thompson G, Hannington MD, Rona PA (1995) Deducing patterns of fluid flow and mixing within the TAG active hydrothermal mound using mineralogical and geochemical data. J Geophys Res Solid Earth 100:12527–12555
Varga RJ, Moores EM (1985) Spreading structure of the Troodos ophiolite, Cyprus. Geology 13:846–850
Von Damm KL (1990) Seafloor Hydrothermal Activity: Black Smoker Chemistry and Chimneys. Annu Rev Earth Planet Sci 18(1):173–204. https://doi.org/10.1146/annurev.ea.18.050190.001133
Von Damm KL (1995) Controls on the chemistry and temporal variability of seafloor hydrothermal fluids. In: SE Humphris, Zierenberg RA, Mullineaux LS, Thomson RE (ed) Seafloor hydrothermal systems: physical, chemical, biological, and geological interactions. American Geophysical Union, Washington D.C., vol.91 pp. 222–247
Von Damm KL, Oosting SE, Kozlowski R, Buttermore LG, Colodner DC, Edmonds HN, Edmond JM, Grebmeier JM (1995) Evolution of East Pacific Rise hydrothermal vent fluids following a volcanic eruption. Nature 375:47–50
Wallace PJ (2005) Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J Volcanol Geotherm Res 140(1–3):217–240. https://doi.org/10.1016/j.jvolgeores.2004.07.023
Wang Y, Han X, Petersen S, Frische M, Qiu Z, Cai Y, Zhou P (2018) Trace metal distribution in sulfide minerals from ultramafic-hosted hydrothermal systems: examples from the Kairei Vent Field, Central Indian Ridge. Minerals 8:526
Woelki D, Regelous M, Haase KM, Romer RHW, Beier C (2018) Petrogenesis of boninitic lavas from the Troodos Ophiolite, and comparison with Izu–Bonin–Mariana fore-arc crust. Earth Planet Sci Lett 498:203–214
Wohlgemuth-Ueberwasser CC, Viljoen F, Petersen S, Vorster C (2015) Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: an in-situ LA-ICP-MS study. Geochim Cosmochim Acta 159:16–41
Woodruff LG, Shanks WC (1988) Sulfur isotope study of chimney minerals and vent fluids from 21°N, East Pacific Rise: hydrothermal sulfur sources and disequilibrium sulfate reduction. J Geophys Res Solid Earth 93:4562–4572
Wortmann UG, Bernasconi SM, Böttcher ME (2001) Hypersulfidic deep biosphere indicates extreme sulfur isotope fractionation during single-step microbial sulfate reduction. Geology 29:647–650
Yamamoto M (1976) Relationship between Se/S and sulfur isotope ratios of hydrothermal sulfide minerals. Mineral Deposita 11:197–209
Yang K, Scott SD (1996) Possible contribution of a metal-rich magmatic fluid to a sea-floor hydrothermal system. Nature 383:420–423
Yang K, Scott SD (2002) Magmatic degassing of volatiles and ore metals into a hydrothermal system on the modern sea floor of the eastern Manus Back-Arc Basin, Western Pacific. Econ Geol 97:1079–1100
Yeats CJ, Parr JM, Binns RA, Gemmell JB, Scott SD (2014) The SuSu Knolls hydrothermal field, Eastern Manus Basin, Papua New Guinea: an active submarine high-sulfidation copper-gold system. Econ Geol 109:2207–2226
You C-F, Bickle MJ (1998) Evolution of an active sea-floor massive sulphide deposit. Nature 394:668–671
Zeng Z, Ma Y, Chen S, Selby D, Wang X, Yin X (2017) Sulfur and lead isotopic compositions of massive sulfides from deep-sea hydrothermal systems: implications for ore genesis and fluid circulation. Ore Geol Rev 87:155–171
Acknowledgements
The authors acknowledge the support of the Geological Survey Department of Cyprus, especially Costas Costantinou and Andreas Zissimos. We thank Michael Green and Ifigenia Gavriel for the discussion and assistance in the field. We thank Stefanie Brueckner and associate editor Mostafa Fayek for their constructive reviews and editor-in-chief Georges Beaudoin for the editorial handling of this manuscript.
Funding
This research was partly funded by the NERC SoS consortium grant NE/M010848/1 “TeaSe: tellurium and selenium cycling and supply” awarded to Cardiff University and by the Canadian Research Chair program awarded to John W. Jamieson.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: M. Fayek
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martin, A.J., McDonald, I., Jamieson, J.W. et al. Mineral-scale variation in the trace metal and sulfur isotope composition of pyrite: implications for metal and sulfur sources in mafic VMS deposits. Miner Deposita 57, 911–933 (2022). https://doi.org/10.1007/s00126-021-01080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-021-01080-1