Abstract
Aims/hypothesis
The aim of this work was to evaluate whether the association of prediabetes with dementia is explained by the intervening onset of diabetes.
Methods
Among participants of the Atherosclerosis Risk in Communities (ARIC) study we defined baseline prediabetes as HbA1c 39–46 mmol/mol (5.7–6.4%) and subsequent incident diabetes as a self-reported physician diagnosis or use of diabetes medication. Incident dementia was ascertained via active surveillance and adjudicated. We quantified the association of prediabetes with dementia risk before and after accounting for the subsequent development of diabetes among ARIC participants without diabetes at baseline (1990–1992; participants aged 46–70 years). We also evaluated whether age at diabetes diagnosis modified the risk of dementia.
Results
Among 11,656 participants without diabetes at baseline, 2330 (20.0%) had prediabetes. Before accounting for incident diabetes, prediabetes was significantly associated with the risk of dementia (HR 1.12 [95% CI 1.01, 1.24]). After accounting for incident diabetes, the association was attenuated and non-significant (HR 1.05 [95% CI 0.94, 1.16]). Earlier age of onset of diabetes had the strongest association with dementia: HR 2.92 (95% CI 2.06, 4.14) for onset before 60 years; HR 1.73 (95% CI 1.47, 2.04) for onset at 60–69 years; and HR 1.23 (95% CI 1.08, 1.40) for onset at 70–79 years.
Conclusions/interpretation
Prediabetes is associated with dementia risk but this risk is explained by the subsequent development of diabetes. Earlier age of onset of diabetes substantially increases dementia risk. Preventing or delaying progression of prediabetes to diabetes will reduce dementia burden.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Diabetes is strongly linked to dementia risk [1,2,3]. Prediabetes is an intermediate stage of hyperglycaemia that confers a high risk of progression to diabetes but is also independently associated with other clinical outcomes [4]. Prior studies have shown that prediabetes is a risk factor for neurocognitive outcomes including cognitive decline and dementia [5,6,7]. The risk of progression to diabetes among people with prediabetes is substantial; among middle-aged adults with prediabetes, 5–10% per year will develop diabetes for a total of 70% during their lifetime [8, 9]. Few studies of dementia have accounted for the transition from prediabetes to diabetes. Thus, it is unclear to what extent the intervening development of diabetes explains the excess risk of dementia in people with prediabetes.
There is also substantial heterogeneity in risk of diabetes complications according to age at diabetes onset [10,11,12,13]. Earlier onset of diabetes is associated with a more severe presentation and confers a higher life-long risk of clinical outcomes compared with later-onset diabetes [10,11,12,13]. The age at diabetes onset is critical to evaluating risk of long-term outcomes such as dementia.
In this study, we used data from a large population-based cohort to characterise the association of prediabetes with dementia risk and examine the extent to which this association is explained by the intervening development of clinical diabetes. We also characterised the association between dementia risk and incident diabetes according to age at diabetes onset.
Methods
Study population
The Atherosclerosis Study populationRisk in Communities (ARIC) study is a prospective cohort that originally recruited 15,792 participants aged 45–64 years in 1987–1989 from four US counties: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Baseline for our analysis was visit 2 (1990–1992), the first visit where HbA1c and cognitive function were measured in the ARIC study. Among 14,348 participants who attended visit 2, we excluded participants according to the following criteria: missing HbA1c measurements (n=279); missing covariates (n=589); prevalent dementia (n=7); died before age 50 years (n=7); or diabetes at baseline based on self-reported physician diagnosis, diabetes medication use or HbA1c≥47 mmol/mol (6.5%) (n=1810). There were 11,656 adults included in our final analytical sample.
Baseline prediabetes and incident diabetes definitions
In our primary analysis, we defined prediabetes based on HbA1c criteria (39–46 mmol/mol [5.7–6.4%]) [14]. Participants without prediabetes (HbA1c<39 mmol/mol [5.7%]) served as the reference group. We also conducted secondary analyses of the association of prediabetes with dementia based on three additional definitions of prediabetes: fasting glucose (FG) 5.55–6.94 mmol/l; elevated HbA1c (39–46 mmol/mol [5.7–6.4%]) or elevated FG (5.55–6.94 mmol/l); and elevated HbA1c (39–46 mmol/mol [5.7–6.4%]) and elevated FG (5.55–6.94 mmol/l) [14].
Incident diabetes was defined as either self-reported physician diagnosis or diabetes medication use reported by participants during in-person visits or annual telephone calls (semi-annually beginning in 2012). The date of incident diabetes was defined as the date on which diabetes was first reported during an in-person visit or telephone call. This definition of diabetes has been shown to be highly reliable and specific [15]. We categorised age at diabetes diagnosis as <60, 60–69, 70–79 or 80–93 years.
Dementia ascertainment
Dementia was defined by expert committee review of cognitive function assessments, informant reports and hospitalisation codes as detailed previously [16]. The cognitive function assessments incorporated data from a three-test cognitive battery administered at visits 2 (1990–1992) and 4 (1996–1998), the expanded neuropsychological ten-test battery [17] administered from visit 5 (2011–2013) onwards and informant interview (Clinical Dementia Rating [CDR] scale [18] and the Functional Activities Questionnaire [FAQ]). The Mini-Mental State Examination (MMSE) was also administered [19]. A computer algorithm generated preliminary diagnoses based on low scores in these tests. Dementia diagnoses were then verified by an expert panel of clinicians and neuropsychologists. For participants who did not return for an in-person follow-up evaluation, the expert panel used information from the Telephone Interview for Cognitive Status-Modified (TICSm) or Six-Item Cognitive Screener administered to the participant and an Ascertain Dementia Eight-Item Informant Questionnaire administered to an informant. Dementia was also identified by discharge hospitalisation ICD-9 (http://www.icd9data.com/2007/Volume1/default.htm) or death certificate dementia codes. Follow-up time for dementia was measured from visit 2 (1990–1992) to the first diagnosis of dementia or censoring due to death, loss to follow-up, or administrative censoring on 31 December 2019.
Demographic and genetic risk factors
All baseline data were collected at visit 2 (1990–1992) unless otherwise stated. Date of birth was self-reported at visit 1 and was used to calculate age at baseline (visit 2). Sex and education level were also self-reported at visit 1. Education level was further categorised into three groups: less than high school; high school graduate or equivalent; and college or above. Race was self-selected from four fixed categories (Asian, Black, American Indian/Alaskan Indian, or White) at visit 1. We modelled race as Black vs White participants, including the small number of Asian and American Indian (n=34) participants in the White category (n=9149). ARIC field centres (Forsyth County, Jackson, Minneapolis Suburbs and Washington County) were included. APOE \(\varepsilon\)4 genotype was performed using the TaqMan assay (Applied Biosystems, Foster City, CA, USA) and defined based on the number of \(\varepsilon\)4 alleles (0, 1 or 2 alleles) at visit 2.
Lifestyle factors
BMI was calculated from weight (kg) divided by height (m2) at visit 2. BMI was categorised into four groups: normal weight <25 kg/m2; overweight 25–29.9 kg/m2; obesity 30–39.9 kg/m2; and morbid obesity ≥40 kg/m2. Smoking history and alcohol use were self-reported as current, former or never smokers and alcohol users at visit 2. Physical activity was assessed at visit 1 using a modified Baecke questionnaire and categorised according to American Heart Association guidelines into recommended, intermediate or poor [20].
Clinical factors
All clinical factors were assessed at visit 2. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of BP-lowering medication. Plasma total cholesterol (mmol/l) was measured using enzymatic methods. HDL-cholesterol (mmol/l) was measured after dextran magnesium precipitation. Prevalent stroke was ascertained from self-report during visits 1 and 2, or hospitalisation or death related to stroke prior to visit 2. Prevalent atrial fibrillation was based on self-report and ECG at visit 2.
Statistical analysis
We compared baseline characteristics of the participants according to prediabetes status at baseline using proportions and mean (SD). We used age 50 years as the time origin for survival analysis. We conducted a Kaplan–Meier analysis to evaluate dementia survival by baseline prediabetes status and by age of onset of incident diabetes. Each curve in the incident diabetes age of onset Kaplan–Meier was conditional on being dementia and death free at the starting point. We used Cox proportional hazards regression models to characterise the association of prediabetes with incident dementia. To examine the degree to which incident diabetes explained the association between baseline prediabetes and incident dementia, we added incident diabetes during follow-up as a time-varying covariate to our models. The incident diabetes variable was also evaluated by diagnosis age categories <60, 60–69, 70–79 and ≥80 years. Demographic and genetic factors were adjusted in Model 1 and lifestyle and clinical factors were additionally adjusted in Model 2. We evaluated the assumption of proportionality using log–log plots.
As a sensitivity analysis, we compared the HRs (95% CIs) of dementia according to the four different definitions of prediabetes, with and without adjusting for incident diabetes as a time-varying variable for both Model 1 and Model 2. Since stroke is a major risk factor for dementia, we also performed a sensitivity analysis excluding prevalent stroke. All analyses were done using Stata/SE 17.0 (StataCorp, College Station, TX, USA). p values <0.05 were considered statistically significant.
Results
Among 11,656 participants without diabetes at baseline, the mean age was 56.8 (SD 5.7) years, 55.3% were female, and 20.0% had prediabetes (HbA1c 39–46 mmol/mol [5.7–6.4%]). Participants with prediabetes (vs without) were more likely to self-report Black race (39.5% vs 16.7%) and were more likely to have lower than a high school education (30.2% vs 17.0%). Participants with prediabetes had a higher burden of lifestyle and clinical risk factors (Table 1).
Overall, there were 3143 participants who developed diabetes during a median of 15.9 (p25–p75: 10.0–21.0) years of follow-up. The mean age of incident diabetes was similar for those with and without prediabetes at baseline (~71.6 years). However, those with (vs without) prediabetes were more likely to develop diabetes (44.6% vs 22.5%) (ESM Table 1).
A total of 2247 participants developed dementia over a median of 24.7 (p25–p75: 17.9–27.1) years of follow-up. The development of dementia varied by prediabetes and incident diabetes status (Fig. 1). For instance, among those with prediabetes, the cumulative incidence of dementia was 16.6% higher in those who developed vs did not develop diabetes (23.9% vs 20.5%).
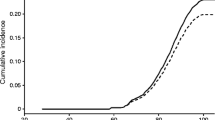
The cumulative incidence of dementia in people with prediabetes was 15% (vs 10% in those without prediabetes) by age 80 years and 63% (vs 53%) by age 90 years (Fig. 2a). Prediabetes was significantly associated with incident dementia (HR 1.19 [95% CI 1.07, 1.31]) after adjusting for demographics and APOE (Table 2, Model 1A). After adjusting for incident diabetes as a time-varying variable, the association of prediabetes and dementia was strongly attenuated and was no longer statistically significant (HR 1.09 [95% CI 0.98, 1.21]) (Model 1B). Results were similar in models that adjusted for other lifestyle and clinical risk factors (HR 1.12 [95% CI 1.01, 1.24] before and HR 1.05 [95% CI 0.94, 1.16] after adjusting for incident diabetes) (Models 2A and 2B).
Risk (cumulative incidence) of dementia (Kaplan–Meier curves) according to baseline prediabetes status (a) and by age at onset of incident diabetes during follow-up (b). For each curve in (a), there were n=9326 participants without prediabetes and n=2330 with prediabetes at the time origin. At the beginning time for each curve in (b), there were n=11,656 participants with no incident diabetes, n=237 with diabetes onset at age <60 years, n=971 with diabetes onset at age 60–69 years, n=1534 with diabetes onset at 70–79 years and n=401 with diabetes onset at 80–93 years
The cumulative incidence of dementia was highest among those who developed diabetes at an earlier age (Fig. 2b). Participants who developed diabetes at age 70–79 or ≥80 years had roughly similar cumulative dementia incidence to those without incident diabetes. In multivariable adjusted models, the strength of the association between incident diabetes and dementia decreased with older age at diabetes onset (Table 2). Individuals who were diagnosed with diabetes at younger than 60 years of age were at the highest risk of dementia (HR 2.92 [95% CI 2.06, 4.14]) (Model 2B). Those diagnosed with diabetes when aged 80 years or older did not have a statistically significantly elevated risk of dementia (HR 1.13 [95% CI 0.94, 1.35]). The HRs were 1.73 (95% CI 1.47, 2.04) for onset at 60–69 years and 1.23 (95% CI 1.08, 1.40) for onset at 70–79 years.
When we compared different definitions of prediabetes, stronger associations with dementia were observed for prediabetes based on HbA1c (ESM Table 2). For all definitions of prediabetes, prediabetes was no longer significantly associated with risk of dementia after adjusting for incident diabetes. Due to the small number of participants with stroke at baseline (N=172 [1.5%]), the results were essentially unchanged after excluding these individuals from analysis.
Discussion
In this community-based study, we found that prediabetes in midlife was modestly associated with dementia risk. After adjusting for the intervening onset of diabetes, however, prediabetes was no longer independently associated with dementia. Results were similar across different definitions of prediabetes. Earlier age at diabetes onset was also associated with substantially greater risk of dementia. Taken together, our findings suggest that preventing prediabetes progression, especially in younger individuals, may be an important way to reduce the dementia burden.
Our study suggests that prediabetes is associated with dementia but this association is primarily explained by the development of clinical diabetes. Prior studies have demonstrated small to moderate relative risks of prediabetes with dementia, ranging from 1.01 to 1.20 [2, 7, 12]. In a meta-analysis, the RR for the association of prediabetes with dementia was 1.18, consistent with our findings [2]. However, no prior studies have formally considered the progression of prediabetes to diabetes in analyses of dementia risk. Our results provide a fuller picture of the association and suggest that prediabetes is not a robust risk factor for dementia in the absence of a subsequent diagnosis of diabetes.
In the USA, up to 96 million adults have prediabetes, accounting for 38% of the adult population [21]. Structured lifestyle intervention programmes (such as the CDC-led National Diabetes Prevention Program) [22] can effectively prevent diabetes progression. However, fewer than 5% of adults with prediabetes receive referrals to these programmes from their healthcare providers [23]. More than 80% of adults with prediabetes are also unaware that they have the condition [22]. Improving early detection and engagement in prediabetes progression to diabetes may have long-term population benefits for dementia prevention, particularly since earlier age of onset of diabetes is associated with the most dramatic risk of dementia.
Our study confirms the strong link between diabetes and dementia risk [1,2,3] but we found strong modification of this association depending on the age of onset of diabetes. Diabetes that was diagnosed earlier in adulthood (age <60 years) was strongly associated with dementia risk whereas old-age-onset diabetes (age >80 years) did not contribute to an excess risk of dementia. There are a few studies regarding dementia risk with diabetes age of onset and our results are in line with the current evidence. The Whitehall II study showed every 5 year earlier onset of diabetes was significantly associated with higher hazard of dementia (HR 1.24 [95% CI 1.06, 1.46]) [12]. The Swedish Twin Registry study reported greater odds of dementia among people whose diabetes age of onset was less than 65 years (OR 2.41 [95% CI 1.05, 5.51]) but not more than 65 years. Our finding suggests the elevated dementia hazard is non-linear. They also suggest that sustained exposure to hyperglycaemia is critical for dementia development. Prevention efforts in people with diabetes diagnosed younger than 65 years should be a high priority [24].
Studies of prediabetes as a risk factor have proposed similar mechanisms to those with diabetes as a risk factor for dementia. Putative mechanisms include acute and chronic hyperglycaemia, glucose toxicity, insulin resistance and microvascular dysfunction of the central nervous system [25,26,27,28,29,30]. Increased peripheral insulin in people with hyperglycaemia results in neuronal insulin receptor desensitisation, which may lead to a decrease in Aβ clearance [25, 28, 29] and an increase in prephosphorylation of τ protein [25, 26, 30]. Glucose toxicity and microvascular dysfunction are associated with increased inflammatory and oxidative stress, leading to increased blood–brain permeability [30,31,32,33]. The combination of these mechanisms has been proposed to explain the link between diabetes and vascular and Alzheimer’s dementia.
Strengths of our study include the large, community-based cohort and long duration of follow-up. We were able to adjust for important confounding factors including APOE and other major dementia risk factors, which were measured by trained personnel using standardised protocols. We identified over 3000 incident cases of diabetes after baseline, and we were able to account for diabetes as a time-varying risk factor in the association between prediabetes and dementia. Our study also benefited from the rigorous ascertainment and adjudication of dementia in the ARIC study.
There are several important limitations of our study. First, we only considered single baseline measurement of HbA1c and fasting glucose. However, we compared different prediabetes definitions with the combination of elevated HbA1c and elevated fasting glucose. Second, we did not have information to differentiate dementia subtypes. Prior studies have shown a higher RR of prediabetes with vascular dementia than with Alzheimer’s disease. Third, the duration of prediabetes at baseline was unknown; we were not able to account for duration of prediabetes in our analyses. Last, we are not able to rule out residual confounding due the observational nature of our study.
In conclusion, prediabetes was associated with dementia risk but this association was explained by the intervening development of diabetes. Earlier age of onset of diabetes was associated with a substantially greater risk of dementia. Among people with prediabetes, preventing and delaying the progression to diabetes is likely to reduce dementia burden.
Abbreviations
- ARIC:
-
Atherosclerosis Risk in Communities
- FG:
-
Fasting glucose
References
Zhang J, Chen C, Hua S et al (2017) An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124:41–47. https://doi.org/10.1016/j.diabres.2016.10.024
Cheng G, Huang C, Deng H, Wang H (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42(5):484–491. https://doi.org/10.1111/j.1445-5994.2012.02758.x
Chatterjee S, Peters SAE, Woodward M et al (2016) Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39(2):300–307. https://doi.org/10.2337/dc15-1588
Echouffo-Tcheugui JB, Selvin E (2021) Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health 42:59–77. https://doi.org/10.1146/annurev-publhealth-090419-102644
Rawlings AM, Sharrett AR, Schneider ALC et al (2014) Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 161(11):785–793. https://doi.org/10.7326/M14-0737
Garfield V, Farmaki A-E, Fatemifar G et al (2021) The relationship between glycaemia, cognitive function, structural brain outcomes and dementia: a Mendelian randomisation study in the UK biobank. Diabetes 70(10):2313–2321. https://doi.org/10.2337/db20-0895
Kim MK, Han K, Koh ES et al (2021) Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus. Diabetes Res Clin Pract 175:108799. https://doi.org/10.1016/j.diabres.2021.108799
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M (2012) Prediabetes: a high-risk state for diabetes development. Lancet 379(9833):2279–2290. https://doi.org/10.1016/S0140-6736(12)60283-9
Hostalek U (2019) Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol 5(1):5. https://doi.org/10.1186/s40842-019-0080-0
Fang M, Echouffo-Tcheugui JB, Selvin E (2020) Burden of complications in U.S. adults with young-onset type 2 or type 1 diabetes. Diabetes Care 43(4):e47–e49. https://doi.org/10.2337/dc19-2394
Ang GY (2020) Age of onset of diabetes and all-cause mortality. World J Diabetes 11(4):95–99. https://doi.org/10.4239/wjd.v11.i4.95
BarbielliniAmidei C, Fayosse A, Dumurgier J et al (2021) Association between age at diabetes onset and subsequent risk of dementia. JAMA 325(16):1640–1649. https://doi.org/10.1001/jama.2021.4001
Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG (2016) Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabetic Med 33(12):1615–1624. https://doi.org/10.1111/dme.13113
American Diabetes Association (2022) Standards of medical care in diabetes—2022 abridged for primary care providers. Clin Diabetes 40(1):10–38. https://doi.org/10.2337/cd22-as01
Schneider ALC, Pankow JS, Heiss G, Selvin E (2012) Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 176(8):738–743. https://doi.org/10.1093/aje/kws156
Knopman DS, Gottesman RF, Sharrett AR et al (2016) Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study. Alzheimers Dement (Amst) 2:1–11. https://doi.org/10.1016/j.dadm.2015.12.002
Schneider ALC, Sharrett AR, Gottesman RF et al (2015) Normative data for 8 neuropsychological tests in older blacks and whites from the atherosclerosis risk in communities (ARIC) study. Alzheimer Dis Assoc Disord 29(1):32–44. https://doi.org/10.1097/WAD.0000000000000042
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(11):2412–2414. https://doi.org/10.1212/wnl.43.11.2412-a
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
American Heart Association Recommendations for Physical Activity in Adults and Kids. Available from www.heart.org. https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults. Accessed 3 Aug 2022
Centers for Disease Control and Prevention (2022) National Diabetes Statistics Report. Available from www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 23 Aug 2022
Centers for Disease Control and Prevention (2021) Prediabetes - Your Chance to Prevent Type 2 Diabetes. Available from http://bit.ly/2hMpYrt. Accessed 23 Aug 2022
Alva ML, Chakkalakal RJ, Moin T, Galaviz KI (2022) The diabetes prevention gap and opportunities to increase participation in effective interventions. Health Affairs 41(7):971–979. https://doi.org/10.1377/hlthaff.2022.00259
Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2009) Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 58(1):71–77. https://doi.org/10.2337/db08-0586
Kellar D, Craft S (2020) Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 19(9):758–766. https://doi.org/10.1016/S1474-4422(20)30231-3
Korte N, Nortley R, Attwell D (2020) Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol 140(6):793–810. https://doi.org/10.1007/s00401-020-02215-w
Li X, Song D, Leng SX (2015) Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. CIA 10:549–560. https://doi.org/10.2147/CIA.S74042
Arnold SE, Arvanitakis Z, Macauley-Rambach SL et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14(3):168–181. https://doi.org/10.1038/nrneurol.2017.185
Launer LJ (2020) Interrelationships among central insulin signalling, diabetes, and cognitive impairment. Lancet Neurol 19(8):640–642. https://doi.org/10.1016/S1474-4422(20)30172-1
Rensma SP, van Sloten TT, Houben AJHM et al (2020) Microvascular dysfunction is associated with worse cognitive performance. Hypertension 75(1):237–245. https://doi.org/10.1161/HYPERTENSIONAHA.119.13023
Ceriello A, Esposito K, Piconi L et al (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57(5):1349–1354. https://doi.org/10.2337/db08-0063
Fischer R, Maier O (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015:e610813. https://doi.org/10.1155/2015/610813
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295(14):1681–1687. https://doi.org/10.1001/jama.295.14.1681
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Data availability
The ARIC data are not publicly available due to confidentiality issues. Investigators can access data from the ARIC study by submitting a manuscript proposal to aricpub@unc.edu.
Funding
The ARIC study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I and HHSN268201700004I. Collection of neurocognitive data was funded by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902 and 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke [NINDS], National Institute on Aging [NIA] and National Institute on Deafness and Other Communications Disorders [NIDCD]); previous brain MRI examinations were funded by R01-HL70825 and biomarkers by R01-HL134320 from the NHLBI. PLL was supported NIH/NHLBI grant K24 HL159246. RFG was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. ES was supported by NIH/NHLBI grants K24 HL152440 and R01 HL158022, NIH/NIDDK grants R01 DK089174 and R01 DK128837, and NIH/NIA grant RF1 AG074044.
Authors’ relationships and activities
ES is on the advisory board for Diabetologia and had no role in peer-review of the manuscript. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
JH, MF, JC and ES designed the study, researched the data and contributed to discussion. JH conducted the analyses and wrote the initial manuscript. All authors provided substantial contributions to the interpretation of data, made critical revisions to the manuscript and approved the final manuscript. JH is the guarantor of this work and, as such, had full access to all the data in the study, controlled the decision to publish and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author, ES, attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, J., Fang, M., Pike, J.R. et al. Prediabetes, intervening diabetes and subsequent risk of dementia: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 66, 1442–1449 (2023). https://doi.org/10.1007/s00125-023-05930-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-05930-7