Abstract
Aims/hypothesis
Glucagon-like peptide 1 (GLP-1) reduces appetite and energy intake in humans, whereas the other incretin hormone, glucose-dependent insulinotropic polypeptide (GIP), seems to have no effect on eating behaviour. Interestingly, studies in rodents have shown that concomitant activation of GIP and GLP-1 receptors may potentiate the satiety-promoting effect of GLP-1, and a novel dual GLP-1/GIP receptor agonist was recently shown to trigger greater weight losses compared with a GLP-1 receptor agonist in individuals with type 2 diabetes. The aim of this study was to delineate the effects of combined GIP and GLP-1 receptor activation on energy intake, appetite and resting energy expenditure in humans.
Methods
We examined 17 overweight/obese men in a crossover design with 5 study days. On day 1, a 50 g OGTT was performed; on the following 4 study days, the men received an isoglycaemic i.v. glucose infusion (IIGI) plus saline (154 mmol/l NaCl; placebo), GIP (4 pmol kg−1 min−1), GLP-1 (1 pmol kg−1 min−1) or GIP+GLP-1 (4 and 1 pmol kg−1 min−1, respectively). All IIGIs were performed in a randomised order blinded for the participant and the investigators. The primary endpoint was energy intake as measured by an ad libitum meal after 240 min. Secondary endpoints included appetite ratings and resting energy expenditure, as well as insulin, C-peptide and glucagon responses.
Results
Energy intake was significantly reduced during IIGI+GLP-1 compared with IIGI+saline infusion (2715 ± 409 vs 4483 ± 568 kJ [mean ± SEM, n = 17], p = 0.014), whereas there were no significant differences in energy intake during IIGI+GIP (4062 ± 520 kJ) or IIGI+GIP+GLP-1 (3875 ± 451 kJ) infusion compared with IIGI+saline (p = 0.590 and p = 0.364, respectively). Energy intake was higher during IIGI+GIP+GLP-1 compared with IIGI+GLP-1 infusion (p = 0.039).
Conclusions/interpretation
While GLP-1 infusion lowered energy intake in overweight/obese men, simultaneous GIP infusion did not potentiate this GLP-1-mediated effect.
Trial registration
ClinicalTrials.gov NCT02598791
Funding
This study was supported by grants from the Innovation Fund Denmark and the Vissing Foundation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
The gut-derived incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), are important regulators of postprandial metabolism and act in concert to promote deposition of nutrients [1]. Infusion studies have established GLP-1 as a potent regulator of appetite and energy intake in humans [2, 3]. Moreover, GLP-1 receptor activation may increase resting energy expenditure (REE) in humans [4, 5]. In contrast, studies with GIP infusions have not revealed any independent effects on appetite, energy intake or REE in humans [6]. Surprisingly, rodent data have shown that GIP and GLP-1 receptor co-activation elicits a synergistic effect with regard to energy intake: potentiating the satiety-promoting and body weight-reducing effects of GLP-1 [7,8,9]. The interest in GIP and GLP-1 receptor co-activation emerged when Finan and colleagues showed, in diet-induced obese mice, that co-administration of GIP and GLP-1 receptor agonists decreased body weight and energy intake to a greater extent than that elicited by either agonist alone [7]. Following these results, unimolecular dual GIP/GLP-1 receptor co-agonists were developed, also showing superior reductions in energy intake and body weight in diet-induced obese mice compared with administration of the GLP-1 receptor agonists exendin-4 and liraglutide, respectively [7]. In line with the results obtained by Finan and colleagues, Gault and colleagues found in ob/ob mice that whereas daily administration of either liraglutide or N-AcGIP(Lys37Myr) alone did not alter body weight or energy intake, the combination (Lira-AcGIP) significantly reduced both body weight and energy intake [8]. In addition, Nørregaard et al showed that co-administration of liraglutide and a novel GIP receptor agonist resulted in significant reductions in energy intake and body weight in diet-induced obese mice compared with liraglutide alone [9]. Only one study has so far evaluated the effects of co-infusion of GIP and GLP-1 on appetite, energy intake and REE in humans and, because of a small sample size and thereby high risk of statistical type 2 error, no firm conclusions could be drawn [10].
In the present study, we evaluated the effects of separate and combined infusions of GIP and GLP-1 on energy intake, appetite and REE in overweight/obese men.
Methods
Research design, approval and ethics
The study was designed as a randomised, double-blind, placebo-controlled, crossover study and was conducted from September 2015 to July 2016 at Clinical Metabolic Physiology, Steno Diabetes Center Copenhagen, Gentofte Hospital, Hellerup, Denmark. The study was approved by the Scientific-Ethical Committee of the Capital Region of Denmark (ID no. H-15008790) and the Danish Data Protection Agency (J. No.: 2012-58-0004) and registered at ClinicalTrials.gov (registration no. NCT02598791). The study was conducted in accordance with the Declaration of Helsinki as revised in 2008. Oral and written consent were obtained from all participants before inclusion.
Study participants
Eligible study participants were white men, aged 25–70 years, with BMI 25–40 kg/m2. Exclusion criteria included diabetes or prediabetes (defined as HbA1c ≥ 6.1% [≥43 mmol/mol]), anaemia, gastrointestinal disease that could interfere with the endpoint variables, anorexia, bulimia or binge-eating disorder, allergy or intolerance to the ingredients included in the standardised meals, tobacco smoking, and any physical or psychological condition that the investigator felt would interfere with trial participation.
Experimental procedures, randomisation and blinding
The study included 5 study days for each participant. On study day 1, a 50 g OGTT was performed. On the following 4 study days glucose was infused, aiming at copying the plasma glucose excursions from the OGTT (isoglycaemic i.v. glucose infusions [IIGIs]) during simultaneous infusion of GIP, GLP-1, GIP+GLP-1 or saline (154 mmol/l NaCl; placebo). All IIGIs were performed in a randomised order with at least 72 h between each study day. The randomisation order followed a prespecified random-numbers table generated from www.random.org. The randomisation order was blinded for the participants and investigators, and generated independently for each study participant by a third person who was not involved in participant enrolment or data collection.
All participants were instructed to abstain from alcohol and strenuous physical exercise for 48 h prior to the study days to ensure filled glycogen stores. To standardise baseline appetite and macronutrient balance, participants were given a standardised meal for dinner the evening before each study day (552 g pasta Bolognese; energy content per 100 g: 616 kJ; 5.9 g fat, 17 g carbohydrates, 5.6 g protein). After the meal, the participants were asked to remain fasting. On the study day, the participants rested in a supine position with a slight elevation of the head. A cannula was inserted in a cubital vein for infusions, and another cannula was inserted in a contralateral vein for blood sampling. The forearm, from which blood samples were drawn, was wrapped in a heating pad for arterialisation of the venous blood.
At time point 0 min, an OGTT or infusion of 20% glucose (wt/vol. solution; Fresenius Kabi, Uppsala, Sweden) was started, as well as infusion of either placebo (saline), GIP (4 pmol kg−1 min−1), GLP-1 (1 pmol kg−1 min−1) or GIP+GLP-1 (4 and 1 pmol kg−1 min−1, respectively). The infusions were ended when the participants had finished eating from the ad libitum meal (see below).
Incretin infusions
Synthetic human GIP(1-42) and GLP-1(7-36) were purchased from PolyPeptide Laboratories (Strasbourg, France). The peptides were ≥96% pure. At the pharmacy of the Capital Region of Denmark, Herlev, Denmark, the hormones were dissolved in sterilised water containing 1% human serum albumin and dispensed into vials with enough peptide per vial for 1 study day. The contents of the vials were tested for endotoxins and sterility. The vials were stored at −20°C until use. On the morning of each study day, the infusions were prepared by a third person diluting the peptide solution or 0.9% saline in 0.9% saline (9 mg/ml; Fresenius Kabi) with 0.5% human albumin (5% solution; CSL Behring, Lyngby, Denmark) to a total of 250 ml each.
Energy intake
The ad libitum meal was served 240 min after glucose ingestion or the start of the infusion and consisted of pasta Bolognese (same energy content per 100 g as the standardised dinner served the night before), served together with 500 ml of water. Participants were instructed to eat until they felt comfortably full. The food was weighed before and after the meal, and the difference in weight was used to calculate the total energy intake (primary endpoint).
Ratings of REE and RQ
REE and RQ were measured by indirect calorimetry using a tight facemask connected to a calorimeter, measuring gas exchange breath by breath (CCM Express, MedGraphics, Medical Graphics Corp, St Paul, MN, USA). Indirect calorimetry was performed for 15 min at baseline (time point −15 to 0 min) and after 210 min of infusion. The calorimeter was calibrated before each measurement.
Appetite ratings
Every 30 min throughout the study day, the participants rated their feelings of hunger, satiety, fullness, prospective food consumption, comfort, nausea and thirst on 100 mm visual analogue scales.
Blood samples and analysis
For glucose measurements, blood was sampled every 5–15 min into fluoride tubes and centrifuged immediately at 7400 g for 45 s at room temperature. Plasma glucose was measured at the bedside using the glucose oxidase method (Yellow Spring Instrument model 2300 STAT Plus analyser; YSI, Yellow Springs, OH, USA). For the remaining blood analyses, samples were drawn at time −30, 0, 15, 30, 45, 60, 90, 120, 150, 180, 210 and 240 min. For analyses of GIP, GLP-1 and glucagon, blood was collected into chilled tubes containing EDTA and a specific dipeptidyl peptidase 4 inhibitor (valine-pyrrolidide, 0.01 mmol/l final concentration; a gift from Novo Nordisk, Måløv, Denmark). The samples were immediately cooled on ice and centrifuged at approximately 2900 g and 4°C for 15 min. The samples were stored at −20°C until analysis by RIAs, as previously described [11, 12]. For analyses of insulin and C-peptide, blood was collected into tubes containing serum clot activator and was left to coagulate for at least 20 min at room temperature before centrifugation at approximately 2900 g and 4°C. Afterwards, the samples were stored at −80°C until analysis using a two-sided electrochemiluminescence immunoassay (ADVIA Centaur CP, Siemens Healthcare, Ballerup, Denmark).
Statistical analysis and calculations
According to our sample-size calculation, 17 participants were needed to detect a difference in energy intake from the ad libitum meal of at least 500 kJ with a power of at least 80%, a two-sided significance level of 5% and a within-subject SD of 734 kJ, based on data from Gregersen et al [13]. To ensure enough power, 18 participants were included and in case of dropouts it was predefined that the participants should be replaced. Unless otherwise stated, data are presented as means ± SEM. p < 0.05 was considered statistically significant. AUC was calculated using the trapezoidal rule. Insulin secretion rates (ISRs) were calculated using the ISEC formula as previously described [14]. Insulin resistance was calculated using HOMA based on fasting plasma glucose and C-peptide values obtained at the screening visit (HOMA2-IR calculator: www.dtu.ox.ac.uk/homacalculator). The combined appetite score was calculated as (hunger + prospective food consumption + [100 – satiety] + [100 – fullness]/4) [14, 15]. For analyses of differences, one-way repeated measures ANOVA (rmANOVA) was performed with Geisser–Greenhouse correction, including the IIGIs. For the REE measurements, we applied a linear mixed model with the intervention as a fixed effect and the individual as a random effect. To correct for multiple comparisons, the Tukey test was applied for both the ANOVAs and the mixed models. Statistical analyses were carried out using GraphPad Prism version 7.02 for Windows (GraphPad Software, San Diego, CA, USA) and SAS version 9.4 for windows (SAS Institute, Cary, NC, USA).
Results
Study participants
Twenty participants were included in the study; 18 completed the five interventions, but one participant was excluded from the data analysis because we could not measure a rise in plasma GLP-1 on the day of supposed GLP-1 infusion. The participants were all white men. None had diabetes or prediabetes defined by HbA1c at the screening visit, none were smokers, and all had stable weight for 3 months prior to inclusion. One participant was undergoing steady treatment with latanoprost eyedrops for glaucoma, which were continued throughout the study. The remaining participants did not take any medication. Detailed participant characteristics are shown in Table 1.
Incretin hormones
At baseline, GIP plasma concentrations were 6.3 ± 0.6 and 7.8 ± 0.7 pmol/l during OGTT and IIGI+saline (p = 0.061) and <5 pmol/l during IIGI+GIP, IIGI+GLP-1 and IIGI+GIP+GLP-1 infusion (elevated limit of quantification due to dilution of the samples to accommodate high concentrations during infusions). During GIP infusion, plasma GIP reached supraphysiological concentrations with mean steady-state (60–240 min) concentrations of 275 ± 11.9 pmol/l (IIGI+GIP) and 265 ± 10.3 pmol/l (IIGI+GIP+GLP-1) (IIGI+GIP vs IIGI+GIP+GLP-1, p = 0.257) (Fig. 1a). Mean baseline GLP-1 levels were 12 ± 0.5, 14 ± 0.7, 10 ± 2.5, 14 ± 0.9 and 14 ± 1.0 pmol/l during OGTT, IIGI+saline, IIGI+GIP, IIGI+GLP-1 and IIGI+GIP+GLP-1, respectively (p = 0.021, all 5 days included, no significant between-day differences according to post hoc multiple testing with Tukey’s correction). During GLP-1 infusion, plasma total GLP-1 levels increased rapidly and reached supraphysiological concentrations with mean steady-state (30–240 min) concentrations of 107 ± 4.3 pmol/l (IIGI+GLP-1) and 99 ± 5.0 pmol/l (IIGI+GIP+GLP-1) (IIGI+GLP-1 vs IIGI+GIP+GLP-1, p = 0.063) (Fig. 1b).
Plasma concentrations of GIP (a), GLP-1 (b) and glucose (c), and administered amount of glucose (d) during a 50 g OGTT, and IIGI+saline, IIGI+GIP, IIGI+GLP-1 and IIGI+GIP+GLP-1 treatments. Key in (a) applies to all figure parts. Data are means ± SEM; n = 17. Statistical analyses in (d): one-way rmANOVA with Geisser–Greenhouse corrections and post hoc Tukey’s test, ***p < 0.001
Plasma glucose and glucose infused
Baseline plasma glucose levels were 5.6 ± 0.1, 5.4 ± 0.2, 5.5 ± 0.2, 5.4 ± 0.2 and 5.4 ± 0.2 mmol/l during OGTT, IIGI+saline, IIGI+GIP, IIGI+GLP-1 and IIGI+GIP+GLP-1, respectively (p = 0.214). During the OGTT, plasma glucose rose to a peak value of 9.8 ± 0.3 mmol/l after 47 ± 4 min and returned to baseline levels after 138 ± 6 min. As evident from Fig. 1c, we obtained isoglycaemic conditions on the other study days (mean CV% compared with the OGTT: 6.4 ± 0.3 [IIGI+saline], 6.2 ± 0.3 [IIGI+GIP], 7.1 ± 0.3 [IIGI+GLP-1] and 7.6 ± 0.3% [IIGI+GIP+GLP-1]). The mean amount of administered glucose varied significantly between all interventions except for IIGI+GLP-1 vs IIGI+GIP+GLP-1, for which statistically similar amounts were infused (p = 0.895; Fig. 1d).
Energy intake
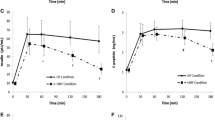
The ad libitum meal was overall evaluated as tasty, with no reported differences in palatability between the interventions (see electronic supplementary material [ESM] Table 1). Energy intake was significantly lower during IIGI+GLP-1 than during IIGI+saline infusion (2715 ± 409 vs 4483 ± 568 kJ, p = 0.014). Furthermore, IIGI+GLP-1 resulted in a significantly lower energy intake compared with IIGI+GIP (4062 ± 520 kJ, p = 0.027) and compared with IIGI+GIP+GLP-1 infusion (3875 ± 451 kJ, p = 0.039). There were no significant differences in energy intake during IIGI+GIP (4062 ± 520 kJ) or IIGI+GIP+GLP-1 infusion (3875 ± 451 kJ) compared with IIGI+saline (p = 0.590 and p = 0.364, respectively) (Fig. 2a).
Energy intake (kJ) from the ad libitum meal (a) and REE (kJ/day) measured at time point −15 to 0 min and 210–225 min (b) during a 50 g OGTT, and IIGI+saline, IIGI+GIP, IIGI+GLP-1 and IIGI+GIP+GLP-1 treatments. Data are means ± SEM; n = 17. Statistical analyses: one-way rmANOVA with Geisser–Greenhouse corrections and post hoc Tukey’s test *p < 0.05
REE and RQ
No significant differences in REE among interventions were observed at baseline (p = 0.269) or at the 210–225 min measure (p = 0.394) (Fig. 2b), or between baseline and the 210–225 min measure for the individual interventions (all p > 0.05). Mean RQ at the 210–225 min measure differed between the interventions and amounted to 0.84 ± 0.02 (OGTT), 0.83 ± 0.02 (IIGI+saline), 0.83 ± 0.01 (IIGI+GIP), 0.96 ± 0.02 (IIGI+GLP-1) and 0.92 ± 0.02 (IIGI+GIP+GLP-1), with an overall difference of p < 0.0001, reflecting the differences in the amount of glucose administered.
Ratings of appetite, comfort, nausea and thirst
At baseline, no significant differences among the interventions were observed for the appetite measures (including hunger, prospective food consumption, satiety, fullness, thirst and a combined appetite score) or for nausea and comfort (Table 2). At the end of the clamp, just prior to the serving of the ad libitum meal, hunger and prospective food consumption were numerically lower during IIGI+GLP-1 and IIGI+GIP+GLP-1 infusion than during IIGI+GIP and IIGI+saline infusion (Table 2, ESM Fig. 1). Comfort and nausea were both stable over time, with a mean high level of comfort and a low level of nausea during all interventions (ESM Fig. 1).
Insulin, C-peptide, ISR and glucagon responses
No significant differences in insulin, C-peptide or ISR were present at baseline. All interventions resulted in significantly greater concentrations of insulin and C-peptide and higher ISR compared with IIGI+saline (Fig. 3a–c, Table 3). IIGI+GLP-1 and IIGI+GIP+GLP-1 resulted in statistically similar insulin and C-peptide responses and ISR. Glucagon concentrations did not differ between interventions at baseline. During IIGI+GLP-1 infusion, glucagon was suppressed and remained at or lower than the detection level throughout the intervention. AUC for glucagon during IIGI+GIP+GLP-1 infusion was higher than during IIGI+GLP-1 infusion. During IIGI+saline and IIGI+GIP, glucagon concentrations reciprocally followed the glucose excursion (Fig. 3d, Table 3).
Discussion
In a randomised, crossover setting we compared the effects of GIP, GLP-1, GIP+GLP-1 and placebo (saline) on energy intake, appetite and REE during isoglycaemic conditions in overweight/obese individuals. The main findings are that while GLP-1 infusion lowered energy intake significantly, co-infusion of GIP+GLP-1 did not lower energy intake more than GLP-1 infusion alone.
The present study is a human proof-of-concept study based on promising rodent studies showing synergistic energy intake and body weight-lowering effects from co-activation of the GIP and GLP-1 receptors, compared with GLP-1 alone [7,8,9]. The clinical data from the present study contrast these rodent findings. In fact, we observed that GIP seemed to repeal some of the energy intake-lowering effect of GLP-1. Regarding REE, we found no differences between the interventions that could explain the weight loss observed in rodents. This is in agreement with a study in mice, where energy expenditure, RQ and locomotor activity were similar among vehicle, liraglutide and GIP/GLP-1 receptor co-agonist administration [7].
To date, only one previous study has evaluated the effects of GIP and GLP-1 co-infusion on energy intake and appetite in humans. Daousi and colleagues infused GIP (2 pmol kg−1 min−1), GLP-1 (1 pmol kg−1 min−1), GIP+GLP-1 (2 and 1 pmol kg−1 min−1, respectively) or placebo (dextrose) for 4 h in six healthy individuals, and found that GIP infusion resulted in greater hunger, desire to eat and prospective food consumption compared with placebo [10]. Furthermore, in six individuals with type 2 diabetes, GIP+GLP-1 co-infusion resulted in a stronger desire to eat compared with placebo [10]. No effect on appetite was found during GLP-1 infusion in either group. Further, no differences were found in energy intake from an ad libitum meal among any of the study days in either group [10], which is in contrast to the majority of studies reporting energy intake and appetite-lowering effects of GLP-1 [1, 2, 16].
Based on the results from the study of Daousi and colleagues, we designed the present study and tried to refine the methods. First, we chose an OGTT/IIGI design to avoid appetite being influenced by differences in plasma glucose concentrations from the insulinotropic abilities of GIP and GLP-1 [17]. We could have clamped the glucose concentration at a fixed glucose concentration, but chose the OGTT/IIGI design as we found it more physiologically relevant. However, as the main purpose of the OGTT was to have a template for the glucose excursions, OGTT results were not included in the data analyses. To decrease the risk of a type 2 statistical error we included more participants than in the study of Daousi and colleagues, who enrolled six healthy individuals and six with diet-controlled type 2 diabetes, compared with the 17 overweight/obese individuals in the present study. We chose to include overweight/obese participants as we wanted to use a group of individuals for whom an appetite-lowering effect would be beneficial. Furthermore, compared with the study by Daousi et al, we chose a higher dose of GIP (4 vs 2 pmol kg−1 min−1) to ensure that the concentration of GIP was sufficiently supraphysiological. Finally, we continued the hormonal infusions until the end of the ad libitum meal, as the half-life of GIP and GLP-1 are only approximately 4 and 2 min, respectively [18, 19]. The differences in design between the two studies might explain why we, in contrast to Daousi et al, were able to reproduce the well-known energy intake-reducing effect of GLP-1 [20].
We observed less glucagon suppression during GIP+GLP-1 infusion than during GLP-1 infusion. This is in line with previous results obtained in individuals with type 2 diabetes co-administered GIP and GLP-1 also in supraphysiological doses [21].
After the promising results in animal models, a dual GIP/GLP-1 receptor co-agonist has been tested in phase 1b [22] and phase 2a trials [23] in individuals with type 2 diabetes, among other measures evaluating changes in plasma glucose and body weight. The phase 1b trial found numerical but not statistically significantly greater improvements in blood glucose and body weight in the dual agonist arm compared with the placebo arm [22]. The phase 2a trial compared the dual GIP/GLP-1 receptor agonist with placebo, and found that participants randomised to the dual agonist achieved significant reductions in body weight from baseline to week 8, but not to week 12. Further, the trial included an open-label liraglutide arm as reference (liraglutide with a 2-week dose escalation uptitrated to 1.8 mg once daily), which resulted in similar body weight reductions compared with the dual GIP/GLP-1 receptor agonist (although not directly comparable) [23]. Recently, treatment with a new dual GIP/GLP-1 receptor agonist was reported to result in significant reductions in body weight compared with dulaglutide in individuals with type 2 diabetes [24]. However, it remains a challenge to evaluate the contribution of GIP receptor engagement in the dual GIP/GLP-1 compounds in humans [25], which seems highly relevant, particularly in the light of the present findings.
The reasons for the observed discrepancies between rodent and human findings regarding reduction in energy intake and body weight need further elucidation. It should be noted that the effect of GIP alone on energy intake and body weight remains equivocal in rodents, and that the pro- vs anti-obesity effects of GIP in rodents are still being discussed [26,27,28,29,30]. However, there are significant differences between the rodent and human GIP systems, which could be responsible for the apparent differences between species [31]. This includes differences in the GIP receptor, which is only 81% conserved between humans and rodents, as well as differences in potency and efficacy of GIP between humans and rodents [31]. Hence, human GIP has been reported to be significantly less potent at the human GIP receptor compared with mouse and rat GIP ligands at their respective GIP receptors, suggesting that the human GIP system is less active than the rodent system [31].
The double-blinded crossover design is an important strength of the present study as, typically, there are large inter-individual differences in energy intake and assessment of appetite. Further, the infused doses of GIP and GLP-1 were based on previous studies examining responses to physiological and supraphysiological doses of the two hormones [6, 32,33,34], and resulted in clear supraphysiological steady-state concentrations of both GIP and GLP-1 compared with the OGTT. Hence, the use of an inappropriately low dose of GIP does not explain the lack of effect. Nonetheless, in healthy individuals, GIP and GLP-1 have previously been shown to induce an additive insulinotropic effect that we did not observe in the present study [35]. Thus, the very robust insulin response to the GLP-1 infusion obtained in the present study may have somewhat masked the effect of GIP during the GIP+GLP-1 co-infusion. If we had used a smaller dose of GLP-1 then the insulinotropic effect of GIP would perhaps have been more evident. Furthermore, the participants included were all overweight or obese. As obese individuals with normal glucose tolerance exhibit a significantly reduced incretin effect compared with lean individuals [36], and as the insulinotropic response to GIP might be impaired in obesity [37], it could be hypothesised that GIP sensitivity in this group of overweight/obese individuals was diminished. Thus, although the findings of Daousi and colleagues in lean individuals are in line with the present findings regarding energy intake [10], we cannot be certain if lean individuals would have a different response to GIP infusion than that observed in the present study.
Because GIP and GLP-1 possess different insulinotropic potencies, insulin concentrations differed among the study days. This impacted the amount of glucose needed to be infused to obtain isoglycaemia, causing differences in intracellular glucose availability among the interventions. Whether such differences influenced our results is uncertain. Importantly, the insulin concentrations, as well as the amount of glucose infused during IIGI+GLP-1 and IIGI+GIP+GLP-1, did not vary. Hence, differences in plasma concentrations of glucose and insulin, respectively, are unlikely to explain the reduced food intake observed during IIGI+GLP-1 infusion compared with IIGI+GIP+GLP-1 infusion.
Finally, we used a relatively short study period with 4 h of intervention, and we cannot rule out the possibility that the effect of GIP and GLP-1 co-receptor activation on energy intake/appetite/REE and ultimately body weight may surface with a longer period of administration.
In conclusion, the present study confirms the energy intake-reducing effect of GLP-1 administration alone, but this was not further enhanced by co-administration of GIP in the supraphysiological dose applied. None of GIP, GLP-1 or GIP+GLP-1 administration changed REE.
Data availability
The datasets are available from the corresponding author on reasonable request and with permission of Steno Diabetes Center Copenhagen and Zealand Pharma.
Abbreviations
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon-like peptide 1
- IIGI:
-
Isoglycaemic i.v. glucose infusion
- ISR:
-
Insulin secretion rate
- REE:
-
Resting energy expenditure
- rmANOVA:
-
Repeated measures ANOVA
References
Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17(6):819–837. https://doi.org/10.1016/j.cmet.2013.04.008
Flint A, Raben A, Astrup A, Holst JJ (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101(3):515–520. https://doi.org/10.1172/JCI990
Flint A, Raben A, Ersbøll AK et al (2001) The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 25(6):781–792. https://doi.org/10.1038/sj.ijo.0801627
Shalev A, Holst JJ, Keller U (1997) Effects of glucagon-like peptide 1 (7-36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Investig 27:10–16
Horowitz M, Flint A, Jones KL et al (2012) Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 97(2):258–266. https://doi.org/10.1016/j.diabres.2012.02.016
Asmar M, Tangaa W, Madsbad S et al (2010) On the role of glucose-dependent insulintropic polypeptide in postprandial metabolism in humans. Am J Physiol Endocrinol Metab 298(3):E614–E621. https://doi.org/10.1152/ajpendo.00639.2009
Finan B, Ma T, Ottaway N et al (2013) Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med 5(209):209ra151. https://doi.org/10.1126/scitranslmed.3007218
Gault VA, Kerr BD, Harriott P, Flatt PR (2011) Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with type 2 diabetes and obesity. Clin Sci (Lond) 121(3):107–117. https://doi.org/10.1042/CS20110006
Nørregaard PK, Deryabina MA, Tofteng Shelton P et al (2017) A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes Metab 20(1):1–9. https://doi.org/10.1111/dom.13034
Daousi C, Wilding JPH, Aditya S et al (2009) Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin Endocrinol 71(2):195–201. https://doi.org/10.1111/j.1365-2265.2008.03451.x
Ørskov C, Rabenhøj L, Wettergren A et al (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43(4):535–539. https://doi.org/10.2337/diab.43.4.535
Lindgren O, Carr RD, Deacon CF et al (2011) Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab 96(8):2519–2524. https://doi.org/10.1210/jc.2011-0266
Gregersen NT, Flint A, Bitz C et al (2008) Reproducibility and power of ad libitum energy intake assessed by repeated single meals. Am J Clin Nutr 87(5):1277–1281. https://doi.org/10.1093/ajcn/87.5.1277
Hovorka R, Soons PA, Young MA (1996) ISEC: a program to calculate insulin secretion. Comput Methods Prog Biomed 50(3):253–264. https://doi.org/10.1016/0169-2607(96)01755-5
Blundell J, Finlayson G, Axelsen M et al (2017) Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab 19(9):1242–1251. https://doi.org/10.1111/dom.12932
Näslund E, Barkeling B, King N et al (1999) Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 23(3):304–311. https://doi.org/10.1038/sj.ijo.0800818
Chaput J-P, Tremblay A (2009) The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes 33(1):46–53. https://doi.org/10.1038/ijo.2008.221
Vilsbøll T, Agersø H, Lauritsen T et al (2006) The elimination rates of intact GIP as well as its primary metabolite, GIP 3-42, are similar in type 2 diabetic patients and healthy subjects. Regul Pept 137(3):168–172. https://doi.org/10.1016/j.regpep.2006.07.007
Vilsbøll T, Agersø H, Krarup T, Holst JJ (2003) Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88(1):220–224. https://doi.org/10.1210/jc.2002-021053
Verdich C, Flint A, Gutzwiller J-P et al (2001) A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389
Mentis N, Vardarli I, Köthe LD et al (2011) GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 60(4):1270–1276. https://doi.org/10.2337/db10-1332
Schmitt C, Portron A, Jadidi S et al (2017) Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes Metab 19(10):1436–1445. https://doi.org/10.1111/dom.13024
Frias JP, Bastyr EJ, Vignati L et al (2017) The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab 26(2):343–352.e2. https://doi.org/10.1016/j.cmet.2017.07.011
Frias JP, Nauck MA, Van J et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193. https://doi.org/10.1016/S0140-6736(18)32260-8
DiMarchi RD (2018) “Let’s stay together”; GIP and GLP-1 dual agonism in the treatment of metabolic disease. Mol Metab 18:1–2. https://doi.org/10.1016/J.MOLMET.2018.10.004
Finan B, Müller TD, Clemmensen C et al (2016) Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med 22(5):359–376. https://doi.org/10.1016/j.molmed.2016.03.005
Miyawaki K, Yamada Y, Ban N et al (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8(7):738–742. https://doi.org/10.1038/nm727
McClean PL, Irwin N, Cassidy RS et al (2007) GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 293(6):E1746–E1755. https://doi.org/10.1152/ajpendo.00460.2007
Althage MC, Ford EL, Wang S et al (2008) Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 283(26):18365–18376. https://doi.org/10.1074/jbc.M710466200
Kim SJ, Nian C, Karunakaran S et al (2012) GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One 7(7):e40156. https://doi.org/10.1371/journal.pone.0040156
Sparre-Ulrich AH, Hansen LS, Svendsen B et al (2016) Species-specific action of (Pro3)GIP – a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. Br J Pharmacol 173(1):27–38. https://doi.org/10.1111/bph.13323
Vilsbøll T, Knop FK, Krarup T et al (2003) The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide – regardless of etiology and phenotype. J Clin Endocrinol Metab 88(10):4897–4903. https://doi.org/10.1210/jc.2003-030738
Rudovich N, Pivovarova O, Gögebakan O et al (2015) Effect of exogenous intravenous administrations of GLP-1 and/or GIP on circulating pro-atrial natriuretic peptide in subjects with different stages of glucose tolerance. Diabetes Care 38(1):e7–e8. https://doi.org/10.2337/dc14-1452
Elahi D, McAloon-Dyke M, Fukagawa NK et al (1994) The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–37) in normal and diabetic subjects. Regul Pept 51(1):63–74. https://doi.org/10.1016/0167-0115(94)90136-8
Nauck MA, Bartels E, Orskov C et al (1993) Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 76:912–917
Knop FK, Aaboe K, Vilsbøll T et al (2012) Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab 14(6):500–510. https://doi.org/10.1111/j.1463-1326.2011.01549.x
Calanna S, Piro S, Di Pino A et al (2013) Beta and alpha cell function in metabolically healthy but obese subjects: relationship with entero-insular axis. Obesity 21(2):320–325. https://doi.org/10.1002/oby.20017
Acknowledgements
The authors would like to thank all study participants and acknowledge invaluable laboratory assistance from A. Rasmussen-Lanng, S. M. Schmidt and I. al Nachar, all from Clinical Metabolic Physiology, Steno Diabetes Center Copenhagen, Gentofte Hospital, Denmark. Part of this work has been presented at the annual meeting of the European Association for the Study of Diabetes, 11–15 September 2017, Lisbon, Portugal, and published as an abstract in Diabetologia 2017;60(Suppl 1):5.
Funding
This study was supported by grants from the Innovation Fund Denmark (grant no. 4135-00091B) and the Vissing Foundation. The funders were not involved in the design of the study, collection, analysis or interpretation of data, preparation of the manuscript; or the decision to publish.
Author information
Authors and Affiliations
Contributions
NCB was involved in planning the study, conducting clinical experiments and statistical analyses, and writing the manuscript. AL was involved in planning the study. LSG was involved in planning the study, conducting clinical experiments, analysing the plasma samples for GIP, GLP-1 and glucagon, and statistical analyses. ECEM, MMA and SB were involved in conducting clinical experiments. BH and JJH analysed plasma samples for GIP, GLP-1 and glucagon. LJ, MC and TV were involved in planning the study. FKK conceptualised the study and was involved in planning the study and writing the manuscript. All authors critically reviewed the manuscript and approved the version to be published. NCB and FKK are the guarantors of the work.
Corresponding author
Ethics declarations
NCB holds an unrestricted grant from the Innovation Fund Denmark and is employed as a PhD student at Zealand Pharma in collaboration with Steno Diabetes Center Copenhagen, Gentofte Hospital. LSG is a minority shareholder in Antag Therapeutics. LJ is employee of Zealand Pharma. JJH has served on advisory panels for GlaxoSmithKline, Novo Nordisk, Zealand Pharma, AstraZeneca, MSD, Intarcia and Hanmi and as a consultant for Novo Nordisk, and has received research support from Merck, Sharp & Dome. FKK has received lecture fees from, participated in advisory boards of, consulted for and/or received research grants from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, Sanofi and Zealand Pharma, and is a minority shareholder in Antag Therapeutics. AL, ECEM, MMA, SB, BH, MC and TV declare no duality of interest associated with their contribution to this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 322 kb)
Rights and permissions
About this article
Cite this article
Bergmann, N.C., Lund, A., Gasbjerg, L.S. et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia 62, 665–675 (2019). https://doi.org/10.1007/s00125-018-4810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4810-0