Abstract
Background
Glucagon-like peptide-1 (GLP-1), an incretin hormone, is released in response to food intake. It is unclear how meals high in protein (HP) and monounsaturated fat (HMF) affect GLP-1 response.
Purpose
To examine the effect of a HP versus a HMF meal on GLP-1 response.
Methods
Twenty-four overweight/obese participants consumed two meals (HP: 31.9 % energy from protein; HMF: 35.2 % fat and 20.7 % monounsaturated fat) in a random order. Both meals contained the same energy and carbohydrate content. GLP-1, insulin, glucagon, C-peptide, and glucose were assessed from blood drawn in the fasting and postprandial states. The effect of meal condition on hormone and glucose responses and appetite ratings were assessed by repeated measures analysis.
Results
Statistically significant (p < 0.01) time by meal condition effect was observed on active GLP-1, total GLP-1, insulin, C-peptide, and glucagon, but not glucose (p = 0.83). Area under the curve was significantly higher during the HP versus the HMF meal conditions for active GLP-1 (23.7 %; p = 0.0007), total GLP-1 (12.2 %; p < 0.0001), insulin (54.4 %; p < 0.0001), C-peptide (14.8 %; p < 0.0001), and glucagon (40.7 %; p < 0.0001). Blood glucose was not different between the HP versus HMF conditions (−4.8 %; p = 0.11). Insulin sensitivity was higher during the HMF versus HP conditions (Matsuda index mean difference: 16.3 %; p = 0.007). Appetite ratings were not different by meal condition.
Conclusions
GLP-1 and insulin responses were higher during the HP condition. However, no difference was found in blood glucose between conditions, and insulin sensitivity was higher during the HMF condition, indicating that a HMF meal may be optimal at regulating blood glucose in overweight/obese individuals without type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by the L cells in the intestine in response to food consumption [1, 2]. GLP-1 plays a major role in insulin secretion [3]. GLP-1 secretion following a meal may be suppressed in healthy overweight compared to normal-weight individuals [4] and in patients with type 2 diabetes [5]. Medications such as GLP-1 receptor agonists and dipeptidyl peptidase-4 inhibitors are used to enhance GLP-1 levels and insulin secretion in patients with type 2 diabetes [6], and GLP-1 agonists may be used in obese patients to induce satiety [6].
There is some evidence that protein and monounsaturated fat may influence postprandial GLP-1 and insulin responses. Lejeune et al. [7] have shown that a high-protein diet resulted in a greater postprandial GLP-1 response after dinner compared to an adequate-protein diet in healthy participants. Belza et al. [8] reported that protein dose dependently increased the postprandial GLP-1 response, but not the insulin response in normal and overweight participants. Beysen et al. [9] have reported that fat feeding with heparin infusion led to higher postprandial GLP-1 and insulin responses following a test meal rich in monounsaturated fatty acids compared with meals rich in saturated or polyunsaturated fatty acids in healthy individuals.
Only three studies have directly compared the effect of test meals varying in fat and protein content on postprandial GLP-1 and insulin release [10–12]. Ryan et al. [10] reported that postprandial GLP-1 response was lipid-load dependent, whereas insulin was protein-load dependent in lean men. Batterham et al. [11], on the other hand, found no difference in GLP-1 or insulin responses following a high-protein or high-fat meal, in either lean or obese individuals, whereas van der Klaauw et al. [12] reported a greater postprandial concentration of GLP-1, but a lower concentration of insulin after a high-protein meal compared to a high-fat meal in healthy men. The above studies had several limitations including a small sample size [12], test meals that were infused into the duodenum rather than oral ingestion [10], and subjects who were largely normal weight [10, 12]. In addition, all three studies administered test meals that were very rich in protein or fat and unsuitable to consume on a regular basis. Also, none of the studies reported how much of the fat in the test meals was in the form of monounsaturated fat.
The purpose of this study was to compare the effect of a high-monounsaturated fat (HMF) meal with a high-protein (HP) meal on postprandial GLP-1, insulin, C-peptide, glucagon, and glucose responses, and appetite ratings in overweight/obese subjects.
Materials and methods
Participants
Twenty-four overweight/obese (OW/O) men and women between the ages of 18 and 65 years were recruited for the study. To be classified as OW/O, the participants had to meet two of the following three criteria: body mass index (BMI) ≥25 kg/m2 (≥23 kg/m2 in Asians), percent body fat of >25 % in men and >35 % in women, and waist circumference >102 cm in men (≥90 cm in Asian men) and >88 cm in women (≥80 cm in Asian women) [13, 14]. Exclusion criteria included use of weight loss or hypoglycemic agents, liver, kidney, or untreated thyroid disease, consumption of ≥14 alcoholic drinks per week in men and ≥7 per week in women, smoking, pregnancy, lactation, previous bowel surgery, documented malabsorption, and dieting to lose weight.
Participants were recruited from Texas Christian University (TCU) and the surrounding community. This study was approved by the TCU Institutional Review Board (IRB) and performed in accordance with the ethical standards laid down in the Declaration of Helsinki guidelines and by the Office for Human Research Protection, US Department of Health and Human Services. All participants signed an informed consent document approved by the TCU IRB before participation. The study was conducted in the Metabolic and Exercise Physiology Laboratories at TCU.
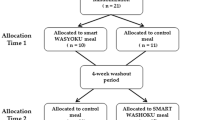
Experimental design
A randomized cross-over design was used to investigate the effect of meal composition (HMF versus HP) on postprandial concentrations of GLP-1, insulin, C-peptide, glucagon, and glucose, and appetite ratings. Eligible participants reported to the laboratories on two separate days for a meal. On the first study day, the participants were randomized to consume either a HMF or HP test meal. On the second study day, the participants consumed the remaining test meal. The two study days were separated by a wash-out period of at least 4 days. Blood samples and appetite ratings were collected in the fasting state immediately prior to meal consumption and for 3 h in the postprandial state.
Test meals
The test meals were in the form of a beverage. The HMF meal was made with plain low-fat yogurt, avocado, and sugar, and contained 35.2 % from total fat, 20.7 % from monounsaturated fat, 12.6 % energy from protein, and 52.3 % from carbohydrates. The HP meal was made with plain nonfat Greek yogurt, plain whole milk yogurt, and sugar and contained 31.9 % energy from protein, 15.5 % energy from total fat, 4.3 % from monounsaturated fat, and 52.6 % energy from carbohydrates. Energy content and volume of the meals were kept constant across the two meals. Men received 840 kcal and women received 700 kcal during each meal. The calorie content was approximately 35 % of the daily needs for men and women, respectively. The macronutrient composition of the meals was calculated using the Food Processor software program (SQL edition, Salem, OR).
Study protocol
The meals were scheduled between 6 and 10 a.m. Each participant consumed the test meals on the two study days at approximately the same time within a window of 30 min. Because GLP-1 response may be influenced by the menstrual cycle, the two study days were scheduled during the follicular phase of the cycle in menstruating women [15].
The participants were instructed to fast for 12 h prior to test meal consumption. They were instructed not to exercise and to keep their diet as similar as possible on the day before each study day. This was verified by asking the participants to recall the amount of exercise that they had performed and the quantity and type of food and drink that they had consumed during the previous day immediately prior to administering the test meals. Participant weight was also measured before the test meals that were consumed.
The participants were randomly assigned to consume one of the two meals on the first study day and consumed the remaining meal on the second study day. The participants were instructed to consume their meals within 20 min and to match the time taken to eat the meal on the second study day with the time taken to eat the meal on the first study day.
Participants received water during the postprandial period. Amount and rate of water consumption were standardized. The participants were asked to consume 40 % of their water allowance during the 1st hour (men: 426 mL; women: 295 mL) and 30 % each during the 2nd (men: 319 mL; women: 221 mL) and 3rd (men: 319 mL; women: 221 mL) hour of the postprandial period. No other food or drink was allowed during the postprandial period.
A venous catheter was inserted into the participant’s antecubital vein, and blood samples were collected in the fasting (0) and postprandial (30, 60, 120, and 180 min from when meal ingestion began) states with the participant lying in a supine position. To keep the catheter clean and viable, a saline solution was flushed through the catheter after each blood draw and every 30 min in between the blood draws. The participants were instructed to stay sedentary in between the blood draws.
Ratings of hunger, fullness, and desire to eat were collected in the fasting state immediately before the meal, and at 30, 60, 120, and 180 min from when the meal ingestion began. The palatability of the meal was assessed at 1 min after the meal began and immediately after meal consumption.
Measures
Demographics and anthropometry
All participants completed a demographics questionnaire. BMI was calculated by dividing measured weight (kg) by measured height (m) squared (kg/m2). Waist circumference was measured in centimeter at the level of the umbilicus. Percent body fat was assessed using dual-energy x-ray absorptiometry (DEXA).
Exercise and diet recall
The participants were asked to recall how much exercise they had performed on the day before the study days to ensure that they had followed the instructions to not exercise on the day before each study day.
The participants were asked to recall the amount and type of food and drink they had consumed during the day before the study days to ensure that the energy intake was similar on the day before each study day. The 24-h diet recall is a valid tool to assess energy intake [16]. The diet recalls were analyzed using the Food Processor software program (SQL edition, Salem, OR).
Appetite and meal palatability ratings
Hunger, fullness, desire to eat, and meal palatability ratings were assessed using validated 100-mm visual analogue scales [17]. Each scale had a question (e.g., “How hungry do you feel?”) and opposite evaluative labels (“Not at all” and “Extremely”) at either end of the scale. A higher score on these scales reflected greater hunger, fullness, and desire to eat, and better meal palatability, respectively.
Blood sampling and biochemical assays
Whole blood samples were immediately treated with dipeptidyl peptidase-4 inhibitor and aprotinin to prevent breakdown of active GLP-1 and glucagon, respectively. Whole blood was then centrifuged, and plasma was stored at −80 °C until analysis. The blood samples were assessed, in duplicate, for active GLP-1, total GLP-1, insulin, glucagon, and C-peptide using the MILLIPLEX® MAP Human Metabolic Hormone Magnetic Bead Panel kits (EMD Millipore Corporation, Billerica, MA). All samples from each participant were assessed in one assay run. The minimum detectable concentrations for active GLP-1, total GLP-1, insulin, C-peptide, and glucagon are 1.2, 2.5, 87, 9.5, and 13 pg/mL, respectively. The intra-assay CV for active GLP-1, total GLP-1, insulin, C-peptide, glucagon, and glucose was 6.9, 12.8, 7.97, 4.3, 8.7, and 2.0 %, respectively.
Insulin resistance and insulin sensitivity
Homeostasis model assessment (HOMA) of insulin resistance (IR) or HOMA-IR was determined using the Oxford calculator [18] and insulin sensitivity using the C-peptide-based Matsuda method [19].
Statistical analysis
A 2 by 5 mixed-model repeated measures analysis with doubly repeated measures was used to evaluate the effect of the meal conditions (HMF and HP), time (0, 30, 60, 120, and 180 min), and the interaction between meal condition and time on active GLP-1, total GLP-1, insulin, C-peptide, glucagon, and glucose concentrations. Differences in the outcome variables by meal condition and time were analyzed by least square mean contrasts. Active GLP-1, insulin, C-peptide, and glucagon concentrations were log-transformed before analysis. Area under the curve (AUC) was computed for each variable using the trapezoidal rule, and the difference in AUC by meal condition was analyzed using a paired t test. A paired t test was also used to analyze HOMA-IR and insulin sensitivity by meal condition. The effect of the meal sequence in this cross-over design study was also modeled in our analysis, and no effect of meal sequence was observed.
A 2 by 5 mixed-model repeated measures analysis with doubly repeated measures was also used to examine the effect of the meal condition, time, and the interaction between these factors on ratings of hunger, fullness, and desire to eat. Differences in the appetite ratings by meal condition and time were analyzed by least square mean contrasts. Difference in meal palatability by meal condition was examined by paired t test.
The difference in body weight on the study days and energy intake on the day before the study days was analyzed by paired t test. The amount of exercise performed on the day before the two study days was compared with the Wilcoxon signed-rank test. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics
Participant characteristics are shown in Table 1. The participants were 38.7 ± 15.3 (mean ± SD) years of age. The male/female ratio was 50:50. Forty-two percent of the participants were Hispanic or Latino, and 75 % were white. Mean BMI was 31.6 ± 4.0 kg/m2. Percent body fat was 47.7 ± 4.5 % in females and 33.2 ± 5.9 % in males. Waist circumference was 106.8 ± 9.8 cm in females and 106.9 ± 11.3 cm in males. Fasting blood glucose was 95.9 ± 7.6 mg/dL, and 29.2 % of the subjects were prediabetic (fasting blood glucose: ≥100 and <126 mg/dL).
Energy intake and amount of exercise on the day before the study days and body weight on the study days
There was no difference in the reported energy intake (HMF: 1973 ± 668 kcal; HP: 1941 ± 693 kcal; p = 0.62) or minutes of exercise performed (HMF and HP: both median 0 min and range 0–60 min; p = 1.0) on the day before the two study days. Body weight was similar on the two study days (HMF: 91.7 ± 15.5 kg; HP: 91.8 ± 14.8 kg; p = 0.71).
Active GLP-1, total GLP-1, insulin, C-peptide, glucagon, and glucose
The postprandial active GLP-1, total GLP-1, insulin, C-peptide, glucagon, and glucose responses by meal condition are shown in Fig. 1. The line graphs show changes over time as geometric means and 95 % confidence intervals for active GLP-1, insulin, C-peptide, and glucagon and arithmetic means and 95 % confidence intervals for total GLP-1 and glucose. AUC for these variables is shown in Table 2. AUC is expressed as medians and 25th and 75th percentiles.
Postprandial active glucagon-like peptide-1 (GLP-1) (a), total GLP-1 (b), insulin (c), C-peptide (d), glucagon (e), and glucose (f) responses by meal condition in 24 participants. The data are shown as geometric means and 95 % confidence intervals for active GLP-1, insulin, C-peptide, and glucagon and arithmetic means and 95 % confidence intervals for total GLP-1 and glucose. Mixed-model repeated measures analysis showed a significant meal condition by time interaction effect for active GLP-1 (p = 0.004), total GLP-1 (p < 0.0001), insulin (p < 0.0001), C-peptide (p < 0.0001), and glucagon (p < 0.0001) but not glucose (p = 0.83). Differences between the high-protein (HP) and high-monounsaturated fat (HMF) meal condition: *p < 0.05; # p < 0.01; † p < 0.001
There was a statistically significant meal condition by time interaction (p = 0.004) effect on plasma active GLP-1 response, indicating different responses over time among the two meal conditions. The concentration of active GLP-1 increased following consumption of both the HMF and HP meals and remained significantly elevated throughout the postprandial period compared to the respective fasting concentration (p < 0.0001). Active GLP-1 concentration was significantly higher during the HP compared to the HMF meal conditions at 120 and 180 min (Fig. 1a). The AUC on active GLP-1 was significantly higher during the HP compared to the HMF conditions (p = 0.0007).
A statistically significant time by meal condition interaction (p < 0.0001) on total GLP-1 response was observed. In both the meal conditions, total GLP-1 was significantly higher during all the postprandial time points compared to respective fasting value (p < 0.0001). Total GLP-1 concentration was significantly higher during the HP versus the HMF meal conditions at 120 and 180 min (Fig. 1b). AUC on total GLP-1 response was significantly higher during the HP compared to the HMF conditions (p < 0.0001).
Also, observed was a significant time by meal condition interaction (p < 0.0001) effect on insulin response. Insulin concentrations increased after the two meals and remained elevated throughout the postprandial period compared to the corresponding fasting concentration (p < 0.0001). Insulin concentration was significantly greater during the HP compared to the HMF meal conditions at 60, 120, and 180 min (Fig. 1c). AUC on insulin response was also significantly higher during the HP condition compared to that during the HMF condition (p < 0.0001).
There was also a statistically significant interaction between time and meal condition (p < 0.0001) on C-peptide response. The concentration of C-peptide increased following consumption of both the HMF and HP meals and remained significantly elevated at all the postprandial time points compared to the respective fasting concentration (p < 0.0001). C-peptide response was significantly higher at 120 and 180 min in the HP compared to the HMF conditions (Fig. 1d). AUC on C-peptide response was also significantly higher during the HP versus the HMF conditions (p < 0.0001).
A statistically significant time by meal composition interaction (p < 0.0001) on glucagon response was observed. During both meal conditions, glucagon concentrations were significantly elevated at each postprandial time point compared to the corresponding fasting value (p < 0.05). Glucagon concentration was significantly higher at 30, 60, 120, and 180 min in the HP compared to the HMF conditions (Fig. 1e). AUC on glucagon response was also significantly higher during the HP versus the HMF conditions (p < 0.0001).
There was a statistically significant effect of time (p < 0.0001) on plasma glucose concentration. Glucose concentration was higher at 30 min following both the HMF and HP meals compared to the respective fasting concentration (Fig. 1f). There was no meal condition (p = 0.14) or meal condition by time interaction (p = 0.83) effect on plasma glucose, however. AUC on glucose response was also not different by meal condition (p = 0.11).
HOMA-IR and insulin sensitivity
HOMA-IR was not different in the HMF compared to the HP meal conditions (mean difference (95 % confidence interval): −0.16 (−0.39 to 0.07); p = 0.17). Insulin sensitivity (Matsuda index) was significantly higher on the HMF compared to the HP meal conditions (mean difference (95 % confidence interval): 0.8 (0.2–1.3); p = 0.007).
Ratings of hunger, fullness, desire to eat, and palatability
Ratings of hunger, fullness, and desire to eat by meal condition are shown in Fig. 2. The data are presented as medians and 25th and 75th percentiles.
Hunger, fullness, and desire to eat ratings by meal condition. The data are presented as medians and 25th and 75th percentiles. Mixed-model repeated measures analysis showed no meal condition or meal condition by time interaction effect on hunger (p = 0.67 and p = 0.11, respectively), desire to eat (p = 0.18 and p = 0.67, respectively), or fullness (p = 0.21 and p = 0.10, respectively)
There was a statistically significant main effect of time (p < 0.0001) on hunger, fullness, and desire to eat. Hunger ratings and desire to eat ratings were lower, and fullness ratings were significantly higher at all the postprandial time points, during both the meal conditions, compared with the respective fasting ratings (p < 0.0001). There was no meal condition or meal condition by time interaction effect on hunger (p = 0.67 and p = 0.11, respectively), desire to eat (p = 0.18 and p = 0.67, respectively), or fullness (p = 0.21 and p = 0.10, respectively), however.
Palatability ratings (average of the ratings at 1 min from when the meal began and when the meal ended) were not different by meal conditions (HMF: median (25th, 75th percentile): 47.8 (33.8, 70.1) mm; HP: 60.5 (32.1, 77.0) mm; p = 0.51).
Discussion
This is the first study to compare the effect of a high-monounsaturated fat versus a high-protein meal on GLP-1 and other hormones that regulate blood glucose in overweight/obese participants. The HP meal resulted in significantly greater postprandial GLP-1 (total and active), insulin, C-peptide, glucagon responses, and AUC compared to the HMF meal. There was no difference in postprandial blood glucose response and appetite ratings by meal condition, however. In addition, insulin sensitivity was greater in the HMF compared to the HP conditions.
Only three studies have compared the effect of meals varying in fat and protein content on GLP-1 release [10–12]. Ryan et al. [10] evaluated the effect of intraduodenal infusion of lipid and protein alone or in combination with postprandial hormone and glycemic responses in lean participants and found that GLP-1 response was lipid-load dependent, whereas insulin and glucagon responses were protein-load dependent and there was no difference in blood glucose response by the composition of the infusion. van der Klaauw et al. [12] evaluated the effect of meals varying in macronutrient composition on postprandial GLP-1 release in healthy subjects and reported that a high-protein meal resulted in greater concentrations of GLP-1, but a lower concentration of insulin than a high-fat meal. Batterham et al. [11], on the other hand, found no difference in GLP-1 or insulin responses following a high-protein or high-fat meal, in either lean or obese participants. How much of the fat was in the form of monounsaturated fat was not reported by any of the above studies, however.
A possible reason for the higher GLP-1 and insulin response during the HP compared to the HMF conditions may be partly due to the fact that the HP meal had higher yogurt content than the HMF meal. Yogurt is rich in casein, whey protein, and branched chain amino acids. Casein has been shown to increase GLP-1 response in vitro in a dose-dependent manner [20] and insulin response in patients with type 2 diabetes [21]. Casein coagulates when exposed to gastric acid and delays gastric emptying [22]. Delayed absorption of casein may stimulate GLP-1 release from the L cells of colon and distal small intestine. Whey protein has also been shown to stimulate the release of GLP-1 and insulin [23] possibly due to the bioactive peptides [24] that are released when whey protein is digested. Branched chain amino acids such as leucine and isoleucine have been shown to induce a dose-dependent increase in GLP-1 in vitro [20].
In the present study, the C-peptide response by meal condition was similar to the insulin response. C-peptide is co-secreted with insulin from the proinsulin molecule following meal ingestion and may be a better indicator of overall insulin secretion since it has a longer half-life compared to insulin [25]. The rise in C-peptide along with the insulin response suggests that the insulin response to the meals was related to insulin secretion rather than insulin clearance.
Despite the higher insulin response to the HP meal, postprandial blood glucose concentration was not different by meal condition. This may be partly due to higher insulin sensitivity during the HMF compared to the HP conditions. This indicates that a HFM meal may attenuate the insulin requirement for glucose uptake compared to a HP meal and may be optimal for blood glucose control in overweight/obese individuals without type 2 diabetes. Another reason for the similar glucose response during the two meal conditions, despite a higher insulin response in the HP condition, may be due to the higher glucagon response to the HP meal. The higher glucagon response during HP meal condition may have been a homeostatic response to offset the glucose-lowering effect of the higher insulin response during that condition. A higher glucagon response along with a higher insulin response to protein intake has also been noted by other studies [10, 26]. Lastly, the high-protein content of the HP meal may have stimulated gluconeogenesis. Linn et al. [27] have reported that a high-protein diet is associated with increased stimulation of gluconeogenesis compared to a normal protein diet.
There were no differences in postprandial hunger, desire to eat, and fullness ratings by meal condition despite the fact that GLP-1 is known to be a hormone that induces satiety [6] and was higher during the HP compared to the HMF condition. This may be partly explained by the fact that a high-fat intake also induces satiety by slowing gastric emptying [28]. The results on appetite ratings in the present study are similar to the findings on hunger and fullness responses reported by van der Klaauw [12] and hunger responses by Ryan et al. [10]. These studies found no difference in appetite ratings following a high-protein or high-fat intake.
The present study had several limitations. Glucose-dependent insulinotropic polypeptide (GIP), an incretin hormone, was not assessed. Alterations in diet composition may also influence GIP [8, 29]. In addition, the participants were fed the meals only once. The effect of chronic feeding of these meals on GLP-1, GIP, insulin, and glucose responses needs to be investigated. The test meals were served as a beverage instead of solid meals. However, GLP-1 secretion has been shown to be greater with liquid meals compared to solid meals [30]. Another limitation is that insulin sensitivity was calculated rather than measured.
The present study had several strengths. It was a randomized cross-over design. A number of variables that could potentially confound the results were controlled: The HP and HMF meals had the same energy content, percent energy from carbohydrates, and added sugar content; water intake and the rate of water consumption and body weight were the same during the two study days; there were no differences in energy intake and the amount of exercise performed on the pre-study days; and females with a menstrual cycle were scheduled during the follicular phase to reduce the potential influence of the menstrual cycle on GLP-1 [15]. Another advantage was that the meal composition for both the test meals was within the range recommended by the Institute of Medicine. The previous three studies comparing high-fat to high-protein intakes administered test meals that were very rich in protein or fat and unsuitable to consume on a regular basis [10–12]. Lastly, the findings from this study are more generalizable because of the diverse group of participants including an equal number of males and females and a relatively high percentage of Hispanics and non-whites.
In conclusion, a HP meal resulted in greater GLP-1, insulin, C-peptide, and glucagon responses compared to a HMF meal. Blood glucose was not different during the two conditions, however, and insulin sensitivity was higher during the HMF condition, suggesting that a HMF meal reduces the insulin requirement for glucose uptake compared to a HP meal. These results imply that a HMF meal may optimally regulate blood glucose despite having lower GLP-1 and insulin responses compared to a HP meal in overweight/obese individuals without type 2 diabetes. These conclusions may not be generalized to patients with type 2 diabetes since they may have a delayed incretin and impaired insulin response which could affect blood glucose control. Future studies need to be conducted in patients with type 2 diabetes and over a longer period of time.
References
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117(1):13–23. doi:10.1172/JCI30227
Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH (2008) Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept 149(1–3):70–78. doi:10.1016/j.regpep.2007.10.008
Vilsboll T, Holst JJ (2004) Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia 47(3):357–366. doi:10.1007/s00125-004-1342-6
Meyer-Gerspach AC, Wolnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, Steinert RE, Beglinger C (2014) Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav 129:265–271. doi:10.1016/j.physbeh.2014.02.043
Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001) Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50(3):609–613
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38(1):140–149. doi:10.2337/dc14-2441
Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS (2006) Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr 83(1):89–94
Belza A, Ritz C, Sorensen MQ, Holst JJ, Rehfeld JF, Astrup A (2013) Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr 97(5):980–989. doi:10.3945/ajcn.112.047563
Beysen C, Karpe F, Fielding BA, Clark A, Levy JC, Frayn KN (2002) Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 45(11):1533–1541. doi:10.1007/s00125-002-0964-9
Ryan AT, Luscombe-Marsh ND, Saies AA, Little TJ, Standfield S, Horowitz M, Feinle-Bisset C (2013) Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 98(2):300–311. doi:10.3945/ajcn.113.061333
Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ (2006) Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4(3):223–233. doi:10.1016/j.cmet.2006.08.001
van der Klaauw AA, Keogh JM, Henning E, Trowse VM, Dhillo WS, Ghatei MA, Farooqi IS (2013) High protein intake stimulates postprandial GLP1 and PYY release. Obesity 21(8):1602–1607. doi:10.1002/oby.20154
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163. doi:10.1016/S0140-6736(03)15268-3
Inoue S, Zimmet P, Caterson I, Chunming C, Ikeda Y et al (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia Pty Limited, Sydney, p 56
Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C (2009) Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol 297(3):G602–G610. doi:10.1152/ajpgi.00051.2009
Coulston AM, Boushey CJ (2008) Nutrition in the prevention and treatment of disease, 2nd edn. Elsevier Academic Press, Burlington
Flint A, Raben A, Blundell JE, Astrup A (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24(1):38–48
HOMA Oxford Calculator. https://www.dtu.ox.ac.uk/homacalculator/
Matsuda M, DeFronzo RA (1999) Insulin sensitivity obtained from oral glucose tolerance testing. Diabetes Care 22(9):1462–1470
Chen Q, Reimer RA (2009) Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 25(3):340–349. doi:10.1016/j.nut.2008.08.012
Brader L, Holm L, Mortensen L, Thomsen C, Astrup A, Holst JJ, de Vrese M, Schrezenmeir J, Hermansen K (2010) Acute effects of casein on postprandial lipemia and incretin responses in type 2 diabetic subjects. Nutr Metab Cardiovasc Dis 20(2):101–109. doi:10.1016/j.numecd.2009.03.019
Billeaud C, Guillet J, Sandler B (1990) Gastric emptying in infants with or without gastro-oesophageal reflux according to the type of milk. Eur J Clin Nutr 44(8):577–583
Jakubowicz D, Froy O, Ahren B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M, Wainstein J (2014) Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia 57(9):1807–1811. doi:10.1007/s00125-014-3305-x
Power O, Hallihan A, Jakeman P (2009) Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 37(2):333–339. doi:10.1007/s00726-008-0156-0
Jones AG, Hattersley AT (2013) The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 30(7):803–817. doi:10.1111/dme.12159
Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, Holst JJ, Chisholm DJ, Greenfield JR (2011) Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr 141(7):1233–1238. doi:10.3945/jn.111.139824
Linn T, Santosa B, Gronemeyer D, Aygen S, Scholz N, Busch M, Bretzel RG (2000) Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 43(10):1257–1265. doi:10.1007/s001250051521
Gentilcore D, Chaikomin R, Jones KL, Russo A, Feinle-Bisset C, Wishart JM, Rayner CK, Horowitz M (2006) Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 91(6):2062–2067. doi:10.1210/jc.2005-2644
Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM (2009) Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr 89(1):106–113. doi:10.3945/ajcn.2008.26362
Brynes AE, Frost GS, Edwards CM, Ghatei MA, Bloom SR (1998) Plasma glucagon-like peptide-1 (7-36) amide (GLP-1) response to liquid phase, solid phase, and meals of differing lipid composition. Nutrition 14(5):433–436
Acknowledgments
This study was funded by the TCU Invests in Scholarship grant. The authors would like to acknowledge Manall Jaffery, Alex Villanueva, Shane Jenke, Justin Repshas, Leighsa Brace, Henry Aleck, Aaron Caldwell, and Elizabeth Sanders for helping with some data collection and/or blood analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Shah, M., Franklin, B., Adams-Huet, B. et al. Effect of meal composition on postprandial glucagon-like peptide-1, insulin, glucagon, C-peptide, and glucose responses in overweight/obese subjects. Eur J Nutr 56, 1053–1062 (2017). https://doi.org/10.1007/s00394-016-1154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1154-8