Abstract
Key message
Genome re-sequencing and recombination analyses identified Capana06g000193 as a strong candidate for the minor male fertility restoration locus Rf2 in chili pepper G164 harboring two dominant male fertility restoration genes.
Abstract
Male fertility restoration genes of chili pepper restorer line G164 (Capsicum annuum L.) were studied using molecular marker genotypes of an F2 population (7G) of G164 crossed with the cytoplasmic male sterility line 77013A. The ratio of sterile to fertile single plants in the F2 population was 1:15. This result indicates that chili pepper G164 has two dominant restoration genes, which we designated as Rf1 and Rf2. An individual plant recessive for Rf1 and heterozygous for Rf2, 7G-112 (rf1rf1Rf2rf2), was identified by molecular marker selection and genetic analysis, and a single Rf2 gene-segregating population with a 3:1 ratio of fertile to sterile plants was developed from the self-pollination of male fertile individuals of 77013A and 7G-112 hybrid progeny. Bulk segregant analysis of fertile and sterile pools from the segregating populations was used to genetically map Rf2 to a 3.1-Mb region on chromosome 6. Rf2 was further narrowed to a 179.3-kb interval through recombination analysis of molecular markers and obtained the most likely candidate gene, Capana06g000193.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cytoplasmic male sterility (CMS), a maternal genetic trait that blocks production of viable or fertile pollen, is widespread in higher plants and has been extensively applied to generate crop hybrid seeds (Kim and Zhang 2018). The use of CMS to produce hybrids requires a maintainer line and a fertility restorer line, collectively termed the three-line breeding system. A stable, strong restorer line containing one or more Restorer-of-fertility (Rf) genes is essential for overcoming male sterility to restore the fertility of hybrids. Such Rf genes encode mitochondrial-localized proteins that modify the transcripts of CMS genes or inhibit protein translation and accumulation (Kim and Zhang 2018). Many Rf genes have been found in grain crops, such as rice (Komori et al. 2004; Wang et al. 2006; Fujii and Toriyama 2009; Itabashi et al. 2011; Hu et al. 2012; Tang et al. 2014; Huang et al. 2015; Liu et al. 2016; Zhang et al. 2016), maize (Cui et al. 1996), wheat (Castillo et al. 2014, 2015) and barley (Ui et al. 2015), oil crop rapeseed (Uyttewaal et al. 2008), fiber crop cotton (Zhang et al. 2005; Yin et al. 2006), and vegetables, such as sugar beet (Matsuhira et al. 2012; Kitazaki et al. 2015), Chinese cabbage (Zhang et al. 2017a), radish (Koizuka et al. 2003), onion (Kim et al. 2015), and chili pepper (Jo et al. 2010, 2016; Wu et al. 2019; Cheng et al. 2020; Wei et al. 2020; Zhang et al. 2020, 2021).
Chili pepper (Capsicum spp. [Solanaceae]) is one of the most widely consumed vegetables and condiments worldwide (Wang and Bosland 2006; Bosland and Votavo 2012). In addition, chili pepper is the largest vegetable crop in China, with a planted area of 2.1 million hm2 and a yield of 64 million tons in 2018 according to China Agricultural Research System statistics (Zou et al. 2020). In China, hybrid varieties account for more than 80% of pepper market production (Wang et al. 2020). The application of the CMS/Rf breeding system to hybrid pepper seed production is an effective way to eliminate reliance on the labor-intensive practice of manual emasculation (Jindal et al. 2019). CMS in pepper was first reported in an Indian accession (PI 164835) by Peterson (1958). The genes coxII and atp6-2 contained in one fragment were first considered to be associated with CMS in chili pepper (Kim et al. 2001; Kim and Kim 2006). Another 456-bp open reading frame gene, orf456, was then found downstream of coxII (Kim et al. 2007), and later study identified that the orf456 gene to be a longer open reading frame and named orf507 (Gulyas et al. 2010). Recently, two OFRs, orf300a and orf314a, were selected as strong candidates causing male sterile in pepper CMS line 138A (Wang et al. 2019). Functional analysis of CMS genes in chili pepper is still lacking, and reports on CMS utilization have not defined sterile types or genes. Most research has been focused on restorer line selection and breeding, genetic mapping of restorer genes, and molecular marker development.
As in the application of CMS/Rf to crops whose seeds are harvested, enhanced fertility restoration of hybrids is required to ensure a higher grain yield. A stable, strong restorer line allows hybrid F1 fruits to generate enough seeds to guarantee normal pepper pod development. Previous studies have focused on restorer-trait inheritance (Novak et al. 1971; Wang et al. 2004) and the development of molecular markers related to restorer genes (Zhang et al. 2000; Gulyas et al. 2006; Kim et al. 2006; Kumar et al. 2007; Lee et al. 2008a, 2008b; Min et al. 2009; Jo et al. 2010, 2016; Wu et al. 2012; Lin et al. 2015). Following the release of pepper genomic data (Kim et al. 2014; Qin et al. 2014), several Rf genes have been isolated from various C. annuum cultivars by exploiting whole genome resequencing and high-efficiency molecular marker development (Jo et al. 2016; Wu et al. 2019; Cheng et al. 2020; Wei et al. 2020; Zhang et al. 2020), namely, CaPPR6 isolated from Chunyang (Jo et al. 2016), Capana06g002967 and Capana06g002969 from 138C (Wu et al. 2019), Capana06g003028 from B702 (Cheng et al. 2020), CaRf032 (CA00g82510) from IVF2014032 (Zhang et al. 2020), and Capana06g002866 from R1 (Wei et al. 2020).
Molecular markers tightly linked to Rf genes of chili pepper are completely consistent when applied to the selection of related restorer genes but exhibit low accuracy when used to analyze the restoration of individuals in natural populations. Jo et al. (2016) reported that the CaPPR6-co-segregated marker Co1Mod1-CAPS had an accuracy rate of 88.0 and 92.2% in 50 and 51 chili pepper groups, respectively. Zhang et al. (2020) found that the accuracy rate of Co1Mod1-CAPS and S1361 (tightly linked to CaRf032) was only 64.4 and 70.3%, respectively, in 101 pepper groups. Chili pepper contains multiple copies of PPR genes and has multiple candidate genes (Jo et al. 2016; Barchenger et al. 2018). The highly repetitive and multiple haplotypes of Rf genes may be responsible for the low accuracy of Rf gene-specific markers in marker-assisted selection among natural groups. S1597 and S1609, two newly developed Kompetitive Allele-Specific PCR (KASP) marker co-segregating with CaPPR6 (Rfm), have been further confirmed to co-segregate with fertile/sterile phenotypes in five segregating populations of commercial varieties (Zhang et al. 2021). This result suggests that the CaPPR6 gene has been relatively widely used in commercial hybrid seed production and that S1597 and S1609 has potential application value in marker-assisted selection for CaPPR6.
Many different types of CMS lines have been classified in various plant species (Bohra et al. 2016; Kim and Zhang 2018). In general, restorer lines are identified according to the fertility of hybrids of the CMS line and other inbred lines. The sterility of CMS lines is generally restored by specific restorer genes, but a single restorer gene can also restore the fertility of different CMS lines of rice (Kim and Zhang 2018). The fertility restorer trait is mostly controlled by a single dominant locus, and each restorer line often has only one gene of major effect; however, restorer lines containing two restorer genes have been reported in pigeonpea (Saxena et al. 2011, 2018) and rice (Zhang et al. 2017b). Restorer genes isolated from different restorer lines can restore the fertility of the same sterile line (Hu et al. 2012; Huang et al. 2015; Zhang et al. 2016; Kim and Zhang, 2018; Melonek et al. 2021). In previous studies, we found that restorer lines IVF2014032 and 0601 M, which carry restorer genes Rf032 (CA00g82510) and Rfm (CaPPR6), respectively, can both restore the fertility of sterile line 77013A (Zhang et al. 2020, 2021).
In the present study, we analyzed the chili pepper C. annuum ‘G164’ containing one major and one minor restorer gene. Using single-gene segregating populations, we fine-mapped the minor fertility restoration gene Rf2 to a 179.3-Kb interval and cloned a candidate for Rf2 through bulked segregant analysis (BSA) and analysis of recombinant plants. Our results should provide insights into the fertility restoration ability of different-effect Rf genes in chili pepper.
Materials and methods
Plant materials
The C. annuum cytoplasmic male-sterile line 77013A and two C. annuum restorer lines—0601 M carrying the restorer gene CaPPR6, and G164 possessing two predicted dominant restorer genes—were used in this study. 77013A and G164 were crossed to obtain an F1 population, and a single F1 plant was then self-crossed to construct an F2 segregating population (7G). A new segregating population (7G7) was developed by crossing 77013A with fertile individual F2 plants determined to be homozygous recessive for CaPPR6 (Jo et al. 2016) according to linked markers S1597 and S1609 (Zhang et al. 2021). In addition, fertile plants from the above-described newly developed segregating populations, which were homozygous recessive for markers S1597 and S1609 and had a 1:1 ratio of fertile and sterile plants, were self-crossed to create mapping populations (Fig. 1). A total of 61 fertile and 58 sterile individuals were selected from the mapping populations and grouped as the fertile pool (F-pool) and the sterile pool (S-pool), respectively. A small segregating population derived from a F1 (77013A × 0601 M) × F1 (77013A × G164) cross was used to detect the separation of the Rf1 (CaPPR6) gene-linked marker S1609 and the Rf2 (Capana06g000193) gene-linked marker S1820. A small piece of a young leaf was taken from each individual plant at the seedling stage as a DNA source for molecular marker analysis.
Breeding scheme used to create single Rf2 gene-segregating populations via hybridization, self-pollination, and molecular marker-assisted selection. Markers S1597 and S1609 (Zhang et al. 2021) were used to select CaPPR6 (Rf1) homozygous recessive fertile single plants (genotypes rf1rf1Rf2Rf2 or rf1rf1Rf2rf2) from an F2 (7G) population, which were then backcrossed with the sterile parent, 77013A. After statistical analysis of the segregation of fertility traits in four backcross progeny populations (Table 2), a single plant, 7G-112 with the genotype rf1rf1Rf2rf2, was identified in the F2 population. Because fruits were immature owing to winter, we were unable to harvest seeds from the 7G-112 plant. Five fertile plants were therefore randomly selected from the backcross population (7G7) to obtain self-crossed seeds and used to develop a population for genetic mapping of the Rf2 gene
Male fertility phenotyping
Phenotypic analysis of male fertility and sterility was carried out by visually checking for the presence or absence of pollen grains and seed-containing fruits according to the methods of Zhang et al. (2020). In addition, the whole anther of a single flower of each plant was collected before flowering in a 1.5-ml centrifuge tube, dried in a drying oven at 50 °C, and stained with 1% acetocarmine in a volume of 100 μl. After mixing with a shaker, pollen grains were observed and counted in a hemocytometer using an optical microscope (model BX51; Olympus, Japan). The total number of pollen grains per flower was obtained from the pollen concentration, which was the hemocytometer count multiplied by the solution volume (100 μl). Anthers were collected every 2 weeks after anthesis of the flower at node 3, and the experiment was repeated three times for each plant.
BSA by genome resequencing
High-throughput sequencing, sequence alignment, and SNP detection were performed by OE Biotechnology (Beijing, China). The F-pool and the S-pool were sequenced on an Illumina HiSeq 4000 system in PE150 mode, and the raw reads were filtered using the NGSQC toolkit (Dai et al. 2010). The clean reads were then mapped to the Zunla-1 v2.0 reference genome (Qin et al. 2014) using Burrows-Wheeler Aligner software (Li and Durbin 2009). SNPs were called using the mpileup function of SAMtools (Li et al. 2009). Finally, homozygous SNPs with a quality score ≥ 20 and read depth ≥ 4 were selected, and the variants were further annotated with the SnpEff tool (Cingolani et al. 2012). The SNP index was estimated from the ratio of the number of reads in each pool to the total number of reads corresponding to the SNPs (Abe et al. 2012), and Δ(SNP index) was calculated by subtracting the two F-pool and S-pool SNP indexes (Takagi et al. 2013). The SNP index and Δ(SNP index) values for the F-pool and the S-pool were plotted against the Zunla-1 genome sequence in 10-kb increments with a 1-Mb sliding window (Qin et al. 2014). Candidate regions of fertility restoration were defined as those locations where the labeled fitted value exceeded the threshold of the 99% confidence interval.
KASP marker development and genetic mapping
Polymorphic SNPs of the F-pool and S-pool were used to develop KASP markers, with the corresponding primers designed with BatchPrimer3 online software at https://wheat.pw.usda.gov/demos/BatchPrimer3/ (You et al. 2008). KASP markers S1597 and S1609, which are tightly linked to CaPPR6 (Jo et al. 2016), were developed by Zhang et al. (2021). KASP assays were carried out on a Roche LightCycler 480 instrument (Roche Diagnostics, Rotkreuz, Switzerland) in 4-μl reaction mixtures consisting of 2 μl template DNA (5 ng μl−1), 2 μl of 2 × KASP Master Mix, and 0.055 μl primer mixture according to Zhang et al. (2020). The genetic linkage map of 179 individual plants in F2 generation was constructed with IciMapping 4.0 software (Meng et al. 2015).
qRT-PCR of Capanga06g00193
Total RNA was isolated from different-sized, mixed flower buds of male-fertile and male-sterile lines using Trizol reagent (Invitrogen, Shanghai, China). First-strand cDNA was synthesized by reverse transcription using 5 × All-In-One RT MasterMix (ABM, Richmond, Canada). Forward (5′-GTTGGGCTCACAGATCTTCTTC-3′) and reverse (5′-CGTGTTGATTGTGCATTTTCGT-3′) primers for amplification of CaPPR6 and forward (5′-AACAATAGAACATCACCCTTTTGAC-3′) and reverse (5′-GTGAAACCTCGAGCAATGCA-3′) primers to amplify Capanga06g00193 were designed using Primer3 online software (Untergrasser et al. 2012). Amplification primers for the Actin gene (5′-GTCCTCTTCCAACCATCCAT-3′ and 5′-TACTTTCTCTCTGGTGGTGC-3′) were from Wang et al. (2012). PCR amplifications were performed in 10-μl reaction volumes containing 1.5 μl of 200 ng μl−1 cDNA, 0.5 μl of each 0.5 μM primer, 5 μl EvaGreen 2 × qPCR MasterMix-No Dye (ABM), and 2.5 μl RNase-free water following the manufacturer’s instructions on a Roche LightCycler 480 instrument. The 2−ΔΔCt method was used for calculating relative expression levels based on Actin as an internal control. Three biological replicates were performed per line, and average transcript levels of each repeat were calculated from three technical replicates.
Sequencing of Capanga06g00193 in male-fertile and -sterile plants
PCR amplification and sequencing of Capanga06g00193 was carried out according to Zhang et al. (2020). In brief, PCR amplification was performed in 50-µl volumes using KOD-Plus-Neo (Toyobo, Osaka, Japan) and gene-specific forward (5′-TCATCTTAAACCACGGACGAAAAT-3′) and reverse (5′-GTAGGTATGCGACTTTCTTGACAG-3′) primers. The amplification protocol was as follows: 5 cycles of 94 °C for 15 s, 58 °C for 30 s, and 68 °C for 2 min; 5 cycles of 94 °C for 15 s, 64 °C for 30 s, and 68 °C for 2 min; 20 cycles of 94 °C for 15 s, 60 °C for 30 s, and 68 °C for 2 min; and a final extension of 68 °C for 5 min. PCR products were cloned into an pEASY-BLUNT vector (Tiangen, Beijing, China) and transformed into Escherichia coli TOP10 (Tiangen) according to Zhang et al. (2021). Sequencing of clones was performed by Sangon Biotech (Shanghai, China). DNAMAN v6 was used for multiple comparisons of the resulting Capanga06g00193 sequences.
Results
Genetic analysis of Rf genes of G164
F1 hybrids of the cross between 77013A and G164 were male fertile, with a large number of pollen production and normal fruit development. In contrast, the F2 population contained both sterile and fertile plants, respectively, with or without pollen grains (Supplementary Fig. 1). The sterile plants had shriveled anthers and no or small seedless fruits. The fertile plants produced pollen and set seeded fruits, but the average number of pollen grains among plants varied from 157.2 × 103 to 430.5 × 103 per flower (Fig. 2). Moreover, the ratio of sterile plants to fertile plants in this population was 1:15 (Table 1). These results indicate that fertility restoration in G164 is controlled by two independent genes with different effects on male fertility restoration, which we designated as Rf1 and Rf2.
The genotypes and phenotypes of F2 (7G) plants. a Comparison of the average production of pollen grains per flower among different genotypes. The genotypes were detected using markers S1597 and S1609 (Zhang et al. 2021) linked to the Rf1 (CaPPR6) gene and the S1820 marker linked to the Rf2 (Capana06g000193) gene. Different lowercase letters indicate a significant difference (Student’s t-test, p < 0.05). b The abundance of pollen grains in male fertile plants dominant for Rf1. c Male fertile plants recessive for Rf1 produce less pollen grains than dominant Rf1 plants. d The absence of pollen grains in male sterile plants
The genotypes of the CaPPR6 (Jo et al. 2016)-linked markers S1597 and S1609, which we recently developed (Zhang et al. 2021), were exactly the same in the F2 population. Plants that were homozygous dominant or heterozygous were all male fertile, whereas plants with homozygous recessive genotypes were fertile or sterile. The genotypic ratio of the two markers corresponding to sterility and fertility was 1:3:4:8 (Table 1), which is consistent with the segregation of one gene among the traits controlled by two genes. Given that markers S1597 and S1609 are tightly linked to the CaPPR6 gene (Jo et al. 2016; Zhang et al. 2021), these results support CaPPR6 as the most likely candidate gene for Rf1 in G164. A genetic linkage map of Rf1 and Rf2 linked markers was constructed using 179 F2 individuals generated from the male-sterile line 77013A and restorer line G164. The markers linked to Rf1 and Rf2 were separated into two parts on two arms of the chromosome 6, respectively (Fig. 3). This further indicated that the two restorer genes of G164 were located at opposite ends of the chromosome and therefore they were considered to be able to be separated independently.
Genetic linkage map of Rf1 and Rf2. The markers S1585, S1597, and S1609 were co-segregated with Rf1, and S1803, S1820, and S1718 were co-segregated with Rf2. On the right side of the genetic map were the physical locations of the markers on the reference genome Zunla-1 v2.0 (Qin et al. 2014). The markers S1585, S1597, S1609, S1350, and S1436 were developed by Zhang et al. (2021). The underlined physical location number was obtained by BLAST searches. The physical location of S1585 was not retrieved in Zunla-1 v2.0 (Qin et al. 2014)
Predicted genotypes of F2 generation plants
Four individual plants in the F2 population, namely 7G-46 and 7G-112, with the Rf1 homozygous recessive fertile genotype (rf1rf1), and 7G-42 and 7G-61, with the Rf1 heterozygous fertile genotype (Rf1rf1), were selected and crossed with 77013A. All progeny derived from 7G-42 and 7G-46 were fertile. The Rf1 genotypes of the 7G-42 progeny population were Rf1rf1 and rf1rf1, whereas all those of 7G-46 progeny were rf1rf1 (Table 2). These results suggest that the restored genotypes of individual 7G-42 and 7G-46 plants were Rf1rf1Rf2Rf2 and rf1rf1Rf2Rf2, respectively. The Rf1rf1 to rf1rf1 genotypic ratio of the 7G-42 progeny population was not 1:1 (Table 2), however, which may be because double heterozygous plants (Rf1rf1Rf2rf2) have higher germination ability compared with single heterozygous plants (rf1rf1Rf2rf2). Further study is needed to confirm this hypothesis.
The 7G-61 and 7G-112 progeny populations contained both fertile and sterile plants. In these populations, the ratio of fertile to sterile plants was 1:1, consistent with traits being controlled by a single gene in a backcrossed population (Table 2). Rf1 genotypes of the fertile and sterile plants in the 7G-61 progeny population were Rf1rf1 and rf1rf1, respectively, in agreement with the 1:1 ratio. We therefore inferred that the restored genotype of the 7G-61 plant was heterozygous for Rf1, i.e., Rf1rf1rf2rf2 (Table 2).
The genotypes of fertile and sterile plants in the 7G-112 progeny population were all rf1rf1, which indicates that the 7G-112 plant was heterozygous for Rf2, with a restored genotype of rf1rf1Rf2rf2 (Table 2). We therefore concluded that Rf2 could be genetically mapped using segregating populations obtained by self-crossing fertile plants in the 7G-112 progeny population (Fig. 1).
Genetic analysis of Rf2 and BSA genetic mapping
We randomly selected the self-pollinated progeny of five individuals from the ‘7G7’ population for genetic mapping of Rf2. In all five populations, the ratio of sterile plants to fertile plants was 1:3 (Table 3), indicative of a single dominant restorer gene, Rf2, in the segregating populations.
A total of 61 fertile and 58 sterile plants were selected from the five ‘7G7’ populations to form fertile and sterile pools for genome resequencing. Average sequencing depths of the fertile and sterile pools were 36 × and 35 × , respectively. According to comparisons with the Zunla-1 reference genome (Qin et al. 2014), 998.08 M and 998.48 M clean reads (Supplementary Table 1) were obtained from the two pools. After comparison with the Zunla-1 reference genome, 16,440,061 SNPs and 1,307,939 InDels were identified in the fertile pool, and 16,426,311 SNPs and 1,280,471 InDels were obtained from the sterile pools. The Rf2 locus was located on chromosome 6 in a 3.1-Mb interval (767.2–3884.6 kb) that exceeded the threshold value (> 0.5) according to SNP-index and Δ(SNP index) calculations (Supplementary Fig. 2).
Fine mapping of Rf2 and candidate gene survey
KASP primers were designed from SNPs between the F- and S-pools in the candidate region, and the polymorphic markers were subsequently applied to genotype the 517 individuals of the five populations used for genetic mapping. The Rf2 gene was initially located in a 419.9-kb interval between markers S1754 and S1719 on the basis of genotype and phenotype comparative analysis (Supplemental Table 1; Fig. 4). The two flanking markers, S1754 and S1719, were also used to select recombinants from the 4,416 plants in the mapping population (Table 3; Fig. 1). As a result, the Rf2 gene was narrowly located, along with seven co-segregated markers, in a 179.3-kb interval between S1736 and S1719 (Supplemental Table 1; Fig. 4). Fifteen genes were annotated in the candidate region of the Zunla-1 reference genome (Qin et al. 2014). Among them, expect Capana06g000193, others were expressed at low levels and were not significantly expressed between the parental lines (Supplemental Table 3). The Capana06g000193 was predicted to encode a PPR protein, thus suggesting this gene as a candidate for Rf2 of G164. The expression of Capana06g000193 (Rf2) in G164 flower buds was significantly higher than that in 77013A and 0601 M, while CaPPR6 (Rf1) was significantly highly expressed in G164 and 0601 M flower buds (Fig. 5). Moreover, the sterile phenotype was detected in F2 (77013A × G164) and F1 (77013A × 0601 M) × F1 (77013A × G164) populations only when the CaPPR6-linked marker S1609 and the Capana06g000193-linked marker S1820 were both homozygous for sterile genotypes at the same time (Fig. 2; Supplementary Fig. 1). These results confirmed CaPPR6 and Capana06g000193, respectively, as positive candidate genes for the Rf1 and Rf2 loci of chili pepper G164.
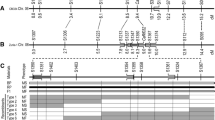
Recombinants between phenotypes and marker genotypes in Rf2 gene-segregating populations. Sixteen marker genotypes for 44 recombinants were detected. Physical locations of the flanking markers on chromosome 6 of the Zunla-1 reference genome are 2,560,823 bp for S1736 and 2,740,136 bp for S1719. Fifteen genes were located in the 179.3-kb candidate interval between the two flanking markers. Red markers indicate co-segregation with the phenotype. A, genotype of sterile line 77013A; B, genotype of restorer line G164; H, heterozygous genotype; MS, male-sterile; MF, male-fertile
Expression analysis of the male fertility restoration genes CaPPR6 and Capana06g000193 in the flower buds of 77013A, 0601 M, and G164. 77013A is a male-sterile line carrying homozygous recessive alleles of CaPPR6 and Capana06g000193, and 0601 M and G164 are male fertility restoration lines harboring CaPPR6 and CaPPR6 + Capana06g000193, respectively. The average standard deviation was determined from three biological replicates; different lowercase letters indicate a significant difference (Student’s t-test, p < 0.05)
Sequence analysis of Capana06g000193
The Capana06g000193 gene comprises 945 nucleotides and encodes 113 amino acids with no introns. Six SNPs responsible for changes in coded amino acids, but not leading to premature stop codons, were located between 77013A and G164 lines (Supplementary Figs. 3, 4). Two of these SNPs, at 14 and 441 bp of Capana06g000193, were converted into KASP markers S1820 and S1814, respectively (Supplementary Table 1; Supplementary Fig. 5). The two markers were found to co-segregate with male-fertile and -sterile phenotypes of the recombinants of mapping populations (Fig. 4) and individual F1 (77013A × 0601 M) × F1 (77013A × G164) population plants (Supplementary Fig. 1).
Further analysis of the Capana06g000193 promoter region uncovered an AT-rich element 243 bp away from the start codon (Supplementary Fig. 6). In this element, the presence of an A/G SNP (at bp −251 of Capana06g000193) between 77013A and G164 parents suggests that this mutation may be involved in the differential expression of Capana06g000193 between 77013A and G164 lines.
Discussion
Multiple candidate Rf genes have been identified in the chili pepper genome (Barchenger et al. 2018). To date, numerous Rf genes have been isolated from different restorer lines of C. annuum, and the fertility restoration of these restorer lines has been found to be due to a single dominant gene (Jo et al. 2010, 2016; Wu et al. 2019; Cheng et al. 2020; Wei et al. 2020; Zhang et al. 2020). In the present study, we found that chili pepper G164 carries two independently inherited Rf genes (Table 1; Fig. 3). The presence of two inherited Rf genes in a single restorer line has been previously reported in pigeonpea (Saxena et al. 2011, 2018) and rice (Zhang et al. 2017b). Saxena et al. (2011) found that pigeonpea hybrids with two dominant genes produce a greater pollen load and have greater stability compared with those carrying a single dominant gene. Zhang et al. (2017b) have reported that the seed setting rate of rice hybrids harboring two restorer genes is more stable than that of F1 plants possessing only one Rf gene. In the present study, we found that chili pepper plants dominant for Rf1 produced more pollen than those dominant for Rf2. In addition, the pollen content of plants homozygous for the recessive Rf1 (rf1rf1Rf2Rf2) gene was far less than that for Rf2 (Rf1Rf1rf2rf2) (Fig. 2), which indicates that Rf1 is the main restorer gene, whereas Rf2 is the minor restorer gene.
The fertility of a CMS system can be restored by multiple Rf genes, and a particular Rf gene can restore the fertility of different CMS-type lines. RFL79 (Rf1) and RFL29a (Rf3), two major fertility restorer genes in wheat, can restore normal pollen production in T-CMS-carrying transgenic wheat plants (Melonek et al. 2021). Rf5 (Rf1b) and Rf6, both identified from CMS-HL-type indica rice, are able to rescue CMS-BT-type rice plants (Hu et al. 2012; Huang et al. 2015; Kim and Zhang, 2018). In regards to chili pepper, we have found that restorer lines IVF2014032 (Zhang et al. 2020), 0601 M (Zhang et al. 2021), and G164 (this study) have different Rf genes, but all restore the fertility of the pepper CMS line 77013A. The discovery of different male fertility restorers and the identification of restorer lines carrying more than one Rf gene are important contributions to the study of the genetic basis of CMS/Rf systems in pepper.
To fine map Rf2, we developed a Rf2 single-gene segregating population through Rf1-linked marker-assisted selection. After selection for the homozygous recessive Rf1 genotype using the marker S1609, we obtained individual plants that were heterozygous or homozygous for Rf2, and the accuracy of the marker selection was verified by test-crossing or self-crossing of individual plants lacking the Rf1 gene (Tables 1, 2; Fig. 1). A previous study has revealed that chili pepper contains multiple restorer candidate genes (Barchenger et al. 2018). Markers for restorer genes of different restorer lines tend to not be highly accurate for screening individual plants of natural populations, but restorer gene-linked markers are highly consistent with fertility restoration phenotypes (Jo et al. 2010, 2016; Wu et al. 2019; Cheng et al. 2020; Wei et al. 2020; Zhang et al. 2020). We have previously reported that the S1609 marker, which is related to CaPPR6, is completely consistent with marker genotypes and fertility phenotypes when selecting among the progeny of plants predicted to contain CaPPR6 (Zhang et al. 2021). KASP assays are a cost-effective, high-throughput option for plant genotyping (Semagn et al. 2014), and the use of S1609 to restore the CaPPR6 gene in this study further illustrates the reliability of applying this technique in assisted selection.
In this study, we obtained the candidate gene of Rf2 by map-based cloning and developed markers S1814 and S1820 derived from the SNP variation of this gene. We also analyzed F2 genotypes using the Rf2-linked marker S1820 and the Rf1-linked marker S1609 and discovered that individual sterile plants were double recessive (data not shown). Plants homozygous for the dominant Rf1 gene had the largest average number of pollen grains, whereas plants homozygous for the recessive Rf1 gene had the lowest. Moreover, plants homozygous dominant for Rf1, regardless of whether Rf2 was dominant or recessive, all produced more pollen, with no significant differences in pollen levels observed among them (Fig. 2). This result is inconsistent with the report of Saxena et al. (2011) that pigeonpea hybrids harboring two dominant fertility restorer genes produce more pollen than plants with only a single dominant gene. Our observations also conflict with the findings of Melonek et al. (2021) that RFL79 (Rf1) and RFL29a (Rf3) act independently in wheat and improve fertility restoration when stacked. In our study, in contrast, we found that the presence of a dominant Rf2 does not promote the production of more pollen grains when Rf1 is dominantly homozygous.
In our gene expression analysis, both CaPPR6 (Rf1) and Capana06g000193 (Rf2) were highly expressed in G164, whereas only CaPPR6 was highly expressed in 0601 M (Fig. 5). In addition, sterile individuals in the F1 (77013A × 0601 M) × F1 (77013A × G164) population were homozygous recessive for both Rf1 and Rf2 genes, and individual plants homozygous for Rf1 were found (Supplementary Fig. 1). These results further confirm that G164 contains two independently segregated candidate restorer genes. It is necessary to use genetic technology such as virus-induced gene silencing to verify the function of the candidate genes. Although sequence variation was found upstream of Capana06g000193 (Supplementary Fig. 6), the relationship between this variation and gene expression needs further study. Moreover, studying the interaction between different restorer genes and the same cytoplasmic sterility gene in the same CMS/Rfs system has served as the basis for revealing the fertility restoration differences of various restorer genes.
Data availability
The reference genome sequences analyzed during the current study are available in the China National GeneBank DataBase (https://ftp.cngb.org/pub/CNSA/data2/CNPhis0000547/pepper/).
References
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M (2012) Genome sequencing reveals agronomically important loci in rice using MutMap. Nature Biotech 30:174–178
Barchenger DW, Said JI, Zhang Y, Song M, Ortega FA, Ha Y, Kang B-C, Bosland PW (2018) Genome-wide identification of chile pepper pentatricopeptide repeat domains provides insight into fertility restoration. J Amer Soc Hort Sci 143(6):418–429
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Bosland PW, Votava EJ (2012) Peppers: vegetable and spice capsicums, 2nd edn. CAB International, Wallingford
Castillo A, Atienza SG, Martín AC (2014) Fertility of CMS wheat is restored by two Rf loci located on a recombined acrocentric chromosome. J Exp Bot 65:6667–6677
Castillo A, Rodríguez-Suárez C, Martín AC, Pistón F (2015) Contribution of chromosomes 1HchS and 6HchS to fertility restoration in the wheat msH1 CMS system under different environmental conditions. PLoS ONE 10:e0121479
Cheng J, Chen Y, Hu Y, Zhou Z, Hu F, Dong J, Chen W, Cui J, Wu Z, Hu K (2020) Fine mapping of restorer-of-fertility gene based on high-density genetic mapping and collinearity analysis in pepper (Capsicum annuum L.). Theor Appl Genet 133:889–902.
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 6:80–92
Cui X, Wise RP, Schnable PS (1996) The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 272:1334–1336
Dai M, Thompson RC, Maher C, Contreras-Galindo R, Kaplan MH, Markovitz DM, Omenn G, Meng F (2010) NGSQC: cross-platform quality analysis pipeline for deep sequencing data. BMC Genomics 11:S7
Fujii S, Toriyama K (2009) Suppressed expression of RETROGRADE-REGULATED MALE STERILITY restores pollen fertility in cytoplasmic male sterile rice plants. Proc Natl Acad Sci USA 106:9513–9518
Gulyas G, Pakozdi K, Lee JS, Hirata Y (2006) Analysis of fertility restoration by using cytoplasmic male-sterile red pepper (Capsicum annuum L.) lines. Breed Sci 56:331–334
Gulyas G, Shin Y, Kim H, Lee JS, Hirata Y (2010) Altered transcript reveals an Orf507 sterility-related gene in chili Pepper (Capsicum annuum L.). Plant Mol Biol Rep 28:605–612
Hu J, Wang K, Huang W, Liu G, Gao Y, Wang J, Huang Q, Ji Y, Qin X, Wan L, Zhu R, Li S, Yang D, Zhu Y (2012) The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycinerich protein GRP162. Plant Cell 24:109–122
Huang W, Yu C, Hu J, Wang L, Dan Z, Zhou W, He C, Zeng Y, Yao G, Qi J, Zhang Z, Zhu R, Chen X, Zhu Y (2015) Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc Natl Acad Sci USA 112:14984–14989
Itabashi E, Iwata N, Fujii S, Kazama T, Toriyama K (2011) The fertility restorer gene, Rf2, for lead rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J 65:359–367
Jindal SK, Dhaliwal MS, Meena OP (2019) Molecular advancements in male sterility systems of Capsicum: A review. Plant Breed 139:42–64
Jo YD, Kim YM, Park MN, Yoo JH, Park M, Kim BD, Kang BC (2010) Development and evaluation of broadly applicable markers for Restorer-of-fertility in pepper. Mol Breed 25:187–201
Jo YD, Ha Y, Lee JH, Park M, Bergsma AC, Choi HI, Gortischnig S, Kloosterman B, van Dijk PJ, Choi D, Kang BC (2016) Fine mapping of Restorer-of-fertility in pepper (Capsicum annuum L.) identified a candidate gene encoding a pentatricopeptide repeat (PPR)-containing protein. Theor Appl Genet 129:2003–2017
Kim DH, Kim BD (2006) The organization of mitochondrial atp6 gene region in male fertile and CMS lines of pepper (Capsicum annuum L.). Curr Genet 49:59–67
Kim Y-J, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Kim DH, Kang JG, Kim S, Kim B-D (2001) Identification of coxII and atp6 region as associated to CMS in Capsicum annuum by using RFLP and long and accurate PCR. J Kor Soc Hort Sci 42:121–127
Kim DS, Kim DH, Yoo JH, Kim BD (2006) Cleaved amplified polymorphic sequence and amplified fragment length polymorphism markers linked to the fertility restorer gene in chili pepper (Capsicum annuum L.). Mol Cells 21:135–140
Kim DH, Kang JG, Kim BD (2007) Isolation and characterization of the cytoplasmic male sterility-associated orf456 gene of chili pepper (Capsicum annuum L.). Plant Mol Biol 63:519–532
Kim S, Park M, Yeom SI, Kim YM, Lee JM, Lee HA, Seo E, Choi J, Cheong K, Kim KT, Jung K, Lee GW, Oh SK, Bae C, Kim SB, Lee HY, Kim SY, Kim MS, Kang BC, Jo YD, Yang HB, Jeong HJ, Kang WH, Kwon JK, Shin C, Lim JY, Park JH, Huh JH, Kim JS, Kim BD, Cohen O, Paran I, Suh MC, Lee SB, Kim YK, Shin Y, Noh SJ, Park J, Seo YS, Kwon SY, Kim HA, Park JM, Kim HJ, Choi SB, Bosland PW, Reeves G, Jo SH, Lee BW, Cho HT, Choi HS, Lee MS, Yu Y, Choi YD, Park BS, van Deynze A, Ashrafi H, Hill T, Kim WT, Pai HS, Ahn HK, Yeam I, Giovannoni JJ, Rose JK, Sørensen I, Lee SJ, Kim RW, Choi IY, Choi BS, Lim JS, Lee YH, Choi D (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46:270–278
Kim S, Kim C, Park M, Choi D (2015) Identification of candidate genes associated with fertility restoration of cytoplasmic male-sterility in onion (Allium cepa L.) using a combination of bulked segregant analysis and RNA-seq. Theor Apple Genet 128:2289–2299
Kitazaki K, Arakawa T, Yui-Kurino MM, R, Matsuhira H, Mikami T, Kubo T (2015) Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J 83:290–299
Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Kawasaki S, Imamura J (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34:407–415
Komori T, Ohta S, Murai N, Takakura Y, Kuraya Y, Suzuki S, Hiei Y, Imaseki H, Nitta N (2004) Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J 37:315–325
Kumar S, Singh V, Singh M, Rai S, Kumar S, Rai SK, Rai M (2007) Genetics and distribution of fertility restoration associated RAPD markers in inbreds of pepper (Capsicum annuum L.). Sci Hortic 111:197–202
Lee J, Yoon JB, Park HG (2008a) Linkage analysis between the partial restoration (pr) and the restorer-of-fertility (Rf) loci in pepper cytoplasmic male sterility. Theor Appl Genet 117:383–389
Lee J, Yoon JB, Park HG (2008b) A CAPS marker associated with the partial restoration of cytoplasmic male sterility in chili pepper (Capsicum annuum L.). Mol Breed 21:95–104
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The Sequence alignment/map (SAM) format and SAMtools. Bioinformatics 25:2078–2079
Lin S-W, Shieh H-C, Wang Y-W, Tan C-W, Schafleitner R, Yang W-J, Kumar S (2015) Restorer breeding in sweet pepper: Introgressing Rf allele from hot pepper through marker-assisted backcrossing. Sci Hortic 197:170–175
Liu Y, Zhao Z, Lu Y, Li C, Wang J, Dong B, Liang B, Qiu T, Zeng W, Cao M (2016) A preliminary identification of Rf*-A619, a novel restorer gene for CMS-C in maize (Zea mays L.). PEERJ 4:e2719
Matsuhira H, Kagami H, Kurata M, Kitazaki K, Matsunaga M, Hamaguchi Y, Hagihara E, Ueda M, Harada M, Muramatsu A, Yui-Kurino R, Taguchi K, Tamagake H, Mikami T, Kubo T (2012) Unusual and typical features of a novel restorer-of-fertility gene of sugar beet (Beta vulgaris L.). Genetics 192:1347–1358
Melonek J, Duarte J, Martin J, Beuf L, Murigneux A, Varenne P, Comadran J, Specel S, Levadoux S, Bernath-Levin K, Torney F, Pichon JP, Perez P, Small I (2021) The genetic basis of cytoplasmic male sterility and fertility restoration in wheat. Nat Commun 12:1036
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Min WK, Kim S, Sung SK, Kim BD, Lee S (2009) Allelic discrimination of the Restorer-of-fertility gene and its inheritance in peppers (Capsicum annuum L.). Theor Appl Genet 119:1289–1299
Novak F, Betlach J, Dubovsky J (1971) Cytoplasmic male sterility in sweet pepper (Casicum annuum L.). I. Phenotype and inheritance of male sterile character. Z Pflanzenzucht 65:129–140
Peterson PA (1958) Cytoplasmically inherited male sterile in Capsicum. Am Nat 92:111–119
Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, Yang Y, Wu Z, Mao L, Wu H, Ling-Hu C, Zhou H, Lin H, González-Morales S, Trejo-Saavedra DL, Tian H, Tang X, Zhao M, Huang Z, Zhou A, Yao X, Cui J, Li W, Chen Z, Feng Y, Niu Y, Bi S, Yang X, Li W, Cai H, Luo X, Montes-Hernández S, Leyva-González MA, Xiong Z, He X, Bai L, Tan S, Tang X, Liu D, Liu J, Zhang S, Chen M, Zhang L, Zhang L, Zhang Y, Liao W, Zhang Y, Wang M, Lv X, Wen B, Liu H, Luan H, Zhang Y, Yang S, Wang X, Xu J, Li X, Li S, Wang J, Palloix A, Bosland PW, Li Y, Krogh A, Rivera-Bustamante RF, Herrera-Estrella L, Yin Y, Yu J, Hu K, Zhang Z (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA 111:5135–5140
Saxena KB, Sultana R, Saxena RK, Kumar RV, Sandhu JS, Rathore A, KaviKishor PB, Varshney RK (2011) Genetics of fertility restoration in A4-based diverse maturing hybrids in pigeonpea [Cajanus cajan (L.) Millsp.]. Crop Sci 51:574–578
Saxena RK, Patel K, Sameer Kumar CV, Tyagi K, Saxena KB, Varshney RK (2018) Molecular mapping and inheritance of restoration of fertility (Rf) in A4 hybrid system in pigeonpea (Cajanus cajan (L.) Millsp.). Theor Appl Genet 131:1605–1614
Semagn K, Babu R, Hearne S, Olsen M (2014) Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): overview of the technology and its application in crop improvement. Mol Breed 33:1–14
Takagi H, Abe A, Yoshida K, Kosugi S, Natsume S, Mitsuoka C, Uemura A, Utsushi H, Tamiru M, Takuno S, Innan H, Cano LM, Kamoun S, Terauchi R (2013) QTL-seq: rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J 74:174–183
Tang H, Luo D, Zhou D, Zhang Q, Tian D, Zheng X, Chen L, Liu Y-G (2014) The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol Plant 7:1479–1500
Ui H, Sameri M, Pourkheirandish M, Chang MC, Shimada H, Stein N, Komatsuda T, Handa H (2015) High-resolution genetic mapping and physical map construction for the fertility restorer Rfm1 locus in barley. Theor Appl Genet 128:283–290
Untergrasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3-new capabilities and interfaces. Nucleic Acids Res 40:e115
Uyttewaal M, Amal N, Quadrado M, Martin-Canadell A, Vrielynck N, Hiard S, Gherbi H, Bendahmane A, Budar F, Mireau H (2008) Characterization of Raphanus sativus pentatricopeptide repeat proteins encoded by a fertility restorer locus for Ogura cytoplasmic male sterility. Plant Cell 20:3331–3345
Wang D, Bosland PW (2006) The genes of Capsicum. HortScience 41:1169–1187
Wang LH, Zhang BX, Lefebvre V, Huang SW, Daubeze AM, Palloix A (2004) QTL analysis of fertility restoration in cytoplasmic male sterile pepper. Theor Appl Genet 109:1058–1063
Wang Z, Zou Y, Li X, Zhang Q, Chen L, Wu H, Su D, Chen Y, Guo J, Luo D, Long Y, Zhong Y, Liu YG (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18:676–687
Wang SB, Liu KW, Diao WP, Zhi L, Ge W, Liu JB, Pan BG, Wan HJ, Chen JF (2012) Evaluation of appropriate reference genes for gene expression studies in pepper by quantitative real-time PCR. Mol Breed 30:1393–1400
Wang L, Zhang B, Zhang Z, Cao Y, Yu H, Feng X (2020) Research progress in genetics and breeding of Capsicum. Acta Horticulturae Sinica 47:1727–1740 (In Chinese)
Wang P, Lu Q, Ai Y, Wang Y, Li T, Wu L, Liu J, Cheng Q, Sun L, Shen H (2019) Candidate gene selection for cytoplasmic male sterility in pepper (Capsicum annuum L.) through whole mitochondrial genome sequencing. Int J Mol Sci 20: 578
Wei B, Bosland PW, Zhang Z, Wang Y, Zhang G, Wang L, Yu J (2020) A predicted NEDD8 conjugating enzyme gene identified as a Capsicum candidate Rf gene using bulk segregant RNA sequencing. Hortic Res 7:210
Wu GP, Yuan W, Liu JB, Diao WP, Wang SB, Pan BG, Ge W, Hou XL (2012) SRAP and SCAR marker linked to restoring gene for cytoplasmic male sterility in sweet pepper. Mol Plant Breed 10(4):446–451 (In Chinese)
Wu L, Wang P, Wang Y, Cheng Q, Lu Q, Liu J, Li T, Ai Y, Yang W, Sun L, Shen H (2019) Genome-wide correlation of 36 agronomic traits in the 287 pepper (Capsicum) accessions obtained from the SLAF-seq-Based GWAS. Int J Mol Sci 20:5675
Yin J, Guo W, Yang L, Liu L, Zhang T (2006) Physical mapping of the Rf1 fertility-restoring gene to a 100 kb region in cotton. Theor Appl Genet 112:1318–1325
You FM, Huo N, Gu YQ, Luo M-C, Ma Y, Hane D, Lazo GR, Dvorak J, Anderson OD (2008) BatchPrimer3: a high throughput web application for PCR and sequencing primer design. BMC Bioinformatics 9:253
Zhang BX, Huang SW, Yang GM, Guo JZ (2000) Two RAPD markers linked to a major fertility restorer gene in pepper. Euphytica 113:155–161
Zhang J, Stewart JM, Wang T (2005) Linkage analysis between gametophytic restorer Rf2 gene and genetic markers in cotton. Crop Sci 45:147–156
Zhang H, Zhang L, Si H, Ge Y, Liang G, Gu M, Tang S (2016) Rf5 is able to partially restore fertility to Honglian-type cytoplasmic male sterile japonica rice (Oryza sativa) lines. Mol Breed 36:102
Zhang H, Wu J, Dai Z, Qin M, Hao L, Ren Y, Li Q, Zhang L (2017a) Allelism analysis of Br Rfp locus in different restorer lines and map-based cloning of a fertility restorer gene, Br Rfp1, for pol CMS in Chinese cabbage (Brassica rapa L.). Theor Appl Genet 130:539–547
Zhang H, Che J, Ge Y, Pei Y, Zhang L, Liu Q, Gu M, Tang S (2017b) Ability of Rf5and Rf6 to restore fertility of chinsurah boro II-type cytoplasmic male sterile Oryza sativa (ssp. japonica) lines. Rice 10:2
Zhang Z, Zhu Y, Cao Y, Yu H, Bai R, Zhao H, Zhang B, Wang L (2020) Fine mapping of the male fertility restoration gene CaRf032 in Capsicum annuum L. Theor Appl Genet 133:1177–1187
Zhang Z, An D, Cao Y, Yu H, Zhu Y, Mei Y, Zhang B, Wang L (2021) Development and application of KASP markers associated with Restorer-of-fertility gene in Capsicum annuum L. Physiol Mol Biol Plants 27:2757–2765
Zou X, Ma Y, Dai X, Li X, Yang S (2020) Spread and industry development of pepper in China. Acta Hortic Sinica 47:1715–1726 (in Chinese)
Acknowledgements
This work was performed at the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, China. We thank Liwen Bianji (Edanz) (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by Beijing Natural Science Foundation (6212029), the Central Public-interest Scientific Institution Basal Research Fund (IVF-BRF2020005), the China Agricultural Research System (CARS-25), and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Science (CAAS-ASTIP-IVFCAAS).
Author information
Authors and Affiliations
Contributions
ZHZ designed the study and developed the KASP markers. DLA and LQS carried out the phenotypic investigation, genetic mapping, and qRT-PCR. YCC and HLY participated in the genetic mapping and the phenotypic investigation. BXZ and LHW contributed to the design of the study and revision of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sanwen Huang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Below is the link to the supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., An, D., Yu, H. et al. Fine mapping of Rf2, a minor Restorer-of-fertility (Rf) gene for cytoplasmic male sterility in chili pepper G164 (Capsicum annuum L.). Theor Appl Genet 135, 2699–2709 (2022). https://doi.org/10.1007/s00122-022-04143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-022-04143-7