Abstract
Key message
The quantitative barley leaf rust resistance gene, Rph26, was fine mapped within a H. bulbosum introgression on barley chromosome 1HL. This provides the tools for pyramiding with other resistance genes.
Abstract
A novel quantitative resistance gene, Rph26, effective against barley leaf rust (Puccinia hordei) was introgressed from Hordeum bulbosum into the barley (Hordeum vulgare) cultivar ‘Emir’. The effect of Rph26 was to reduce the observed symptoms of leaf rust infection (uredinium number and infection type). In addition, this resistance also increased the fungal latency period and reduced the fungal biomass within infected leaves. The resulting introgression line 200A12, containing Rph26, was backcrossed to its barley parental cultivar ‘Emir’ to create an F2 population focused on detecting interspecific recombination within the introgressed segment. A total of 1368 individuals from this F2 population were genotyped with flanking markers at either end of the 1HL introgression, resulting in the identification of 19 genotypes, which had undergone interspecific recombination within the original introgression. F3 seeds that were homozygous for the introgressions of reduced size were selected from each F2 recombinant and were used for subsequent genotyping and phenotyping. Rph26 was genetically mapped to the proximal end of the introgressed segment located at the distal end of chromosome 1HL. Molecular markers closely linked to Rph26 were identified and will enable this disease resistance gene to be combined with other sources of quantitative resistance to maximize the effectiveness and durability of leaf rust resistance in barley breeding. Heterozygous genotypes containing a single copy of Rph26 had an intermediate phenotype when compared with the homozygous resistant and susceptible genotypes, indicating an incompletely dominant inheritance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated barley (Hordeum vulgare L.) was one of the first domesticated cereals and served as a founder crop of modern agriculture (Park et al. 2015). Today, barley ranks fourth in global production among all cereals after maize (Zea mays L.), wheat (Triticum spp. L.) and rice (Oryza sativa L.) and twelfth for all crops (Food and Agriculture Organization of the United Nations 2014). In New Zealand, barley is one of the most important cereal crops. In 2016, the cultivation area of barley was the largest among all cereal crops and the production was the second highest with 364,200 metric tons (mt), following wheat (459,300 mt) (Statistics New Zealand 2016).
However, like most cultivated crops, there are various abiotic and biotic stresses, such as nutrient, water, pest and disease stress, that limit the commercial yield of barley. Barley leaf rust, caused by Puccinia hordei, is one of the most important diseases of barley and is widely distributed in major growing areas all over the world (Clifford 1985; Park et al. 2015). In recent decades, the incidence of leaf rust has increased in barley-growing regions mainly because of more intensive cultivation (Park et al. 2015).
To control the damage caused by P. hordei, the application of plant resistance through breeding programmes is considered the most effective method (Park et al. 2015). Host resistance to leaf rust in cultivated barley has been characterized into two types: (1) Hypersensitive or major resistance, and (2) quantitative or partial resistance (Parlevliet 2002). Hypersensitive resistance can inhibit rust development through localized cell death, and results in highly resistant infection types (IT) to barley leaf rust. In this resistance process, R genes in host plants are involved in recognition events for a corresponding avirulence (Avr) effector protein from the rust pathotype (Bettgenhaeuser et al. 2014). Thus, the hypersensitive resistance conditioned by R genes is only effective to the rust isolates carrying the compatible Avr effector proteins, making this type of resistance race-specific. In addition, the monogenic nature of hypersensitive resistance is often non-durable through the appearance of new rust pathotypes lacking the target avirulence factor (Paulitz and Steffenson 2011). Another approach to develop cultivars with more durable resistance to barley leaf rust is the use of quantitative, partial, adult plant or ‘slow rusting’ resistance. Unlike the complete elimination of disease symptoms by hypersensitive resistance genes, partial resistance is characterized by a susceptible infection type but coupled with a reduced rate of epidemic build-up or reduced disease severity by the pathogen (Parlevliet 2002). Partial resistance is normally conditioned by a number of quantitative trait loci (QTL) with minor effect (sometimes prefixed by Rphq), of which 20 QTLs have been identified from barley and its wild relatives (Qi et al. 1998, 2000; Marcel et al. 2007, 2008). The polygenic nature makes partial resistance more durable than hypersensitive resistance, but also more difficult to utilize in breeding programmes (Johnston et al. 2013).
The combination of reduced genetic diversity from intensive breeding for improved yield and quality, and the evolution of new leaf rust pathotypes, has resulted in a limit to the resistance resources available in elite barley cultivars. Hordeum bulbosum L., the only member of the secondary gene pool of cultivated barley, has long been investigated as a source of novel disease resistances which are not available in H. vulgare (Fetch et al. 2009; Jie and Snape 1989; Pickering et al. 1995, 2006; Ruge et al. 2003; Scholz et al. 2009; Shtaya et al. 2007; Toubia-Rahme et al. 2003; Walther et al. 2000; Xu and Kasha 1992). Because of incompatibility barriers between H. bulbosum (2n = 2x or 4x) and H. vulgare (2n = 2x), H. bulbosum has historically been used to produce barley double haploids through chromosome elimination (Kasha and Kao 1970). Introgression lines (ILs) with genomic segments from H. bulbosum introduced into H. vulgare genetic backgrounds were first produced from interspecific hybrids 30 years ago (Szigat and Pohler 1982) and were confirmed by using in situ hybridization and Southern blotting (Pickering et al. 1995; Xu and Kasha 1992). These ILs provide important genetic resources to explore novel traits from H. bulbosum and enable the utilization of diverse genes outside the primary gene pool (cultivated barley and the wild barley H. vulgare subsp. spontaneum) to improve cultivated barley (Johnston et al. 2013).
In this study, the IL called 200A12 (also coded as E-1HL in Pickering et al. 2004) was investigated, which features the very end of chromosome 1HL transferred from H. bulbosum into the cultivated barley genome. This introgression from the H. bulbosum genome was first identified in field trials as possessing a partial or ‘slow rusting’ resistance to P. hordei that is designated here as Rph26. In a previous study, the presence of this resistance gene in 200A12 resulted in a 16% longer latency period of P. hordei relative to its barley genetic background cultivar ‘Emir’, and 43% and 15% reductions in infection frequency compared with the cultivars ‘Emir’ and ‘Vada’, respectively (Pickering et al. 2004). A prolonged latency period was considered to be the most crucial parameter used to identify partial resistance (Neervoort and Parlevliet 1978). In addition, 200A12 had a higher percentage of early abortion and smaller established P. hordei colonies than ‘Vada’ and ‘Emir’, indicating a higher degree of partial resistance. Early aborted colonies were normally associated with the defence mechanism by blocking rust haustorium formation at penetration sites on plant cell walls, but not hypersensitivity (Pickering et al. 2004).
The overall goal of this project was to develop an F2 population from a backcross between 200A12 and its own barley parental cultivar ‘Emir’. This population was used to first identify interspecific recombinant genotypes within the original introgression for genetic mapping of Rph26 and second to determine the genetic inheritance of the resistance gene.
Materials and methods
Plant and pathogen material
The line 200A12 was developed (Fig. 1) from a cross between the tetraploid H. bulbosum genotype A17 (from the Botanical Garden of Montevideo, Uruguay, Scholz et al. 2009) (BBBB) and the diploid barley cultivar ‘Emir’ (VV) to produce a partially fertile triploid hybrid (VBB) designated 178M, where V and B represent the genome constitution of H. vulgare and H. bulbosum, respectively. This triploid hybrid was then used as the pollen parent to backcross with the same barley parent ‘Emir’. From the resulting progeny, the diploid 200A12 was identified as possessing resistance to leaf rust and a homozygous introgression from H. bulbosum on the distal end of chromosome 1HL (Pickering et al. 2004). The cultivar ‘Emir’ is also thought to carry the adult plant resistance (APR) gene Rph20 (5HS) from molecular marker analysis (bPb-0837-PCR) performed by Hickey et al. (2012).
A New Zealand P. hordei isolate, pathotype (pt) 5457P+, was used for all phenotypic evaluations of ‘Emir’, 200A12 and recombinant genotypes against barley leaf rust. This isolate was evaluated in a separate pathotyping test against different genotypes (Park 2003) to be virulent upon the resistance genes Rph1, Rph2, Rph3, Rph4, Rph6, Rph9, Rph10, Rph12, Rph19 and RphP.
Development of interspecific markers
Based on the H. bulbosum-specific single nucleotide polymorphisms (SNPs) identified from 200A12 (Wendler et al. 2015), 12 cleaved amplified polymorphic sequences (CAPS), 28 high-resolution melting (HRM) and four size-polymorphic markers within the introgressed region were developed to discriminate between H. vulgare and H. bulbosum alleles (Kong 2015). By including the previously developed markers KS240con, H31_5700 (Johnston, unpublished) and H31_14212 (Johnston et al. 2009), a total of 47 interspecific markers located at the distal end of chromosome 1HL were used to map the resistance gene Rph26, in 200A12.
Marker names, marker types, primer sequences and variations from the basic polymerase chain reaction (PCR) conditions can be found in Supplementary Table 1. Size-polymorphic and CAPS markers were amplified in a 10-μL reaction volume which contained 1 X ReddyMix PCR buffer (Thermo Fisher), 0.2 mM dNTPs (Fermentas), 2.5 mM MgCl2 (Thermo Fisher), 0.3 μM each primer (Bioneer), 0.2 U ThermoPrime Taq DNA polymerase (Thermo Fisher) and 20 ng DNA template. The basic PCR conditions were 94 °C for 2 min, 40 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, with a final extension of 72 °C for 5 min. HRM markers were also amplified using a 10-μL reaction volume with 2 μL 5X HOT FIREPol® EvaGreen® HRM mix-no ROX (Solis BioDyne), 0.25 μM each primer and approximately 20 ng DNA template. To prevent evaporation losses, 20 μL of PCR-grade mineral oil (SIGMA) was added to each reaction. Basic touchdown PCR conditions were 95 °C for 15 min, 10 cycles of 95 °C for 30 s, 65 °C for 30 s reducing 1 °C per cycle and 72 °C for 30 s, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, with a final cycle of 95 °C for 30 s and 28 °C for 30 s to maximize the formation of heteroduplex PCR products if the DNA sample was heterozygous. All PCRs were conducted on either a C1000 Thermal Cycler (BioRad) or a Mastercycler Pro S (Eppendorf).
Population development for gene mapping and determining genetic inheritance
To map the resistance gene, Rph26, within the introgressed segment and determine its genetic inheritance, the IL 200A12 was backcrossed to its own barley parent ‘Emir’ to produce F1 seeds. The F1 plants were screened with the marker H31_14212 to confirm that they were heterozygous for the 1HL introgression from 200A12. F1 plants with the correct genotype were allowed to self-fertilize to produce seeds for the F2 mapping population (377B population).
As the barley genetic background cultivar ‘Emir’ was consistent throughout the development of this population, all subsequent mapping was focused solely on interspecific recombination events within the original H. bulbosum introgression on chromosome 1HL. Genomic DNA was extracted from a total of 1368 F2 plants from freeze-dried leaf material using the ‘Wheat and Barley DNA Extraction in 96-well plates’ method (Chao and Somers 2012). The F2 population was then genotyped with the markers CM_1186 and H31_14212, which were located at the proximal and distal ends of the introgression, respectively. Interspecific recombinant genotypes were identified when F2 individuals had different genotypes for the markers CM_1186 and H31_14212. F3 seeds from each F2 recombinant genotype were obtained via self-fertilization. To allow identification of homozygous (true breeding) F3 recombinant genotypes prior to seed germination, endosperm powder was scraped from between 20 and 40 F3 seeds per F2 recombinant with a scalpel blade and genomic DNA was extracted using a quick seed extraction protocol (von Post et al. 2003). The DNA from each F3 seed was screened with the same flanking markers to identify the seeds which were homozygous for each interspecific recombination event. The identified F3 homozygous recombinant seeds from each F2 recombinant genotype were retained for pathological and molecular marker screening. In this way, pathology and genotyping were simplified by using F3 homozygous recombinant lines instead of F2 heterozygous recombinant genotypes and the time period used to obtain homozygous recombinant lines for genetic mapping was shortened.
To determine the genetic inheritance of Rph26, a separate set of 60 F2 plants derived from the 377B population (‘Emir’ × 200A12) was marker genotyped with CM_1186 and H31_14212, using the same DNA extraction method by Chao and Somers (2012). These markers identified which plants had two copies (BB), one copy (VB) or none (VV) of the H. bulbosum introgression on chromosome 1HL.
Inoculation and evaluation of infection
Two separate experiments were conducted under greenhouse conditions to evaluate the phenotypic response of these genotypes to inoculation with P. hordei. The first experiment was used to determine the genetic location of Rph26 within the H. bulbosum introgression on chromosome 1HL. Homozygous F3 seeds from recombinant lines identified in the marker testing of seed DNA extracts plus parental lines were pre-germinated on damp filter paper in petri dishes at 4 °C for 1 week and then transferred to room temperature. Once germinated, three healthy-looking F3 seedlings per F2 recombinant were transferred into 15-cm-diameter pots containing potting mix, with one seedling per pot. The layout of the experiment was a 2-latinized row–column design generated using CycDesign 5.1 (VSN International Ltd 2013) and consisted of three replicates of 28 plants (4 × 7) featuring one plant (pot) each of ‘Emir’, 200A12 and 18 of the homozygous F3 recombinant lines (but only two replicates of 377B_708_F3). Owing to an unusual genotyping result in F2 and F3 marker testing for 377B_794, eight plants per replicate were included. When most plants in the experiment reached tillering stage (growth stage (GS) 21, Zadoks et al. 1974), leaf rust inoculations were carried out for three successive days, with a complete replicate inoculated each day. Prior to inoculation, the third leaf of each plant, counted from the bottom, was folded down over a plastic board and secured using adhesive tape. Water agar slides (2%) were evenly distributed on the plastic board, with one slide placed between every four secured leaves, to estimate urediniospore densities and germination rate. Leaf rust inoculations were performed by atomising a urediniospore–mineral oil suspension (1 mg of urediniospores in 2 mL of mineral oil (Pegasol, Mobil Oil) per eight plants) over the secured plant leaves. After incubation at 20 °C and 100% relative humidity in the dark for 24 h, the plants were maintained at 22 °C, 14/10 h light/dark photoperiod in a greenhouse. The urediniospore densities (urediniospores/cm2) and germination rate (%) were estimated by counting the number of urediniospores (germinated and non-germinated) within a 1 cm2 area of water agar slides under a compound microscope. The urediniospores with germ tubes which were equal to or greater than the width of the urediniospores were determined as germinated.
For the phenotypic evaluation of the F3 recombinants, leaf rust infection was assessed by visually counting the number of uredinium, by observing infection type (IT) and by using a quantitative method of assessing fungal growth in the infected leaf tissues (Ayliffe et al. 2014). At 4–5 days post-inoculation (DPI) before mature uredinia appeared, a 5-cm long area of each inoculated leaf containing pale flecks, which indicated the early signs of uredinia formation or resistance response, was selected and marked. Once visible, the mature uredinia within the marked areas were counted daily until 14 DPI, at which point no further uredinia developed. The IT was assessed at 10 DPI using a 0–4 scale as described by Park (2003). Inoculated plants with ITs of three and above were considered to be compatible (i.e. virulent pathogen/susceptible host). Once the uredinium count was completed, the marked 5-cm-long leaf segments were collected, and all the replicated leaf tissues from a same test line were pooled and used for evaluation of fungal biomass using the protocol described by Ayliffe et al. (2014). Briefly, samples were extracted in 1M KOH containing 0.1% (vol/vol) Silwet L-77 by autoclaving at 121 °C and 15 psi for 20 min. The leaf tissue was washed and neutralized with 50 mM Tris-HCl (pH 7.0), and then immersed in Tris buffer at a rate of 8 ml of 50 mM Tris-HCl (pH 7.0) per 1 g fresh leaf tissue for sonication. Three replicate aliquots of 200 μL per sample were each mixed with 10 μL of a 1 mg/mL solution of lectin wheat germ agglutinin conjugated to fluorescein isothiocyanate (WGA-FITC, Sigma Aldrich). After staining for 10 min, the samples were centrifuged at 600×g for 3 min. The pelleted tissue was washed in 50 mM Tris-HCl (pH 7.0) and centrifuged twice more before being resuspended in 100 μL of 50 mM Tris-HCl (pH 7.0). Fluorescence units (FU) were measured using a SpectraMax M2 (Molecular Devices) under 485 nm absorption and 535 nm emission wavelengths at 1.0 s measurement time in black 96-well microtiter plates.
The second experiment was established to test the genetic inheritance of Rph26 and to re-examine the phenotypes of key lines 377B_348_F3 and 377B_708_F3 from the first experiment using the visual assessments only. Plants were raised in 20-cm-diameter pots containing potting mix, with four plants of the same genotype per pot, resulting in three replicates of nine pots featuring one pot of the BB genotype, two or three pots of the VB genotype, one or two pots of the VV genotype, one pot each of 377B_348_F3 and 377B_708_F3 and two parental controls (‘Emir’ and 200A12). Pots were arranged randomly within each replicate. Inoculations were carried out when most plants had their flag leaf visible on the main stem (GS37, Zadoks et al. 1974) by using the same inoculation techniques described above (but with 2 mg of urediniospores in 3 mL of mineral oil per replicate). The same method for estimating urediniospore deposition and germination, and incubation conditions was used. The observed mean germinated spore deposition rates for each experimental replicate were 98, 107 and 129 per cm2.
Genotyping and linkage mapping
Genomic DNA was extracted from freeze-dried leaf material from one homozygous F3 recombinant line of the three used in the pathology experiment using the DNeasy Plant Mini Kit (Qiagen). These nineteen homozygous F3 recombinants (one from each F2 interspecific recombinant) collectively known as “200A12_F3_Popn”, and parents, were genotyped with 47 interspecific markers to determine the extent of the H. bulbosum introgression in each recombinant line. A genetic linkage map was developed using the R package ASMap (Taylor and Butler 2017; version 0.4) and run through RStudio (version 1.0.136; R version 3.3.3). The genotype data for all 1368 F2 plants were used in the genetic mapping. This was done by imputing the non-recombinant F2 genotypes [either homozygous H. vulgare (VV), homozygous H. bulbosum (BB) or heterozygous (VB)] for all markers using only the data obtained from the flanking markers. For example, plants that were genotyped as VB for CM_1186 and VB for H31_14212 were assumed to be VB for all of the intervening markers. The reconstituted F2 genotypes for the detected interspecific recombinants were included in the F2 mapping by combining the genotypes obtained from the F2 flanking marker screening with the genotyping results of the F3 homozygous recombinant lines for all the remaining markers (Fig. 2). This mapping scheme both reduced the workload and cost associated with genotyping the entire F2 population (of which the majority were non-recombinant) and enabled the genetic distance between markers to remain accurate for the complete mapping population. QTLs representing five pathological traits (mean uredinium count 6 DPI, mean uredinium count 14 DPI, mean time, latency period and fungal biomass) measured on the homozygous F3 recombinant lines (‘200A12_F3_Popn’) were mapped onto the F2-derived map using the R package “qtl’ (Broman et al. 2003), with population type ‘dh’ (doubled haploid) to represent the homozygous F3 lines and the function ‘scanone’ using Haley–Knott regression with the calc.genoprob parameters of step = 0.01 cM and error.prob = 0.001. Logarithm of the odds (LOD) significance thresholds were set at alpha = 0.01 with 10,000 permutations.
An example of how the reconstituted F2 genotypes were derived from the flanking marker data obtained during the F2 screening and the final F3 homozygous genotyping of all markers (BB, VV, VB and ?? denote a homozygous H. bulbosum, a homozygous barley genotype, a heterozygous genotype and the absence of genotyping, respectively)
Statistical analysis
Analyses of uredinium counts at selected assessments
The uredinium count data were separately analysed at two time points (6 and 14 DPI for the first experiment and 7 and 13 DPI for the second experiment) using a hierarchical generalized linear model approach (HGLM, Lee et al. 2006), with a Poisson distribution for the fixed effects (lines) and a gamma distribution for random effects (replicates), and logarithmic links for both. The dispersion was estimated for both assessment dates. The importance of the random effects was assessed with a χ2 test of the change in deviance on dropping the term, as implemented in GenStat’s HGRTEST procedure (VSN International Ltd 2015). An overall test for differences between the lines was carried out similarly, using GenStat’s HGFTEST procedure. In the results, means and associated 95% confidence limits are presented. These were obtained on the link (logarithmic) scale and back-transformed for presentation.
Modelling the distribution of time until uredinium formation
The number of uredinia at each assessment included both newly formed uredinia and the existing uredinia from the previous time point. Thus, the data are in the form of ‘accumulated counts’ (O’Neill et al. 2004; Hunter et al. 1984) and are suitable for analysis by methods that estimate the distribution of times until uredinium formation (DPI for each uredinium to appear), as described in O’Neill et al. (2004). The analysis was carried out in two parts: Firstly, the total numbers of uredinia per line at each assessment were analysed. Secondly, the total numbers of uredinia per line in each replicate and during each assessment were analysed. When combined with the results from the first analysis, this second analysis allowed an assessment of whether there was substantial variation between replicates within lines. The data were modelled using the logistic distribution of the log DPI, as implemented in GenStat’s CUMDISTRIBUTION procedure (Butler et al. in VSN International Ltd 2015). It was assumed that no further uredinia would be formed after the final assessment (14 DPI; ‘ALLRESPOND = yes’ selected for the fitting process). The cumulative distribution function (c.d.f) is F(z):
where z = log(DPI–Lag) and b, m, Lag are estimated parameters.
The primary results of these analyses were estimates of the lag before uredinia began to appear (Lag), the mean time to uredinium formation (Mean), the DPI to 50% uredinium formation (median or latency period, LP) and the standard deviation of the distribution of DPI to uredinium formation (SD). Mean, LP and SD were calculated from Lag, b and m, as follows:
Note that if tan(π/b) < (π/b), the SD could not be calculated (since it would require the calculation of the square root of a negative number). Some ‘analysis of parallelism’ (Ross 1984) was carried out to assess whether any of the parameters b, m or Lag varied substantially between lines. The analyses were all carried out with GenStat (Payne et al. 2015).
Results
Genetic mapping of Rph26
Development of ‘200A12_F3_Popn’
After screening with the flanking markers CM_1186 and H31_14212, 19 F2 interspecific recombinant genotypes were identified from a total F2 mapping population of 1368 plants. One of these genotypes, 377B_794, was detected as displaying a homozygous H. bulbosum genotype for the proximal marker and a homozygous H. vulgare genotype for the distal marker as an F2 plant, indicating that it was the rare product of two recombinant gametes. Marker testing of the F3 seed DNA identified between two and twelve F3 homozygous recombinant seeds from each of the F2 recombinant genotypes (8–45% of tested progeny, Mean = 24.5%).
Phenotypic evaluation
Visual assessment
The mean number of uredinia varied among lines at both assessments; ‘Emir’ had the highest number of uredinia, at 55 and 77 per plant (6 DPI and 14 DPI, respectively), whilst 200A12 had one of the lowest, at 2 and 15 (6 DPI and 14 DPI, respectively). Uredinium numbers of the 377B_794_F3 plants tended to be at the bottom half of the range of numbers, whilst numbers for the other recombinants covered the whole range (0.4–50.8 at 6 DPI and 9.0–65.1 at 14 DPI, Table 1). The final number of uredinia (14 DPI) was very highly correlated with the numbers at 6 DPI (r = 0.96 for individual pot data and r = 0.97 for the means).
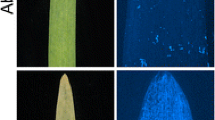
Using the ITs alone (Table 1), it was possible to classify all the ‘200A12_F3_Popn’ lines into two qualitative groups: one group having ITs similar to the resistant parent 200A12, with small- to medium-sized uredinia surrounded by chlorotic and/or necrotic tissue (Fig. 3b), and another group having infection types similar to the susceptible parent ‘Emir’, with medium to large uredinia with or without surrounding chlorosis (Fig. 3a). The exception was line 377B_708_F3 which was considered to be an intermediate type, with IT similar to 200A12 but with higher uredinium counts at both time points (Table 1).
Statistical modelling
The model which estimated different values for all three of the main parameters (Lag, b, m) fitted substantially better than models with either a single Lag, b or m parameter (\(\chi_{30}^{2} = 54.2\), p = 0.004; \(\chi_{30}^{2} = 51.7\), p = 0.008; \(\chi_{30}^{2} = 117.7\), p < 0.001, respectively). The model-derived estimates for mean time and latency period differed strongly between the parental lines ‘Emir’ (shorter mean time and latency period) and 200A12 (longer mean time and latency period) (Fig. 4), with the results from the entire ‘200A12_F3_Popn’ forming a continuous distribution (Table 1).
Statistical modelling of uredinium (pustule) development in the parental lines ‘Emir’ (red circles) and 200A12 (blue stars) combined from all three replicate plants. The left-hand figure shows a distinct difference in the cumulative number of uredinium between the susceptible parent ‘Emir’ (red circles) and the resistant parent 200A12 (blue stars). The right-hand figure shows the percentage uredinium development, with uredinia developing more rapidly on ‘Emir’ (red circles, shorter latency period) than on 200A12 (blue stars, longer latency period). The vertical dotted line shows the end of the lag phase. The solid black lines indicate the appearance of 50% of eventual uredinium number, thus indicating the latency period (median time to uredinium formation) (color figure online)
Fungal biomass assay
As the inoculated leaf tissue from each line was pooled across the biological replicates, only the mean FU and standard deviation for the three technical replicates are shown (Table 1). The resistant parent 200A12 and susceptible parent ‘Emir’ gave clearly different mean FU at 533 and 816, respectively, with the mean FU for all of the recombinant lines falling between 343 and 970 (Table 1). The assignment of the recombinant lines into resistant or susceptible groups based only on IT (Table 1) was also reflected in the mean fungal biomass data (resistant < 580 FU and susceptible > 680 FU), with the exception of line 377B_708_F3, which gave a resistant IT but a high mean fungal biomass of 744.
Genetic linkage map
From the 47 interspecific markers used for genotyping, a total of 29 markers consisting of 14 CAPS, 11 HRM and four size-polymorphic markers gave clear and consistent genotype results (Table 2). By combining the flanking marker genotypes of the F2 interspecific recombinants (CM_1186 and H31_14212) and the genotyping results of ‘200A12_F3_Popn’ for the remaining markers (from KS240con to CM_1199 in Table 2), the genotypes of F2 interspecific recombinants for the remaining markers were reconstituted.
The genetic linkage map of the H. bulbosum-introgressed region of 200A12 was constructed by using the reconstituted genotypes from the nineteen F2 interspecific recombinants, combined with the remaining 1349 non-recombinant F2 genotypes (homozygous H. vulgare, homozygous H. bulbosum or heterozygous for all markers) using the R package ASMap (Taylor and Butler 2017). The barley leaf rust resistance, Rph26, was mapped as five separate QTLs (uredinium number 6dpi and 14dpi, mean time, latency period and fungal biomass) within the H. bulbosum introgression on chromosome 1HL using the results of the pathological evaluation (first experiment). QTL peaks for all traits were significant at the alpha = 0.01 level (LOD thresholds were 2.7, 3.0, 2.5, 2.0 and 2.8, respectively) and the QTL peak for all five pathological traits was located at 0.10–0.11 cM, proximal of a cluster of four markers CM_1143, CM_1144, CM_1191 and CM_1194 (Fig. 5).
Genetic linkage map of the Hordeum bulbosum introgression within 200A12 on barley chromosome 1HL. DNA markers CM_1186 and H31_14212 (bold) were the flanking markers used to detect interspecific recombinants. The graphs on the right-hand side show LOD scores for five pathological traits; mean uredinium number at six (‘ured_6DPI’, red) and fourteen (‘ured_14DPI’, green) days post-inoculation (DPI), mean time (‘mean_time’, dark blue), latency period (‘latency_period’, brown) and fungal biomass (‘fung_biomass’, black). The thick, vertical QTL bars indicate the area covered by a drop of one LOD from the QTL peak, and the thin vertical lines, a drop of two LOD. The crosshatched region indicates the likely position of Rph26. Numbers to the left of the linkage map indicate genetic distance (cM) from the most proximal marker (CM_1186). Linkage map and QTL graphs were constructed using MapChart v2.2 (Voorrips 2002) (color figure online)
Analysis of the allelic effects for all five quantitative traits, at the near-peak marker CM_1194, revealed a clear separation between ‘Emir’ and 200A12 genotypes (data not shown). However, the data for line 377B_708_F3 gave inconsistencies between the resistant IT scores and the more susceptible values for uredinium count at 14 DPI and fungal biomass.
Genetic inheritance of Rph26
In order to determine the genetic inheritance of Rph26, a set of 60 F2 plants from the 377B population were genotyped using the same flanking markers CM_1186 and H31_14212. These markers identified 12 plants homozygous for the H. bulbosum introgression (377B_BB), 32 heterozygous plants (377B_VB) and 16 plants without the introgression (377B_VV). Inoculations at flag leaf emergence with P. hordei revealed an intermediate uredinium number for the heterozygous (377B_VB) genotypes when compared with the homozygous presence (377B_BB) and absence (377B_VV) genotypes (Table 3). The additional lines 377B_348_F3 and 377B_708_F3 gave responses similar to the resistant parent in the first two replicates; however, 377B_708_F3 gave a susceptible response in the third replicate (Fig. 6), resulting in a higher overall mean (Table 3). The third experimental replicate gave higher uredinium counts for both of the susceptible genotypes 377B_VV and ‘Emir’ (Fig. 6) which was reflected in the higher mean germinated spore deposition rate for the third replicate.
Discussion
Access to new sources of genetic disease resistance is key to the future of barley breeding. H. bulbosum, as the sole member of the secondary genepool of Hordeum, is an important donor of disease resistance (and potentially other traits) for cultivated barley. However, moving traits from H. bulbosum into barley is not trivial and is exacerbated by suppressed interspecific recombination and the linkage drag of undomesticated alleles. In this study, the quantitative leaf rust resistance gene, Rph26, was genetically mapped within the H. bulbosum introgression on 1HL in the line 200A12 (also coded as E-1HL, Pickering et al. 2004). This not only helps to identify markers closely linked to Rph26, but also reduces the size of the introgression around the trait of interest and hence reduces the potential for linkage drag. A major impediment to working with a wild species such as H. bulbosum is usually the lack of marker resources. This study benefitted considerably from the availability of a large number of SNPs identified in genotype by sequencing (GBS) experiments between a set of introgression lines and their barley parents (Wendler et al. 2015). This sequence resource enabled markers to be quickly developed, with good coverage over the entire introgressed region on 1HL in 200A12 (Kong 2015).
There are three observations from our experiments which help to reveal something of the nature of Rph26 resistance. The first is that in the inheritance experiment, Rph26 was found to display incomplete dominance, with the heterozygous genotypes (VB) possessing an intermediate level of resistance to P. hordei when compared with the parental (homozygous) genotypes (the presence or absence of the H. bulboum introgression on chromosome 1HL). This effect is generally caused by the different dosage of the resistance gene/allele; thus, there would be a higher amount of the resistance gene product (protein) in the homozygous resistant genotype possessing two copies of the gene/allele when compared with the heterozygous genotype carrying only one copy (Griffiths et al. 2000). A second observation is that the difference in response of ‘Emir’ and 200A12 to infection with P. hordei was very clear under low inoculum pressure (Fig. 3); however, there was evidence from our experiments that the effect of Rph26 was reduced under higher inoculum pressure. This suggests that Rph26 resistance can become saturated at higher inoculum pressure. The third observation is that Rph26 shows an increase in resistance over the course of plant development reaching full effect at flag leaf stage (data not shown). This is perhaps due to the expression of Rph26 also increasing over plant development as with Lr34 in wheat (Krattinger et al. 2009). These three observations collectively suggest that the level of Rph26 expression is critical for effective resistance as gene/allele dosage (incomplete dominance effect) and plant developmental stage have large impacts on plant resistance. Although currently untested, it seems likely that the amount of inoculum required to saturate Rph26 resistance would also change in response to allele/gene dosage and developmental stage.
For the genetic mapping of Rph26, F3 lines with homozygous introgressions of reduced size were used to simplify the pathological characterization once F2 interspecific recombinants were identified within the H. bulbosum introgression using flanking markers. These F3 recombinant lines were characterized using a combination of visual assessments (uredinium count and ITs), statistical modelling (mean time and LP) and a fungal biomass assay. A qualitative assignment resistant or susceptible to all the recombinants was possible using only IT, which resulted in Rph26 co-segregating with the markers CM_1143, CM_1144, CM_1191 and CM_1194.
The remaining pathological data (uredinium count at 6 DPI, uredinium count at 14 DPI, mean time, latency period and fungal biomass) gave continuous distributions, and thus, these five traits were mapped as separate QTLs. The peaks of all five QTLs were located at the proximal end of the original introgression between 0.10 and 0.11 cM and just proximal to the same cluster of four markers CM_1143, CM_1144, CM_1191 and CM_1194 at 0.15 cM. Twelve of the 19 recombinant lines in ‘200A12_F3_Popn’ possessed the ‘BB’ or 200A12 genotype for this cluster of markers, whilst the remaining seven recombinants possessed the ‘VV’ or ‘Emir’ genotype, and there were clear differences in the phenotypic distributions of these two genotypic classes. Nevertheless, there were some conflicting data for two lines, 377B_348_F3 and 377B_708_F3, which shared the same IT classification and identical marker genotype, but clearly differed for their uredinium counts and fungal biomass data (Table 1). These last two traits are intimately linked together, as the leaf material from the uredinium evaluation at 14 DPI was directly used in the fungal biomass assay. Further evaluation of these two lines against P. hordei in the second phenotypic experiment resulted in further ambiguity with 377B_708_F3 appearing resistant in response to P. hordei in two of the replicates but susceptible in the third (Fig. 6). All lines were marker tested both before and after the experiment to eliminate the chance of mislabelling. There are at least two possible explanations for the unusual results observed for 377B_708_F3. One hypothesis is that the Rph26 resistance is conditioned by more than one closely linked gene and that the recombination event in 377B_708_F3 has occurred within this locus making 377B_708_F3 less resistant or more sensitive to the applied levels of inoculum or other changes to experimental conditions. An alternative hypothesis is that during the development of 377B_708_F3, a mutation has occurred within Rph26 which has reduced the efficacy of Rph26 under certain experimental conditions. Further clarification with a larger number of 377B_708_F3 may help to determine the correct phenotype of 377B_708_F3 or to confirm whether this genotype has a particular sensitivity to changes in experimental conditions such as inoculum load. Clarity around the phenotype 377B_708_F3 would also help to refine the location of Rph26 to a much smaller genetic interval. For instance, if the line 377B_708_F3 was confirmed to give a susceptible response to leaf rust, this would imply that the introgression 377B_348_F3 extends further than 377B_708_F3 in the proximal direction (to include Rph26). Additional marker development in this region could help to determine a much shorter interval for the gene Rph26.
Additional replicates of 377B_794_F3 were included in the pathological characterization as a result of the F2 genotyping (homozygous H. bulbosum genotype for CM_1186 and homozygous H. vulgare genotype for H31_14212), which revealed that 377B_794 was the product of two separate recombinant gametes (egg and pollen). The extra replicates were to protect against the chance that the smaller introgressions on each chromosome were of different sizes and that Rph26 may be located in that intervening region and thus would be phenotypically segregating in the F3 progeny. However, the pathological data (uredinium count at 6 DPI, IT, LP and mean time) of all of the 377B_794_F3 plants were consistent with the results of the parental line 200A12. Three of the 24 plants did show slightly raised uredinium counts at 14 DPI, but all 24 plants gave the same IT as 200A12. The location of the Rph26 resistance was determined to be proximal of this region, so no further analysis was undertaken for these plants.
This study shows the value of using multiple pathological assessments during the phenotyping of subtle resistance gene effects to ensure that phenotypic evaluations are consistent. Visual IT scores based on uredinium size, chlorosis and necrosis are external expressions of the host plant when its defence mechanisms are triggered by the rust pathogen penetrating into the apoplast and plant cells. Uredinium count can also be used to denote the pathological phenotypes of test lines; however, these data alone are unable to truly reflect the resistance responses of the plants, since other important characteristics like uredinium size are not taken into account by uredinia counts. Perhaps for cereal rusts, where 97% of fungal biomass is located within the plant tissue (Ayliffe et al. 2013), a more quantitative assessment of internal fungal growth, such as fungal biomass, may reflect host response to P. hordei more accurately. Time permitting, the use of multiple pathological assessments will ultimately give a greater understanding of resistance gene effect.
The introgressed region containing the leaf rust resistance locus Rph26 has been narrowed to within a 0.37-cM region or 50% of the genetic length of original introgression region. The goal of this study and other fine mapping studies, where agronomic alleles of interest have usually been confined to genomic regions of about 2–5 cM (Kumar et al. 2016; Qin et al. 2011; Schmalenbach et al. 2011; Zhang et al. 2013), is to have markers sufficiently close to the preferred gene/allele of the target trait to maximize the selection accuracy during marker-assisted breeding. The smallest proximal introgression containing Rph26 has been identified in the lines 377B_794_F3, 377B_953_F3, 377B_1079_F3, 377B_1122_F3 and 377B_1296_F3. The markers flanking this smaller introgressed region are tightly linked to the resistance locus and would facilitate the use of the Rph26 in barley breeding through marker-assisted selection (MAS). Based on the 200A12 F2 mapping population, the original introgression between the flanking markers CM_1186 and H31_14212 covered a genetic distance of 0.75 cM. In contrast, the Morex genome released by the International Barley Genome Sequencing Consortium (2012) indicated that the genetic distance between makers CM_1186 and H31_14212 was 6.5 cM. Thus, an eightfold reduction in recombination frequency was identified between H. bulbosum and barley compared with the barley intraspecific recombination frequency in the same region of chromosome 1HL. This high degree of suppressed recombination in H. vulgare/H. bulbosum introgression lines has also been reported by other studies, with up to a 14-fold reduction in interspecific recombinant frequency having been observed (Johnston et al. 2013; Ruge-Wehling et al. 2006). Suppressed recombination can be advantageous during MAS, as it reduces the likelihood of further recombination events between markers and traits but can also make it difficult to separate traits of interest from deleterious traits (linkage drag) in the same introgression. In order to map or clone genes of interest in any H. bulbosum introgression, a much larger mapping population is required compared with the same task within barley germplasm.
The closely linked markers identified in this study can be used to select the smallest introgression required to transfer Rph26 into better adapted barley breeding lines. Removing extraneous portions of the introgression helps to reduce the probability of linkage drag caused by undomesticated genes or alleles that are genetically linked to the resistance locus. Although no unwanted linkage drag has been identified in 200A12, a significant yield penalty was previously identified and separated from Rph22 and Rym16Hb in an introgression of H. bulbosum on chromosome 2HL (Johnston et al. 2015). Although Rph26 may not confer a high level of resistance to leaf rust on its own, the markers closely linked to Rph26 will allow for its efficient combination with other resistance genes in the same genotype (pyramiding of multiple resistance alleles) within a short period of time (Park et al. 2015). Finding sources of potentially durable resistance to rust diseases in barley germplasm is an important objective for breeding programmes. However, the disease resistance conferred by a single, hypersensitive reaction-based, resistance gene (R-gene) has repeatedly proven non-durable as a result of the rapid evolution of new rust pathotypes which can overcome that gene (Park 2003). A better breeding strategy is the one that involves pyramiding multiple resistance genes into the same barley cultivar. The use of multiple resistance genes, especially involving different defence mechanisms, is likely to increase the effectiveness and durability of that resistance over time (Golegaonkar et al. 2009). With the help of closely linked markers, pyramiding of multiple resistance genes can be accelerated and validated through genotypic selection instead of the complicated and time-consuming selection based on subtle differences in the phenotypes for a combination of several resistance genes (Boopathi 2012). Based on the source of this resistance gene (H. bulbosum) and its chromosomal position on 1HL, we consider it to be a novel and detectable locus. Following consultation with Dr Frank Ordon, Julius Kuehn Institute, Germany and Dr Jerome Franckowiak, University of Minnesota, USA, it has been officially assigned the locus Rph26 with the resistant allele Rph26.ap, in line with international protocols for disease resistance locus designation in barley.
Germplasm resources
A collection of 154 ILs (including 200A12) featuring chromosomal segments of H. bulbosum within a barley genetic background has been deposited with NordGen for preservation in the Svalbard Global Seed Vault (Pickering et al. 2010). All other genotypes discussed in this paper are available for distribution under material transfer agreement (MTA) from the corresponding author.
Author contribution statement
Study was conceived by PAJ, XY, SCa, EEJ and SCh; plants materials were initially developed by RP; molecular markers were developed by HYK and PAJ; marker genotyping and fungal biomass were performed by XY and VM; linkage mapping by XY and PAJ; plant phenotyping was performed by XY; experimental designs, trial plans, statistical analyses and figures were done by RCB; and manuscript was written by PAJ and XY with contributions and editing from all authors.
References
Ayliffe M, Periyannan SK, Feechan A, Dry I, Schumann U, Wang M-B, Pryor A, Lagudah E (2013) A simple method for comparing fungal biomass in infected plant tissues. Mol Plant-Microbe Interact 26(6):658–667
Ayliffe M, Periyannan SK, Feechan A, Dry I, Schumann U, Lagudah E, Pryor A (2014) Simple quantification of in planta fungal biomass. In: Birch P, Jones JT, Bos JIB (eds) Plant-pathogen interactions: methods and protocols. Humana Press, Totowa, pp 159–172
Bettgenhaeuser J, Gilbert B, Ayliffe M, Moscou MJ (2014) Nonhost resistance to rust pathogens—a continuation of continua. Front Plant Sci 5:664
Boopathi NM (2012) Genetic mapping and marker assisted selection: basics, practice and benefits. Springer, New Delhi. https://doi.org/10.1007/978-81-322-0958-4
Broman KW, Wu H, Sen Ś, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Chao S, Somers D (2012) Wheat and barley DNA extraction in 96-well plates. MAS wheat. http://maswheat.ucdavis.edu/protocols/general_protocols/DNA_extraction_003.htm. Accessed June 2017
Clifford BC (1985) Barley Leaf Rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts volume II: diseases, distribution and control. Academic Press, New York
Fetch T Jr, Johnston P, Pickering R (2009) Chromosomal location and inheritance of stem rust resistance transferred from Hordeum bulbosum into cultivated barley (H. vulgare). Phytopathology 99:339–343
Food and Agriculture Organization of the United Nations (2014) FAOSTAT. http://www.fao.org/faostat/en/#data/QC. Accessed June 2018
Golegaonkar PG, Karaoglu H, Park RF (2009) Molecular mapping of leaf rust resistance gene Rph14 in Hordeum vulgare. Theor Appl Genet 119(7):1281–1288. https://doi.org/10.1007/s00122-009-1132-0
Griffiths AJF, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM (2000) An introduction to genetic analysis, 7th edn. W. H. Freeman & Co Ltd., New York. https://www.ncbi.nlm.nih.gov/books/NBK21920/
Hickey LT, Lawson W, Platz GJ, Dieters M, Franckowiak J (2012) Origin of leaf rust adult plant resistance gene Rph20 in barley. Genome 55:396–399
Hunter EA, Glasbey CA, Naylor REL (1984) The analysis of data from germination tests. J Agric Sci 102(1):207–213
International Barley Genome Sequencing Consortium (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491:711–717
Jie X, Snape JW (1989) The resistance of Hordeum bulbosum and its hybrids with H. vulgare to common fungal pathogens. Euphytica 41:273–276
Johnston PA, Timmerman-Vaughan GM, Farnden KJ, Pickering R (2009) Marker development and characterisation of Hordeum bulbosum introgression lines: a resource for barley improvement. Theor Appl Genet 118(8):1429–1437
Johnston PA, Niks RE, Meiyalaghan V, Blanchet E, Pickering R (2013) Rph22: mapping of a novel leaf rust resistance gene introgressed from the non-host Hordeum bulbosum L. into cultivated barley (Hordeum vulgare L.). Theor Appl Genet 126(6):1613–1625
Johnston PA, Meiyalaghan V, Forbes ME, Habekuß A, Butler RC, Pickering R (2015) Marker assisted separation of resistance genes Rph22 and Rym16Hb from an associated yield penalty in a barley: Hordeum bulbosum introgression line. Theor Appl Genet 128(6):1137–1149
Kasha K, Kao K (1970) High frequency haploid production in barley (Hordeum vulgare L.). Nature 225(5235):874–876
Kong HY (2015) Development of a SNP validation toolset for wheat. Unpublished Honours dissertation, Lincoln University, Lincoln, New Zealand. https://researcharchive.lincoln.ac.nz/handle/10182/6777. Accessed June 2018
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360–1363
Kumar P, He Y, Singh R, Davis RF, Guo H, Paterson AH, Peterson DG, Shen X, Nichols RL, Chee PW (2016) Fine mapping and identification of candidate genes for a QTL affecting Meloidogyne incognita reproduction in Upland cotton. BMC Genom 17(1):567
Lee Y, Nelder JA, Pawitan Y (2006) Generalized linear models with random effects: unified analysis via H-likelihood. Chapman & Hall/CRC Press, London, p 416
Marcel T, Varshney R, Barbieri M, Jafary H, de Kock M, Graner A, Niks R (2007) A high-density consensus map of barley to compare the distribution of QTLs for partial resistance to Puccinia hordei and of defence gene homologues. Theor Appl Genet 114:487–500
Marcel TC, Gorguet B, Ta MT, Kohutova Z, Vels A, Niks RE (2008) Isolate specificity of quantitative trait loci for partial resistance of barley to Puccinia hordei confirmed in mapping populations and near-isogenic lines. New Phytol 177:743–755
Neervoort W, Parlevliet J (1978) Partial resistance of barley to leaf rust, Puccinia hordei. V. Analysis of the components of partial resistance in eight barley cultivars. Euphytica 27(1):33–39
O’Neill ME, Thompson PC, Jacobs BC, Brain P, Butler RC, Turner H, Mitakda B (2004) Fitting and comparing seed germination models with a focus on the inverse Gaussian distribution. Aust N Z J Stat 46(3):349–366
Park R (2003) Pathogenic specialization and pathotype distribution of Puccinia hordei in Australia, 1992 to 2001. Plant Dis 87(11):1311–1316
Park RF, Golegaonkar PG, Derevnina L, Sandhu KS, Karaoglu H, Elmansour HM, Dracatos PM, Singh D (2015) Leaf rust of cultivated barley: pathology and control. Annu Rev Phytopathol 53:565–589
Parlevliet J (2002) Durability of resistance against fungal, bacterial and viral pathogens; present situation. Euphytica 124(2):147–156
Paulitz TC, Steffenson BJ (2011) Biotic stress in barley: disease problems and solutions. In: Ullrich SE (ed) Barley: production, improvement, and uses. Blackwell Publishing, Hoboken, pp 307–354. https://doi.org/10.1002/9780470958636.ch11
Payne R, Murray D, Harding S (2015) The Guide to the Genstat® Command Language (Release 18). VSN International, Hemel Hempsted, Hertfordshire, United Kingdom
Pickering RA, Hill AM, Michel M, Timmerman-Vaughan GM (1995) The transfer of a powdery mildew resistance gene from Hordeum bulbosum L. to barley (H. vulgare L.) chromosome 2 (2I). Theor Appl Genet 91(8):1288–1292. https://doi.org/10.1007/bf00220943
Pickering R, Niks RE, Johnston PA, Butler RC (2004) Importance of the secondary genepool in barley genetics and breeding. II. Disease resistance, agronomic performance and quality. Czech J Genet Plant Breed 40(3):79–85
Pickering R, Ruge-Wehling B, Johnston P, Schweizer G, Ackermann P, Wehling P (2006) The transfer of a gene conferring resistance to scald (Rhynchosporium secalis) from Hordeum bulbosum into H. vulgare chromosome 4HS. Plant Breed 125:576–579
Pickering R, Johnston P, Meiyalaghan V, Ebdon S, Morgan E (2010) Hordeum vulgare–H. bulbosum introgression lines. Barley Genet Newsl 40:1
Qi X, Niks R, Stam P, Lindhout P (1998) Identification of QTLs for partial resistance to leaf rust (Puccinia hordei) in barley. Theor Appl Genet 96:1205–1215
Qi X, Fufa F, Sijtsma D, Niks R, Lindhout P, Stam P (2000) The evidence for abundance of QTLs for partial resistance to Puccinia hordei on the barley genome. Mol Breed 6:1–9
Qin B, Cao A, Wang H, Chen T, You FM, Liu Y, Ji J, Liu D, Chen P, Wang XE (2011) Collinearity-based marker mining for the fine mapping of Pm6, a powdery mildew resistance gene in wheat. Theor Appl Genet 123(2):207–218. https://doi.org/10.1007/s00122-011-1577-9
Ross GJS (1984) Parallel model analysis: fitting non-linear models to several sets of data. In: COMPSTAT 1984—sixth symposium on computational statistics, pp 458–463
Ruge B, Linz A, Pickering R, Proeseler G, Greif P, Wehling P (2003) Mapping of Rym14 Hb, a gene introgressed from Hordeum bulbosum and conferring resistance to BaMMV and BaYMV in barley. Theor Appl Genet 107:965–971
Ruge-Wehling B, Linz A, Habekuß A, Wehling P (2006) Mapping of Rym16 Hb, the second soil-borne virus-resistance gene introgressed from Hordeum bulbosum. Theor Appl Genet 113(5):867–873
Schmalenbach I, March TJ, Bringezu T, Waugh R, Pillen K (2011) High-resolution genotyping of wild barley introgression lines and fine-mapping of the threshability locus thresh-1 using the Illumina GoldenGate assay. G3 Genes Genom Genet 1(3):187–196
Scholz M, Ruge-Wehling B, Habekuß A, Schrader O, Pendinen G, Fischer K, Wehling P (2009) Ryd4 Hb: a novel resistance gene introgressed from Hordeum bulbosum into barley and conferring complete and dominant resistance to the barley yellow dwarf virus. Theor Appl Genet 119:837–849
Shtaya M, Sillero J, Flath K, Pickering R, Rubiales D (2007) The resistance to leaf rust and powdery mildew of recombinant lines of barley (Hordeum vulgare L.) derived from H. vulgare x H. bulbosum crosses. Plant Breed 126:259–267
Statistics New Zealand (2016) Agricultural Production Statistics: June 2016. http://www.stats.govt.nz/browse_for_stats/industry_sectors/agriculture-horticulture-forestry/AgriculturalProduction_final_HOTPJun16final.aspx. Accessed June 2018
Szigat G, Pohler W (1982) Hordeum bulbosum × H. vulgare hybrids and their backcrosses with cultivated barley. Cereal Res Commun 10:73–78
Taylor and Butler (2017) R Package ASMap: efficient genetic linkage map construction and diagnosis. J Stat Softw https://arxiv.org/abs/1705.06916. Accessed June 2017
Toubia-Rahme H, Johnston P, Pickering R, Steffenson B (2003) Inheritance and chromosomal location of Septoria passerinii resistance introgressed from Hordeum bulbosum into Hordeum vulgare. Plant Breed 122:405–409
von Post R, von Post L, Dayteg C, Nilsson M, Forster BP, Tuvesson S (2003) A high-throughput DNA extraction method for barley seed. Euphytica 130(2):255–260
Voorrips R (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78
VSN International Ltd. (2013) CycDesigN 5.1 A package for the computer generation of experimental designs. (Version 4.0): VSN International Ltd, Hertfordshire, United Kingdom
VSN International Ltd. (2015) Genstat Reference Manual (Release 18), Part 3: procedures. VSN International Ltd, Hertfordshire, United Kingdom, p 1519
Walther U, Rapke H, Proeseler G, Szigat G (2000) Hordeum bulbosum—a new source of disease resistance—transfer of resistance to leaf rust and mosaic viruses from H. bulbosum into winter barley. Plant Breeding 119:215–218
Wendler N, Mascher M, Himmelbach A, Johnston P, Pickering R, Stein N (2015) Bulbosum to go: a toolbox to utilize Hordeum vulgare/bulbosum introgressions for breeding and beyond. Mol Plant 8(10):1507–1519
Xu J, Kasha KJ (1992) Transfer of a dominant gene for powdery mildew resistance and DNA from Hordeum bulbosum into cultivated barley (H. vulgare). Theor Appl Genet 84(7):771–777. https://doi.org/10.1007/bf00227383
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14(6):415–421
Zhang X, Han D, Zeng Q, Duan Y, Yuan F, Shi J, Wang Q, Wu J, Huang L, Kang Z (2013) Fine mapping of wheat stripe rust resistance gene Yr26 based on collinearity of wheat with Brachypodium distachyon and rice. PLoS One 8(3):e57885
Acknowledgements
The authors would like to thank Merle Forbes and Rachael Warren for technical support. Funding provided by The New Zealand Institute for Plant and Food Research Limited Strategic Science Investment Fund (Cropping Systems). Thanks to Dr Samantha Baldwin, Jamie Macalister and Dr Sathiyamoorthy Meiyalaghan for critical reading of this manuscript. Many thanks to Donna Gibson for taking the photographs and helping with the figure and table designs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Communicated by Xiaoquan Qi.
Richard Pickering: Retired, formerly of the New Zealand Institute for Plant & Food Research Limited, Lincoln 7608, Canterbury, New Zealand.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, X., Kong, H.Y., Meiyalaghan, V. et al. Genetic mapping of a barley leaf rust resistance gene Rph26 introgressed from Hordeum bulbosum. Theor Appl Genet 131, 2567–2580 (2018). https://doi.org/10.1007/s00122-018-3173-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-018-3173-8