Abstract
Key message
Recent advances in Setaria genomics appear promising for genetic improvement of cereals and biofuel crops towards providing multiple securities to the steadily increasing global population.
Abstract
The prominent attributes of foxtail millet (Setaria italica, cultivated) and green foxtail (S. viridis, wild) including small genome size, short life-cycle, in-breeding nature, genetic close-relatedness to several cereals, millets and bioenergy grasses, and potential abiotic stress tolerance have accentuated these two Setaria species as novel model system for studying C4 photosynthesis, stress biology and biofuel traits. Considering this, studies have been performed on structural and functional genomics of these plants to develop genetic and genomic resources, and to delineate the physiology and molecular biology of stress tolerance, for the improvement of millets, cereals and bioenergy grasses. The release of foxtail millet genome sequence has provided a new dimension to Setaria genomics, resulting in large-scale development of genetic and genomic tools, construction of informative databases, and genome-wide association and functional genomic studies. In this context, this review discusses the advancements made in Setaria genomics, which have generated a considerable knowledge that could be used for the improvement of millets, cereals and biofuel crops. Further, this review also shows the nutritional potential of foxtail millet in providing health benefits to global population and provides a preliminary information on introgressing the nutritional properties in graminaceous species through molecular breeding and transgene-based approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Setaria belongs to the tribe Paniceae in the subfamily Panicoideae which includes around 125 species distributed all over the world. Importantly, this genus comprises of the ancient domesticated crop, foxtail millet (Setaria italica) and its wild ancestor green foxtail (S. viridis). Both foxtail millet and green foxtail are genetically close to several millets and cereals, such as pearl millet (Pennisetum glaucum), common millet (Panicum miliaceum), maize (Zea mays), sugarcane (Saccharum officinarum), sorghum (Sorghum bicolor), etc. Further, they are also closely related to candidate biofuel grasses with complex genomes, such as switchgrass (Panicum virgatum), napier grass (Pennisetum purpureum) and pearl millet (Pennisetum glaucum) (Doust et al. 2009). These attributes of S. italica and S. viridis (henceforth denoted as ‘Setaria’) along with their small diploid genomes (~500 Mb), self-fertilization and short cycling times (6 weeks, seed to seed) have accentuated Setaria as ideal model for functional genomic studies in millets, cereals and bioenergy grasses (Doust et al. 2009; Li and Brutnell 2011; Lata et al. 2013). In addition, Setaria uses C4 mode of photosynthesis, in which phosphoenolpyruvate carboxylase (PEPC) assists the immediate uptake of carbon dioxide and delivers it to RuBisCO for photosynthesis. Hence, being a C4 crop, Setaria possesses higher photosynthesis than C3 plants even under high light intensity and high temperature. The immediate quenching and delivery of carbon dioxide by PEPC do not require keeping stomata open for a long period and hence less water is lost by transpiration, resulting in better water use efficiency (Lata et al. 2013).
It was documented that foxtail millet is a domesticated version of green foxtail (Doust et al. 2009), and the domestication of foxtail millet originated in Yellow River valley and other regions of north China around the 5th/4th millennium B.C. (Gumelnitsa culture; Hunt et al. 2008). Subsequently, it has become a major crop adapted to arid and dry areas of India, China, and other parts of Asia, North Africa, and the Americas (Lata and Prasad 2012) and noteworthy, it is the world’s second largest cultivated millet (CGIAR Factsheet; http://www.cgiar.org/our-research/crop-factsheets/millets). Recently, foxtail millet genome has been sequenced by two independent groups, namely the Beijing Genomics Institute, China (Zhang et al. 2012) and the US Department of Energy––Joint Genome Institute (Bennetzen et al. 2012). The availability of high-quality reference genome of foxtail millet (Lata and Prasad 2013a; Muthamilarasan et al. 2013a), high-density haplotype map of foxtail millet genome variation (Jia et al. 2013a) and other genomic data has accelerated many high-throughput studies in the aspect of both structural and functional genomics in Setaria. Thus, the accumulated genomic information has reached the level where this foxtail millet–green foxtail pair can now truly be considered a novel model system. In this context, the present review summarizes the recent advancements in structural and functional genomics of Setaria and its applicability in genetic improvement of millets, cereals and biofuel grasses.
Progresses of structural genomics in pre-genome sequencing era of Setaria
Structural genomics denote the study of sequence organization in the genome, where the role of DNA markers is inevitable in various applications, such as investigating genetic diversity and phylogenetic relationships, constructing high-density genome maps, mapping of useful genes, comparative genome mapping and marker-assisted selection for crop improvement. Structural genomics of foxtail millet has been well studied than its wild progenitor green foxtail since it is a cultivated species. Wang et al. (1998) was the first to report markers in foxtail millet, where an RFLP-based map consisting of 160 loci was constructed in an intervarietal cross. These markers were proven useful in constructing comparative genetic maps of foxtail millet and rice (Devos et al. 1998). Later, Jia et al. (2007) demonstrated the importance of EST-derived simple sequence repeat (EST-SSR) markers in foxtail millet. Being the primary report, 30 SSRs were identified in 1213 foxtail millet EST sequences and primers were designed for 26 SSRs. Of the 26 primers, 2 primers showed polymorphism in 12 foxtail millet and 1 green foxtail accessions with 10 alleles detected for four loci at an average of 2.5 alleles per locus (Jia et al. 2007). The first SSR-linkage map of foxtail millet was reported by Jia et al. (2009). They constructed two genomic libraries enriched for (GA)n and (CA)n and identified 100 polymorphic SSR markers. Further, the SSR-linkage map was constructed by integrating 81 developed SSR markers with 20 RFLP anchored markers and the genetic diversity was analyzed in 40 foxtail millet accessions (Jia et al. 2009).
Considering the importance of intron length polymorphic (ILP) markers in expediting molecular breeding, Gupta et al. (2011) reported 98 potential ILP markers exploiting the EST sequences of dehydration- and salinity-stressed suppression subtractive hybridization (SSH) libraries (Lata et al. 2010; Puranik et al. 2011a). They also demonstrated the high level of cross-species transferability and the utility of these ILP markers in germplasm characterization and in studying genomic relationships in millet and non-millet species (Gupta et al. 2011). Recently, Muthamilarasan et al. (2013a, b) have developed 5123 ILP markers and demonstrated their utilities in germplasm characterization, transferability, phylogenetics and comparative mapping studies in millets, cereals and bioenergy grass species. Moreover, Heng et al. (2011) reported 45 polymorphic SSR markers from RAPD-enriched library and showed their applications in genetic diversity and cross-species transferability analyses. Using a drought- and salt-tolerant foxtail millet cv. ‘Prasad’, Gupta et al. (2012) constructed (CA)n, (AAC)n and (ATG)n enriched library to develop around 172 novel genomic SSRs. In addition to demonstrating their use in genetic studies, comparative mapping of the developed genomic SSRs onto the genomes of rice, maize and sorghum had also been performed. Hence, this report substantiated the importance of SSR markers in (i) translating information from one species to other, (ii) enriching marker numbers at defined genetic loci, and (iii) assisting map-based cloning of important genes through comparative mapping (Gupta et al. 2012). The same group had also constructed a (GA/CT)n microsatellite-enriched library to develop 78 SSR markers and substantiated the role of these markers in diverse genotyping applications, resolving QTL, establishing phylogenetic relationships and transferability among several important grass species (Gupta et al. 2013).
Advancement of structural genomics in post-genome sequencing era of foxtail millet
A milestone in the area of Setaria genomics is the release of first assembled reference genome of foxtail millet and green foxtail in the year 2012 by two independent groups (Zhang et al. 2012; Bennetzen et al. 2012). Zhang et al. (2012) sequenced the foxtail millet cv. ‘Zhang gu’ using Illumina second generation sequencers (~86 % genome coverage). The project generated a total of 16,903 contigs and 439 scaffolds covering a total length of 423 Mb with 28 Mb (6.6 %) gaps and a predicted genome size of ~485 Mb (Zhang et al. 2012). A photo-thermo-sensitive male sterile line ‘A2’ was also sequenced and comparison of both the genomes revealed several thousand single nucleotide polymorphisms (SNPs), insertion/deletion polymorphisms (InDels) and structural variations (SVs) between the two cultivars. The sequence analysis also showed the presence of 38,801 genes, out of which ~82 % were expressed. Further, a genetic linkage map was constructed from a cross of Zhang gu and A2, mapped with 751 markers including 118 SNPs and 641 SVs (Zhang et al. 2012). Foxtail millet accession ‘Yugu1’ and green foxtail accession ‘A10’ were sequenced by Bennetzen et al. (2012) using ABI3730xl capillary sequencer. A total of 5,736,559 reads were generated and assembled to achieve genome coverage of ~80 %. The analysis showed the presence of 24,000–29,000 expressed genes and identified the genome of 396.7 Mb present in nine chromosomes. In addition, 4.2 Mb in 327 scaffolds (predominantly <50 kb in size) was also identified. The recombinant inbred lines (RILs) from the S. italica ‘B100’ X S. viridis ‘A10’ populations were sequenced and 3,149,093 SNPs were identified. Of these, 992 SNPs were scored for segregation in the RIL population and a high-resolution genetic map was constructed (Bennetzen et al. 2012).
The availability of foxtail millet genome sequence in public domain has facilitated large-scale development of genomic resources. Pandey et al. (2013) scanned the whole genome sequence of foxtail millet and identified a total of 28,342 microsatellite repeat-motifs (SSRs) spanning 405.3 Mb of foxtail millet genome. These SSRs were mapped onto the nine chromosomes of foxtail millet to generate a high-density physical map (Pandey et al. 2013). Noticeably, these markers showed a high percentage (~90 %) of cross-genera transferability across millets including green foxtail, cereals and bioenergy grasses. In silico comparative mapping of the physically mapped foxtail millet SSR markers with the physical location on the chromosomes of other related grass genomes of sorghum, maize and rice had also been performed, which would be useful in investigating the transferability of these markers and in studying the evolution. In a similar study, Zhang et al. (2014) scanned the foxtail millet whole genome and identified 5,020 highly repetitive microsatellite motifs. Based on sequence comparison between foxtail millet and green foxtail, 788 SSR primer pairs were designed. Out of these, 733 were polymorphic, and a physical map was constructed based on these highly polymorphic SSR markers.
Kumari et al. (2013) used the 66,027 ESTs of foxtail millet from NCBI EST database to develop functional SSR markers. The study identified 495 EST-SSRs, and primer pairs were designed for 447 markers. The developed markers were validated successfully in green foxtail and in other millets, cereals and bioenergy grasses, and an average transferability of ~88 % was observed (Kumari et al. 2013). The study also showed the utility of conserved orthologous set (COS) markers in the genome analysis of foxtail millet, sorghum, maize and rice. Synteny analysis of EST-SSRs from foxtail millet, rice, maize and sorghum suggested the nested chromosome fusion frequently observed in grass genomes (Kumari et al. 2013). Considering the usefulness of microRNA (miRNA)-based molecular markers in animal systems and in Brassica sp, foxtail millet genome sequence was analyzed for predicting potential miRNAs and 176 miRNA-based markers were developed (Yadav et al. 2014a). In view of the highly polymorphic nature of transposable elements (TEs), a genome-wide analysis for identification of TEs and development of ~30,000 TE-based markers were performed to generate 5 types of markers, namely Retrotransposon-Based Insertion Polymorphisms (RBIP), Inter-Retrotransposon Amplified Polymorphisms (IRAP), Repeat Junction Markers (RJM), Repeat Junction–Junction Markers (RJJM), Insertion-Site-Based Polymorphisms (ISBP) and Retrotransposon-Microsatellite Amplified Polymorphisms (RMAP). In addition, the utility of these markers in studying the genetic variation and analyzing the genome structure was also demonstrated (Unpublished data). Of note, all the developed markers (SSRs, EST-SSRs, ILPs and miRNA-based markers) showed a higher percentage of cross-genera transferability across millets, cereals and bioenergy grasses, which signify the importance of these markers in large-scale genotyping applications in all these species.
The availability of high-throughput sequencing technology along with the release of high-quality genome sequence (Zhang et al. 2012; Bennetzen et al. 2012) had facilitated the rapid investigation of genomic variation in a large-scale collection of foxtail millet cultivars and transcriptome sequencing of green foxtail cultivars. Jia et al. (2013a) sequenced 916 diverse foxtail millet varieties and identified 2,584,083 SNPs and used 845,787 common SNPs (minor allele frequency >0.05) to construct a haplotype map of the foxtail millet genome. Through genome-wide association studies (GWAS), the 916 varieties were phenotyped under five different environments to identify 512 loci associated with 47 agronomic traits. These studies would promisingly serve as fundamental resources for the improvement of foxtail millet using genetic and genomic approaches. Similarly, Bai et al. (2013) resequenced a foxtail millet landrace ‘Shi-Li-Xiang’ (SLX) and compared with the two reference genome sequences to investigate the patterns of genetic variations. The study showed 762,082 SNPs, 26,802 InDels of 1–5 bp in length, and 10,109 SVs between SLX and Yugu1 (Bennetzen et al. 2012); 915,434 SNPs, 28,546 InDels and 12,968 SVs between SLX and Zhang gu (Zhang et al. 2012; Bai et al. 2013). The SNPs, InDels and SVs reported by Bai et al. (2013) would expedite the identification of agronomically important genes in foxtail millet and green foxtail, and thus serve as invaluable tools in molecular breeding. Xu et al. (2013) used Illumina RNA-seq technology to perform a comprehensive transcriptome analysis using RNA sample pooled from multiple developmental stages and different tissues of green foxtail. Reference-based assembly using foxtail millet genome as a reference generated 42,754 transcripts, whereas de novo assembly generated 60,751 transcripts, from which 9,576 and 7,056 potential SSRs were developed, respectively (Xu et al. 2013).
Efficacy of developed marker resources in genomics-assisted breeding
Few reports are available on the demonstration of the utility of developed genomic resources in analyzing genetic associations. Le Thierry et al. (2000) developed AFLP markers and used them to assess the genetic relationships between foxtail millet and green foxtail. Similarly, Li et al. (2012) developed and used 27 ISSR markers in analyzing domestication-related geographical structure and phylogenetic relationship of foxtail millet, green foxtail and other Setaria species. Wang et al. (2012) used 77 SSR markers developed by Jia et al. (2007, 2009) and Gupta et al. (2012) to examine the genetic diversity and population structure of Chinese landraces of foxtail millet. The study showed a high degree of molecular diversity among the Chinese foxtail millet landraces (Wang et al. 2012). Likewise, Jia et al. (2013b) used the same set of SSR markers developed in foxtail millet (Jia et al. 2007, 2009; Gupta et al. 2012) in evaluating genetic diversity, population structure and linkage disequilibrium (LD) in a large sample size of 288 green foxtail accessions collected from all geographical regions of China. The analysis revealed a high genetic diversity of green foxtail, which highlights its applicability in genetic marker development, construction of segregating populations, functional gene cloning, and association mapping. Both the reports by Jia et al. (2013b) and Wang et al. (2012) showed that Setaria was first domesticated in the Yellow River valley, which is in accordance to Hunt et al. (2008). In addition, the study showed a lower level of LD in green foxtail than foxtail millet, which provides prospects for identification of potential markers/genes in trait association mapping controlling agronomical traits in green foxtail (Jia et al. 2013b).
A study by Mauro-Herrera et al. (2013) demonstrated the utility of SSR markers reported by Wang et al. (2012) in investigating the genetic control of flowering in Setaria spp. The report indicated the co-localization of quantitative trait loci (QTL) that control differences in flowering time. It also revealed the robustness of genetic control of flowering in Setaria over a range of photoperiod and other environmental factors. A comprehensive comparison of QTL for flowering in Setaria, sorghum, and maize suggested that the difference in flowering time in individual grass lineages is controlled by a combination of conserved as well as lineage-specific genes (Mauro-Herrera et al. 2013). Recently, Vetriventhan et al. (2014) genotyped the ICRISAT foxtail millet core collection consisting of 155 accessions using 72 SSR markers and investigated genetic diversity, population structure and LD. A high degree of molecular diversity and LD decay of less than 40 cM of genetic distance in the accessions were observed and the study proved that the foxtail millet core collection of ICRISAT is an invaluable resource for trait association mapping, crop breeding and germplasm management.
In a similar study, 184 foxtail millet accessions from diverse geographical locations were genotyped using 50 SSR markers reported by Jia et al. (2009) and Pandey et al. (2013). The LD analysis considering population structure and relative kinship revealed significant association (R 2 = 18 %) of 8 SSR markers (p < 0.01) with nine agronomic traits (overall 18 % association potential) (Gupta et al. 2014). These trait-associated SSR markers, once validated, can be used for identification of genes/QTL regulating the agronomic traits and eventually for marker-assisted genetic enhancement of foxtail millet. The SSR b129 along with SSR p75 on chromosome 5 showed significant association with grain weight and yield traits. Also, SSRs b225 and p61 showed association with inflorescence compactness and grain shape, respectively, which could also be considered as candidate markers to study grain quality and yield. Thus, association mapping of markers and QTL expands our understanding of the genes that influence agronomically/economically important traits and reveals the candidates which could be beneficial for targeted breeding.
Role of functional genomics in dissecting the stress physiology of foxtail millet
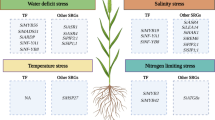
Being naturally tolerant to abiotic stresses, particularly drought, foxtail millet and green foxtail have higher water use efficiency than other cereals such as maize, wheat and sorghum (Lata et al. 2013). This urged the plant research community to examine the molecular biology of stress tolerance of Setaria, particularly in foxtail millet. Interestingly, the genome sequencing and annotation project by Beijing Genomics Institute (Zhang et al. 2012) identified a total of 1,517 foxtail millet-specific gene families. Out of these, 586 genes were predicted to have roles in stress responses and were annotated as ‘response to water’. This supports the fact that foxtail millet is a rich source of genes that might be responsible for stress tolerance behavior and adaptation to arid and semi-arid environments.
Investigating the molecular mechanism of drought tolerance
Lata et al. (2011a) were the first to examine the genetic diversity of drought-induced oxidative stress tolerance in foxtail millet cultivars. A total of 107 foxtail millet cultivars were screened for their dehydration tolerance on the basis of lipid peroxidation (LP) activity. The study showed lower levels of lipid peroxidation and electrolyte leakage along with higher activity of catalase, ascorbate peroxidase and glutathione reductase in tolerant cultivars. The results demonstrated the existence of sophisticated antioxidant machinery with efficient ascorbate–glutathione pathway in dehydration-tolerant foxtail millet cultivar, which enables the crop to cope with drought-induced oxidative stress (Lata et al. 2011a).
To understand the genetic network of stress tolerance, Zhang et al. (2007) investigated the differentially expressed genes induced during drought stress in foxtail millet. Using suppression subtracted hybridization (SSH) and cDNA microarray, the study identified 95 and 57 ESTs up-regulated in roots and shoots of seedlings, respectively. The expression profile analysis showed that genes induced in roots were different from those in shoots and most of the genes were found to participate in protein degradation pathway (Zhang et al. 2007). A similar study performed by Lata et al. (2010) identified the differentially expressed transcripts in dehydration stress-tolerant foxtail millet cultivar. The validation of expression pattern using Reverse Northern and quantitative real-time PCR (qRT-PCR) showed the up-regulation of 86 transcripts. Further, qRT-PCR analysis indicated a 5–11-fold induction of DREB2 (a Dehydration-Responsive Element-Binding type) proteins (Lata et al. 2010). In another report, Li et al. (2014) identified an abscisic acid (ABA)-responsive DREB-binding protein gene (SiARDP) to be highly expressed during drought, salinity and low temperature stresses, and ABA treatment in foxtail millet seedlings. Constitutive expression of SiARDP in Arabidopsis enhanced drought and salt tolerance during seed germination and seedling development (Li et al. 2014). As the DREBs regulate the expression of many stress-inducible genes mostly in an abscisic acid-independent manner and play a critical role in improving the abiotic stress tolerance of plants by interacting with a DRE/CRT cis-element present in the promoter region of various abiotic stress-responsive genes (Lata and Prasad 2011), functional characterization of the highly expressed SiDREB2 gene has been performed.
Characterization of SiDREB2 gene evidenced a synonymous SNP associated with dehydration tolerance at the 558th base pair (an A/G transition) in a set of 45 foxtail millet accessions (Lata et al. 2011b). Based on this SNP, an Allele-Specific Marker (ASM) for dehydration tolerance was developed (Lata et al. 2011b). The ASM was validated in a core set of 170 foxtail millet accessions and the regression of LP and relative water content (RWC) on the ASM suggested that the SiDREB2-associated trait contributed to ~27 % and ~20 %, respectively, of the total variation in LP and RWC (Lata and Prasad 2012, 2013b). These results demonstrated the importance of this gene for dehydration tolerance. Currently, this ASM is now being used for allele mining and marker-aided breeding of foxtail millet by the Tamil Nadu Agricultural University (TNAU), Coimbatore, Tamil Nadu, India. Encouragingly, this ASM would serve as a rapid, inexpensive and more reproducible tool for genotyping, and also enhance marker-aided breeding of foxtail millet for dehydration tolerance.
Recently, Qi et al. (2013) used the next generation deep sequencing technology to analyze the whole transcriptome of foxtail millet. The study identified a total of 2,824 genes with drought-responsive expression patterns, of which 48.2 % were up-regulated and 51.8 % were down-regulated (Qi et al. 2013). Of the up-regulated genes, late embryogenesis abundant protein (LEA), dehydrin, heat shock protein (HSP), aquaporin and phosphatase 2C (PP2C) were the most abundant which suggested the possible role of these genes in dehydration tolerance behavior of foxtail millet. Yin et al. (2014) have performed transcript profiling in foxtail millet exposed to drought stress and identified that an F-box protein is involved in the response to drought stress or abscisic acid (ABA) treatment. Very recently, Zhu (2014) identified a small molecule ABA mimic (AM1) which acts as a potent activator of multiple members of the family of ABA receptors. In Arabidopsis, AM1 activates a gene network that is highly similar to that induced by ABA. Treatments with AM1 reduce leaf water loss and promote drought resistance in Arabidopsis and protect plants from water loss and drought stress. Experiments are underway to understand the role of AM1 in drought tolerance of Setaria. Despite these efforts, little is known about the mechanism of drought tolerance and the genes responsible for tolerance in Setaria. For effective identification of genes linked to drought tolerance, Zhi et al. (2014) had attempted to construct RIL population in foxtail millet. These RILs would enable the rapid measurement of drought tolerance under different stress conditions and produce accurate phenotype data for identification of genes linked to drought tolerance. Although the experimental outcomes of international efforts have provided some novel insights into the mechanisms of dehydration tolerance, further detailed investigations are required to identify the underlying tolerance-related gene regulatory networks in planta.
Deciphering the cellular machinery of salinity tolerance
In addition to natural tolerance towards drought stress, Setaria is also known for its superior salt-tolerance ability. Salinity tolerance of foxtail millet was extensively studied, but no effort has been invested in understanding the tolerance mechanism of green foxtail. First systematic study to identify the differentially expressed transcripts accumulated during salinity stress was conducted by Jayaraman et al. (2008) using cDNA-AFLP where the transcripts accumulation was validated through qRT-PCR. A total of 90 transcript-derived fragments (TDFs) were reported to be differentially expressed, of which 86 TDFs were classified based on their complete presence or absence (qualitative variants) and four based on differential expression pattern levels (quantitative variants) in the two contrasting foxtail millet varieties [cv. ‘Prasad’ (tolerant); cv. ‘Lepakshi’ (sensitive)]. Noteworthy, 27 non-redundant differentially expressed cDNAs were unique to salt-tolerant cultivars (Jayaraman et al. 2008). These represented different groups of genes involved in metabolism, cellular transport, cell signaling, transcriptional regulation, mRNA splicing, seed development and storage, etc. Further, qRT-PCR analysis revealed a considerable up-regulation of seven out of nine genes in tolerant cultivar after 1 h of salt stress when compared to susceptible cultivar (Jayaraman et al. 2008).
Puranik et al. (2011a) compared the transcriptome of salinity tolerant and sensitive foxtail millet cultivars by constructing SSH library. The study identified SiNAC (Setaria italica NAM, ATAF, and CUC) to be strongly up-regulated during salinity stress in the tolerant cultivar (cv. ‘Prasad’). Molecular cloning and characterization of this SiNAC gene showed that this membrane-associated NAC family gene has a novel DNA-binding site (Puranik et al. 2011b) and they may function as a transcriptional activator in response to stress and developmental regulation in foxtail millet (Puranik et al. 2011c). This NAC gene family has emerged as an important transcription factor (TF) in plants, which plays a significant role in biotic and abiotic stress tolerance in addition to their routine functions in orchestration of organ, fiber and secondary wall development, cell cycle control, and senescence (Puranik et al. 2012). Hence, considering the importance of NAC TFs, a genome-wide study along with expression profiling and evolutionary analysis was conducted by Puranik et al. (2013). This study identified 147 NAC proteins encoded in the foxtail millet genome. The NAC proteins were evidenced to be highly varied in length and are predominantly nuclear localized. The structural analysis showed a highly conserved N-terminal (having NAC domain) and a highly diversified C-terminal (responsible for transcription activation/repression) regions. The foxtail millet physical map of NAC TFs showed an uneven distribution of SiNAC genes on the chromosomes, with 19 genes found to be tandem repeats. The phylogenetic analysis classified SiNAC proteins into 11 sub-families based on their domain composition. In silico comparative mapping of SiNAC genes onto the genome of rice, maize and sorghum showed highest orthology between foxtail millet–sorghum (~77 %) and foxtail millet–maize (72 %), thus supporting their close evolutionary relationships. The duplication and divergence rates (Ka/Ks) of SiNAC genes demonstrated that SiNAC gene family had strong purifying selection pressure (Ka/Ks < 1). Expression profiling of candidate SiNAC genes in response to different stress conditions showed that cold stress induced relatively drastic changes in SiNAC transcript abundance than dehydration or salinity (Puranik et al. 2013).
Comparison of the transcript profiles at different time points of dehydration (Lata et al. 2010) and salinity stress (Puranik et al. 2011a) showed that only 10 % of these genes were regulated under both the stresses, which evidently indicates the presence of gene sets that are unique to respective stresses. This strongly suggests that foxtail millet is equipped with distinct tolerance mechanisms to perceive and respond to salt- and dehydration-stress conditions (Lata et al. 2010; Puranik et al. 2011a). Further, a comparative analysis of transcriptional profiling under dehydration and salinity stress in foxtail millet with the available datasets of other plant systems revealed that >40 % of the transcripts were unique to foxtail millet and have not been identified in any other species. This highlights the individuality of foxtail millet in terms of its response to dehydration and salinity stress.
In a similar study by Mishra et al. (2012a), a differential expression of WD40 proteins in salinity and dehydration stress SSH library was observed. These WD40 proteins were identified to play a crucial role in diverse protein–protein interactions by acting as scaffolding molecules and thus assisting the proper activity of the proteins (Mishra et al. 2012b). Molecular cloning and characterization of SiWD40 gene showed the protein architecture, cellular localization and most importantly a putative regulation of SiWD40 expression by dehydration-responsive elements (DREs) during abiotic stress (Mishra et al. 2012a). Hence, in view of the importance of WD40 proteins in abiotic stress response, genome-wide analysis and expression profiling were performed for SiWD40 genes of foxtail millet (Mishra et al. 2013), which proffer added knowledge to the complex molecular mechanisms responsible for abiotic stress tolerance in foxtail millet. Since genome-wide identification and expression profiling of gene families participating in abiotic stress tolerance unlock new avenues for systematic functional analysis of respective gene family candidates, the outcomes of these efforts could promisingly be applied for improvising stress adaptation in plants. In addition, under-expression and over-expression studies of candidate genes would provide further insights in identifying the precise molecular mechanism of stress tolerance. Recently, the procedure for virus-induced gene silencing (VIGS) has been standardized in foxtail millet (http://cdm16483.contentdm.oclc.org/cdm/ref/collection/p15481coll2/id/3763), which would seamlessly assist the millet research community in performing functional characterization of stress-related genes in foxtail millet. Moreover, Kumar et al. (2013) validated a set of housekeeping genes to identify stable internal controls for qRT-PCR analyses and reported that Act2 and RNA POL II are apt controls for salinity-stress-related expression analyses, whereas EF-1α and RNA POLII are suitable for dehydration-stress-related expression analyses. These findings would encourage the transcriptomics and expression profiling studies equitably.
Molecular characterization of stress-responsive proteins
Dicer-like (DCL), Argonautes (AGO) and RNA-dependent RNA polymerases (RDR) assist in post-transcriptional control of gene expression by playing pivotal roles in RNA interference mechanism. In view of this, Yadav et al. (2014b) examined the DCL, AGO and RDR gene family members in foxtail millet and identified 8, 19 and 11 genes, respectively. In addition to physical mapping and phylogenetic analysis, expression profiling of these genes during salinity, dehydration and ABA treatments was investigated in two foxtail millet cultivars with contrasting tolerance to salinity and dehydration [acc. ‘IC04’ (tolerant); acc. ‘IC41’ (sensitive)]. The analysis showed a differential expression pattern of these genes at different time points of stresses in both the cultivars (Yadav et al. 2014b). Considering the reports on the involvement of zinc finger proteins in plant stress response and hormone signal transduction, Muthamilarasan et al. (2014) performed a genome-wide analysis of C2H2-type zinc finger proteins in foxtail millet and showed that these proteins may define response to abiotic stresses. The study identified 124 C2H2-type of zinc finger protein encoding genes in foxtail millet and characterized these proteins at a molecular level. Of note, 47 SiC2H2 genes were shown to possess molecular markers, of which nine had more than one marker. Among the three marker types, SSRs were found to be predominant (49), followed by ILPs and EST-SSR (2 and 1, respectively). These markers are functionally relevant and have intense utility in various applications of structural, functional and comparative genomics, including marker-assisted genetic improvement of foxtail millet.
The expression profiling of Aldehyde Dehydrogenase (ALDH) gene family members in foxtail millet seedlings subjected to salinity, dehydration, oxidative, heat and cold stresses was analyzed (Zhu et al. 2014). The study showed a differential tissue-specific expression of the genes in response to these stresses. Further, bacterial cells transformed with candidate SiALDH genes showed enhanced salinity tolerance. A similar investigation was performed by Wang et al. (2014), where the genes encoding cytokinin oxidase/dehydrogenase (CKX) were identified and characterized. The report showed that SiCKX genes are stress-inducible and a high up-regulation of certain candidate genes in response to abiotic stresses hints the putative functioning of CKX proteins in stress response mechanisms. All these studies except SiCKX gene family analysis have identified the respective orthologous genes in related grass genomes, such as sorghum, maize and rice and the evolutionary relationships between these orthologous gene pairs were derived (Yadav et al. 2014b; Muthamilarasan et al. 2014; Zhu et al. 2014). Taken together, these genome-wide investigations of stress-responsive proteins provide a foundation for evolutionary and functional characterization of the above-mentioned proteins in foxtail millet and related grass species to dissect their functions in response to environmental stimuli.
Advances in foxtail millet small RNA biology
Small RNAs are a group of noncoding RNAs reported to play a significant role in gene regulation. These regulatory small RNAs are broadly classified into small interfering RNA (siRNA) and microRNA (miRNA) according to their length (Bartel 2004; Sahu et al. 2010; Sharma et al. 2013; Muthamilarasan and Prasad 2013). With the advent of next generation sequencing platforms and high-throughput analysis technologies, the speed of small RNA discovery and characterization has increased dramatically. As mentioned, Qi et al. (2013) generated a genome-wide transcriptome of drought stressed foxtail millet using deep sequencing approach. Besides the identification of differentially expressed transcripts, the study also revealed the potential roles of siRNAs in drought stress response. Further, the study showed that the decrease in levels of 24-nt siRNA flanking genes was associated mostly with proximal up-regulated genes, thus demonstrating a potential effect of these siRNAs on drought-regulated gene expression (Qi et al. 2013).
A genome-wide analysis for identification of miRNAs in foxtail millet was performed by Khan et al. (2014). The study identified 355 mature miRNAs encoded in the foxtail millet genome, and the secondary structures as well as corresponding targets of identified miRNAs were analyzed. Further, the miRNAs were mapped onto the foxtail millet genome and a comparative map between foxtail millet miRNAs and miRNAs of sorghum, maize, rice and Brachypodium was constructed for assisting miRNA-mediated gene regulation studies in foxtail millet as well as in related grasses. Expression profiling was performed for eight candidate miRNAs under diverse abiotic stresses in foxtail millet, which unraveled the putative involvement of these miRNAs in stress tolerance (Khan et al. 2014). Similarly, Yi et al. (2013) constructed 2 small RNA libraries and identified 43 known miRNAs, 172 novel miRNAs and 2 mirtron precursor candidates. Moreover, a comparative genomic analysis with sorghum had also been performed to understand the evolutionary dynamics of miRNA family expansions.
To identify miRNAs responsive to drought stress, four small RNA libraries (in two replicates) were constructed and sequenced using Illumina HighSeq 2000 sequencer. The differential expression patterns of miRNAs across different tissues with respect to stress treatments and time points suggested the functions of miRNAs in response to drought stress (Unpublished data). Further characterization of these differentially expressed siRNAs and miRNAs is a requisite, which could facilitate the development of new strategies for alleviating the adverse effects of drought and salinity stress on foxtail millet growth and development.
Online resources for foxtail millet genomics
The release of foxtail millet genome sequence necessitated the establishment of web-based databases with unrestricted public access to the global scientific community (Table 1). The Joint Genome Institute of U.S. Department of Energy and the Center for Integrative Genomics had constructed a public domain database PHYTOZOME (http://phytozome.jgi.doe.gov/pz/portal.html). Presently, the PHYTOZOME v10 provides access to 46 sequenced and annotated plant genomes including foxtail millet (annotation version 2.1) (Bennetzen et al. 2012). The whole genome sequence information of foxtail millet annotated by Bennetzen et al. (2012) is also available in Gramene v39 (http://www.gramene.org/; Monaco et al. 2013) and PlantGDB (SiGDB; Duvick et al. 2008). The genome data generated by Beijing Genomics Institute (Zhang et al. 2012) are available in Foxtail millet Database (http://foxtailmillet.genomics.org.cn/). Further, online databases were also developed for the genomic and genetic resources generated in foxtail millet. The Foxtail millet Marker Database (FmMDb; http://www.nipgr.res.in/foxtail.html) is the first comprehensive database for structural and comparative genomics in millet and bioenergy grass species (Suresh et al. 2013) (Fig. 1a). It encompasses all the molecular marker information generated in foxtail millet for the easy access of breeders and researchers working towards crop improvement. Further, Foxtail millet miRNA Database (FmMiDNADb; http://59.163.192.91/FmMiRNADb/index.html) comprises of the complete set of miRNAs identified in foxtail millet along with the information of miRNA-based molecular markers (Khan et al. 2014) (Fig. 1b). Foxtail millet Transcription Factor Database (FmTFDb; http://59.163.192.91/FmTFDb/index.html) contains the comprehensive information of transcription factors (2297 TFs in 55 families) encoded in foxtail millet genome along with the details of their physical position, phylogeny, expression pattern and sequence information (Bonthala et al. 2014) (Fig. 1c). With the availability of green foxtail transcriptome data, transcriptome-wide mining of TFs was performed in green foxtail using foxtail millet TFs as reference. The analysis revealed 1037 TFs belonging to 45 families and the data were assembled into a simple open-access database (Setaria viridis Transcription Factor Database; Unpublished data). The orthologous genes of these TFs were identified in foxtail millet and physical map was constructed using CMAP interface. A TF-based comparative map (between the genomes of foxtail millet –sorghum, -maize, -rice, and -Brachypodium) was also incorporated (Unpublished data). Further, the Foxtail millet Transposable Elements-based Marker Database (FmTEMDb; http://59.163.192.83/ltrdb/index.html) comprises the data of RBIP, IRAP, RJM, RJJM, ISBP and RMAP markers (Unpublished data) (Fig. 1d). These databases would serve as a valuable resource for the researchers and breeders and expedite the improvement of Setaria and related grass species through conventional breeding and transgene-based approaches.
Screenshot of different unique web-based resources developed in foxtail millet genomics. a Foxtail millet Marker Database (FmMDb; http://www.nipgr.res.in/foxtail.html), b Foxtail millet miRNA Database (FmMiRNADb; http://59.163.192.91/FmMiRNADb/), c Foxtail millet Transcription Factor Database (FmTFDb; http://59.163.192.91/FmTFDb/), and d Foxtail millet Transposable Elements-based Marker Database (FmTEMDb; http://59.163.192.83/ltrdb/index.html)
Nutritional genomics of foxtail millet: a new thrust area
Being a versatile crop, foxtail millet is also known for its nutritional and therapeutic potential and is nutritionally superior to conventional food grains. Grains of foxtail millet have higher micro and macronutrient contents than other cereals and more importantly, they have low glycaemic index (GI) and high fiber content (Geervani and Eggum 1989; Pawar and Pawar 1997). The protein content of foxtail millet (305.8 mg/g) is the highest among millets and major cereals (Rao et al. 2011) and in addition, it contains high amount of fibers (as β-glucans; 42.6 %) (Amadou et al. 2011). These β-glucans increase the metabolism of sugar and cholesterol to induce hypoglycemic and hypocholesterolaemic effects and hence are beneficial for prevention of diabetes and cardiovascular diseases (Krishnakumari and Thayumanvan 1997; Itagi et al. 2012). Therefore, foxtail millet is used in the preparation of low GI foods for treating diabetics, particularly type 2 diabetes (Anju and Sarita 2010; Thathola et al. 2010; Itagi et al. 2012; Chhavi and Sarita 2012; Jali et al. 2012). Noteworthy, foxtail millet has higher amount of minerals (3.3 mg/g), iron (0.3 mg/g) and calcium (0.3 mg/g) when compared to rice which has 0.06, 0.07 and 0.1 mg/g of minerals, iron and calcium, respectively (http://www.swaraj.org/shikshantar/millets.pdf).
Regardless of these enormous health benefits, no attempt has been made to understand the genetics and genomics of nutritional traits in foxtail millet. National and international efforts need to be invested in exploring grain characteristics, and studying genetic variations of grain characteristics in the foxtail millet germplasm using genomics and biochemical phenotyping. Further, combining the strength of phenomics and metabolomics platform with GWAS will assist in identification of SNPs and candidate genes controlling nutritional content in the grains. Once identified, these genes in the diverse germplasm can be introgressed into other graminaceous species through comparative genomics (Fig. 2). The nutritionally enhanced crops would serve as a source for healthy diet for the global population and contributing to evade nutritional insecurity. In addition, genetic enhancement of foxtail millet landraces will provide health benefits not only for diabetic and cardiovascular patients, but also for all healthy individuals by promoting a healthy digestive system and delaying/avoiding diabetes in vulnerable people.
Concluding remarks and future prospects
Since the declaration as a model crop, both foxtail millet and green foxtail have gained momentum in three prime areas viz, stress biology, C4 photosynthesis and bioenergy research. But compared to green foxtail, foxtail millet has invited considerable attention in terms of both structural and functional genomics research and the release of its genome sequence has further accelerated the research on foxtail millet genomics. Notably, foxtail millet research has now provided numerous scientific leads to proceed further towards crop improvement of millets, cereals and bioenergy grasses. Though many candidate stress-responsive genes were identified in foxtail millet, overexpression of these genes in Setaria has not been possible till date due to the lack of an efficient transformation system. All the transformation techniques reported in Setaria have very low transformation efficiency (not more than 10 %) (Dong and Duan 2000; Liu et al. 2005; Fang et al. 2008; Liu et al. 2007; Wang et al. 2011). The major research areas in Setaria genomics which need to be explored are (i) in terms of structural genomics, all possible genomic resources (molecular markers) need to be mined in large-scale, analyzed and provided to the breeders and researchers for conducting molecular breeding and marker-assisted selection; (ii) genome-wide studies of gene families, especially those which play crucial role in abiotic stress tolerance need to be conducted as they would provide more insight into the complex regulatory mechanism of stress response; (iii) epigenetic mode of gene expression regulation still remains elusive, even though certain preliminary data on this topic were available in cereal crops (Shaik and Ramakrishna 2012; Sahu et al. 2013); and (iv) studies on nutritional genomics of Setaria need to be initiated to investigate its nutritional potential.
Further, resequencing of diverse collection of foxtail millet cultivars and its direct wild progenitors would assist in studying the genetic changes underlying phenotypic variations and mining of alleles for agronomic traits. In spite of these hitches, Setaria has now gained immense scientific importance after being regarded as the model plant. Unlike major cereals, which can prosper in conferring food security, millets such as foxtail millet can ensure multiple securities including food, nutrition, fodder, fiber, health, livelihood and ecology. Further, the potential abiotic stress tolerance characteristics of foxtail millet qualify it to be a climate change-compliant crop and of note, their nutritional quality appears promising in evading malnutrition globally. Large-scale development of genetic and genomic resources and providing their unrestricted access via web-based databases would certainly accelerate crop improvement programs. The first International Setaria Genetics Conference (ISGC 2014) was held on March 10–12, 2014 at Beijing, China for promoting Setaria as a novel model for grasses functional genomics (http://www.Setariadb.cn/meetabout.php?cid=11). The conference brought together a broad spectrum of researchers working on different aspects of Setaria biology including germplasm collection, genomic resource development, characterization of genome variation, trait mapping, gene expression analysis, genetic tool development, C4 photosynthesis biology, gene transformation, and foxtail millet breeding. It provided the researchers a forum to exchange research experiences, discuss future cooperation, and establish an International Setaria Community.
Author contributions
MP conceived and outlined the review. MM wrote the manuscript.
References
Amadou I, Amza T, Yong-Hui S, Guo-Wei L (2011) Chemical analysis and antioxidant properties of foxtail millet bran extracts. Songklanakarin J Sci Technol 33:509–515
Anju T, Sarita S (2010) Suitability of Foxtail Millet (Setaria italica) and Barnyard Millet (Echinochloa frumentacea) for Development of Low Glycemic Index Biscuits. Malays J Nutr 16:361–368
Bai H, Cao Y, Quan J, Dong L, Li Z, Zhu Y, Zhu L, Dong Z, Li D (2013) Identifying the genome-wide sequence variations and developing new molecular markers for genetics research by re-sequencing a Landrace cultivar of foxtail millet. PLoS ONE 8:e73514
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, Estep M, Feng L, Vaughn JN, Grimwood J, Jenkins J, Barry K, Lindquist E, Hellsten U, Deshpande S, Wang X, Wu X, Mitros T, Triplett J, Yang X, Ye CY, Mauro-Herrera M, Wang L, Li P, Sharma M, Sharma R, Ronald PC, Panaud O, Kellogg EA, Brutnell TP, Doust AN, Tuskan GA, Rokhsar D, Devos KM (2012) Reference genome sequence of the model plant Setaria. Nat Biotechnol 30:555–561
Bonthala VS, Muthamilarasan M, Roy R, Prasad M (2014) FmTFDb: a foxtail millet transcription factors database for expediting functional genomics in millets. Mol Biol Rep. doi:10.1007/s11033-014-3574-y
Chhavi A, Sarita S (2012) Evaluation of composite millet breads for sensory and nutritional qualities and glycemic response. Malays J Nutr 18:89–101
Devos KM, Wang ZM, Beales J, Sasaki T, Gale MD (1998) Comparative genetic maps of foxtail millet (Setaria italica) and rice (Oryza sativa). Theor Appl Genet 96:63–68
Dong Y, Duan S (2000) Production of transgenic millet plants via particle bombardment. Acta Bot Boreal-Occident Sin 20:175–178
Doust AN, Kellogg EA, Devos KM, Bennetzen JL (2009) Foxtail millet: a sequence-driven grass model system. Plant Physiol 149:137–141
Duvick J, Fu A, Muppirala U, Sabharwal M, Wilkerson MD, Lawrence CJ, Lushbough C, Brendel V (2008) PlantGDB: a resource for comparative plant genomics. Nucleic Acids Res 36:D959–D965
Fang FQ, Qian Z, Ao GM, Yu JJ (2008) Co-suppression of Si401, a maize pollen specific Zm401 homologous gene, results in aberrant anther development in foxtail millet. Euphytica 163:103–111
Geervani P, Eggum BO (1989) Nutrient composition and protein quality of minor millets. Plant Foods Hum Nutr 39:201–208
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186
Gupta S, Kumari K, Das J, Lata C, Puranik S, Prasad M (2011) Development and utilization of novel intron length polymorphic markers in foxtail millet [Setaria italica (L.) P. Beauv.]. Genome 54:586–602
Gupta S, Kumari K, Sahu PP, Vidapu S, Prasad M (2012) Sequence based novel genomic microsatellite markers for robust genotyping purposes in foxtail millet [Setaria italica (L.) P. Beauv.]. Plant Cell Rep 31:323–337
Gupta S, Kumari K, Muthamilarasan M, Subramanian A, Prasad M (2013) Development and utilization of novel SSRs in foxtail millet [Setaria italica (L.) P. Beauv.]. Plant Breed 132:367–374
Gupta S, Kumari K, Muthamilarasan M, Parida SK, Prasad M (2014) Population structure and association mapping of yield contributing agronomic traits in foxtail millet. Plant Cell Rep 33:881–893
Heng L, Chih C, Song C, Chang K (2011) Development of Simple Sequence Repeats (SSR) Markers in Setaria italica (Poaceae) and Cross-Amplification in Related Species. Int J Mol Sci 12:7835–7845
Hunt HV, Vander Linden M, Liu X, Motuzaite-Matuzeviciute G, Colledge S, Jones MK (2008) Millets across Eurasia: chronology and context of early records of the genera Panicum and Setaria from archaeological sites in the Old World. Veg Hist Archaeobot 17:5–18
Itagi S, Naik R, Bharati P, Sharma P (2012) Readymade foxtail millet mix for diabetics. Int J Sci Nat 3:131–134
Jali M, Kamatar M, Jali S, Hiremath M, Naik R (2012) Efficacy of value added foxtail millet therapeutic food in the management of diabetes and dyslipidamea in type 2 diabetic patients. Recent Res Sci Tech 4:7
Jayaraman A, Puranik S, Rai NK, Vidapu S, Sahu PP, Lata C, Prasad M (2008) cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol Biotechnol 40:241–251
Jia XP, Shi YS, Song YC, Wang YG, Wang TY, Li Y (2007) Development of EST-SSR in foxtail millet (Setaria italica). Genet Res Crop Evol 54:233–236
Jia X, Zhang Z, Liu Y, Zhang C, Shi Y, Song Y, Wang T, Li Y (2009) Development and genetic mapping of SSR markers in foxtail millet [Setaria italica (L.) P. Beauv.]. Theor Appl Genet 118:821–829
Jia G, Huang X, Zhi H, Zhao Y, Zhao Q, Li W, Chai Y, Yang L, Liu K, Lu H, Zhu C, Lu Y, Zhou C, Fan D, Weng Q, Guo Y, Huang T, Zhang L, Lu T, Feng Q, Hao H, Liu H, Lu P, Zhang N, Li Y, Guo E, Wang S, Wang S, Liu J, Zhang W, Chen G, Zhang B, Li W, Wang Y, Li H, Zhao B, Li J, Diao X, Han B (2013a) A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat Genet 45:957–961
Jia G, Shi S, Wang C, Niu Z, Chai Y, Zhi H, Diao X (2013b) Molecular diversity and population structure of Chinese green foxtail [Setaria viridis (L.) Beauv.] revealed by microsatellite analysis. J Exp Bot 64:3645–3656
Kersey PJ, Allen JE, Christensen M, Davis P, Falin LJ, Grabmueller C, Hughes DS, Humphrey J, Kerhornou A, Khobova J, Langridge N, McDowall MD, Maheswari U, Maslen G, Nuhn M, Ong CK, Paulini M, Pedro H, Toneva I, Tuli MA, Walts B, Williams G, Wilson D, Youens-Clark K, Monaco MK, Stein J, Wei X, Ware D, Bolser DM, Howe KL, Kulesha E, Lawson D, Staines DM (2014) Ensembl genomes 2013: scaling up access to genome-wide data. Nucleic Acids Res 42:D546–D552
Khan Y, Yadav A, Suresh BV, Muthamilarasan M, Yadav CB, Prasad M (2014) Comprehensive genome-wide identification and expression profiling of foxtail millet [Setaria italica (L.)] miRNAs in response to abiotic stress and development of miRNA database. Plant Cell Tiss Organ Cult 118:279–292
Krishnakumari S, Thayumanvan B (1997) Comparative study of resistant starch from minor millets on intestinal response of blood glucose, serum cholesterol and triglycerides in rats. J Sci Food Agric 73:296–302
Kumar K, Muthamilarasan M, Prasad M (2013) Reference genes for quantitative Real-time PCR analysis in the model plant foxtail millet (Setaria italica L.) subjected to abiotic stress conditions. Plant Cell Tiss Organ Cult 115:13–22
Kumari K, Muthamilarasan M, Misra G, Gupta S, Subramanian A, Parida SK, Chattopadhyay D, Prasad M (2013) Development of eSSR-markers in Setaria italica and their applicability in studying genetic diversity, cross-transferability and comparative mapping in millet and non-millet species. PLoS ONE 8:e67742
Lata C, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot 14:4731–4748
Lata C, Prasad M (2012) Validation of an allele-specific marker associated with dehydration stress tolerance in a core set of foxtail millet accessions. Plant Breed 132:496–499
Lata C, Prasad M (2013a) Setaria genome sequencing: an overview. J Plant Biochem Biotechnol 22:257–260
Lata C, Prasad M (2013b) Association of an allele-specific marker with dehydration stress tolerance in foxtail millet suggests SiDREB2 to be an important QTL. J Plant Biochem Biotechnol 23:119–122
Lata C, Sahu PP, Prasad M (2010) Comparative transcriptome analysis of differentially expressed genes in foxtail millet (Setaria italica L.) during dehydration stress. Biochem Biophy Res Commun 393:720–727
Lata C, Jha S, Dixit V, Sreenivasulu N, Prasad M (2011a) Differential antioxidative responses to dehydration-induced oxidative stress in core set of foxtail millet cultivars [Setaria italica (L.)]. Protoplasma 248:817–828
Lata C, Bhutty S, Bahadur RP, Majee M, Prasad M (2011b) Association of an SNP in a novel DREB2-like gene SiDREB2 with stress tolerance in foxtail millet [Setaria italica (L.)]. J Exp Bot 62:3387–3401
Lata C, Gupta S, Prasad M (2013) Foxtail millet: a model crop for genetic and genomic studies in bioenergy grasses. Crit Rev Biotechnol 33:328–343
Le Thierry d’Ennequin M, Panaud O, Toupance B, Sarr A (2000) Assessment of genetic relationship between Setaria italica and its wild relative S. viridis using AFLP markers. Theor Appl Genet 100:1061–1066
Li P, Brutnell TP (2011) Setaria viridis and Setaria italica, model genetic systems for Panicoid grasses. J Exp Bot 62:3031–3037
Li W, Zhi H, Wang Y-F, Li H-Q, Diao X (2012) Assessment of genetic relationship of foxtail millet with its wild ancestor and close relatives by ISSR markers. J Integr Agric 11:556–566
Li C, Yue J, Yu J (2014) An ABA responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, played critical role under drought stress. In: International Setaria Genetics Conference, pp 62
Liu Y, Yu J, Zhao Q, Zhu D, Ao G (2005) Genetic transformation of millet (Setaria italica) by Agrobacterium. J Agric Biotechnol 13:32–37
Liu Y, Yu J, Ao G, Zhao Q (2007) Factors influencing Agrobacterium mediated transformation of foxtail millet (Setaria italica). Chin J Biochem Mol Biol 23:531–536
Mauro-Herrera M, Wang X, Barbier H, Brutnell TP, Devos KM, Doust AN (2013) Genetic control and comparative genomic analysis of flowering time in Setaria (Poaceae). G3 (Bethesda) 3:283–295
Mishra AK, Puranik S, Bahadur RP, Prasad M (2012a) The DNA-binding activity of an AP2 protein is involved in transcriptional regulation of a stress-responsive gene, SiWD40, in foxtail millet. Genomics 100:252–263
Mishra AK, Puranik S, Prasad M (2012b) Structure and regulatory networks of WD40 protein in plants. J Plant Biochem Biotechnol 21:32–39
Mishra AK, Muthamilarasan M, Khan Y, Parida SK, Prasad M (2013) Genome-wide investigation and expression analyses of WD40 protein family in the model plant foxtail millet (Setaria italica L.). PLoS ONE 9:e86852
Monaco MK, Stein J, Naithani S, Wei S, Dharmawardhana P, Kumari S, Amarasinghe V, Youens-Clark K, Thomason J, Preece J, Pasternak S, Olson A, Jiao Y, Lu Z, Bolser D, Kerhornou A, Staines D, Walts B, Wu G, D’Eustachio P, Haw R, Croft D, Kersey PJ, Stein L, Jaiswal P, Ware D (2013) Gramene 2013: comparative plant genomics resources. Nucleic Acids Res 42:D1193–D1199
Muthamilarasan M, Prasad M (2013) Cutting-edge research on plant miRNAs. Curr Sci 104:287–289
Muthamilarasan M, Theriappan P, Prasad M (2013a) Recent advances in crop genomics for ensuring food security. Curr Sci 105:155–158
Muthamilarasan M, Venkata Suresh B, Pandey G, Kumari K, Parida SK, Prasad M (2013b) Development of 5123 intron-length polymorphic markers for large-scale genotyping applications in foxtail millet. DNA Res 21:41–52
Muthamilarasan M, Bonthala VS, Mishra AK, Khandelwal R, Khan Y, Roy R, Prasad M (2014) C2H2-type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Funct Integr Genomics 14:531–543
Pandey G, Misra G, Kumari K, Gupta S, Parida SK, Chattopadhyay D, Prasad M (2013) Genome-wide development and use of microsatellite markers for large-scale genotyping applications in foxtail millet [Setaria italica (L.)]. DNA Res 20:197–207
Pawar VS, Pawar VD (1997) Malting characteristics and biochemical changes of foxtail millet. J Food Sci Technol 34:416–418
Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, McGarvey KM, Murphy MR, O’Leary NA, Pujar S, Rajput B, Rangwala SH, Riddick LD, Shkeda A, Sun H, Tamez P, Tully RE, Wallin C, Webb D, Weber J, Wu W, Dicuccio M, Kitts P, Maglott DR, Murphy TD, Ostell JM (2014) RefSeq: an update on mammalian reference sequences. Nucleic Acids Res 42:D756–D763
Puranik S, Bahadur RP, Srivastava PS, Prasad M (2011a) Molecular cloning and characterization of a membrane associated NAC family gene, SiNAC from foxtail millet [Setaria italica (L.) P. Beauv.]. Mol Biotechnol 49:138–150
Puranik S, Jha S, Srivastava PS, Sreenivasulu N, Prasad M (2011b) Comparative transcriptome analysis of contrasting foxtail millet cultivars in response to short-term salinity stress. J Plant Physiol 168:280–287
Puranik S, Kumar K, Srivastava PS, Prasad M (2011c) Electrophoretic Mobility Shift Assay reveals a novel recognition sequence for Setaria italica NAC protein. Plant Signal Behav 6:1588–1590
Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17:369–381
Puranik S, Sahu PP, Mandal SN, Venkata Suresh B, Parida SK, Prasad M (2013) Comprehensive genome-wide survey, genomic constitution and expression profiling of the NAC transcription factor family in Foxtail Millet (Setaria italica L.). PLoS ONE 8:e64594
Qi X, Xie S, Liu Y, Yi F, Yu J (2013) Genome-wide annotation of genes and noncoding RNAs of foxtail millet in response to simulated drought stress by deep sequencing. Plant Mol Biol 83:459–473
Rao BR, Nagasampige MH, Ravikiran M (2011) Evaluation of nutraceutical properties of selected small millets. J Pharm Bioallied Sci 3:277–279
Sahu PP, Rai NK, Chakraborty S, Singh M, Prasanna HC, Ramesh B, Chattopadhyay D, Prasad M (2010) Tomato cultivar tolerant to Tomato leaf curl New Delhi virus infection induces virus-specific siRNA accumulation and defense associated host gene expression. Mol Plant Pathol 11:531–544
Sahu PP, Pandey G, Sharma N, Puranik S, Muthamilarasan M, Prasad M (2013) Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep 32:1151–1159
Shaik R, Ramakrishna W (2012) Bioinformatic analysis of epigenetic and microRNA mediated regulation of drought responsive genes in rice. PLoS ONE 7:e49331
Sharma N, Sahu PP, Puranik S, Prasad M (2013) Recent advances in plant-virus interaction with emphasis on small interfering RNAs (siRNAs). Mol Biotech 55:63–77
Suresh BV, Muthamilarasan M, Mishra G, Prasad M (2013) FmMDb: a versatile database of foxtail millet markers for millets and bioenergy grasses research. PLoS ONE 8:e71418
Thathola A, Srivastava S, Singh G (2010) Effect of foxtail millet (Setaria italica) supplementation on serum glucose, serum lipids and glycosylated hemoglobin in type 2 diabetics. Diabetologia Croatica 40:23–28
Vetriventhan M, Upadhyaya HD, Anandakumar CR, Senthilvel S, Varshney RK, Parzies HK (2014) Population structure and linkage disequilibrium of ICRISAT foxtail millet (Setaria italica (L.) P. Beauv.) core collection. Euphytica 196:423–435
Wang ZM, Devos KM, Liu CJ, Wang RQ, Gale MD (1998) Construction of RFLP-based maps of foxtail millet, Setaria italica (L.) P. Beauv). Theor Appl Genet 96:31–36
Wang L, Peterson RB, Brutnell TP (2011) Regulatory mechanisms underlying C4 photosynthesis. New Phytol 190:9–20
Wang C, Jia G, Zhi H, Niu Z, Chai Y, Li W, Wang Y, Li H, Lu P, Zhao B, Diao X (2012) Genetic diversity and population structure of Chinese foxtail millet [Setaria italica (L.) Beauv.] landraces. G3 (Bethesda) 2:769–777
Wang Y, Liu H, Xin Q (2014) Genome-wide analysis and identification of cytokinin oxidase/dehydrogenase (CKX) gene family in foxtail millet (Setaria italica). Crop J 2:244–254
Xu J, Li Y, Ma X, Ding J, Wang K, Wang S, Tian Y, Zhang H, Zhu XG (2013) Whole transcriptome analysis using next-generation sequencing of model species Setaria viridis to support C4 photosynthesis research. Plant Mol Biol 83:77–87
Yadav CB, Muthamilarasan M, Pandey G, Prasad M (2014a) Identification, characterization and expression profiling of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in foxtail millet. Plant Mol Biol Rep doi:10.1007/s11105-014-0736-y
Yadav CB, Muthamilarasan M, Pandey G, Khan Y, Prasad M (2014b) Development of novel microRNA-based genetic markers in foxtail millet for genotyping applications in related grass species. Mol Breed. doi:10.1007/s11032-014-0137-9
Yi F, Xie S, Liu Y, Qi X, Yu J (2013) Genome-wide characterization of microRNA in foxtail millet (Setaria italica). BMC Plant Biol 13:212
Yin H, Yu Q, Dai Y, An L, Li W (2014) Transcript profiling of foxtail millet seedlings under drought condition and discovery of a new drought- tolerant gene. In: International Setaria Genetics Conference, pp 24
Zhang J, Liu T, Fu J, Zhu Y, Jia J, Zheng J, Zhao Y, Zhang Y, Wang G (2007) Construction and application of EST library from Setaria italica in response to dehydration stress. Genomics 90:121–131
Zhang G, Liu X, Quan Z, Cheng S, Xu X, Pan S, Xie M, Zeng P, Yue Z, Wang W, Tao Y, Bian C, Han C, Xia Q, Peng X, Cao R, Yang X, Zhan D, Hu J, Zhang Y, Li H, Li H, Li N, Wang J, Wang C, Wang R, Guo T, Cai Y, Liu C, Xiang H, Shi Q, Huang P, Chen Q, Li Y, Wang J, Zhao Z, Wang J (2012) Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol 30:549–554
Zhang S, Tang C, Zhao Q, Li J, Yang L, Qie L, Fan X, Li L, Zhang N, Zhao M, Liu X, Chai Y, Zhang X, Wang H, Li Y, Li W, Zhi H, Jia G, Diao X (2014) Development of highly polymorphic simple sequence repeat markers using genome-wide microsatellite variant analysis in Foxtail millet [Setaria italica (L.) P. Beauv]. BMC Genom 2:15
Zhi H, Jia G, Niu Z, Liu X, Ge Y, Chai Y, Diao X (2014) Construction of an RIL population and segment introgression lines via interspecific cross between Setaria italica and S. viridis. In: International Setaria Genetics Conference, pp 50
Zhu JK (2014) An ABA mimicking ligand that reduces water loss and promotes drought resistance in plants. In: International Setaria Genetics Conference, pp 9
Zhu C, Ming C, Zhao-Shi X, Lian-Cheng L, Xue-Ping C, You-Zhi M (2014) Characteristics and expression patterns of the Aldehyde Dehydrogenase (ALDH) Gene Superfamily of Foxtail Millet (Setaria italica L.). PLoS ONE 9:e101136
Acknowledgments
The authors’ work in the area of millet genomics was supported by the core grant of National Institute of Plant Genome Research (NIPGR), New Delhi and Department of Biotechnology, Government of India. MM acknowledges the award of Research Fellowship from University Grants Commission, New Delhi, India. Authors are grateful to Dr. Swarup K Parida, NIPGR for critically reading the manuscript. Authors also thank the reviewers for their constructive comments.
Conflict of interest
No conflict of interest declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rajeev K. Varshney.
Authors sincerely bestow this article to Prof. Arun Kumar Sharma, Botany Department, Calcutta University, Kolkata, India, on the occasion of his 90th birthday.
Rights and permissions
About this article
Cite this article
Muthamilarasan, M., Prasad, M. Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses. Theor Appl Genet 128, 1–14 (2015). https://doi.org/10.1007/s00122-014-2399-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2399-3