Abstract
Exceptional natural phenomena, such as those that occur during a total solar eclipse, provide unique opportunities to study animal behavior outside the naturally evolved context, which can be informative in more general terms. Circumstantial descriptions of abnormal animal behavior during solar eclipses abound, although scientific studies conducted during an eclipse are relatively rare due to inherent logistical difficulties. Here, honey bee foraging and homing behavior were studied during the total solar eclipse of August 21, 2017. In the first experiment, we studied foraging behavior of honey bees during the progression of the solar eclipse and found that the foraging activity drastically decreased but did not completely cease during the totality of the eclipse, in contrast to previous reports of complete cessation. The data indicate that the level of ambient light can largely overrule the internal circadian rhythm of foraging honey bees. Furthermore, colonies with a higher need for foraging decreased their foraging activity less than satiated colonies, consistent with the hypothesis that individual foraging decisions may be influenced by colony state, which affects cost-benefit analyses. In a second experiment, the temporal dynamics of homing of released workers and drones was compared in periods before, during, and after the solar eclipse. During the totality of the eclipse, very few bees arrived back at their hive, while homing before the total eclipse was accelerated, particularly in drones. The results suggest that, while the homing abilities of honey bees are not compromised until the sun is completely eclipsed, they may still interpret the diminishing light as an indicator of deteriorating flight conditions. Our unique study provides some insight into the control of honey bee foraging behavior when external cues and internal circadian rhythms are at odds, lent support to the notion that food deprivation can lead to riskier foraging, and indicated that homing in honey bees is possible even with very small amounts of sunlight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daylight from the sun is critical for many biological processes in most organisms. The regular alternation between day and night has led to the evolution of circadian clocks that allow animals to make anticipatory adjustments in physiology and behavior (Jagannath et al. 2017). Accordingly, the endogenous molecular mechanisms that control the circadian clock are entrained by light as the most important external zeitgeber (Roenneberg et al. 2003). Circadian rhythms persist in constant light or darkness, and the internal circadian clock can slowly adjust to shifted dark-light rhythms (Golombek and Rosenstein 2010). Experimental perturbations of a species’ light regime have been frequently employed to conduct detailed studies of circadian rhythms under laboratory conditions (Pilorz et al. 2018). However, aberrant light conditions in nature are rare, leaving a void of chronobiological field studies, particularly those that are relevant for understanding animal behavior. A total solar eclipse, the phenomenon during which the moon casts a shadow onto the earth by fully blocking the sun, provides a rare opportunity to study animal behavior under abnormal lack-of-light conditions in nature. The eclipse creates conditions in which a species’ internal circadian clock is suddenly in strong conflict with the external zeitgeber. However, total solar eclipses are very rare and occur in limited geographic areas, imposing logistical difficulties for scientific studies. These limitations may explain the paucity of published reports of natural phenomena during total solar eclipses, despite strong public interest and abundant circumstantial observations of nature during such events.
The sun also aids many animals, including birds and insects, in orienting and navigating their environments. The position of the sun and the resulting polarization pattern of the sky provide directional information for compass orientation (Homberg 2004). Homing behavior is an important function of orientation and can occur via landmark orientation and path integration, depending on the species and the familiarity of an individual with the terrain (Collett et al. 2013). The sun compass is integrated with the circadian clock to compensate for the angular movement of the sun (Scapini et al. 2005) and it is typically combined with further navigational strategies to reduce errors (Collett and Graham 2004). On overcast days or other partial blockage of sunlight, the light polarization pattern is sufficient to navigate for most insects, including honey bees (Evangelista et al. 2014). However, missing directional information when the sun is completely blocked can severely disrupt animal orientation, despite the potential use of backup systems (Dyer and Could 1983). A solar eclipse temporarily eliminates the directional information usually provided by the sun and also reduces ambient light to levels where general visual orientation may be impossible (Ugolini et al. 2004), as has been demonstrated for the ant Cataglyphis bicolor (Délye 1974). Successful orientation is required for all actively moving animals and is particularly important for central place foragers that return to a nest. Honey bees and other insects use path integration to compute a straight path back to the nest after foraging and have to combine information about compass direction and distance. In the absence of the celestial compass, honey bee foragers must rely on estimates of body rotations to compute a homing vector, a process that can lead to errors (Heinze et al. 2018). Conditions, such as dawn or dusk, can also compromise homing abilities, therefore making foraging riskier (Rivera et al. 2015).
The profitability of foraging may increase in risky conditions because competition is decreased when most animals are risk-sensitive and decrease foraging activity. Risk sensitivity of foraging behavior is generally studied in the context of variable versus constant rewards (Kacelnik and Bateson 1996). The animals’ energy budget also plays a critical role for the risk-sensitivity (McNamara and Houston 1992), influencing decisions that balance an animal’s food intake and mortality risk (Nonacs and Dill 1990). In social insects, many individuals integrate a colony’s foraging efforts and stored resources buffer against short-term fluctuations in food supply, making these species less sensitive to individual foraging variability (Banschbach and Waddington 1994; Hübner and Czaczkes 2017). However, the overall energy budget of the colony affects risk sensitivity in other regards, such as making riskier foraging decisions to increase colony food intake (Fewell and Winston 1992; Schulz et al. 1998). In honey bees, the nurse bees determine and inform foragers about the colony’s nutritional needs and foragers respond accordingly by trading off the risks associated with foraging with benefits gained from addressing the colony’s needs (Camazine 1993). Foraging during adverse weather is one form of risky foraging (Woyciechowski and Kozlowski 1998). Honey bees do not forage at night, presumably to avoid the risk of not finding food sources or failing to return home. However, this has not been directly tested and alternative explanations, such as primarily diurnal nectar production by flowers, may explain the absence of night foraging (Bloch et al. 2017). Moreover, it is unknown how the perceived risk of disorientation in the dark is influenced by a colony’s nutritional status. Interestingly, control of circadian foraging patterns and sensitivity to disorientation risk can be explored independently of nectar availability during the unusual circumstances of a solar eclipse.
Limited information is available about animal behaviors during total solar eclipses due to the rare frequency of these events. Birds, cattle, bees, and horses exhibited extreme and unusual behaviors during the 1999 total solar eclipse in Turkey (Özbey et al. 2004) and the buzzing of pollinators is significantly reduced (Galen et al. 2018). Honey bees are among the most commonly observed animals during solar eclipses, but most observations remain anecdotal and have led to a scientific consensus that foraging activity is only reduced during partial solar eclipses and stops completely during full eclipses (Briceno and Ramirez 1993; Roonwal 1956). Some compensatory increases in foraging activity may occur during the time directly before and after an eclipse (Briceno and Ramirez 1993), which may indicate a conflict between the bees’ circadian clock and external conditions. However, the effects on foraging behavior of honey bees at the beginning of an eclipse differ from the effects towards the end (Woyke et al. 2000). This directionality in the foraging behavior suggests that the diminishing light at the beginning of the eclipse elicits behaviors normally exhibited during sunset, while the rapidly increasing light at the end of the eclipse may simulate foraging behaviors more commonly performed at sunrise. It is unclear how general these previous observations are, however, given the rarity of observations and considerable variation among honey bee colonies (Woyke et al. 2000). We hypothesized that colony energy budgets may be responsible for variable foraging decisions during solar eclipses according to the foragers’ perceived risk-to-benefit ratio. Based on this hypothesis, we predicted that starved colonies would be more risk-prone and would be more willing to engage in riskier, yet potentially more rewarding foraging activities during the conditions of a solar eclipse, compared to satiated colonies. We tested this prediction in a field experiment, in which we monitored the foraging activities of starved and fed colonies before, during, and after the total solar eclipse on 21 August 2017 in Clemson, SC, USA. In addition, we studied the homing behavior of released workers and drones to test our assumption that foraging during a solar eclipse is riskier than during regular times.
Materials and methods
Two experiments were performed at the Cherry Farm Insectary at Clemson University (34.67° N, 82.84° W), located in the center of the path of the total solar eclipse on 21 August 2017. Eleven experimental colonies of the Western honey bees (Apis mellifera) were selected from the Clemson Apiary for the experiment. We chose 20 August as a control day, which was comparable to the following day, except for the solar eclipse. On 21 August, the solar eclipse started at 13:08 and ended at 16:02 local time, with the period of total eclipse between 14:37 and 14:39 (for a total of 2 min and 37 s). Ambient light, temperature, and relative humidity were monitored every 30 min using an air thermometer (GSP-6, Elitech Technology Inc., CA, USA), a light meter (Fisherbrand Traceable, Fisher Scientific Inc., PA, USA), and a RH meter (GSP-6, Elitech Technology Inc., CA, USA), respectively.
Experiment 1
To test how the energy budget of a colony affects the modulation of foraging activity during a solar eclipse, five pairs of colonies in standard 10-frame Langstroth hives were set up 2 days before the onset of the experiment. The paired hives were located next to each other and each pair contained one colony that was manipulated to create a strong need for foraging through the addition of brood (B+) and removal of food (F−) and one colony with little need for foraging through the removal of brood (B−) and addition of food (F+). The “B+F−” colonies contained four frames of brood, two frames of honey and pollen, and four empty frames and the “B−F+” colonies contained two frames of brood, six frames of honey and pollen, and two empty frames. The colonies were paired according to their initial size and composition. Colony treatments were assigned randomly and observers remained blind to the treatment of each colony for the duration of the experiments. The composition (number of brood frames, number of honey frames, and approx. number of total bees) of these hives was assessed prior to and after the experiment and relative changes in colony composition were calculated (Table S1).
Thirty-minute observation periods were spread out during the control and eclipse day at identical times (Fig. 1), such that one observation was made before the start of the solar eclipse (pre-O), five observations were made within eclipse window (O1–O5), and one observation was made after the completion of the solar eclipse (post-O). This design was symmetrically centered on the brief period of complete darkness in the middle of O3 which took place between 14:37 and 14:39 local time.
Observational timeline for the eclipse day. Five 30-min observation periods were determined to symmetrically cover the total solar eclipse (O1 to O5). The 2:37 min period of totality fell exactly in the middle of O3. Two additional observation periods were also performed before and after the eclipse (designated as pre-O and post-O). The same timeline was followed for the control day observations on 20 August 2017
Each colony pair was monitored by one observer. Separate manual hand counters were used to record the number of departing and returning individuals simultaneously for one of the two colonies in a pair. During each 30-min observation period, the observers alternated ten times between the two paired colonies, monitoring first one hive entrance and then the other for 1 min each, separated by 30-s intervals between observations. The raw counts of the number of departing and returning foragers were summed to determine overall foraging activity per minute. The ten measures per colony were averaged to calculate an overall foraging activity value. These values for each colony and observation period were computed into relative foraging activities on the eclipse day by subtracting the values of the control day from the corresponding values on the eclipse day (∆F = FEclipse − FControl). Overall foraging activity and relative foraging activity were compared between “B+F−” and “B−F+” colonies.
Experiment 2
A separate colony that was isolated from experiment 1 was used for comparing the pattern of homing behavior of released workers and drones in 40-min intervals before, during, and after the eclipse. The first observation period was performed from 10:36 to 11:16 (pre-eclipse), the second observation from 14:27 to 15:07 PM was done during the solar eclipse and included the total eclipse period, and the final observation was made post-eclipse in the evening from 17:15 to 17:55. The two-story hive contained approximately 40,000 bees, including several thousand drones. At each observation period, 500–750 bees were collected by gently brushing one frame of bees from the hive’s periphery into a large plastic jar. The exact number of bees could not be determined due to variable numbers of drones and workers in the jar. The bees were transported 192 m away from their hive and coated in fluorescent powder (Glominex LLC, Irving, TX, USA), using a different color for each observation period. Just before the bees were released, one observer was stationed at the hive and continuously monitored the entrance for returning bees marked with fluorescent powder. The number of painted drones and workers was counted in 1-min intervals for a total duration of 40 min.
Results
Experiment 1
The patterns of foraging activity during the control and eclipse days are shown in Fig. 2. Foraging activity was not significantly different at the beginning of the eclipse day, but it was significantly reduced during the eclipse day compared with the control day during the middle (O3) and second half (O4 to Post-O) of the total solar eclipse (Fig. 2, Table 1). Across all observation periods on both days, foraging activity was only significantly related to relative humidity (R2 = 0.58, n = 14, p < 0.001) but not to ambient light (R2 = 0.19, n = 14, p < 0.065) or temperature (R2 = 0.17, n = 14, p = 0.078). Foraging activity was most severely reduced during and directly after the total eclipse but did not stop completely. After the eclipse was completely over, foraging on the eclipse day (post-O) was increased relative to the day before (Table 1).
Foraging activity of ten honey bee colonies and abiotic variables compared between eclipse and control day. a The numbers of departing and returning honey bees was recorded in 3-min intervals separately for each colony pair and averaged across all five pairs to illustrate the overall foraging patterns throughout the control (dashed line) and eclipse day (solid line). The overall impact of the eclipse is strongest near the period of total eclipse (indicated by the vertical dashed line in O3), but also depressed foraging for several hours afterwards. During the post-eclipse interval, foraging was higher on the eclipse day than on the control day, possibly indicating compensatory foraging. The data are not continuous because observation intervals alternated with non-observation intervals. b Concomitant to the foraging counts, light intensity, temperature, and relative humidity were measured on the control and eclipse days
The number of departing and returning forager numbers was not significantly different between the control day and the eclipse day before and at the beginning of the eclipse (pre-O to O2) and during the post-eclipse observation interval (post-O), while significant differences were observed during O3 to O5 (Table S2). To test more specifically for directionality effects, foraging activity during the eclipse day was compared before and after the period of total eclipse. Foraging activity was significantly lower directly after than directly before the eclipse (second half of the O3 versus first half of the O3: t = 2.8, n = 8, p = 0.023), but it was not significantly different between the O4 and O2 (t = 1.72, n = 19, p = 0.1). Across all colonies, the number of departing workers relative to returning foraging was also significantly lower directly before the eclipse (first half of O3) compared to the period directly after the eclipse (second half of O3; Fisher’s exact test, p = 0.001) whereby 69.8% of foragers were observed returning home during the first half while only 62.1% of foragers were returning foragers in second half of O3. We found no significant difference between O4 and O2 for the number of departing and returning foragers (Fisher’s exact test, p value = 0.93).
During the control and eclipse day, the average foraging activity of “B+F−” colonies (control day = 30.14 ± 11.03; eclipse day = 23.61 ± 7.85) was lower than that of “B−F+” colonies (control day = 38.38 ± 15.00, eclipse day = 30.97 ± 10.26), although the difference was only significant for the eclipse day (paired t test: t = 4.4, n = 5, p = 0.011). Relative foraging on the eclipse day relative to the control day (∆F) was also affected by the hive manipulations: The “B+F−” colonies exhibited a significantly smaller reduction in foraging activity than the “B−F+” colonies during O4 (paired t test: t = 4.8, n = 5, p = 0.001) and O5 (t = 5.0, n = 5, p = 0.001) on eclipse day. Additionally, they exhibited a significantly smaller increase during post-O (t = 4.1, n = 5, p = 0.003) (Fig. 3). The “B+F−” and “B−F+” colonies did not differ in changes of colony composition over the course of the experiment, except that nectar/honey quantities in the “satiated” colonies increased overall by 167%, while they decreased in the “food deprived” colonies by 16% on average, which was significantly different (t = 3.7, n = 10, p = 0.006).
Impact of colony food need on relative foraging activity during the solar eclipse. Colonies with added brood and removed food (B+F−, black bars) adjusted their total foraging activity less than colonies with removed brood and added food (B−F+, gray bars) in response to the solar eclipse. The difference between eclipse and control day (∆F) was not as negative during and directly after the peak of the eclipse (O3–O5) and not as positive after the solar eclipse was over (post-O) in B+F− than in B−F+ colonies. Means with 95% confidence intervals are shown
Experiment 2
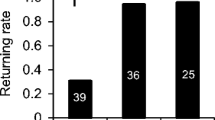
The arrival patterns of returning bees differed significantly among the three observation periods for workers (Fig. 4a; log-rank test, χ2 = 128.8, n = 3, p < 0.001) and drones (Fig. 4b; Log-rank test, χ2 = 24.4, n = 3, p < 0.001). Overall, workers returned to the test hive fastest during the evening period (16.37 ± 0.77 min), followed by the morning period (21.36 ± 0.91 mins) and then the mid-day eclipse period (24.29 ± 1.27 min). Drones also returned fastest in the evening (13.30 ± 1.42 min) but returned faster during the eclipse (16.29 ± 1.47 min) than during the morning period (23.18 ± 3.39 min). The number of workers and drones during the eclipse compared to the other observation periods increased 10 min before total darkness and decreased thereafter (Fig. 4). This effect was more pronounced in drones, with over 35% returning to the hive before total darkness. The unknown number of released bees precluded a calculation of individuals that did not return to their colony during the observation period.
Homing dynamics of workers and drones before, during, and after the solar eclipse. Cumulative arrival of workers (a) and drones (b) in the homing experiment differed among all three periods. The overall return of both castes was fastest in the evening period (observation period 3). On average, workers returned faster during the morning than during the mid-day eclipse period, but for drones the opposite was true. The number of individuals returning during the eclipse period was increased just before total darkness and decreased during and after total darkness for workers and drones, although the effects were much more pronounced in drones. Across the three observation periods the number of returning individuals varied for drones between 38 and 221 and for workers between 309 and 562. These numbers were used to standardize each curve
Discussion
Extraordinary natural events provide unique opportunities to carry out experiments that can help explain general biological phenomena. Our systematic studies of honey bee homing and foraging behavior during the 2017 total solar eclipse provided information on how (1) conflicts between the internal circadian rhythm of honey bees, abiotic external conditions, and food availability are resolved in the control of foraging activity; (2) the lack of celestial orientation cues affects the homing abilities of honey bees; and (3) food deprivation at the colony level can lead to relatively higher risk tolerance in foraging decisions.
Our observations of colony foraging rates revealed a drastic but incomplete reduction of foraging activity when the sun was fully covered and a subsequent depression of foraging activity until the end of the eclipse. Our results were consistent with earlier behavioral studies that reported either a strong reduction or complete cessation of foraging activity during solar eclipses in honey bees (Briceno and Ramirez 1993; Woyke et al. 2000). A concomitant acoustic monitoring of the 2017 eclipse reported an almost complete cessation of buzzing among flowers (Galen et al. 2018). The distinction between complete cessation and strong reduction of foraging may largely be a question of sample size. We used ten hives and almost continuous sampling with five observers, which enabled us to detect a few departing and returning foragers in almost complete darkness. Apis mellifera and particularly its relative, the giant honey bee (A. dorsata), regularly forage at low light conditions, enabled by special visual adaptations (Warrant et al. 1996). Our observations of sporadic foraging activity during the total solar eclipse are therefore not trivial because they suggest that foraging is possible, even though most workers elect to cease foraging during the phase of complete darkness.
The decision to cease foraging at an unusual time of day may be triggered by several environmental variables. To test the effects of some of these variables on foraging decisions during an eclipse, we measured light intensity, temperature, and relative humidity as potential external cues that might change with a total eclipse of the sun. These variables are normally correlated (Clarke and Robert 2018) but only relative humidity showed a significant correlation with foraging activity across both days. This contrast to previous studies that identify temperature and light as most significant influences on honey bee foraging (Devillers et al. 2004; Clarke and Robert 2018) may be due to the exceptional temporal pattern of change during the solar eclipse. Although we did not measure nectar or pollen availability in the food plants of our honey bees and some flowers close during the solar eclipse (Kullenberg 1955), it is possible that some plants fail to adjust to the short, exceptional event of the eclipse. Thus, the eclipse observations may demonstrate a foraging reduction regardless of nectar and pollen availability, which may confound temporal foraging patterns under normal circadian circumstances (Corbet 1990; Bloch et al. 2017). It is possible that the environmental changes affected the internal clocks of the workers due to decreasing light serving as a major zeitgeber for their circadian rhythm (Moore and Rankin 1985; Moore and Rankin 1993; Ish-Am and Eisikowitch 1998; Puškadija et al. 2007; Lee et al. 2016). A more likely explanation is that the increasing relative humidity, as well as decreasing light and temperature overruled the bees’ internal clock and other influences, such as recruitment dances that may have continued during the actual eclipse within the hives. Such short-term adjustments of foraging behavior are not likely to have evolved in response to solar eclipse conditions due to the rarity of these events. However, flexible adjustment of foraging behavior may be important in other circumstances, such as inclement weather (Riddell Pearce et al. 2013) and relative humidity may be an accurate predictor of such events in the Southeastern USA.
Our observations did confirm previous work reporting that the temporal dynamics and directionality, of the solar eclipse event matters to honey bees when making foraging decisions (Woyke et al. 2000). Overall foraging activity was more strongly decreased after the period of total darkness than it was before the event. Furthermore, the ratio of the number of departing versus returning foragers was significantly lower before the total eclipse than it was after the eclipse ended. These and other before-and-after comparisons are typically conducted under the assumption that light intensity decreases before the period of total eclipse and increases after the total eclipse in a similar fashion. However, our measurements demonstrated that this was not the case. Thus, we cannot exclude the possibility that the differences in foraging activity before and after the total eclipse were due to the absolute values of environmental variables rather than their relative change (Woyke et al. 2000). In general, relative change of environmental variables has not been sufficiently studied in the context of foraging activity, even though it may be an important environmental cue that can indicate the approach of nightfall or inclement weather. The higher ratio of the number of departing versus returning foragers before and after the eclipse could be interpreted as the bees responding to relative changes. However, the different ratios of departing and returning foragers are also a logical consequence of a temporary interruption in foraging activity. Interestingly, bees foraged significantly more after the eclipse was over (Fig. 2: post-O). Thus, our experiment fails to provide conclusive support for the argument that directional changes in light intensity or other environmental variables, such as relative humidity, guide honey bee foraging behavior but indicates that this possibility deserves further testing.
Our test of the assumption that homing behavior is more difficult during a solar eclipse and thus foraging can be considered riskier, yielded inconclusive results during the second experiment. Relative to the control conditions before and after the eclipse, released bees were returning faster to their source hive just before the total eclipse occurred, suggesting that they are capable of successfully orienting back to their hive under low light conditions. The accelerated rate of returns just before the total eclipse, which was particularly pronounced in drones compared to workers, cannot be explained by a difference in flight velocity because workers can fly faster than drones (Hrassnigg and Crailsheim 2005). While workers can survive a night outside their hive, such survival is unlikely for drones (O. Rueppell, pers. observation). Therefore, we interpret the eclipse-induced faster return of the drones to their hive as a more targeted homing of drones than workers during unfavorable conditions. During the period of near total darkness, both worker and drone returns were considerably slower than during the other periods and during the remainder of the eclipse period (Fig. 4). This effect was also more pronounced in drones than in workers, indicating their greater sensitivity to environmental conditions. Overall, the reduced speed of return provided at least partial support for our assumption that flight and foraging during the an eclipse period could be perceived as more hazardous by the bees, which is important for the interpretation of the foraging differences between the food- and brood-manipulated colonies in the first experiment.
Contrary to our expectations, colonies with more stored food and less brood (B−F+) exhibited a higher overall foraging activity than colonies with more brood and less food (B+F−). Our drastic and simultaneous manipulation of food stores and brood quantities may explain these results. Normally, the presence of brood increases foraging in honey bee colonies, and brood pheromones specifically increase pollen foraging (Pankiw et al. 1998; Peso and Barron 2014; Traynor et al. 2015; Ma et al. 2018). Our “B+F−” colonies not only had more brood to feed but also experienced a strong reduction in food stores prior to the experimental observations. This relative change may have been interpreted by the bees as an indicator of a generally unfavorable foraging environment, leading these manipulated hives to reduce their foraging efforts despite the presence of needy larvae. The “B+F−” colonies may have been forced to switch from a rate-maximizing to an efficiency-maximizing foraging strategy. A behavioral reversal from foraging to nursing in workers may be an additional explanation of their lower overall foraging effort. Furthermore, “B+F−” colonies may have started to cannibalize brood (Schmickl and Crailsheim 2001) to meet their nutritional needs. Regardless of mechanism, the colony inspections indicated that the “B+F−” colonies further lost nectar/honey stores over the experimental period, while the “B−F+” colonies gained more nectar/honey stores. This difference corroborates the observed differences in foraging activity, although a lack of brood feeding in the “B−F+” colonies possibly contributed also to their gain of food stores. In contrast, the colonies’ pollen stores, that are typically tightly regulated (Fewell and Winston 1992), did not significantly change.
Despite the decreased foraging effort of the “B+F−” colonies overall, their relative foraging activity was higher than that of the “B−F+” colonies during the intervals O3–O5 during the eclipse day. These time intervals included the period when foraging activity generally was depressed compared to the control day and included the total eclipse period. Thus, “B+F−” colonies did not reduce their foraging activity as much as “B−F+” colonies during unfavorable conditions. This observation confirmed our prediction that higher food demand forces honey bee workers to be more risk prone (Nonacs and Dill 1990; Schulz et al. 1998). Foraging under risky conditions can be energetically more rewarding because nectar and pollen sources are less depleted by potential competitors that are more risk averse. Thus, the unusual conditions of a solar eclipse provided us with a unique opportunity for an unambiguous demonstration of the modulation of individual risk sensitivity in response to colony food requirements. This adjustment of foraging behavior represents a behavioral strategy of social insect colonies to overcome food shortages in addition to energy-saving decreases in overall activity (Rueppell and Kirkman 2005) and flexible brood rearing behavior (Willard et al. 2011).
References
Banschbach VS, Waddington KD (1994) Risk-sensitive foraging in honey bees: no consensus among individuals and no effect of colony honey stores. Anim Behav 47:933–941
Bloch G, Bar-Shai N, Cytter Y, Green R (2017) Time is honey: circadian clocks of bees and flowers and how their interactions may influence ecological communities. Philos Trans R Soc B 372:20160256
Briceno R, Ramirez W (1993) Activity of Apis mellifera (Hymenoptera: Apidae) and some spiders (Araneidae) during the 1991 total solar eclipse in Costa Rica. Rev Biol Trop 41:291–293
Camazine S (1993) The regulation of pollen foraging by honey bees: how foragers assess the colony’s need for pollen. Behav Ecol Sociobiol 32:265–272
Clarke D, Robert D (2018) Predictive modelling of honey bee foraging activity using local weather conditions. Apidologie 49:386–396
Collett TS, Graham P (2004) Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol 14:R475–R477
Collett M, Chittka L, Collett TS (2013) Spatial memory in insect navigation. Curr Biol 23:R789–R800
Corbet SA (1990) Pollination and the weather. Isr J Bot 39:13–30
Délye G (1974) Observations sur le comportement de la fourmi Cataglyphis bicolor (Fabricius) lors d'une éclipse totale de soleil. Insect Soc 21:369–379
Devillers J, Doré JC, Tisseur M, Cluzeau S, Maurin G (2004) Modelling the flight activity of Apis mellifera at the hive entrance. Comput Electron Agric 42:87–109
Dyer FC, Could JL (1983) Honey bee navigation: the honey bee's ability to find its way depends on a hierarchy of sophisticated orientation mechanisms. Am Sci 71:587–597
Evangelista C, Kraft P, Dacke M, Labhart T, Srinivasan M (2014) Honeybee navigation: critically examining the role of the polarization compass. Philos Trans R Soc B 369:20130037
Fewell JH, Winston ML (1992) Colony state and regulation of pollen foraging in the honey-bee, Apis mellifera L. Behav Ecol Sociobiol 30:387–393
Galen C, Miller Z, Lynn A, Axe M, Holden S, Storks L, Ramirez E, Asante E, Heise D, Kephart S, Kepart J (2018) Monitoring reveals impacts of a total solar eclipse on flight behavior and activity schedule of foraging bees. Ann Entomol Soc Am say035. https://doi.org/10.1093/aesa/say035
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90:1063–1102
Heinze S, Narendra A, Cheung A (2018) Principles of insect path integration. Curr Biol 28:R1043–R1058
Homberg U (2004) In search of the sky compass in the insect brain. Naturwissenschaften 91:199–208
Hrassnigg N, Crailsheim K (2005) Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 36:255–277
Hübner C, Czaczkes TJ (2017) Risk preference during collective decision making: ant colonies make risk-indifferent collective choices. Anim Behav 132:21–28
Ish-Am G, Eisikowitch D (1998) Mobility of honey bees (Apidae, Apis mellifera L.) during foraging in avocado orchards. Apidologie 29:209–219
Jagannath A, Taylor L, Wakaf Z, Vasudevan SR, Foster RG (2017) The genetics of circadian rhythms, sleep and health. Hum Mol Genet 26:R128–R138
Kacelnik A, Bateson M (1996) Risky theories—the effects of variance on foraging decisions. Am Zool 36:402–434
Kullenberg B (1955) Biological observations during the solar eclipse in southern Sweden (province of Öland) on 30th June 1954. Oikos 6:51–60
Lee KY, Yim SH, Seo HJ, Kim SY, Yoon HJ (2016) The influence of insect pollination and artificial pollination on fruit quality and economic profit in the ‘Niitaka’pear (Pyrus pyrifolia Nakai). Korean J Org Agric 24:759–771
Ma R, Villar G, Grozinger CM, Rangel J (2018) Larval pheromones act as colony-wide regulators of collective foraging behavior in honeybees. Behav Ecol 29:1132–1141
McNamara JM, Houston AI (1992) Risk-sensitive foraging: a review of the theory. Bull Math Biol 54:355–378
Moore D, Rankin MA (1985) Circadian locomotor rhythms in individual honeybees. Physiol Entomol 10:191–197
Moore D, Rankin MA (1993) Light and temperature entrainment of a locomotor rhythm in honeybees. Physiol Entomol 18:271–278
Nonacs P, Dill LM (1990) Mortality risk vs. food quality trade-offs in a common currency: ant patch preferences. Ecology 71:1886–1892
Özbey O, Aysondu MH, Özer H, Şimsek ÜG (2004) The effects of a solar eclipse on animals behavior. Turk J Vet Anim Sci 28:55–61
Pankiw T, Page RE, Fondrk MK (1998) Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). Behav Ecol Sociobiol 44:193–198
Peso M, Barron AB (2014) The effects of brood ester pheromone on foraging behaviour and colony growth in apicultural settings. Apidologie 45:529–536
Pilorz V, Helfrich-Förster C, Oster H (2018) The role of the circadian clock system in physiology. Pflügers Arch Eur - J Physiol:1–13
Puškadija Z, Štefanić E, Mijić A, Zdunić Z, Parađiković N, Florijančić T, Opačak A (2007) Influence of weather conditions on honey bee visits (Apis mellifera carnica) during sunflower (Helianthus annuus L.) blooming period. Poljoprivreda 13:230–233
Riddell Pearce FC, Couvillon MJ, Ratnieks FLW (2013) Hive relocation does not adversely affect honey bee (hymenoptera: Apidae) foraging. Psyche 2013:693856
Rivera MD, Donaldson-Matasci M, Dornhaus A (2015) Quitting time: when do honey bee foragers decide to stop foraging on natural resources? Front Ecol Evol 19:50
Roenneberg T, Daan S, Merrow M (2003) The art of entrainment. J Biol Rhythm 18:183–194
Roonwal M (1956) Behaviour of the rock bees, Apis dorsata Fabr., during a partial solar eclipse in India. Proc Natl Inst Sci India B 22:281–286
Rueppell O, Kirkman RW (2005) Extraordinary starvation resistance in Temnothorax rugatulus (Hymenoptera, Formicidae) colonies: demography and adaptive behavior. Insect Soc 52:282–290
Scapini F, Rossano C, Marchetti GM, Morgan E (2005) The role of the biological clock in the sun compass orientation of free-running individuals of Talitrus saltator. Anim Behav 69:835–843
Schmickl T, Crailsheim K (2001) Cannibalism and early capping: strategy of honeybee colonies in times of experimental pollen shortages. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 187:541–547
Schulz DJ, Huang ZY, Robinson GE (1998) Effects of colony food shortage on behavioral development in honey bees. Behav Ecol Sociobiol 42:295–303
Traynor KS, Le Conte Y, Page RE (2015) Age matters: pheromone profiles of larvae differentially influence foraging behaviour in the honeybee, Apis mellifera. Anim Behav 99:1–8
Ugolini A, Castellini C, Tiribilli B (2004) The orientation of the sandhopper Talitrus saltator during a partial solar eclipse. J Comp Physiol A 190:855–859
Warrant E, Porombka T, Kirchner WH (1996) Neural image enhancement allows honeybees to see at night. Proc R Soc Lond B 263:1521–1526
Willard LE, Hayes AM, Wallrichs MA, Rueppell O (2011) Food manipulation in honeybees induces physiological responses at the individual and colony level. Apidologie 42:508–518
Woyciechowski M, Kozlowski J (1998) Division of labor by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.). Apidologie 29:191–205
Woyke J, Jasinski Z, Cezary F, Woyke H (2000) Flight activity of Apis mellifera foragers at the hive entrance during 86% eclipse of sun. Pszczel Ćesz Nauk 44:239–249
Acknowledgements
We would like to thank Clemson University for facilitating the research. The exact timing of the eclipse was calculated by NASA and helped in the advance experimental design.
Funding
Financial support was provided by the University of North Carolina at Greensboro, the US Army Research Office (W911NF1520045), Clemson University, and the USDA National Institute of Food and Agriculture, Hatch project 1005512.
Author information
Authors and Affiliations
Contributions
The project idea was conceived by OR and PW. SB, JT, and OR designed the experiment. Colonies were prepared and provided by JT. All authors participated in the data collection. Data analysis and interpretation was performed by PW, SB, JT, and OR. PW and SB wrote the initial draft of the manuscript, which was significantly revised by OR, before going through a final set of revisions and approvals from all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by: Sean O'Donnell
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waiker, P., Baral, S., Kennedy, A. et al. Foraging and homing behavior of honey bees (Apis mellifera) during a total solar eclipse. Sci Nat 106, 4 (2019). https://doi.org/10.1007/s00114-018-1597-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-018-1597-2