Abstract
Inferences of function and ecology in extinct taxa have long been a subject of interest because it is fundamental to understand the evolutionary history of species. In this study, we use a quantitative approach to investigate the locomotor behaviour of Simocyon batalleri, a key taxon related to the ailurid family. To do so, we use 3D surface geometric morphometric approaches on the three long bones of the forelimb of an extant reference sample. Next, we test the locomotor strategy of S. batalleri using a leave-one-out cross-validated linear discriminant analysis. Our results show that S. batalleri is included in the morphospace of the living species of musteloids. However, each bone of the forelimb appears to show a different functional signal suggesting that inferring the lifestyle or locomotor behaviour of fossils can be difficult and dependent on the bone investigated. This highlights the importance of studying, where possible, a maximum of skeletal elements to be able to make robust inferences on the lifestyle of extinct species. Finally, our results suggest that S. batalleri may be more arboreal than previously suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reconstruction of the ecology, behaviour and lifestyle of extinct species of mammals is a subject that has been of interest to many evolutionary biologists and palaeontologists (Bock and von Wahlert 1965; Gonyea 1978; Damuth 1981; Gould and Vrba 1982; Van Valkenburgh 1984, 1985, 1987, 1988; Ewer 1973; Taylor 1989; Damuth and MacFadden 1990; Vrba 1992; Janis and Wilhelm 1993; Jones and Stoddart 1998; Iwaniuk et al. 1999, 2000; Yalden 1999; Ruff 2000; Argot 2001, 2003a, b, 2004; Andersson 2003, 2004a, b, 2005; Schmitt 2003; Salesa et al. 2005, 2010; Schutz and Guralnick 2007; Webb and Sparrow 2007; Boyer and Bloch 2008; Boyer et al. 2010a; b; Polly 2008; Polly and Macleod 2008; Samuels and Van Valkenburgh 2008; Flores and Díaz 2009; Meachen-Samuels and Van Valkenburgh 2009; Figueirido et al. 2011; Figueirido and Janis 2011; Halenar 2011; Ercoli et al. 2012; Samuels et al. 2013; Meachen-Samuels 2012; Walmsley et al. 2012; Meloro et al. 2013; Janis and Figueirido 2014; Martín-Serra et al. 2014). Indeed, the investigation of fossil organisms is fundamental to understanding the evolutionary history of species (Simpson 1953; Slater et al. 2012). Unfortunately, a fossil is rarely well preserved with all its soft and hard tissues, and thus teeth, bones or parts of bones are the main material with which palaeontologists work. The reconstruction of the palaeobiology of an extinct species with its skeleton as principal evidence is a difficult exercise. As such, one first needs to understand the adaptive nature of the morphology of the skeleton in living species and its relation to ecology, locomotion or behaviour, while taking into account potential effects of shared ancestry.
The main methods used for palaeobiological reconstructions are ecomorphological ones (Wainwright 2007). Although the relationships between form and function are intuitively appealing, they are often not straightforward due to behavioural filters and processes such as many-to-one mapping (Alfaro et al. 2005; Wainwright et al. 2005; Polly 2008). Form and function are, however, linked at a fundamental level, and bones are functionally important (Bock and von Wahlert 1965). They allow movement and, whilst supporting loads, also need to respond and resist to muscular forces (Hildebrand 1985; Bryant and Seymour 1990; Bryant and Russell 1992; Demes et al. 1994; Witmer 1995; Jungers et al. 1998; Biknevicius et al. 2004; Alexander 2006; Polly 2008; Fabre et al. 2014). As bones are shaped by force and motion, they are intimately related to the movements executed and, by inference, also the lifestyle of a species. Following these principles, different approaches can be used to reconstruct the palaeobiology of a species. Most commonly, qualitative approaches consisting of the anatomical comparison of a fossil with living relatives or analogous species are used. The main problem of this approach is the impossibility to test the hypotheses of the inferences that are made. On the other hand, quantitative approaches including morphometrics (e.g. Van Valkenburgh 1984, 1985, 1987, 1988; Andersson 2003, 2004a, b, 2005; Polly 2008; Polly and Macleod 2008; Samuels and Van Valkenburgh 2008; Ercoli et al. 2012; Janis and Figueirido 2014), functional morphology (e.g. Argot 2001, 2003a, b, 2004; Salesa et al. 2005, 2010) and biomechanics (e.g. Schmitt and Lemelin 2002; Hutchinson 2004, 2011; Alexander 2006; Hutchinson and Gatesy 2006; Hutchinson et al. 2007) can be used to reconstruct the locomotor modes of fossils. However, reducing the shape of a bone to linear and angular measurement may lead to a loss of information and a lack of precision. Geometric morphometric approaches allow for a detailed description of the shape of a bone and accurate tests and comparisons of the shape of a fossil to its living relatives and analogues.
In the present study, we aim to quantitatively infer the locomotor strategy of an extinct carnivoran, the ailurid Simocyon batalleri, from the Late Miocene of Spain. The nearly complete skeleton of the extinct species S. batalleri was previously described by Salesa et al. (2008) as a generalist terrestrial carnivore able to climb trees: ‘Such locomotor abilities are consistent with a palaeobiological model of a generalised carnivore that foraged mainly on the ground but could readily climb to trees for safety if faced with the threat of larger competing carnivore’. Several anatomical features of the postcranial skeleton of S. batalleri points towards this lifestyle: for example, this species had an enlarged radial sesamoid (‘false thumb’) that likely allowed for considerable climbing ability; the lumbar region was likely adapted to produce strong vertical forces whilst climbing; the hand showed a higher degree of pronation–supination capability than other carnivorans; the scapula had an increased attachment areas for those muscles involved in producing strong adduction–abduction forces at the shoulder (Antón et al. 2006; Salesa et al. 2008).
In order to quantitatively test the hypothesis that S. batalleri was semi-arboreal, we used 3D surface geometric morphometric approaches performed on the three long bones of the forelimb for a large sample of musteloids and explored the position of S. batalleri in the morphospace of the living species. Moreover, we compare the morphology of S. batalleri to the mean morphologies of extant species with different types of locomotion. Finally, we test the locomotor strategy of S. batalleri using a leave-one-out cross-validated linear discriminant analysis. Our predictions follow those of Salesa et al. (2008), and we predict that S. batalleri will fall in the morphospace of the living species that have a generalist terrestrial or semi-arboreal locomotor style.
Materials and methods
Materials
The sample is composed of the three long bones of the forelimb of 77 individuals belonging to 20 extant species of mustelids, one extant species and one extinct species of ailurid, eight extant species of procyonids and four extant species of mephitids. For each species, the number of specimens ranged from 1 to 7 (Table 1; Supplementary Table S1). All specimens were adults and predominantly of wild-caught origin. Equal numbers of males and females were included where possible. Specimens were obtained from the following collections: Mammifères et Oiseaux, Muséum National d’Histoire Naturelle, Paris, France; the Naturhistorisches Museum, Basel, Switzerland; the Harvard Museum of Comparative Zoology, Cambridge, Massachusetts; the Smithsonian National Museum of Natural History, Washington, District of Columbia, USA; the MNCN, Museo Nacional de Ciencias Naturales—CSIC, Madrid. See Supplementary Table S1 for a complete list of the specimens used in the analyses.
Bones of extant specimens were scanned using a Breuckmann 3D surface scanner at the Muséum National d’Histoire Naturelle, Paris (white light fringe StereoSCAN3D model with a camera resolution of 1.4 megapixels). Bones of the fossil were scanned using a Philips Brilliance 64 CT Scan at the Hospital Nuestra Señora de América (Madrid, Spain; Fig. 1).
Geometric morphometrics
The shape of the long bones of the forelimb is complex and cannot be adequately represented using a traditional landmark-based approach. Consequently, a 3D sliding-landmark procedure (Bookstein 1997; Gunz et al. 2005; Gunz and Mitteroecker 2013) was used to better describe and quantify the morphology of these long bones, and especially their articulations given the importance of the articulations in determining limb segment excursions important during locomotion. The acquisition of the morphometric data was done using the software Idav Landmarks (Wiley et al. 2005), while Edgewarp3D 3.31 (Bookstein and Green 2002) was used to obtain the sliding landmarks (Supplementary Fig. S1 and Supplementary Tables S2, S3 and S4). Once all landmark data were obtained, a generalised Procrustes superimposition (Rohlf and Slice 1990) was performed using the package Rmorph (Baylac 2012) in R (R Development Core 2011). Finally, a principal component analysis (PCA) was performed on the shape data to evaluate the distribution of the specimens in morphospace. A PCA was also performed on the combined shape data of the forelimb using the whole tangent dataset derived from the Procrustes analyses for each long bone. For more information concerning the protocol and methods of 3D surface geometric morphometric approaches used in this paper, we refer to Fabre et al. (2013a, b; 2014, 2015).
Locomotor category attribution of S. batalleri
A leave-one-out cross-validated linear discriminant analysis (LDA) was performed on each long bone of the forelimb as well as on the combined shape data of the entire forelimb. The LDA was performed using the ‘lda’ function from the ‘MASS’ package in the software R, performed on the principal components of the Generalised Procrustes Superimposition (GPA) for each bone and for the whole forelimb of the living specimens. The leave-one-out cross-validation procedure removes one specimen at a time and predicts its classification using LDA function computed on all the remaining specimens. At the end, a classification accuracy of the locomotor category of each living specimen is given by the percentage of specimens correctly assigned by the cross-validated LDA. Finally, the fossil is added to the analysis and assigned to a locomotor category. We defined five categories of locomotion (Table 2) following Nowak (2005), Wilson and Mittermeier (2009), Hunter and Barrett (2011b), Samuels et al. (2013) and Fabre et al. (2013a, 2015) (Tables 1 and 2): arboreal, semi-arboreal, terrestrial, aquatic and semi-fossorial. Note, however, that while animals were classified as belonging to a single lifestyle, they may occasionally also show other locomotor behaviours. For example, most musteloids will swim when needed, and many will dig in the leaf litter and top soil to find food.
Results
Geometric morphometrics
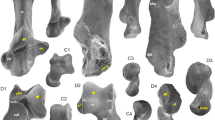
The first two principal component axes of the analysis on the shape of the humerus account for 61.1 % of the total shape variation (Fig. 2a, Supplementary Fig. S2). The overall distribution of the different taxa in the morphospace shows the arboreal and semi-arboreal species clustering together, which tend to overlap with the terrestrial species on these axes. Semi-fossorial and aquatic species are at the opposite side of the arboreal and semi-arboreal species, and semi-fossorial species tend also to overlap with the terrestrial species on these axes. In this scatter plot, S. batalleri falls in the morphospace of the arboreal and semi-arboreal species. This suggests that the shape of the humerus of S. batalleri is similar to that of arboreal and semi-arboreal musteloids. Indeed, S. batalleri falls in the morphospace of extant species (Supplementary Fig. S2) close to the arboreal red panda (Ailurus fulgens), kinkajou (Potos flavus), the semi-arboreal stone marten (Martes foina) and the pine marten (Martes martes).
Results of the principal components analyses performed on the morphometric data of a the humerus, b the ulna, c the radius and d the forelimb. Symbols are as follows: green circles and polygon indicate arboreal species; yellow circles and polygon indicate semi-arboreal species; red circles and polygon indicate terrestrial species; brown circles and polygon indicate semi-fossorial species; blue circles and polygon indicate aquatic species; black circle indicates Simocyon batalleri
The first two principal components axes accounted for 63.2 % of the total shape variation of the ulna (Fig. 2b, Supplementary Fig. S3). The overall distribution of the locomotor categories shows that the arboreal species are included within the morphospace of the semi-arboreal species when considering these two axes. Both of them tend to be separated from terrestrial, semi-fossorial and aquatic species which fall in the opposite part of the morphospace. Aquatic species tend to be separated from all the other locomotor styles. In this scatter plot, S. batalleri falls in the morphospace of the terrestrial species, not so far of the semi-arboreal and semi-fossorial ones. This suggests that the ulna of S. batalleri is morphologically similar to some terrestrial, semi-arboreal and semi-fossorial musteloids. More specially, S. batalleri tends to cluster with the generalist terrestrial wolverine (Gulo gulo) and the semi-arboreal tayra (Eira barbara).
The first two principal component axes represent 71.8 % of the total shape variation of the radius (Fig. 2c, Supplementary Fig. S4). This scatter plot displays the arboreal and semi-arboreal species in the same part of the morphospace. They tend to overlap with a few of the species on these first two axes. The terrestrial species tend to overlap with the semi-fossorial species on these axes. Aquatic species are in the same part of the morphospace as semi-fossorial and terrestrial species, but they are completely separated from other kinds of locomotor types. In this scatter plot, S. batalleri falls in the morphospace of semi-arboreal and terrestrial species. This suggests that S. batalleri has a radius that is morphologically similar to semi-arboreal and terrestrial musteloids. In this scatter plot, S. batalleri tends to be similar to the semi-arboreal coatis (Nasua nasua and Nasua narica), the terrestrial wolverine (Gulo gulo) and the American mink (Neovison vison).
The first two principal components axes represent 61.5 % of the overall shape variation of the combined data set for the forelimb (Fig. 2d, Supplementary Fig. S5). This scatter plot shows the arboreal species are included in the morphospace of the semi-arboreal species on the plot defined by the first two PC axes. The terrestrial species are in the middle of the morphospace and overlap with some semi-arboreal species and some semi-fossorial species on these axes. The semi-fossorial species have a large distribution and they overlap with a large part of the aquatic species on the first two PC axes. Both of them are located at the opposite side of the arboreal and semi-arboreal species. In this scatter plot, S. batalleri is included within the morphospace of the semi-arboreal living species of musteloids which means that S. batalleri display an overall morphology of the long bones of the forelimb similar to that of semi-arboreal musteloids. In this scatter plot, S. batalleri tends to group with the semi-arboreal coatis (N. nasua and N. narica), the tayra (Eira barbara) and the ringtail (Bassariscus astutus).
Locomotor categories discrimination and attribution of S. batalleri
The results obtained after the LDA for the humerus shows that 80 % of the aquatic specimens are well classified and 20 % is classified as semi-fossorial (Table 3). Concerning the arboreal specimens, 86.7 % are well classified and 13.3 % of them are classified as semi-arboreal. For the semi-arboreal group, 88.9 % specimens are well classified and 7.4 % of them are classified as arboreal, and 3.7 % of them as terrestrial. One hundred percent of the semi-fossorial specimens are well classified. Finally, 75 % of the terrestrial specimens are well classified and 16.7 % is attributed to the semi-arboreal group and 8.3 % to the semi-fossorial group. The results of the leave-one-out cross-validated linear discriminant analysis performed on the shape data of the humerus attribute S. batalleri to the arboreal category at 97.5 %. The shape of the humerus of S. batalleri appears similar to that of the arboreal musteloids.
For the ulna, the LDA classified 80 % of the aquatic specimens correctly and misclassified 20 % as terrestrial species (Table 3). Eighty six percent (86.7 %) of the arboreal specimens are correctly attributed; thirty percent (13.3 %) of them are attributed to the semi-arboreal group. Concerning the semi-arboreal category, 18 specimens are well attributed, and three are classified as arboreal. For the semi-fossorial category, 14 specimens are well classified whereas two are attributed to the semi-arboreal group. Finally, all the specimens of the terrestrial category are well classified. The results of the LDA performed on the ulnar shape data attribute S. batalleri to the semi-arboreal category at 48 % to semi-fossorial at 23.4 % and to the terrestrial one at 19.8 %. This suggests that the ulna of S. batalleri is morphologically similar to that of semi-arboreal species and some semi-fossorial and terrestrial ones.
For the radius, the result of the classification for the aquatic specimens after an LDA resulted in 40 % being classified correctly, 40 % attributed to the terrestrial group and 20 % attributed to the semi-fossorial group (Table 3). Ninety three percent (93.3 %) of the arboreal specimens are well classified and 6.7 % of them are attributed to the semi-arboreal group. Concerning the semi-arboreal specimens, 88.9 % of them are well attributed whereas 3.7 % of them are attributed to the arboreal specimens and 7.4 % of them to the semi-fossorial group. Seventy six percent (76.5 %) of the semi-fossorial specimens are well classified; 17.6 % are attributed to the semi-arboreal group and 5.9 % to the terrestrial category. For the terrestrial category, 66.7 % of the species are well classified, 16.65 % are classified as semi-fossorial and 16.65 % as semi-arboreal. The results of the LDA predictions using the radial shape data attribute S. batalleri to the arboreal locomotor strategy at 68.2 % and to the terrestrial locomotor strategy at 24.8 %. This result means that the shape of the radius of S. batalleri is similar to that of arboreal and some terrestrial specimens of musteloids.
The results of the LDA for the combined data set of the forelimb show a good classification for all the specimens in the aquatic category (Table 3). Ninety three percent (93.3 %) of the specimens of the arboreal category are well attributed whereas only 6.7 % are classified as semi-arboreal. For the semi-arboreal category, all the specimens are well classified. Eighty eight percent (88.2 %) of the specimens are well attributed to the semi-fossorial category, whereas 11.8 % of them are attributed to the terrestrial category. Among the terrestrial category, 66.7 % of the specimens are well classified, 16.7 % are classified among the semi-fossorial category, 8.3 % among the semi-arboreal category and 8.3 % to the arboreal one. Finally, the result of the LDA performed on the discriminant function of the whole forelimb shape attributed S. batalleri to the arboreal locomotor group at 99.9 % probability. This suggests that the shape of the long bones of the forelimb in S. batalleri is morphologically similar to that of the arboreal species of musteloids.
Discussion
The first interesting result of this analysis is that S. batalleri falls within the morphospace of the living species, which means that its forelimb morphology is similar to that of extant musteloids. The PCA performed on the forelimb shape data set showed different results depending on the bone analysed. S. batalleri falls in the morphospace of the arboreal and semi-arboreal species when evaluating the humerus shape (Fig. 2a). However, when examining the ulna, S. batalleri falls in the morphospace of the terrestrial species (Fig. 2b), and for the radius, S. batalleri falls in the morphospace of the terrestrial and semi-arboreal species (Fig. 2c). The results obtained for the humerus, the ulna and the radius are relatively congruent with the locomotor hypothesis of Salesa et al. (2008). Importantly, these results show that the functional signal can be different from one bone to another. When taking into account the whole forelimb in the shape analysis (Fig. 2d), the results of the PCA show that S. batalleri fall in the morphospace of the semi-arboreal musteloids. This result confirms the prediction of Salesa et al. (2008) that S. batalleri tends to be a generalist with a semi-arboreal locomotor strategy. This result shows that including the different bones together in the analysis may provide better results than when treating each bone separately.
The results of the cross-validation test of the LDA of the living musteloids for each bone show that the species tend to be generally well assigned to the locomotor categories based on literature data (Table 3). For the humerus, the locomotor assignment is perfect for the semi-fossorial specimens and particularly high for the aquatic, the terrestrial and the semi-fossorial specimens in comparison to those of the other categories. The results are similar for the ulna with good attribution for aquatic and semi-fossorial specimens. However, for the radius, terrestrial specimens appear to be better assigned than those of other categories. Nevertheless, the assignment is better when the three bones are used together, except for the semi-fossorial specimens where the humerus by itself provides better results.
The results of the LDA attribute S. batalleri to the arboreal category for the humerus. Yet, when using the ulnar shape, this fossil ailurid is similar to the semi-fossorial species and some terrestrial species. The results of the LDA for the radius suggest that S. batalleri is similar to the arboreal and some terrestrial specimens of musteloids. Finally, the result for the whole forelimb suggests very strongly that S. batalleri is morphologically similar to arboreal specimens of musteloids. For each bone taken individually, the results are similar to those of the PCA performed on the forelimb data set and highlight the fact that each bone can have a different functional signal. This highlights the difficulty to infer the palaeobiology of an extinct species based on single bones. However, these results tend to be in accordance with the terrestrial and semi-arboreal locomotor category attribution of S. batalleri in the study of Salesa et al. (2008). Concerning the result obtained for the whole forelimb, it is somewhat different than the previous results, suggesting that S. batalleri is more arboreal than expected. The results, with S. batalleri attributed to the arboreal locomotion for the humerus, radius and the total forelimb, whereas it is attributed to semi-arboreal category for the ulna, may reflect the fact that there are multiple morphologic (phenotypic) solutions to the same functional or ecological problem (Alfaro et al. 2005: Wainwright et al. 2005; Wainwright 2007; Losos 2011).
These results highlight the importance to study the whole skeleton, at least where possible, and to be careful in inferring lifestyle when studying isolated bones, even when using quantitative methods as was done here. Our results also show that some bones better capture functional signal than others, which implies that some bones can be more informative for inferring locomotor and behavioural strategies in extinct species. For example (Table 3), the humerus appears to be a good indicator of aquatic and semi-fossorial adaptations, the ulna for the arboreal and semi-fossorial adaptations, and the radius for arboreal adaptations based on our LDA analysis. Nevertheless, it is important to note that the discriminant analysis forces the group classification by decreasing the intragroup variability and increasing the intergroup variability which may influence the result that we obtained. To overcome this problem, it would be interesting to use alternative classification methods such as Gaussian Mixture Model that allow one to test the presence of groups without a priori assignments.
These results tend also to corroborate the previous study made on the anatomy of the postcranial skeleton of S. batalleri. Indeed, several anatomical features point towards this as an arboreal/semi-arboreal lifestyle: for example, the forearm of S. batalleri shows a higher degree of pronation–supination capability compared to other carnivorans as shown by the study of Fabre et al. (2015). The olecranon process of the ulna is short which allows a full extension of the elbow (Samuels and Van Valkenburgh 2008). The radial notch is also oriented laterally in arboreal species and in S. batalleri. This has been interpreted in previous studies as increasing the degree of pronation and supination of the forelimb, thus allowing a wider range of rotation at the elbow (Hildebrand 1988; Andersson 2003, 2004b; Peigné et al. 2008; Ercoli et al. 2012; Fabre et al. 2015). Furthermore, several other anatomical features of the postcranial skeleton of S. batalleri points towards this lifestyle: for example, this species has an enlarged radial sesamoid (‘false thumb’) that likely allowed for considerable climbing ability, refining the grasping capacity of the hand and allowing this species to reach the highest parts of trees. Moreover, the configuration of the carpals shows adaptations to a semi-arboreal lifestyle: the pisiform, for example, has a strong and ridged articular facet for the transverse carpal ligament (or flexor retinaculum), which is markedly different from that in other musteloids (in which the facet is smooth and lacking ridged borders). This ligament is the attachment surface of the muscles abductor pollicis brevis and opponens pollicis (Davis 1964; Antón et al. 2006), the main flexors of the pollex and the palm. The morphology of the pisiform thus suggests a strong development of this ligament in S. batalleri, pointing towards the presence of very strong muscles (Antón et al. 2006; Salesa et al. 2008). Previous studies have also shown that the spinous processes of the lumbar vertebrae of S. batalleri are triangular instead of rectangular which is common in other musteloids. This indicates the presence of well-developed interspinal muscles between the spinous processes, typical of carnivorans that bound, but do not trot or gallop. These muscles may play an important role when climbing trees. Finally, the scapula has an increased attachment area for those muscles involved in producing adduction–abduction at the shoulder (Antón et al. 2006; Salesa et al. 2008).
Conclusion
Our results show that S. batalleri is included in the morphospace of the living musteloids for each bone of the forelimb. However, our results also show that different bones may show a different functional signal, which indicates that inferring lifestyle of extinct taxa can be difficult. This implies that one has to be careful when reconstructing the palaeobiology of an extinct species when studying only isolated bones. Our results also highlight the importance to study, when possible, a maximum of skeletal elements to infer the lifestyle of an extinct species. Our results suggest that S. batalleri may be more arboreal than previously suggested, although the different methods employed provided slightly different results. This fossil ailurid shared its habitat with other larger carnivorans, such as the sabre-toothed felids Machairodus aphanistus and Promegantereon ogygia, or the lion-sized amphicyonid Magericyon anceps (Antón et al. 2004; Peigné et al. 2008; Salesa et al. 2006; Siliceo et al. 2015). In this context, it seems reasonable that a generalised carnivoran such as S. batalleri, lacking large canines and being smaller than other large members of the predator guild, developed strong climbing abilities for escape from these larger species, but likely also for some foraging on trees (Salesa et al. 2008). A quantitative assessment of behaviour in extant species rather than qualitative categories may provide better congruence among methods, yet this remains to be tested.
References
Afflerbaugh K (2002) "Conepatus chinga" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Conepatus_chinga/
Aguilar W (2003) "Ictonyx striatus" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Ictonyx_striatus/
Alexander RMN (2006) Principles of animal locomotion. Princeton University Press, New Jersey
Alfaro ME, Bolnick DI, Wainwright PC (2005) Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am Nat 165:E140–54
Allegra J, Rath R, Gunderson A (2012) "Enhydra lutris" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Enhydra_lutris/
Andersson K (2003) Locomotor evolution in the Carnivora (Mammalia): evidence from the elbow joint. University of Uppsala, Sweden
Andersson K (2004a) Predicting carnivoran body mass from a weight-bearing joint. J Zool 262:161–172
Andersson K (2004b) Elbow-joint morphology as a guide to forearm function and foraging behaviour in mammalian carnivores. Zool J Linn Soc 142:91–104
Andersson K (2005) Were there pack-hunting canids in the Tertiary, and how can we know? Paleobiology 31:56–72
Antón M, Salesa MJ, Morales J, Turner A (2004) First known complete skulls of the scimitar-toothed cat Machairodus aphanistus (Felidae, Carnivora) from the Spanish late Miocene site of Batallones-1. J Vertebr Paleontol 24:957–969
Antón M, Salesa MJ, Pastor JF, Peigné S, Morales J (2006) Implications of the functional anatomy of the hand and forearm of Ailurus fulgens (Carnivora, Ailuridae) for the evolution of the ‘false-thumb’ in pandas. J Anat 209:757–764
Argot C (2001) Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol 247:51–79
Argot C (2003a) Functional adaptations of the postcranial skeleton of two Miocene borhyaenoids (Mammalia, Metatheria), Borhyaena and Prothylacinus, from South America. Palaeontology 46:1213–1267
Argot C (2003b) Functional-adaptive anatomy of the axial skeleton of some extant marsupials and the paleobiology of the Paleocene Marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol 255:279–300
Argot C (2004) Functional-adaptive features and palaeobiologic implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid Thylacosmilus atrox (Metatheria). Alcheringa 28:229–266
Baylac M (2012) Rmorph: an R geometric and multivariate morphometrics library. Available from the author: baylac@mnhn.fr
Bender J (2001) "Pteronura brasiliensis" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Pteronura_brasiliensis/
Berger L (2004) "Bassaricyon gabbii" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Bassaricyon_gabbii/
Biknevicius AR, Mullineaux DR, Clayton HM (2004) Ground reaction forces and limb function in tölting Icelandic horses. Equine Vet J 36:743–747
Bock WJ, von Wahlert G (1965) Adaptation and the form-function complex. Evolution 19:269–299
Bookstein FL (1997) Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal 1:225–243
Bookstein FL, Green WDK (2002) Users Manual, EWSH3.19. http://brainmap.stat.washington.edu/edgewarp/
Boyer DM, Bloch JI (2008) Evaluating the Mitten-Gliding hypothesis for Paromomyidae and Micromomyidae (Mammalia, “Plesiadapiformes”) using comparative functional morphology of new Paleogene skeletons. In: Sargis EJ, Dagosoto M (eds), Mammalian evolutionary morphology: a tribute to Frederick S. Szalay. Springer, Dordrecht, the Netherlands, pp 233–284
Boyer DM, Seiffert ER, Simons EL (2010a) Astragalar morphology of Afradapis, a large adapiform primate from the earliest late Eocene of Egypt. Am J Phys Anthropol 143:383–402
Boyer DM, Prasad GVR, Krause DW, Godinot M, Goswami A, Verma O, Flynn JJ (2010b) New postcrania of Deccanolestes from the Late Cretaceous of India and their bearing on the evolutionary and biogeographic history of euarchontan mammals. Naturwissenschaften 97:365–377
Braddy S (2003) "Nasua nasua" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Nasua_nasua/
Brilliant T (2000) "Poecilogale albinucha" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Poecilogale_albinucha/
Bryant HN, Russell AP (1992) The role of phylogenetic analysis in the inference of unpreserved attributes of extinct taxa. Phil Trans R Soc London B 337:405–418
Bryant HN, Seymour KL (1990) Observation and comments on the reliability of muscle reconstruction in fossil vertebrates. J Morphol 206:109–117
Carter K (2004) "Martes foina" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Martes_foina/
Damuth J (1981) Population density and body size in mammals. Nature 290:699–700
Damuth J, MacFadden BJ (1990) Body size in mammalian paleobiology: estimation and biological implications. Cambridge University Press, Cambridge
Davis DD (1964) The giant panda: a morphological study of evolutionary mechanisms. Fieldiana: Zool Mem 3:1–339
Demes B, Larson S, Stern JT Jr, Jungers WL, Biknevicius AR, Schmitt D (1994) The kinetics of primate quadrupedalism: “Hind limb drive” reconsidered. J Hum Evol 26:353–374
Dubbelde E (2011) "Mustela eversmanii" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Mustela_eversmanii/
Ercoli MD, Prevosti FJ, Alvarez A (2012) Form and function within a phylogenetic framework: locomotory habits of extant predators and some Miocene Sparassodonta (Metatheria). Zool J Linn Soc 165:224–251
Ewer RF (1973) The carnivores. Cornell University Press, Ithaca, NY
Fabre A-C, Cornette R, Peigné S, Goswami A (2013a) Influence of body mass on the shape of forelimb in musteloid carnivorans. Biol J Linn Soc 110:91–103
Fabre A-C, Cornette R, Slater G, Argot C, Peigné S, Goswami A, Pouydebat E (2013b) Getting a grip on the evolution of grasping in musteloid carnivorans: a three-dimensional analysis of forelimb shape. J Evol Biol 26:1521–1535
Fabre A-C, Goswami A, Peigné S, Cornette R (2014) Morphological integration in the forelimb of musteloid carnivorans. J Anat 225:19–30
Fabre A-C, Cornette R, Goswami A, Peigné S (2015) Do constraints associated with the locomotor habitat drive the evolution of forelimb shape? A case study in musteloid carnivorans. J Anat. doi:10.1111/joa.12315
Figueirido B, Janis CM (2011) The predatory behavior of the thylacine: Tasmanian tiger or marsupial wolf? Biol Lett 7:937–940
Figueirido B, Pérez-Claros JA, Hunt RM Jr, Palmqvist P (2011) Body mass estimation in amphicyonid carnivoran mammals: a multiple regression approach from the skull and skeleton. Acta Palaeontol Pol 56:225–246
Flores DA, Díaz MM (2009) Postcranial skeleton of Glironia venusta (Didelphimorphia, Didelphidae, Caluromyinae): Description and Functional Morphology. Zoosyst Evol 85:311–339
Fox R (2001) "Procyon lotor" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Procyon_lotor/
Goldberg J (2003) "Bassariscus astutus" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Bassariscus_astutus/
Gonyea WJ (1978) Functional implications of felid forelimb morphology. Acta Anat 102:111–121
Gould SJ, Vrba ES (1982) Exaptation—a missing term in the science of form. Paleobiology 8:4–15
Gregg M (2013) "Galictis vittata" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Galictis_vittata/
Gunz P, Mitteroecker P (2013) Semilandmarks: a method for quantifying curves and surfaces. Hystrix 24:1–7
Gunz P, Mitteroecker P, Bookstein FL (2005) Semilandmarks in three dimensions. In: Slice SE (ed) Modern morphometrics in physical anthropology. Kluwer Academic/Plenum Publishers, New York, pp 73–98
Halenar LB (2011) Reconstructing the locomotor repertoire of Protopithecus brasiliensis. II. Forelimb morphology. Anat Rec 294:2048–2063
Heath T, Platnick J (2008) "Ailurus fulgens" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Ailurus_fulgens/
Hildebrand M (1985) Functional vertebrate morphology. Harvard University Press, Cambridge, Massachusetts
Hildebrand M (1988) Analysis of vertebrate structure, 3th edition. Wiley & Sons: New York, NY
Hoffman Z (2014) "Mellivora capensis" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Mellivora_capensis/
Hunter L, Barrett P (2011a) Carnivores of the world. Princeton University Press, Princeton, NJ
Hunter L, Barrett P (2011b) A Field Guide to the Carnivores of the World. New Holland Publishers: London, UK.
Hutchinson JR (2004) Biomechanical modeling and sensitivity analysis of bipedal running ability. II. Extinct taxa. J Morphol 262:441–461
Hutchinson JR (2011) On the inference of function from structure using biomechanical modelling and simulation of extinct organisms. Biology Letters http://rsbl.royalsocietypublishing.org/content/early/2011/06/08/rsbl.2011.0399.full.html
Hutchinson JR, Gatesy SM (2006) Beyond the bones. Nature 440:292–294
Hutchinson JR, Ng-Thow-Hing V, Anderson FC (2007) A 3D interactive method for estimating body segmental parameters in animals: application to the turning and running performance of Tyrannosaurus rex. J Theor Biol 246:660–680
Iwaniuk AN, Pellis SM, Whishaw IQ (1999) The relationship between forelimb morphology and behaviour in North American carnivores (Carnivora). Can J Zool 77:1064–1074
Iwaniuk AN, Pellis SM, Whishaw IQ (2000) The relative importance of body size, phylogeny, locomotion, and diet in the evolution of forelimb dexterity in fissiped carnivores (Carnivora). Can J Zool 78:1110–1125
Janis CM, Figueirido B (2014) Forelimb anatomy and the discrimination of the predatory behaviour of carnivorous mammals: the thylacine as a case study. J Morphol 275:1321–1338
Janis CM, Wilhelm PB (1993) Were there mammalian pursuit predators in the Tertiary? Dances with wolf avatars. J Mammal Evol 1:103–125
Jones ME, Stoddart DM (1998) Reconstruction of the predatory behaviour of the extinct marsupial thylacine (Thylacinus cynocephalus). J Zool (Lond) 246:239–246
Jungers WL, Burr DB, Cole MS (1998) Body size and scaling of long bone geometry, bone strength and positional behavior in cercopithecoid primates. In: Strasser E, Fleagle JG, Rosenberger A, McHenry H (eds) Primate locomotion: recent advances. Plenum, New York, pp 309–330
Kennedy S (2003) "Lutra lutra" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Lutra_lutra/
Kiiskila J (2014) "Mephitis mephitis" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Mephitis_mephitis/
Larivière S (2002) Ictonyx striatus. Mamm Species 698:1–5
Losos JB (2011) Convergence, adaptation and constraint. Evolution 65–7:1827–1840
Lundrigan B, Conley M (2001) "Mustela putorius" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Mustela_putorius/
Marceau J (2001) "Nasua narica" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Nasua_narica/
Martín-Serra A, Figueirido B, Palmqvist P (2014) A three-dimensional analysis of morphological evolution and locomotor performance of the carnivoran forelimb. PLoS ONE 9:e85574
McClearn D (1992) Locomotion, posture, and feeding behavior of kinkajous, coatis, and raccoons. J Mammal 73:245–261
Meachen-Samuels JA (2012) Morphological convergence of the prey-killing arsenal of sabertooth predators. Paleobiology 38:1–14
Meachen-Samuels JA, Van Valkenburgh B (2009) Forelimb indicators of prey-size preference in the Felidae. J Morphol 270:729–744
Meloro C, Elton S, Louys J et al (2013) Cats in the forest: predicting habitat adaptations from humerus morphometry in extant and fossil Felidae (Carnivora). Paleobiology 39:323–344
Nowak RM (2005) Walker’s carnivores of the world. The Johns Hopkins University Press, Baltimore, MD
Patsy V, Sygo M (2009) "Gulo gulo" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Gulo_gulo/
Peigné S, Salesa MJ, Antón M, Morales J (2008) A new amphicyonine (Carnivora: Amphicyonidae) from the Upper Miocene of Batallones-1, Madrid, Spain. Palaeontology 51:943–965
Pennington S (2002) "Spilogale putorius" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Spilogale_putorius/
Petroelje T (2011) "Vormela peregusna" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Vormela_peregusna/
Phillips N (2005) "Procyon cancrivorus" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Procyon_cancrivorus/
Polly PD (2008) Adaptive zones and the pinniped ankle: a 3D quantitative analysis of carnivoran tarsal evolution. In: Sargis E, Dagosto M (eds) Mammalian evolutionary morphology: a tribute to Frederick S. Szalay. Springer, Dordrecht, The Netherlands, pp 165–194
Polly PD, Macleod N (2008) Locomotion in fossil carnivora: an application of eigensurface analysis for morphometric comparison of 3D surfaces. Palaeontol Electron 11(2):8A
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: R Foundation for Statistical Computing, ISBN 3-900051-07-0. Available at: http://www.R-project.org
Rehder D (2007) "Potos flavus" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Potos_flavus/
Roberts MS, Gittleman JL (1984) Ailurus fulgens. Mamm Species 222:1–8
Rohlf FJ, Slice DE (1990) Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Ruff CB (2000) Biomechanical analyses of archeological human skeletons. In: Katzenberg MA, Saunders SR (eds) Biological anthropology of the human skeleton. Wiley-Liss, New York, pp 71–102
Salesa MJ, Antón M, Turner A, Morales J (2005) Aspects of the functional morphology in the cranial and cervical skeleton of the sabre-toothed cat Paramachairodus ogygia (Kaup, 1832) (Felidae, Machairodontinae) from the Late Miocene of Spain: implications for the origins of the machairodont killing bite. Zool J Linn Soc 144:363–377
Salesa MJ, Antón M, Turner A, Morales J (2006) Inferred behaviour and ecology of the primitive sabre-toothed cat Paramachairodus ogygia (Felidae, Machairodontinae) from the Late Miocene of Spain. J Zool 268:243–254
Salesa MJ, Antón M, Peigné S, Morales J (2008) Functional anatomy and biomechanics of the postcranial skeleton of Simocyon batalleri (Viret, 1929) (Carnivora, Ailuridae) from the Late Miocene of Spain. Zool J Linn Soc 152:593–621
Salesa MJ, Antón M, Turner A, Morales J (2010) Functional anatomy of the forelimb in Promegantereon ogygia (Felidae, Machairodontinae, Smilodontini) from the Late Miocene of Spain and the origins of the sabre-toothed felid model. J Anat 216:381–396
Samuels JX, Van Valkenburgh B (2008) Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol 269:1387–1411
Samuels JX, Meachen JA, Sakai SA (2013) Postcranial morphology and the locomotor habits of living and extinct carnivorans. J Morphol 274:121–146
Savage M (2000) "Lontra felina" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Lontra_felina/
Schlimme K (2003) "Neovison vison" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Neovison_vison/
Schmitt D (2003) Substrate size and primate forelimb mechanics: implications for understanding the evolution of primate locomotion. Int J Primatol 24:1023–1036
Schmitt D, Lemelin P (2002) Origins of primate locomotion: gait mechanics of the woolly opossum. Amer J Phys Anthropol 118(3):231–238
Schreffler C (2003) "Eira barbara" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Eira_barbara/
Schutz H, Guralnick RP (2007) Postcranial element shape and function: assessing locomotor mode in extant and extinct mustelid carnivorans. Zool J Linn Soc 150:895–914
Schwanz L (2000) "Martes martes" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Martes_martes/
Seefeldt R (2003) "Melogale moschata" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Melogale_moschata/
Shalu T (2001) "Mustela lutreola" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Mustela_lutreola/
Shefferly N (1999) "Taxidea taxus" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Taxidea_taxus/
Siliceo G, Salesa MJ, Antón M, Pastor JF, Morales J (2015) Comparative anatomy of the shoulder region in the Late Miocene amphicyonid Magericyon anceps (Carnivora): functional and paleoecological inferences. J Mammal Evol
Simpson GG (1953) The major features of evolution. Columbia biological series; no. 17 Columbia Univ. Press, New York
Slater GJ, Harmon LJ, Alfaro ME (2012) Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution 66–12:3931–3944
Taylor ME (1989) Locomotor adaptation by carnivores. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Cornell University Press, New York, NY, pp 383–409
Trapp GR (1972) Some anatomical and behavioural adaptations of ringtails, Bassariscus astutus. J Mammal 53:549–557
Van Valkenburgh B (1984) A morphological analysis of ecological separation within past and present predator guilds. Ph.D. dissertation, The Johns Hopkins University. University Microfilms, Ann Arbor
Van Valkenburgh B (1985) Locomotor diversity in past and present guilds of large predatory mammals. Paleobiology 11:406–428
Van Valkenburgh B (1987) Skeletal indicators of locomotor behaviour in living and extinct carnivores. J Vert Paleontol 7:162–182
Van Valkenburgh B (1988) Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14:155–173
Vrba ES (1992) Mammals as a key to evolutionary theory. J Mammal 73:1–28
Wade-Smith J, Verts B (1982) Mephitis mephitis. Mamm Species 173:1–7
Wainwright PC (2007) Functional versus morphological diversity in macroevolution. Annu Rev Evol Syst 38:381–401
Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD (2005) Many-to-one mapping of form to function: a general principle in organismal design? Integr Comp Biol 45:256–262
Walmsley A, Elton S, Louys J et al (2012) Humeral epiphyseal shape in the felidae: the influence of phylogeny, allometry, and locomotion. J Morphol 273:1424–1438
Wang A (2011) "Meles meles" (On-line), Animal Diversity Web. Accessed February 3, 2015 at http://animaldiversity.ummz.umich.edu/accounts/Meles_meles/
Webb D, Sparrow WA (2007) Description of joint movements in human and non-human primate locomotion using Fourier analysis. Primates 48:277–292
Wiley DF, Amenta N, Alcantara DA, Ghosh D, Kil YJ, Delson E et al (2005) Evolutionary morphing. In: Proceedings of IEEE Visualization 2005 (VIS’05), 23–28 October 2005, Minneapolis, pp 431–438
Williams RC (1955) The osteology and myology of the ranch mink (Mustela vison). PhD thesis, Cornell University Press, New York
Wilson DE, Mittermeier RA (2009) Handbook of the mammals of the world, Lynx Edicions: Barcelona
Witmer LM (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 19–33
Yalden DW (1999) The history of British mammals. T and AD Poyser, London
Acknowledgments
We thank P. D. Polly, J. Samuels, R. Asher and an anonymous reviewer for helpful and constructive comments on an earlier version of this paper; we thank María Jesús Siliceo and Hospital Nuestra Señora de América (Madrid, Spain) for CT scanning the bones of S. batalleri. We thank J. Cuisin, G. Véron, J. Villemain and C. Bens for the access to specimens from the collections Mammifères et Oiseaux, MNHN, Paris; L. Costeur, from the Naturhistorisches Museum, Basel, J. Chupasko from the Harvard Museum of Comparative Zoology, Cambridge, Massachusetts, and S. Peurach from the Smithsonian National Museum of Natural History, Washington, District of Columbia for allowing us to scan the comparative material. We thank the ‘plate-forme de morphométrie’ of the UMS 2700 (CNRS, MNHN) for access to the surface scanner. A-C Fabre thanks the Fondation Fyssen, the doctoral school FdV, the Fondation Bettencourt-Schueller, and A. Murrayn and M. Collins to help her to obtain a UCL IMPACT scholarship for funding. MJS and JM belong to the Research Group UCM-BSCH 910607. We also thank A. Herrel, L. Bascher, F. Goussard, M. Randau, S. Moulin, C. Houssin and three anonymous reviewers for their helpful discussions and comments on this manuscript. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 9733 kb)
Rights and permissions
About this article
Cite this article
Fabre, AC., Salesa, M.J., Cornette, R. et al. Quantitative inferences on the locomotor behaviour of extinct species applied to Simocyon batalleri (Ailuridae, Late Miocene, Spain). Sci Nat 102, 30 (2015). https://doi.org/10.1007/s00114-015-1280-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-015-1280-9