Abstract
The association of cancer and diabetes mellitus (DM) has been studied for decades. Hyperglycemia and the imbalance of hormones are factors that contribute to the molecular link between DM and carcinogenesis and cancer progression. Hyperglycemia alone or in combination with hyperinsulinemia are key factors that promote cancer aggressiveness. Many preclinical studies suggest that high glucose induces abnormal energy metabolism and aggressive cancer via several mechanisms. As evidenced by clinical studies, hyperglycemia is associated with poor clinical outcomes in patients who have comorbid DM. The prognoses of cancer patients with DM are improved when their plasma glucose levels are controlled. This suggests that high glucose level maybe be involved in the molecular mechanism that causes the link between DM and cancer and may also be useful for prognosis of cancer progression. This review comprehensively summarizes the evidence from recent pre-clinical and clinical studies of the impact of hyperglycemia on cancer advancement as well as the underlying molecular mechanism for this impact. Awareness among clinicians of the association between hyperglycemia or DM and cancer progression may improve cancer treatment outcome in patients who have DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM), a chronic and progressive disease diagnosed with excessive plasma glucose, is one of the most prevalent non-communicable diseases worldwide. DM is associated with substantial comorbidities and mortality. The International Diabetes Federation reported that approximately 463 million people, aged 20–79 years old, were diagnosed with DM worldwide in 2019. The number of patients with DM is predicted to rise up to 700 million people by 2045 [1]. Moreover, the pre-diabetic population is also significant. Approximately 374 million people are now diagnosed with either impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), a condition of abnormally high plasma glucose but still below diabetic criteria [1]. These people are at increased risk of developing type 2 DM and other long-term complications [2].
Diabetes-related long-term complications are categorized into macrovascular such as stroke and microvascular types such as diabetic retinopathy [3, 4]. Beside serious long-term complications, DM also increases the risk of tumorigenesis and accelerates cancer progression. The association between DM and cancer has been reported in several cancers including colorectal, breast, and pancreatic cancer [5]. Furthermore, cancer patients who have DM or abnormal plasma glucose are prone to a worse prognosis [6]. Tumor progression in DM patients is also associated with the hormonal effects from insulin and other cytokines [7] and those cancer cells exposed to a supraphysiological concentration of glucose show intracellular signaling alteration and result in more aggressive phenotypes [6, 8, 9].
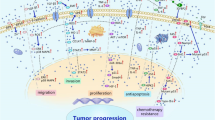
Diabetogenic glucose levels promote the progression of several cancer cell types by various mechanisms. The mechanisms underlying high glucose-induced aggressiveness of cancer are different among each type of cancers and likely cancer-type specific effects. Increase of aerobic glycolysis (Warburg effect)-dependent pathways and activation of several intracellular signaling have been shown in cancer cells under high glucose condition (Fig. 1). Glucose is transported intracellularly via glucose transporter-1 (GLUT1) and stimulates several metabolic pathways. Many key rate-limiting enzymes in glycolysis, e.g., hexokinase-II, phosphofructokinase-1, and pyruvate kinase M2, are upregulated, resulting in the increasing of metabolites needed for synthesis of several macromolecules. In parallel, glucose can activate many signal pathways, e.g., ERK, STAT3, and NF-ĸB, involving cell proliferation, metastatic ability, and chemoresistance of cancer cells [6, 8, 9]. The activations of these intracellular pathways regulate the transcription of their specific downstream target genes that promote the aggressive phenotypes. In addition, at the clinical level, long-term clinical outcome and survival are poor in cancer patients with DM whose plasma glucose are poorly controlled [10, 11]. Therefore, high glucose has been proposed and identified as a mechanism by which DM is linked to cancer progression. In this article, the mechanisms by which glucose dysregulation induces tumor progression and the potential agents that hold promise in cancer treatment under hyperglycemic conditions are reviewed.
The effects of high glucose on energy metabolism and intracellular signaling pathways. The mechanisms of high glucose-induced aggressiveness of cancer are different among cancer types. Glucose upregulates key enzymes in glucose metabolism resulting in the increase of glycolysis, O-GlcNAcylation, lactic acid production, and lipid synthesis. In parallel, glucose also activates several signal pathways that promote aggressive phenotypes of cancer. Red arrows, upregulation; solid arrows, evidence reported in various cancers; dash arrows, unclear mechanisms. Abbreviations: GLUT glucose transporter, HKII hexokinase II, G6P glucose-6-phosphate, PFK1 phosphofructokinase 1, ENO enolase, PKM2 pyruvate kinase M2, LDHA lactate dehydrogenase A, FASN fatty acid synthase, GFAT glutamine fructose-6-phosphate amidotransferase, HIF1α hypoxia-inducible factor 1α, EGF epidermal growth factor, EGFR epidermal growth factor receptor, VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor, CXCR4 C-X-C motif chemokine receptor 4, CXCL12 C-X-C motif chemokine ligand 12
Patients with cancer and hyperglycemia prone to have a worse prognosis
A number of studies demonstrate that clinical outcome is solely dependent on glycemic status of patients regardless DM [7, 12, 13]. A retrospective analysis of 715 clinical stage I–III patients with colon adenocarcinoma suggests that hyperglycemia and obesity are correlated with a poorer prognosis. Hyperglycemia is associated with a decreased 5-year disease-free survival to 66% compared with 80% for the normoglycemic group (HR: 1.87; 95% CI: 1.29–2.72; P=0.001). Consistently, another study observed a 5-year overall survival in patients with hyperglycemia of 73% compared to 83% for patients with normoglycemia (HR: 1.46; 95% CI: 1.002–2.14; P=0.047) [10]. Villarreal-Garza et al. reported that among 265 patients with advanced breast cancer, overall survival is not different between DM and non-DM subjects. However, DM patients with controlled glucose survive significantly longer than patients with uncontrolled glucose (> 130 mg/dL). Regardless of DM history, patients with mean glucose level more than 130 mg/dL are at higher risk of death compared to the normoglycemic group [14]. Compiling 11,091 patients in 11 studies, a systematic review and meta-analysis on DM and a prognosis of cervical cancer was conducted by Chen et al. They reported that DM is a useful prognostic factor for cervical cancer, in which DM is associated with poorer overall survival (HR: 1.59; 95% CI: 1.35–1.87; P < 0.001) and recurrence-free survival (HR: 1.98; 95% CI: 1.47–2.66; P <0.001) compared with non-DM patients [15]. Wright et al. investigated the biochemical recurrence among 1,734 patients with localized prostate cancer who underwent radical prostatectomy or radiotherapy. Patients with fasting plasma glucose from 100 mg/dL have a 50% higher risk of recurrence compared with patients whose plasma glucose was below 100 mg/dL at the time of diagnosis [16, 17].
Post-operative hyperglycemia also affects treatment outcomes. Fiorillo et al. reported that non-DM gastric cancer patients with plasma glucose more than 200 mg/dL after gastrectomy have poorer post-operative outcomes and a higher rate of complications [7]. Moreover, post-operative patients with hyperglycemia also had a worse overall survival rate (45% vs 57%; P=0.05) and disease-free survival rate (46% vs 68%; P =0.02) in another retrospective study [17]. Simon et al. enrolled 301,948 volunteers who came for a regular health checkup and categorized them based on fasting plasma glucose. Following up for several years, patients with high plasma glucose suffer significantly more cancer-related deaths (HR: 1.17; 95% CI: 1.03–1.34; P <0.05), especially gastrointestinal cancer and leukemia. With applications of glucose-lowering medications, the risk is significantly decreased [18]. Table 1 summarizes the effect of hyperglycemia on clinical outcomes of patients with various types of cancer.
As demonstrated by clinical outcome, most patients with cancers who have uncontrolled plasma glucose have a worse prognosis and shorter survival time. Thus, many preclinical studies have been conducted both in vitro and in vivo to elucidate the molecular mechanisms by which high glucose increases the aggressiveness of cancer. Both the metabolic utility of glucose and other glucose’s roles have been investigated to suggest the therapeutic window for the improvement of clinical outcomes.
Warburg effect: a malignant hallmark of glucose metabolism
Deregulation of cellular energetics is one of the 10 cancer hallmarks summarized by Hanahan and Weinberg [19]. However, the aberrant energy metabolism in cancer cells was observed by Warburg almost a century ago [20]. Warburg reported that, even in an oxygen-sufficient environment, cancer cells often utilize glucose via glycolysis instead of oxidative phosphorylation. It is speculated that this so-called aerobic glycolysis or Warburg effect results from impaired mitochondrial function in cancer cells. Although a reasonable assumption, the explanation of the root cause of Warburg effect has been challenged. One criticism is that not all cancers with Warburg effect have impaired mitochondria [21]. Moreover, this phenomenon is also found in highly proliferative normal cells as well as in embryonic cells which have high anabolic rate. Many glycolytic intermediates can be shunted to other anabolic pathways serving precursors for biomolecules syntheses and used as building blocks for cell proliferation. Thus, the Warburg effect provides an advantage for cancer cell survival and advancement rather than being the result of ineffective energetic machinery. To achieve high rate of aerobic glycolysis, some cancer types adapt themselves to have specific glycolytic enzyme isoforms [22]. For example, hexokinase II (HKII) and pyruvate kinase (PK) M2 promote glycolysis and increase glycolytic intermediates accumulation in the glycolytic pathway [23]. This metabolic adaptation then provides cancer cells the building blocks that allow for higher proliferation and more invasiveness. The overexpression of these specific isoforms of proteins or enzymes potentiates cancer cells to survive and compete with normal cells in particular microenvironments.
Glucose is not only a major cellular energy source, but itself also a regulator of the energy metabolism pathways. Glucose upregulates several glycolytic genes and genes involved in glucose utilization, e.g., glucose transporter (GLUT), HKII, and PKM2 in both normal and cancer cells [24, 25]. Studies in pancreatic cancer demonstrate that high glucose upregulates the expression of hypoxia-inducible factor 1α (HIF1α) regardless of oxygenic condition. The increased HIF1α protein results in upregulation of its downstream targets that function in glycolysis, i.e., HKII, platelet-type of phosphofructokinase (PFKP), and lactate dehydrogenase A (LDHA) [26, 27]. A study in breast cancer also demonstrated that high glucose upregulates the expression of HKII and PK [28]. In addition to promoting glycolysis, upregulation of glycolytic enzymes by high glucose facilitates other aggressive phenotypes of cancer cells as well. High glucose upregulates enolase 1 (ENO1), a glycolytic enzyme, in gastric cancer. ENO1 expression in gastric cancer in turn regulates the expression of Snail, which is a transcription factor in the epithelial-mesenchymal transition (EMT) process and, hence, promotes migration of cancer cells apart from its regular role in glycolysis [29]. Altogether, the evidence suggests that high glucose not only fuels abnormal glycolysis but also provides a competitive advantage for cancer cells in the other ways. Therefore, studies of high glucose and other cancer hallmarks are still being investigated.
High glucose induces cancer cell proliferation by modulating signaling pathways
Most studies on the effects on cancer of high glucose and other diabetic conditions are carried out in pancreatic and breast cancers [30,31,32]. However, the effects of high glucose on cancer cell proliferation and growth are reported in almost every organ system. Therefore, in this review, we discuss the effects of high glucose on cancer growth in groups according to the organ in which the cancer occurs.
Pancreatic cancer
Since one etiology of DM is insufficient insulin production, one could speculate that DM may be a consequence of pancreatic cancer. The malignancy or treatment might destroy the Islet of Langerhans and result in type 1 DM in some patients [33, 34]. However, the epidemiological studies indicate that DM is associated with increased risk of pancreatic cancers in some cohort studies [35]. A recent molecular study also reported that high glucose level is associated with aberrant glycosylation in pancreatic cells, especially O-GlcNAcylation. Increased O-GlcNAcylated ribonucleotide reductase results in less enzymatic activity and leads to a deficiency of deoxynucleotide triphosphate (dNTP). The abnormally low dNTP causes genomic DNA alterations in several genes, including in KRAS—an oncogene frequently mutated in pancreatic cancer. This study pointed out a possible mechanism underlying the linkage between DM and the transformation of pancreatic cells toward malignancy [36]. Furthermore, hyperglycemia is also significantly associated with poor clinicopathological characteristics, e.g., higher TNM staging, metastatic status, and shorter survival of patients with pancreatic cancer [37].
A study of pancreatic tissues from patients found that sterol regulatory element-binding protein 1 (SREBP1), an important transcription factor involved in lipid metabolism, is increased in tumor tissue compared with normal tissue. In vitro and in vivo experiments also showed an increased proliferation rate of pancreatic cancer cells in high glucose medium and increased size of implanted tumor in diabetic mice in which the mechanism was proven via the induction of SREBP1 expression [37]. The study with effect of short-term treatment of high glucose to pancreatic cancer cells also showed the consistent results. Han et al. reported that pancreatic cancer cells cultured in high glucose have increased epidermal growth factor (EGF) expression which in turn activates epidermal growth factor receptor (EGFR). They also demonstrated that high glucose can transactivate EGFR independently from the EGF and stimulate the proliferation of pancreatic cancer cells [38]. Another report by Ito et al. [39] revealed high glucose-enhanced pancreatic cell proliferation by increasing the expression of osteopontin. The effect of osteopontin on cell proliferation is augmented by insulin, which is usually increased in patients with insulin resistance or type 2 DM. They also showed that high glucose can induce intracellular oxidative stress, a tumor promoting condition, as indicated by increased level of 8-hydroxy-2’-deoxyguanosine (8-OHdG). A later study by Luo et al. [40] showed that an increase of intracellular reactive oxygen species (ROS) in high glucose conditions is associated with an accelerated proliferation rate in pancreatic cancer cells. Glucose-induced ROS in pancreatic cancer has an anti-apoptotic effect via inhibition of JNK pathway. In addition to its direct effect on cancer cells, high glucose alters the tumor microenvironment and indirectly promotes cancer cell proliferation. High glucose upregulates CXCL12 expression in pancreatic stellate cells and upregulates CXCR4 which is a CXCL12 receptor in pancreatic cancer cells. The interaction of CXCL12-CXCR4 thus accelerates the proliferation of pancreatic cancer cells in a high glucose environment [41]. All studies in pancreatic cancer suggest that diabetic condition, in particular high glucose, can aggravate the carcinogenesis as well as cancer cell proliferation. Hyperglycemia in patients with pancreatic cancer is not likely a simple complication of the malignant progression but indeed a poor prognostic factor that need to be addressed.
Breast cancer
The association between DM and breast cancer has been studied as extensively as it has for pancreatic cancer. Breast cancer is significantly associated with DM and a metabolic syndrome [42, 43]. Epidemiological studies indicate that DM is associated with increased risk of breast cancer and promotes tumor progression once the malignancy is established [44, 45]. An in vivo study supports these epidemiological findings. Mice fed with high glycemic index food and carcinogens developed breast cancer more rapidly and had more severe tumor burden than those fed with lower glycemic index food. The study also found that levels of two adipokines, leptin and adiponectin, are increased in mice fed with high glycemic index food [46]. High glucose and metabolic hormones from adipose tissues are suspected to be involved in the carcinogenesis of breast cancer. This study also demonstrated the importance of lipid metabolism on the progression of breast cancer, as the high expression of the enzyme fatty acid synthase (FASN) is essential for breast cancer cell proliferation and can be upregulated by high glucose [47].
As breast cancer cells are mostly dependent on the activation of hormones and growth factors, it was originally hypothesized that the excessive insulin and insulin-like growth factor (IGF) may underlie this association. The report of Yamamoto et al. [48], in fact, proved that insulin and high glucose play a reciprocal and critical role in stimulation of breast cancer cell proliferation. In addition, it has consistently been shown that high glucose and insulin synergistically promote the proliferation of breast cancer via increased ROS production [49], which activates insulin receptor substrate (IRS) and in turn activates the mitogen-activated protein kinase (MAPK) pathway [50]. The direct effect of high glucose on breast cancer cells has also been shown. High glucose increases ROS level independently from insulin, as reported by Nasir Kansestani et al. [51]. High glucose stimulates ROS production in breast cancer cells and then promotes growth and anti-apoptosis. An in vitro and in vivo study by Wu et al. [52] also reported that high glucose increases ROS level in breast cancer cells. ROS then triggers nuclear factor-κB (NF-κB) pathway to activate nuclear type 2 C protein phosphatase (PP2Cδ) and promotes breast cancer cell proliferation. The expression of PP2Cδ in tumor tissue from patients with breast cancer who had DM is consistently higher than those with normoglycemia. The effect of high glucose on other signaling pathways has also been reported. Hou et al. [53] showed that stimulated EGFR by high glucose activates the Rho family GTPase, Rac1, and Cdc42, resulting in activation of breast cancer cells’ growth. Additionally, Adham et al. [54] also showed the effect of high glucose on another class of growth factors. Vascular endothelial growth factor (VEGF) and its receptor, vascular endothelial growth factor receptor (VEGFR), are increased in breast cancer cells cultured in high glucose by stabilization of the VEGFR protein. The binding of VEGF to VEGFR then promotes the growth of breast cancer cells. The activation of protein kinase Cδ (PKCδ)-dependent ubiquitin proteasome system by high glucose is another mechanism reported in breast cancer cells cultured in high glucose [55]. Aside from promoting cell proliferation in adherent conditions, high glucose also supports the viability of breast cancer cells in anchorage-independent environment and promotes the growth as demonstrated by soft agar colony-forming assay. The mechanism by which high glucose promotes anchorage-independent growth is signaling through the activation of Akt [56] and decreased expression of angiotensinogen [57]. These results suggest that growing in a non-adherent environment not only provides a survival benefit but also supports the growth of circulating cancer cells during their metastatic process in the blood stream.
Gastrointestinal and hepatobiliary tract cancers
The positive effect of high glucose on cancer cell proliferation of gastrointestinal tract and hepatobiliary systems have also been demonstrated. DM and hyperglycemia are associated with poor prognoses of patients with colorectal cancer (CRC). The study of tumor tissues from patients with CRC who had hyperglycemia shows the decreased methylation of phosphatase 1 regulatory subunit 3C (PPP1R3C), a transcriptional protein primarily involved glycogen metabolism. PPP1R3C also has a functional role in cell growth. Consistently with CRC cells cultured in high glucose, the methylation level of PPP1R3C is repressed and resulted in higher proliferation of CRC cells than those in normal glucose medium [58]. High glucose triggers the signaling pathway in CRC cells as demonstrated by the study of Chen et al. [59]. Cultured in high glucose medium, the insulin-like growth factor receptor (IGFR) is highly activated in CRC cells. The activated IGFR then further signals to Src and Erk and increases CRC cell proliferation. High glucose also modulates the expression and activity of the enzyme glutamine-fructose-6-phosphate amidotransferase (GFAT) to enhance the activity of hexosamine biosynthetic pathway (HBP). HBP is necessary for the synthesis of precursors for glycosylation, which is associated with abnormal post-translational modification of proteins in CRC cells. High glycosylation is found in CRC cells cultured in high glucose and thus results in increased proliferation [60].
DM also affects the risk of cancer in the organ all along the hepatobiliary system, i.e., hepatocellular carcinoma (HCC), intrahepatic and extrahepatic cholangiocarcinoma (CCA), and carcinoma of gall bladder. However, the association of DM and the carcinoma of ampulla of Vater and periampullary carcinoma remains unclear [61]. High glucose can promote HCC cell proliferation by increasing O-GlcNAcylation of proteins and modulating expression of miRNA. The O-GlcNAcylation of a transcription factor zinc finger protein 410 (APA1) is increased in HCC cells cultured in high glucose as well as in tumor tissues from patients who had DM. O-GlcNAcylation of APA1 stabilizes and enhances its transcriptional function. APA1 upregulates the expression of gap junction protein gamma 1, a proto-oncogene, which promotes proliferation of HCC cells [62]. High glucose also upregulates miRNA-483-3p and suppresses the expression of endoplasmic reticulum protein 29, resulting in increased proliferation of HCC cells [63]. In addition, our previous report showed the proliferative effect of high glucose in intrahepatic CCA cells by activation of signal transducer and activator of transcription 3 (STAT3) pathway. The in vitro effect was confirmed by immunohistochemistry of tumor tissues from patients, which found higher nuclear expression of STAT3 and p-STAT3 in patients with CCA who had DM. Controlling glucose level to within normal range thus reverses STAT3 effect and reduces the proliferation of CCA cells, suggesting the vital roles of high glucose in CCA progression [64].
Reproductive organs and genitourinary tract cancer
The promoting effect of high glucose on cancers in reproductive organs and genitourinary tract has been studied in both male and female types. Since the function of reproductive organs, i.e., ovaries and testes are under regulation of hormones, the interaction of sex hormones and insulin in patients with DM is hypothesized. The effect of DM on the risk of prostate cancer carcinogenesis are widely studied; however, their association remains controversial [65]. Most reports show that DM is a protective factor for prostate cancer development; in contrast, many reports emphasized the effect of hyperglycemia on cancer cell progression rather than the effect of hormones [66, 67]. Once malignantly transformed, prostate cancer cells show a dependence on high glucose for growth promotion, and high glucose results in worse prognosis [68]. The study by Rezende et al. [69] showed that high glucose alone or in combination with palmitate-a fatty acid increases proliferation of prostate cancer cells by regulating the metabolic sensor AMP-activated protein kinase (AMPK). AMPK is a multifunctional kinase which can inhibit cell proliferation. Therefore, suppression of AMPK by high glucose results in increased proliferation of prostate cancer cells. Supporting these findings, Li et al. [70] found that high glucose upregulates the expression of miRNA-301, which suppresses the translation of p21 and Smad4, resulting in the acceleration of cell cycle progression of prostate cancer both in vitro and in vivo. Recruited patients with prostate cancer who had high plasma glucose also showed a higher severity of prostate cancer burden as indicated by Gleason score. In contrast, an in vitro study from Chen et al. [71] showed the opposite result that high glucose suppresses the proliferation and promotes apoptosis of prostate cancer cells. The underlying mechanism implicated in the latter study was suppression of genes involving the anti-oxidative stress and increase expression of inflammatory cytokines which trigger the apoptosis cascade. However, the study of Chen et al. used only one prostate cancer cell line and is therefore difficult to generalize to prostate cancer biology. The available evidence is inconclusive with respect to the effects of DM and hyperglycemia on the carcinogenesis and progression, especially effect on cell proliferation, in prostate cancer. More epidemiological and molecular investigations are needed to make a concrete conclusion.
The effect of high glucose on proliferation of cancers of female reproductive organs, e.g., ovary, endometrium, and cervix, have also been reported. An in vitro and in vivo study by Kellenberger and Petrik [72] showed that high glucose induces proliferation of ovarian epithelial cancer cells independently from insulin. They also demonstrated the promoting effect of hyperglycemia on tumor growth in insulin deficient type 1 DM mice. The studies of endometrial cancer cells reported the same effect of high glucose. Han et al. [73] showed that in addition to increased glycolytic activity in endometrial cancer cells, high glucose also modulates the signaling of AMPK/mTOR/S6 and MAPK pathways. The modulations of these pathways, hence, results in increased endometrial cancer cell proliferation. Another report also showed that the activation of STAT3 by high glucose is another mechanism underlying the accelerated growth of endometrial cancer [74]. Moreover, the effect of high glucose on STAT3 activation and promoting cell proliferation is also reported in HeLa cells, a cervical cancer cell line, by downregulating genes associated with retinoid-interferon-induced mortality 19 (GRIM-19), a mitochondria-related protein, interacting and inhibiting phosphorylation of STAT3 [75].
Apart from the cancers of male and female reproductive organs, the proliferative effect of high glucose in cancers of the genitourinary tract has also been shown. High glucose increases the proliferation of bladder cancer cells via activation of Wnt/β-catenin signaling pathway [76]. In a combination treatment with insulin, another report showed that ERK and p38 MAPK pathway are activated. Activated ERK and p38 pathways result in high expression of cell cycle machinery and anti-apoptotic proteins in bladder epithelial cells. In patients with DM, glucose levels are increased in not only in the blood but also in urine. High glucose levels in urine may increase risk of bladder cancer development in DM patients and may accelerate tumor growth in DM patients with bladder cancer [77].
Cancer in other systems
While there are relatively fewer number of studies, high glucose also exerts the enhancing effect on cancer cell proliferation in other organs and systems. A study in glioblastoma, a malignancy of glial cells in central nervous tissue, revealed that exposure to high glucose upregulates EGFR and formyl peptide receptor 1 (FPR1), a G-protein-coupled chemoattractant receptor. The upregulation of EGFR and FPR1 results in high proliferation of glioblastoma cells in high glucose conditions [78]. Studies in two lung cancer subtypes, lung adenocarcinoma and non-small cell lung carcinoma (NSCLC), also demonstrated the effect of high glucose on cancer cell growth. High glucose upregulates the expression of the receptor for advanced glycation end-products (RAGE) and nicotinamide adenine dinucleotide phosphate oxidases (NOXs) in lung adenocarcinoma cells. The authors showed that NOXs are possibly a downstream target of RAGE that can activate the expression of VEGF and HIF1α. The upregulation of RAGEs/NOXs/VEGF/HIF1α was proven as the underlying mechanism of high glucose induced lung adenocarcinoma cell proliferation [79]. NSCLC, another subtype of lung cancer, is also affected by high glucose condition. High glucose activates the growth of NSCLC cells by stimulating JNK, ERK, and p38 MAPK pathways and promotes the proliferation and anti-apoptotic properties in NSCLC. The activated JNK downregulates p53, a well-known tumor suppressor gene, leads to promote the survival of NSCLC cells in high glucose [80]. Lastly, high glucose inhibits the expression of growth arrest-specific 5 (GAS5) but elevates tribbles homolog 3 (TRIB3) level. The altered levels of GAS5 and TRIBE3 then result in higher proliferation and promote anti-apoptosis of NSCLC cells [81].
Taken together, this evidence emphasizes the crucial roles of diabetogenic glucose level that not only associates with increased risk of carcinogenesis in almost organ systems but also later becomes a factor that promotes tumor growth. Aside from promoting the growth of the primary tumor, high glucose can also promote an aggressive phenotype which negatively affects patient survival, and enhancement of metastatic activities.
High glucose-induced metastatic potential of cancer cells
Metastasis is a complex process that requires a multistep alteration of cell biology. Epithelial cancer cells need to adapt themselves to have more mesenchymal characteristics, by an epithelial-mesenchymal transition (EMT) process which facilitates their mobility [82]. They need to produce enzymes for degradation of extracellular matrix, express the adhesion molecules for the invasion through the endothelium, and adhere to the secondary sites. Anchorage-dependent cells also need to resist the apoptosis induced by non-adherent status or anoikis resistance [83]. Studies in the past decade demonstrated that high glucose contributes to the promotion of these steps throughout metastatic cascade. In several cancers, high glucose promotes the EMT process, a key step of cell migration, by various mechanism, e.g., upregulation of transcription factors of EMT-related genes. High glucose also upregulates enzymes in both the metalloproteinases (MMPs) and plasminogen activators (PAs) families that are involved in the degradation of extracellular matrix proteins. The reported mechanisms are different from one cancer to another, and in some cancer, further study is required.
Pancreatic cancer
Hyperglycemia is usually associated with a poor prognosis, i.e., the metastatic disease in patients with pancreatic cancer. In vitro and in vivo studies of metastasis induced by high glucose showed that increased ROS mainly contributes to the EMT process and invasiveness of pancreatic cancer. An in vitro study demonstrated that high glucose increases the level of hydrogen peroxide and results in upregulation of urokinase plasminogen activator (uPA), the essential enzyme for invasive ability. The invasiveness of pancreatic cancer cells, thus, could be reversed with the ROS scavenging agents, e.g., superoxide dismutase (SOD) [84]. A later in vivo study by xenograft model in streptozotocin (STZ)-induced type 1 diabetic nude mice by Li et al. discovered several pathways underlying high glucose-induced migration, invasion, and then in vivo metastasis of pancreatic cancer [85]. Most of the signaling pathways are triggered by the increased level of ROS. Hyperglycemic mice contain a higher plasma hydrogen peroxide level than those mice with normoglycemia. Increased hydrogen peroxide is associated with the upregulation of EMT transcription factors and markers such as Snail, N-cadherin, and vimentin, whereas the epithelial marker, E-cadherin, is downregulated. These proteins cause the higher metastatic burden in the hyperglycemic mice. The injection of hydrogen peroxide scavenging agent, polyethylene glycol-conjugated catalase (PEG-CAT) suppresses hyperglycemia-induced metastasis in mice with DM.
The increased production of hydrogen peroxide by high glucose also triggers signaling of ERK and p38 MAPK pathways as well as the transcription factors NF-κB and AP-1. Activation of these signaling pathways results in higher expression of uPA and increases invasiveness of pancreatic cancer cells. The in vivo experiment also revealed more invasion in the renal capsule of pancreatic cancer xenografts, which could be reversed by PEG-CAT treatment [86]. Another study using resveratrol to suppress ROS production also supported these findings that the metastatic potential of pancreatic cancer cells is suppressed in cells cultured in high glucose condition [87]. Apart from the ROS generation, high glucose also mediates the migration and invasion of pancreatic cancer cells by other mechanisms. A study of tumor tissue from patients with pancreatic cancer who had hyperglycemia showed that the expression of HIF1α was significantly higher than those who had normoglycemia. An in vivo study in nude mice with pancreatic cancer xenografts also showed higher expression of HIF1α in type 1 diabetic mice, which results in upregulation of MMP9. Thus, the tissue invasion and distant metastasis are much more occurred in hyperglycemic mice [88]. Another study also showed that high glucose promotes EMT process in premalignant H6c7-kras pancreatic cells by stimulating the secretion of transforming growth factor-β1 (TGFβ1). The TGFβ1 signaling then suppresses the expression of E-cadherin and induces nestin expression. Moreover, the same study also demonstrated that pancreatic cancer cells exposed to high glucose exhibit cancer stem cell properties and increased Nanog-a cancer stem cell marker expression [89].
Breast cancer
A study in patients with breast cancer found that those who had type 2 DM had significantly more lymphatic and distant metastasis [90]. The expressions of GLUT1, MMP2, and MMP9 in patients with type 2 DM are significantly higher than patients without DM. The suppression of GLUT1 expression in breast cancer cells by siRNA suppresses the expression of MMP2 and MMP9, which results in decreased in vitro invasion. The synergistic effects of high glucose and insulin are demonstrated in several studies. For example, Flores-López et al. [49] showed that high glucose and insulin increase the migration and invasion of a highly metastatic breast cancer cell line, MDA-MB-231, via ROS production. The increased expression of uPA, uPAR, and PAI-1, as well as an increase in activity of uPA were shown as the underlying mechanisms. Moreover, the later study by Viedma-Rodriguez et al. [91] showed that using ε-aminocaproic acid, an inhibitor of the binding of plasminogen to cell surface, suppresses the migration and invasion of MDA-MB-231 induced by high glucose and insulin. The EMT markers and expression of uPA, uPAR, PAI-1, MMP2, and MMP9 are accordingly attenuated, emphasizing the importance of the plasminogen systems in invasiveness of breast cancer under the diabetic condition. Another study suggested that high glucose and insulin activate the signaling through the classical pathway of insulin. The IRS1 expression is higher in breast cancer cells cultured in high glucose and high insulin concentration compared with those cultured in normal glucose and normal insulin supplement. The downstream signaling molecules, i.e., Ras/Raf/MEK/ERK, are then activated and result in increased migration and invasion ability of breast cancer cells when exposed to high glucose and high insulin [50].
The direct effects of high glucose on metastatic potential of breast cancer have also been studied. Wu et al. [52] found that increased PP2Cδ expression by activating ROS/NF-κB also promotes migration and invasion of breast cancer cells, in addition to promoting cancer cell growth. The activation of PKCδ phosphorylation under high glucose also increases invasion of breast cancer cells via activation of the proteasome [55]. The alterations of EMT markers, i.e., increased vimentin and Slug and decreased E-cadherin, are promoted by high glucose [47]. The upregulation of cellular transporter proteins by high glucose are other mechanisms involved in the migration and invasion activity of breast cancer cells. Matsui et al. [56] found that GLUT12 expression is increased in breast cancer cells cultured in high glucose. The suppression of GLUT12 expression hence suppresses the migration activity. The same group also showed that the transporters of Zn2+ ion in breast cancer cells are also increased by high glucose. The authors proved that the high level of intracellular Zn2+ ion is essential for the mobility of breast cancer cells. Thus, increased intracellular Zn2+ level by upregulation of Zn2+ transporter facilitates the migration of breast cancer cells [92]. High glucose also indirectly promotes the metastatic potential of breast cancer cells by inducing cytokine production in tumor microenvironment. Kallens et al. [93] reported that high glucose favors a pro-inflammatory and pro-oxidant environment, as indicated by the induction of the cyclooxygenase/prostaglandin E2 (COX-2/PGE2) axis. PGE2 produced by tumor microenvironment cells thus activates the synthesis of interleukin-1β which stimulates EMT and migration of breast cancer cells. A study in orthotopic tumor grafts in type 2 diabetic mice also showed similar results. Hyperglycemic mice bearing breast cancer had a higher lung metastatic burden compared with normal mice or diabetic mice with controlled glucose levels. The treatment of hyperglycemia using insulin reverses the effect of hyperglycemia-induced metastasis, emphasizing the direct effect of high plasma glucose rather than the effect of insulin signaling. The study showed that hyperglycemia suppresses the expression of granulocyte colony-stimulating factor, a cytokine that triggers neutrophil function. Thus, mobilization suppression of lung neutrophils in high glucose results in the attenuation of their anti-metastatic activity [94]. These studies firmly suggest the promoting effect of high glucose on breast cancer metastasis in addition to the effect of insulin in patients with type 2 DM.
Gastrointestinal and hepatobiliary tract cancers
A study by Xu et al. [29] found that the effect of high glucose on the upregulation of ENO1 is associated with increased expression of Snail, in addition to promoting glycolysis. Overexpression of Snail, thus, enhances the EMT process and the migration of gastric cancer cells under high glucose conditions. In vitro and in vivo studies in CRC cells also demonstrated similar results. High glucose promotes the EMT process of CRC cells as evidenced by decreased E-cadherin and increased vimentin which associate with the higher migration ability of CRC cells. High glucose also upregulates high-mobility group A protein 2 to promote the mobility of the CRC cells [95]. High glucose can alter the expression of miRNA, e.g., downregulating miRNA-9. As miRNA-9 normally controls the expression of EMT genes in CRC cells, the suppression of miRNA-9 results in increased N-cadherin and reduced E-cadherin levels and thus enhances the migration of CRC cells [59]. High glucose upregulates GFAT, which is involved with the invasiveness of CRC cells by promoting the aberrant glycosylation of proteins. The alteration of glycosylation of proteins, then, disrupts their normal functions and leads to tumor progression [60]. A study in a rat CRC cell line revealed that high glucose activates STAT3 pathway and upregulates MMP9, which increase invasion activity [96]. High glucose also increases the phosphorylation and promotes nuclear localization of STAT3 in CCA. Activated STAT3 increases expression of vimentin and MMP2 and increases the in vitro migration and invasion of CCA cells [64]. Moreover, in highly metastatic sublines of CCA cells, high glucose additionally increases CCA cells’ migration activity by increased O-GlcNAcylation of NF-ĸB transcription factor, which in turn stimulates the transcription of EMT-related genes [97].
Reproductive and genitourinary tract cancer
A study of bladder cancer found that high glucose can induce cancer cell migration and invasion. However, the laminar shear stress generated from the flowing of blood or fluid containing glucose can suppress the migration of cells by physical force, which may be a protective factor for metastasis in patients with DM [98]. The study in prostate cancer also showed that high glucose and high palmitate promote the migration of cells as well as their proliferation. The mechanism may involve the suppression of AMPK and also an increase activity of catalase, which alters the ROS balance in cells [69]. The effect of DM on risk and progression of endometrial cancer is known by the effect of hormone level. However, the direct effect of glucose on both proliferation and metastatic potential has been demonstrated in recent years [72]. High glucose promotes the migration and invasion of endometrial cells by various mechanisms. Gu et al. [99] reported that high glucose increases the expression of GLUT4 and estrogen receptor (ER) in endometrial cancer cells, which is associated with the expression of VEGF and VEGFR. The suppression GLUT4 not only inhibits the growth of endometrial cancer both in vitro and in vivo but also decreases the invasion. Another study revealed that high glucose disturbs the function of dynamin-related protein 1 in the mediation of mitochondrial homeostasis. The mitochondrial disturbance thus enhances the alteration of cellular metabolism as well as the EMT process of endometrial cancer cells. As a result, the migration and invasion of endometrial cancer cells in high glucose condition is increased by stimulation of EMT process [100].
Lung cancer
A study in lung cancer cells showed that exposure to high glucose, either for short or long time, increases the expression of p-selectin on the cancer cell surface. p-Selectin is an adhesion molecule promoting the attachment of lung cancer cells to the endothelial cells which facilitates their mobility and distant metastasis [101]. As in other cancers, ROS also plays a role in high glucose-induced invasion of lung cancer cells. A study by Kang et al. [102] reported that high glucose increases expression of heme oxygenase 1, an enzyme involved in ROS generation. Concordantly with increased ROS, the expression of TGFβ is also upregulated by high glucose, which then triggers the signaling of PI3K/Akt pathway. The activated PI3K/Akt signal, hence, enhances the invasion of lung cancer cells. The study in NSCLC cell lines demonstrated that in addition to promoting cell proliferation, high glucose also promotes the migration as well. The activation of RAGE-NOXs-VEGF/HIF1α axis or downregulation of GAS5 are associated with increased migration and invasion of NSCLC cell lines, as shown by several studies [79, 81].
High glucose promotes angiogenesis
As shown in several studies, high glucose can upregulate the expression of VEGF and VEGFR in many kinds of cancers to promote their proliferation. The interaction of VEGF and VEGFR not only promotes the proliferation of cancer cells themselves but also promotes other tumor microenvironments that encourage cell proliferation, including angiogenesis. High glucose increases the expression of VEGF and VEGFR in breast cancer via NF-ĸB activation [51, 54]. An in vivo study using a diabetic mice model of breast cancer found that high glucose upregulates the expression of miRNA-467. miRNA-467 is known as a translational suppressor of thrombospondin-1, a potent anti-angiogenic protein in microvascular endothelium and in breast cancer. The overexpression of miRNA-467 thus promotes neovascularization in breast cancer tissues and also underlies the pathogenicity of other microvasculopathy in patients with DM [103]. The systemic injection of miRNA-467 antagonist into diabetic mice with breast cancer also resulted in decreased angiogenesis in the tumor area. This study confirms the effect of high glucose via the action of the miRNA system [104]. Since the effect of high glucose on the vasculopathy in vivo is suspected to be tissue-specific, further study is needed to determine the effect of high glucose in pathological conditions, e.g., in cancer.
High glucose-induced chemoresistance of cancer cells
During cancer treatment, plasma glucose level is a direct determinant of therapeutic outcome for post-chemotherapeutic status. As evidenced in preclinical and clinical studies, hyperglycemia usually influences the response to chemotherapeutic agents in various cancers such as colorectal, breast, pancreatic, and prostate cancer. After CRC cell inoculation in STZ-induced hyperglycemic and non-diabetic mice and following the regular FOLFOX chemotherapy administration; a combination of oxaliplatin, fluorouracil (5-FU), and levofolinate, Ikemura and Hashida observed larger tumor volumes and shorter survival in hyperglycemic mice compared with non-diabetic counterparts. The study reported that the combination of oxaliplatin and 5-FU is less effective in hyperglycemic state [105]. Based on in vitro studies, Ma et al. reported that human CRC cells are more susceptible to the growth inhibition by 5-FU in low glucose conditions [106]. Ideno et al. showed that bromopyruvate uptake by its transporter is suppressed by high glucose in human CRC cell lines, which contributes to a decrease in chemotherapy-induced apoptosis [107]. Another study by Xu et al. pointed out in HCC model that high glucose inhibits AMPK and results in adriamycin resistance in vitro. The resistance to adriamycin of HCC cells thus could be overcome with the co-treatment with the AMPK activator in high glucose condition [108].
Al Qahtani et al. reported that breast cancer cells acquire doxorubicin resistance via upregulation of insulin-like growth factor binding protein-2 (IGFBP-2) and FASN in high glucose conditions. Using siRNA to silence ER-dependent IGFBP-2 expression, sensitivity to doxorubicin is restored. However, hypoxia is able to downregulate IGFBP-2 and FASN which result in chemosensitivity of cancer cells. Hence, negated by hypoxia, IGFBP-2-mediated pathway is involved in chemosensitivity in high glucose conditions [109]. Biernacka et al. also studied the same model in prostate cancer cell lines. They confirmed that high glucose actually increases IGFBP-2 expression via histone acetylation. Upregulated IGFBP-2 leads to significant reduction of docetaxel-induced apoptosis in prostate cancer cells [110]. The other studies also support the involvement of FASN-mediated pathway in chemosensitivity of cancer cells. Zeng et al. revealed that hyperglycemia activates ERα and downstream of FASN and finally enhances chemoresistance in breast cancer cells. When ERα is silenced, all of the effects are abolished. The results indicate that anti-estrogens may possibly be beneficial in ERα-positive breast cancer patients with DM [111]. Another study by Zeng et al. compared the efficacy of several chemotherapeutic agents in breast cancer cells. Chemotherapy-induced cell death is attenuated in cancer cells with hyperglycemic state by upregulated FASN under the modulation of IGF1 [112].
In clinical studies, Yang et al. found that overall survival of stage III colorectal cancer patients receiving adjuvant oxaliplatin depended on plasma glucose level, not diabetic status. Hyperglycemic patients obviously had poorer clinical outcomes, possibly via SMAD3 and MYC phosphorylation, compared to the normoglycemic group [113]. Li et al. retrospectively reviewed records of early-stage cervical cancer patients who received neoadjuvant chemotherapy and underwent radical hysterectomy. Patients with fasting plasma glucose more than 126 mg/dL were less likely to achieve complete response after treatment [114]. Zhao et al. indicated that patients with gastric cancer and DM responded poorly to 5-FU and had shorter survival time. In hyperglycemic state, Nampt1, Sirt1, and mutated p53 were upregulated following by increase in p-gp level, a multi-drug resistant marker, and decrease in Topo-IIα level [115]. Therefore, it is clearly evident both in preclinical and clinical studies that high glucose level and hyperglycemia associate with the chemoresistance. The plasma glucose level, hence, should not be neglected during the therapeutic course of any cancer.
Targeting high glucose-activated pathway to improve cancer treatment in patients with DM
Hyperglycemia is known to drive cancer cells aggressiveness through many different signaling pathways. Apart from preventing hyperglycemia, targeting particular molecules in the pathways may help suppressing tumor progression after treatment completion. Based on recent studies, glucose-lowering agents and targeted drugs have been shown to possess an inhibitory effect on cancer progression [116, 117]. Metformin, a well-known antidiabetic medication, has been proven to interfere tumor growth in various cancer types [118]. As reported by Rogalska et al., metformin induces more cytotoxic effect in an ovarian cancer cell line (SKOV-3) in normoglycemic (5 mM) compared to hyperglycemic condition (25 mM). The underlying mechanism is disruption of HIF1α and O-GlcNAcylation pathways by hyperglycemia. Genes under regulation of both pathways are modified by metformin in high glucose conditions, resulting in suppressed cell proliferation [119]. A similar study was conducted by Litchfield et al. in four ovarian cancer cell lines: HeyA8, Tyk-nu, DOV13, and ID8. The cytotoxic effect of metformin is lower in hyperglycemic conditions (25 mM) [120]. The study of Wallbillich et al. pointed out that STAT3 upregulation in endometrial cancer cells is induced by high glucose and the addition of metformin significantly suppresses the activation of STAT3. Tumor apoptosis and tumor weight reduction are observed in vitro and in vivo, respectively [74]. Valaee et al. evaluated the effect of metformin on EMT process and metastatic potential of gastric cancer cells under high glucose condition. The mesenchymal cell markers, vimentin and β-catenin, are downregulated when metformin is introduced in both normoglycemic (7.8 mM) and hyperglycemic (17.5 mM) conditions. Phenotypic effects of cell migration and invasion in these conditions are also in accord with gene expressions [121]. Lv et al. investigated effects of metformin on cell viability and apoptosis of human hepatocellular cancer cell line (HepG2). In a dose-dependent manner, metformin stimulates cell apoptosis and decreases cell viability of high glucose-induced HepG2 cells. Moreover, these effects are subjected to activation of AMPK phosphorylation and deactivation of mTOR phosphorylation [122]. Results from Varghese et al. in two triple-negative breast cancer cell lines, MDA-MB-468 and MDA-MB-231, also reported the same observation. Metformin exerts a more anti-proliferative effect, via mTOR suppression, in non-glucose and normal glucose conditions (5.5 mM) compared to high glucose condition (25 mM). Apart from glucose level, this effect also varies between cell lines [118]. In addition to metformin, thiazolidinediones has been shown to slow down cancer progression by its glucose-lowering effect [77, 123, 124]. Previous data confirms that the therapeutic effect of 5-FU is attenuated by hyperglycemia [117]. Lau et al. revealed that rosiglitazone and 5-FU synergistically increase apoptotic rate of glucose-treated human CRC cells in two studies [123, 124]. In high glucose condition, reduced form of glutathione level is significantly increased and prevented cell apoptosis. When the combination of 5-FU and rosiglitazone is administered, both in normal and high glucose condition, the apoptosis mediated by HCT116 and HT29 is increased.
A previous study by Cao et al. reported that EMT process in pancreatic cancer cell line (Panc-1) could be inhibited by curcumin [116]. Later, the same group explored the effect of curcumin in reducing hyperglycemia-induced invasive and migratory potential in another pancreatic cancer cell line. By suppressing EGF/EGFR signaling pathway, as well as its downstream ERK and Akt, curcumin inhibits invasive and migratory potential caused by high glucose condition [88]. The same group also introduced another candidate attenuating glucose-driven invasive and migratory potential in Panc-1 cells. By suppressing ERK and p38 MAPK signaling pathway, as well as NF-κB transcriptional factor, resveratrol lowers ROS and hydrogen peroxide levels in Panc-1 cells induced by high glucose conditions. Thus, glucose-driven ROS-induced invasion and migration are repressed [87]. These agents might be alternative approach for the treatment of cancer in patients whose blood glucose are difficult to control. The effects of aforementioned promising agents against high glucose-modulated cancer progression at preclinical level are summarized in Table 2. Although these agents showed a promising effect at preclinical level, the clinical study in cancer patients with DM is limited. One observational study of patients with pancreatic cancer comparing between those who used metformin for DM treatment vs other antidiabetic medications did not found any add-on benefit of metformin in term of overall and recurrent free survival [125].
It has been demonstrated that glucose-lowering agents and targeted drugs play an important role in inhibiting tumor progression via diverse mechanisms. The effects of metformin, resveratrol, and curcumin on the inhibition of glucose-activated pathways are depicted in Fig. 2. These studies, however, are mainly focused on in vitro models. Further animal models and clinical trials are required for better confidence in application of these medications. Along with regular monitoring of plasma glucose level, antidiabetic and targeted drugs may help improve clinical outcomes in cancer patients.
Metformin, curcumin, and resveratrol reverse the high glucose effects on cancer cell progression. Metformin, curcumin, and resveratrol are highly promising agents for cancer treatment. They exert the inhibitory effects on the signaling molecules and pathways activated by high glucose condition resulting in the inhibition of cancer progression. Red lines, inhibition; AMPK AMP-activated protein kinase, ROS reactive oxygen species, GLUT1 glucose transporter 1, EGF epidermal growth factor, EGFR epidermal growth factor receptor
DM: a risk factor or a consequence of cancer development?
Regarding available evidence, DM is associated with the increased risk of a malignancy of many organs [126], e.g., pancreas, liver, colorectum, biliary tract (including bile duct and gallbladder), kidney, breast, ovary, endometrium, urinary bladder, stomach, esophagus, thyroid, meningioma, multiple myeloma, and non-Hodgkin lymphoma. On the other hand, the meta-analysis of observational studies indicates that DM is a protective factor for prostate cancer development [127]. The effects of DM on the increased risk of cancer have been recently reviewed [128, 129].
Opposite to the increased risk of cancers, several studies demonstrated DM as a potential consequence of cancers. The effects of pancreatic cancer on the development of DM have been reported and postulated as a result of pancreatic β cells destruction causing insulin deficiency and also a complication of pancreatectomy that decreased pancreatic mass [130, 131]. Cancers influence DM development which have been shown in the cancers of metabolically active organs, i.e., liver and kidney. The consequence was shown to be resulted from the disturbance of glucose metabolisms and insulin sensitivity [132, 133]. A recent retrospective cohort study in Korea following 524,089 healthy subjects until DM was diagnosed, indicated that people with preceding cancers were significantly associated with the increased risk of DM with a hazard ratio (HR) of 1.35 [95% confident interval (CI): 1.26–1.45; P < .001] [134]. Considering each type of cancer in the same study, the association of malignancy and the risk of DM development was increased in patients with cancer of the pancreas (HR: 5.15; 95% CI: 3.32–7.99), kidney (HR: 2.06; 95% CI: 1.34–3.16), liver (HR: 1.95; 95% CI: 1.50–2.54), gallbladder (HR: 1.79; 95% CI: 1.08–2.98), lung (HR, 1.74; 95% CI, 1.34–2.24), blood (HR: 1.61; 95% CI:1.07–2.43), breast (HR: 1.60; 95% CI: 1.27–2.01), stomach (HR: 1.35; 95% CI, 1.16–1.58), and thyroid (HR, 1.33; 95% CI, 1.12–1.59). The effects of cancers on DM development are suspected as a result of both tumor itself and the therapeutic modalities, for example, the use of corticosteroids [135], chemotherapeutic drugs [136], irradiation [137], and immunosuppressive agents [138]. Cancer cachexia, a common complication of cancer, is also a factor that associated with insulin resistance in cancer patients leading to the increased risk of DM development [139]. To date, the studies on the effects of cancer on DM development are limited and the underlying mechanism is largely unknown. More investigations are needed to clarify the main factors that contribute for DM development in cancer patients. Even if DM is a risk factor or a consequence of cancers, available evidence clearly indicates that concurrent DM and cancer almost always results in the worse prognoses of patients. DM and hyperglycemia are critical factors needed to be concerned to improve therapeutic outcomes of cancers.
Concluding remarks and the future directions of study
The association between DM and cancers as well as the effects of high glucose as a linker for promoting aggressiveness of cancer cells are well evident. The promoting effects of high glucose on cancer hallmarks and their related mechanisms are summarized in Table 3. The molecular mechanisms by which high glucose mediates the aggressive phenotypes, however, are not fully understood. Several models are proposed as responsible mechanisms of high glucose activating cancer cells—namely, the activation of intracellular pathways, post-translational glycosylation and glycation of proteins, and also modification of the glucose metabolism. The mechanisms that high glucose promotes cancer cell progression in each cancer are also varied and seems to be cancer type specific. The aggressive phenotypes of cancer cells activated by high glucose in each cancer type are addressed in Table 4. Searching for the general “master sensor” molecule of high glucose in cancer cells might reveal the target and provide the opportunity to inhibit the aggressiveness of cancers in patients who have DM. The signaling pathways responsible for the aggressiveness of cancer are probably good targets for the development of therapeutic agents. However, one might argue that the easier and more practical way is to control the progression of cancer in patient with DM with the controlling of plasma glucose level. In fact, many patients with critical ill and have underlying DM are difficult to control glucose level within the normal range due to various factors [140]. Therefore, the in-depth understanding of molecular linkage of how high glucose modulates biology of cancer cells is ultimately needed and might direct the research field of DM, hyperglycemia, and cancer treatment. With more in-depth evidence, combining glucose control and cancer therapy may optimize the response of patients to cancer treatment and improve the clinical outcomes.
Data availability
Not applicable
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care 43(Supplement 1):S14–S31
American Diabetes Association (2020) 11. Microvascular complications and foot care: standards of medical care in diabetes−2020. Diabetes Care 43(Supplement 1):S135–S151
American Diabetes Association (2020) 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2020. Diabetes Care 43(Supplement 1):S111–S134
Satija A, Spiegelman D, Giovannucci E, Hu FB (2015) Type 2 diabetes and risk of cancer. Bmj 350:g7707
Ramteke P, Deb A, Shepal V, Bhat MK (2019) Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers (Basel) 11(9):1402
Fiorillo C, Rosa F, Quero G, Menghi R, Doglietto GB, Alfieri S (2017) Postoperative hyperglycemia in nondiabetic patients after gastric surgery for cancer: perioperative outcomes. Gastric Cancer 20(3):536–542
Ryu TY, Park J, Scherer PE (2014) Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J 38(5):330–336
Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, Wu E, Ma Q (2014) Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int 2014:461917–461910
Calderillo-Ruiz G, Lopez H, Herrera M, Trejo E, Carbajal B, Ramos-Ramirez M, Albarran A (2019) Obesity and hyperglycemia as a bad prognosis factor for recurrence and survival in colon cancer. Ann Oncol 30:iv40–iv41
Zylla D, Gilmore G, Eklund J, Richter S, Carlson A (2019) Impact of diabetes and hyperglycemia on health care utilization, infection risk, and survival in patients with cancer receiving glucocorticoids with chemotherapy. J Diabetes Complicat 33(4):335–339
de Beer JC, Liebenberg L (2014) Does cancer risk increase with HbA1c, independent of diabetes? Br J Cancer 110(9):2361–2368
Hosokawa T, Kurosaki M, Tsuchiya K, Matsuda S, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Nakata T, Nishimura T, Suzuki S, Ueda K, Nakanishi H, Itakura J, Takahashi Y, Izumi N (2013) Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol 19(2):249–257
Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, Bacon L, Rivera D, Urzua L, Aguila C, Ramirez-Morales R, Santamaria J, Bargallo E, Mohar A, Herrera LA (2012) Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res 2012:732027–732028
Chen S, Tao M, Zhao L, Zhang X (2017) The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: a systematic review and meta-analysis. Medicine (Baltimore) 96(40):e7981
Wright JL, Plymate SR, Porter MP, Gore JL, Lin DW, Hu E, Zeliadt SB (2013) Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer Prostatic Dis 16(2):204–208
Fiorillo C, Quero G, Laterza V, Mascagni P, Longo F, Menghi R, Razionale F, Rosa F, Mezza T, Boskoski I, Giaccari A, Alfieri S (2020) Postoperative hyperglycemia affects survival after gastrectomy for cancer: a single-center analysis using propensity score matching. Surgery 167(5):815–820
Simon J-M, Thomas F, Czernichow S, Hanon O, Lemogne C, Simon T, Pannier B, Danchin N (2018) Hyperglycaemia is associated with cancer-related but not non-cancer-related deaths: evidence from the IPC cohort. Diabetologia 61(5):1089–1097
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452(7184):230–233
Rempel A, Mathupala SP, Perdersen PL (1996) Glucose catabolism in cancer cells: regulation of the type II hexokinase promoter by glucose and cyclic AMP. FEBS Lett 385(3):233–237
Kahn A (1997) Transcriptional regulation by glucose in the liver. Biochimie 79(2-3):113–118
Liu Z, Jia X, Duan Y, Xiao H, Sundqvist KG, Permert J, Wang F (2013) Excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol Ther 14(5):428–435
Cheng L, Qin T, Ma J, Duan W, Xu Q, Li X, Han L, Li W, Wang Z, Zhang D, Ma Q, Lei J (2019) Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis. Anti Cancer Agents Med Chem 19(12):1503–1512
Santos JM, Hussain F (2020) Higher glucose enhances breast cancer cell aggressiveness. Nutr Cancer 72(5):734–746
Xu X, Chen B, Zhu S, Zhang J, He X, Cao G, Chen B (2019) Hyperglycemia promotes snail-induced epithelial-mesenchymal transition of gastric cancer via activating ENO1 expression. Cancer Cell Int 19:344
Zhang JJ, Jia JP, Shao Q, Wang YK (2019) Diabetes mellitus and risk of pancreatic cancer in China: a meta-analysis based on 26 case-control studies. Prim Care Diabetes 13(3):276–282
Tan J, You Y, Guo F, Xu J, Dai H, Bie P (2017) Association of elevated risk of pancreatic cancer in diabetic patients: a systematic review and meta-analysis. Oncol Lett 13(3):1247–1255
Papa V, Pezzino V, Costantino A, Belfiore A, Giuffrida D, Frittitta L, Vannelli GB, Brand R, Goldfine ID, Vigneri R (1990) Elevated insulin receptor content in human breast cancer. J Clin Invest 86(5):1503–1510
Beger HG, Poch B, Mayer B, Siech M (2018) New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy for benign and malignant tumors: a systematic review and meta-analysis of long-term results. Ann Surg 267(2):259–270
De Bruijn KM, van Eijck CH (2015) New-onset diabetes after distal pancreatectomy: a systematic review. Ann Surg 261(5):854–861
Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M (2014) Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 21(7):2453–2462
Hu CM, Tien SC, Hsieh PK, Jeng YM, Chang MC, Chang YT, Chen YJ, Chen YJ, Lee EYP, Lee WH (2019) High glucose triggers nucleotide imbalance through O-GlcNAcylation of key enzymes and induces KRAS mutation in pancreatic cells. Cell Metab 29(6):1334–1349.e1310
Zhou C, Qian W, Li J, Ma J, Chen X, Jiang Z, Cheng L, Duan W, Wang Z, Wu Z, Ma Q, Li X (2019) High glucose microenvironment accelerates tumor growth via SREBP1-autophagy axis in pancreatic cancer. J Exp Clin Cancer Res 38(1):302
Han L, Ma Q, Li J, Liu H, Li W, Ma G, Xu Q, Zhou S, Wu E (2011) High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS One 6(11):e27074
Ito M, Makino N, Matsuda A, Ikeda Y, Kakizaki Y, Saito Y, Ueno Y, Kawata S (2017) High glucose accelerates cell proliferation and increases the secretion and mRNA expression of osteopontin in human pancreatic duct epithelial cells. Int J Mol Sci 18(4). https://doi.org/10.3390/ijms18040807
Luo J, Xiang Y, Xu X, Fang D, Li D, Ni F, Zhu X, Chen B, Zhou M (2018) High glucose-induced ROS production stimulates proliferation of pancreatic cancer via inactivating the JNK pathway. Oxidative Med Cell Longev 2018:6917206–6917210
Kiss K, Baghy K, Spisák S, Szanyi S, Tulassay Z, Zalatnai A, Löhr JM, Jesenofsky R, Kovalszky I, Firneisz G (2015) Chronic hyperglycemia induces trans-differentiation of human pancreatic stellate cells and enhances the malignant molecular communication with human pancreatic cancer cells. PLoS One 10(5):e0128059
Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D (2012) Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35(11):2402–2411
Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Rafaniello C, Giugliano D (2013) Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause 20(12):1301–1309
Zhou Y, Zhang X, Gu C, Xia J (2015) Diabetes mellitus is associated with breast cancer: systematic review, meta-analysis, and in silico reproduction. Panminerva Med 57(3):101–108
Zhou Y, Zhang X, Gu C, Xia J (2015) Influence of diabetes mellitus on mortality in breast cancer patients. ANZ J Surg 85(12):972–978
Thompson HJ, Neuhouser ML, Lampe JW, McGinley JN, Neil ES, Schwartz Y, McTiernan A (2016) Effect of low or high glycemic load diets on experimentally induced mammary carcinogenesis in rats. Mol Nutr Food Res 60(6):1416–1426
Zielinska HA, Holly JMP, Bahl A, Perks CM (2018) Inhibition of FASN and ERα signalling during hyperglycaemia-induced matrix-specific EMT promotes breast cancer cell invasion via a caveolin-1-dependent mechanism. Cancer Lett 419:187–202
Yamamoto M, Patel NA, Taggart J, Sridhar R, Cooper DR (1999) A shift from normal to high glucose levels stimulates cell proliferation in drug sensitive MCF-7 human breast cancer cells but not in multidrug resistant MCF-7/ADR cells which overproduce PKC-betaII. Int J Cancer 83(1):98–106
Flores-López LA, Martínez-Hernández MG, Viedma-Rodríguez R, Díaz-Flores M, Baiza-Gutman LA (2016) High glucose and insulin enhance uPA expression, ROS formation and invasiveness in breast cancer-derived cells. Cell Oncol (Dordr) 39(4):365–378
Wei ML, Duan P, Wang ZM, Ding M, Tu P (2017) High glucose and high insulin conditions promote MCF-7 cell proliferation and invasion by upregulating IRS1 and activating the Ras/Raf/ERK pathway. Mol Med Rep 16(5):6690–6696
Nasir Kansestani A, Mansouri K, Hemmati S, Zare ME, Moatafaei A (2019) High glucose-reduced apoptosis in human breast cancer cells is mediated by activation of NF-κB. Iran J Allergy Asthma Immunol 18(2):153–162
Wu K, Yu X, Huang Z, Zhu D, Yi X, Wu YL, Hao Q, Kemp KT 2nd, Elshimali Y, Iyer R, Nguyen KT, Zheng S, Chen G, Chen QH, Wang G, Vadgama JV, Wu Y (2019) Targeting of PP2Cδ by a small molecule C23 inhibits high glucose-induced breast cancer progression in vivo. Antioxid Redox Signal 30(17):1983–1998
Hou Y, Zhou M, Xie J, Chao P, Feng Q, Wu J (2017) High glucose levels promote the proliferation of breast cancer cells through GTPases. Breast Cancer (Dove Med Press) 9:429–436
Adham SA, Al Rawahi H, Habib S, Al Moundhri MS, Viloria-Petit A, Coomber BL (2014) Modeling of hypo/hyperglycemia and their impact on breast cancer progression related molecules. PLoS One 9(11):e113103
Zhu S, Yao F, Li WH, Wan JN, Zhang YM, Tang Z, Khan S, Wang CH, Sun SR (2013) PKC?-dependent activation of the ubiquitin proteasome system is responsible for high glucose-induced human breast cancer MCF-7 cell proliferation, migration and invasion. Asian Pac J Cancer Prev 14(10):5687–5692
Matsui C, Takatani-Nakase T, Maeda S, Nakase I, Takahashi K (2017) Potential roles of GLUT12 for glucose sensing and cellular migration in MCF-7 human breast cancer cells under high glucose conditions. Anticancer Res 37(12):6715–6722
Sun S, Sun Y, Rong X, Bai L (2019) High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci Rep 39(6). https://doi.org/10.1042/bsr20190436
Lee SK, Moon JW, Lee YW, Lee JO, Kim SJ, Kim N, Kim J, Kim HS, Park SH (2015) The effect of high glucose levels on the hypermethylation of protein phosphatase 1 regulatory subunit 3C (PPP1R3C) gene in colorectal cancer. J Genet 94(1):75–85
Chen YC, Ou MC, Fang CW, Lee TH, Tzeng SL (2019) High glucose concentrations negatively regulate the IGF1R/Src/ERK Axis through the microRNA-9 in colorectal cancer. Cells 8(4). https://doi.org/10.3390/cells8040326
Vasconcelos-Dos-Santos A, Loponte HF, Mantuano NR, Oliveira IA, de Paula IF, Teixeira LK, de Freitas-Junior JC, Gondim KC, Heise N, Mohana-Borges R, Morgado-Díaz JA, Dias WB, Todeschini AR (2017) Hyperglycemia exacerbates colon cancer malignancy through hexosamine biosynthetic pathway. Oncogenesis 6(3):e306
Saengboonmee C, Seubwai W, Wongkham C, Wongkham S (2015) Diabetes mellitus: possible risk and promoting factors of cholangiocarcinoma: association of diabetes mellitus and cholangiocarcinoma. Cancer Epidemiol 39(3):274–278
Chen Y, Liu R, Chu Z, Le B, Zeng H, Zhang X, Wu Q, Zhu G, Chen Y, Liu Y, Sun F, Lu Z, Qiao Y, Wang J (2018) High glucose stimulates proliferative capacity of liver cancer cells possibly via O-GlcNAcylation-dependent transcriptional regulation of GJC1. J Cell Physiol 234(1):606–618
Li X, Cheng T, He Y, Zhou S, Wang Y, Zhang K, Yu P (2019) High glucose regulates ERp29 in hepatocellular carcinoma by LncRNA MEG3-miRNA 483-3p pathway. Life Sci 232:116602
Saengboonmee C, Seubwai W, Pairojkul C, Wongkham S (2016) High glucose enhances progression of cholangiocarcinoma cells via STAT3 activation. Sci Rep 6:18995
Jayedi A, Djafarian K, Rezagholizadeh F, Mirzababaei A, Hajimohammadi M, Shab-Bidar S (2018) Fasting blood glucose and risk of prostate cancer: a systematic review and meta-analysis of dose-response. Diabetes Metab 44(4):320–327
Feng X, Song M, Preston MA, Ma W, Hu Y, Pernar CH, Stopsack KH, Ebot EM, Fu BC, Zhang Y, Li N, Dai M, Liu L, Giovannucci EL, Mucci LA (2020) The association of diabetes with risk of prostate cancer defined by clinical and molecular features. Br J Cancer 123:657–665
Jian Gang P, Mo L, Lu Y, Runqi L, Xing Z (2015) Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocr Res 40(1):54–61
Cai H, Xu Z, Xu T, Yu B, Zou Q (2015) Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev 31(4):336–343
Rezende LP, Galheigo MRU, Landim BC, Cruz AR, Botelho FV, Zanon RG, Góes RM, Ribeiro DL (2019) Effect of glucose and palmitate environment on proliferation and migration of PC3-prostate cancer cells. Cell Biol Int 43(4):373–383
Li X, Li J, Cai Y, Peng S, Wang J, Xiao Z, Wang Y, Tao Y, Li J, Leng Q, Wu D, Yang S, Ji Z, Han Y, Li L, Gao X, Zeng C, Wen X (2018) Hyperglycaemia-induced miR-301a promotes cell proliferation by repressing p21 and Smad4 in prostate cancer. Cancer Lett 418:211–220
Chen JY, Wang FB, Xu H, Xu LF, Chen D, Liu WH, Mu X, Wen YQ (2019) High glucose promotes prostate cancer cells apoptosis via Nrf2/ARE signaling pathway. Eur Rev Med Pharmacol Sci 23(3 Suppl):192–200
Kellenberger LD, Petrik J (2018) Hyperglycemia promotes insulin-independent ovarian tumor growth. Gynecol Oncol 149(2):361–370
Han J, Zhang L, Guo H, Wysham WZ, Roque DR, Willson AK, Sheng X, Zhou C, Bae-Jump VL (2015) Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol 138(3):668–675
Wallbillich JJ, Josyula S, Saini U, Zingarelli RA, Dorayappan KD, Riley MK, Wanner RA, Cohn DE, Selvendiran K (2017) High glucose-mediated STAT3 activation in endometrial cancer is inhibited by metformin: therapeutic implications for endometrial cancer. PLoS One 12(1):e0170318
Li YG, Han BB, Li F, Yu JW, Dong ZF, Niu GM, Qing YW, Li JB, Wei M, Zhu W (2016) High glucose induces down-regulated GRIM-19 expression to activate STAT3 signaling and promote cell proliferation in cell culture. PLoS One 11(4):e0153659
Gao L, Xu FM, Shi WJ, Zhang S, Lu YL, Zhao DK, Long YF, Teng RB, Ge B (2018) High-glucose promotes proliferation of human bladder cancer T24 cells by activating Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci 22(23):8151–8160
Kim D, Ahn BN, Kim Y, Hur DY, Yang JW, Park GB, Jang JE, Lee EJ, Kwon MJ, Kim TN, Kim MK, Park JH, Rhee BD, Lee SH (2019) High glucose with insulin induces cell cycle progression and activation of oncogenic signaling of bladder epithelial cells cotreated with metformin and pioglitazone. J Diabetes Res 2019:2376512–2376510
Bao Z, Chen K, Krepel S, Tang P, Gong W, Zhang M, Liang W, Trivett A, Zhou M, Wang JM (2019) High glucose promotes human glioblastoma cell growth by increasing the expression and function of chemoattractant and growth factor receptors. Transl Oncol 12(9):1155–1163
Liao YF, Yin S, Chen ZQ, Li F, Zhao B (2018) High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGE-NOXs pathway. Mol Med Rep 17(6):8536–8541
Wang L, Zhong N, Liu S, Zhu X, Liu Y (2017) High glucose stimulates proliferation and inhibits apoptosis of non-small-cell lung cancer cells by JNK-mediated downregulation of p53 pathway. Acta Biochim Biophys Sin Shanghai 49(3):286–288
Ding CZ, Guo XF, Wang GL, Wang HT, Xu GH, Liu YY, Wu ZJ, Chen YH, Wang J, Wang WG (2018) High glucose contributes to the proliferation and migration of non-small cell lung cancer cells via GAS5-TRIB3 axis. Biosci Rep 38(2). https://doi.org/10.1042/bsr20171014
Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18(2):128–134
Buchheit CL, Weigel KJ, Schafer ZT (2014) Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer 14(9):632–641
Li W, Ma Q, Li J, Guo K, Liu H, Han L, Ma G (2011) Hyperglycemia enhances the invasive and migratory activity of pancreatic cancer cells via hydrogen peroxide. Oncol Rep 25(5):1279–1287
Li W, Zhang L, Chen X, Jiang Z, Zong L, Ma Q (2016) Hyperglycemia promotes the epithelial-mesenchymal transition of pancreatic cancer via hydrogen peroxide. Oxidative Med Cell Longev 2016:5190314–5190319
Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen X, Lv Y, Zhou S, Wu E, Ma Q, Huo X (2015) Hydrogen peroxide mediates hyperglycemia-induced invasive activity via ERK and p38 MAPK in human pancreatic cancer. Oncotarget 6(31):31119–31133
Cao L, Chen X, Xiao X, Ma Q, Li W (2016) Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int J Oncol 49(2):735–743
Li W, Wang Z, Xiao X, Han L, Wu Z, Ma Q, Cao L (2019) Curcumin attenuates hyperglycemia-driven EGF-induced invasive and migratory abilities of pancreatic cancer via suppression of the ERK and AKT pathways. Oncol Rep 41(1):650–658
Rahn S, Zimmermann V, Viol F, Knaack H, Stemmer K, Peters L, Lenk L, Ungefroren H, Saur D, Schäfer H, Helm O, Sebens S (2018) Diabetes as risk factor for pancreatic cancer: hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett 415:129–150
Sun XF, Shao YB, Liu MG, Chen Q, Liu ZJ, Xu B, Luo SX, Liu H (2017) High-concentration glucose enhances invasion in invasive ductal breast carcinoma by promoting Glut1/MMP2/MMP9 axis expression. Oncol Lett 13(5):2989–2995
Viedma-Rodríguez R, Martínez-Hernández MG, Flores-López LA, Baiza-Gutman LA (2018) Epsilon-aminocaproic acid prevents high glucose and insulin induced-invasiveness in MDA-MB-231 breast cancer cells, modulating the plasminogen activator system. Mol Cell Biochem 437(1-2):65–80
Takatani-Nakase T, Matsui C, Maeda S, Kawahara S, Takahashi K (2014) High glucose level promotes migration behavior of breast cancer cells through zinc and its transporters. PLoS One 9(2):e90136
Kallens V, Tobar N, Molina J, Bidegain A, Smith PC, Porras O, Martínez J (2017) Glucose promotes a pro-oxidant and pro-inflammatory stromal microenvironment which favors motile properties in breast tumor cells. J Cell Biochem 118(5):994–1002
Fainsod-Levi T, Gershkovitz M, Völs S, Kumar S, Khawaled S, Sagiv JY, Sionov RV, Grunewald M, Keshet E, Granot Z (2017) Hyperglycemia impairs neutrophil mobilization leading to enhanced metastatic seeding. Cell Rep 21(9):2384–2392
Wu J, Chen J, Xi Y, Wang F, Sha H, Luo L, Zhu Y, Hong X, Bu S (2018) High glucose induces epithelial-mesenchymal transition and results in the migration and invasion of colorectal cancer cells. Exp Ther Med 16(1):222–230
Lin CY, Lee CH, Huang CC, Lee ST, Guo HR, Su SB (2015) Impact of high glucose on metastasis of colon cancer cells. World J Gastroenterol 21(7):2047–2057
Phoomak C, Vaeteewoottacharn K, Silsirivanit A, Saengboonmee C, Seubwai W, Sawanyawisuth K, Wongkham C, Wongkham S (2017) High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via O-GlcNAcylation. Sci Rep 7:43842
Lee YH, Yeh CH (2018) Laminar shear stress inhibits high glucose-induced migration and invasion in human bladder cancer cells. In Vitro Cell Dev Biol Anim 54(2):120–128
Gu CJ, Xie F, Zhang B, Yang HL, Cheng J, He YY, Zhu XY, Li DJ, Li MQ (2018) High glucose promotes epithelial-mesenchymal transition of uterus endometrial cancer cells by increasing ER/GLUT4-mediated VEGF Secretion. Cell Physiol Biochem 50(2):706–720
Guo J, Ye F, Jiang X, Guo H, Xie W, Zhang Y, Sheng X (2020) Drp1 mediates high glucose-induced mitochondrial dysfunction and epithelial-mesenchymal transition in endometrial cancer cells. Exp Cell Res 389(1):111880
Malek-Zietek KE, Targosz-Korecka M, Szymonski M (2017) The impact of hyperglycemia on adhesion between endothelial and cancer cells revealed by single-cell force spectroscopy. J Mol Recognit 30(9). https://doi.org/10.1002/jmr.2628
Kang X, Kong F, Wu X, Ren Y, Wu S, Wu K, Jiang Z, Zhang W (2015) High glucose promotes tumor invasion and increases metastasis-associated protein expression in human lung epithelial cells by upregulating heme oxygenase-1 via reactive oxygen species or the TGF-β1/PI3K/Akt signaling pathway. Cell Physiol Biochem 35(3):1008–1022
Bhattacharyya S, Sul K, Krukovets I, Nestor C, Li J, Adognravi OS (2012) Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J Am Heart Assoc 1(6):e005967
Krukovets I, Legerski M, Sul P, Stenina-Adognravi O (2015) Inhibition of hyperglycemia-induced angiogenesis and breast cancer tumor growth by systemic injection of microRNA-467 antagonist. FASEB J 29(9):3726–3736
Ikemura M, Hashida T (2017) Effect of hyperglycemia on antitumor activity and survival in tumor-bearing mice receiving oxaliplatin and fluorouracil. Anticancer Res 37(10):5463–5468
Ma YS, Yang IP, Tsai HL, Huang CW, Juo SH, Wang JY (2014) High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol 33(2):64–72
Ideno M, Sasaki S, Kobayashi M, Futagi Y, Narumi K, Iseki K (2016) Influence of high glucose state on bromopyruvate-induced cytotoxity by human colon cancer cell lines. Drug Metab Pharmacokinet 31(1):67–72
Xu X, Si M, Lou H, Yan Y, Liu Y, Zhu H, Lou X, Ma J, Zhu D, Wu H, Yang B, Wu H, Ding L, He Q (2018) Hyperglycemia decreases anti-cancer efficiency of Adriamycin via AMPK pathway. Endocr Relat Cancer 25(11):955–966
Al Qahtani A, Holly J, Perks C (2017) Hypoxia negates hyperglycaemia-induced chemo-resistance in breast cancer cells: the role of insulin-like growth factor binding protein 2. Oncotarget 8(43):74635–74648
Biernacka KM, Uzoh CC, Zeng L, Persad RA, Bahl A, Gillatt D, Perks CM, Holly JM (2013) Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat Cancer 20(5):741–751
Zeng L, Zielinska HA, Arshad A, Shield JP, Bahl A, Holly JM, Perks CM (2016) Hyperglycaemia-induced chemoresistance in breast cancer cells: role of the estrogen receptor. Endocr Relat Cancer 23(2):125–134
Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, Morgan A, Winters ZE, Foulstone EJ, Shield JP, Perks CM (2010) Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer 17(2):539–551
Yang IP, Miao ZF, Huang CW, Tsai HL, Yeh YS, Su WC, Chang TK, Chang SF, Wang JY (2019) High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage III colorectal cancer receiving adjuvant FOLFOX6 chemotherapy. Ther Adv Med Oncol 11:1758835919866964. https://doi.org/10.1177/1758835919866964
Li J, Wu MF, Lu HW, Zhang BZ, Wang LJ, Lin ZQ (2016) Impact of hyperglycemia on outcomes among patients receiving neoadjuvant chemotherapy for bulky early stage cervical cancer. PLoS One 11(11):e0166612
Zhao W, Chen R, Zhao M, Li L, Fan L, Che XM (2015) High glucose promotes gastric cancer chemoresistance in vivo and in vitro. Mol Med Rep 12(1):843–850
Cao L, Xiao X, Lei J, Duan W, Ma Q, Li W (2016) Curcumin inhibits hypoxia-induced epithelial-mesenchymal transition in pancreatic cancer cells via suppression of the hedgehog signaling pathway. Oncol Rep 35(6):3728–3734
Gerards MC, van der Velden DL, Baars JW, Brandjes DPM, Hoekstra JBL, Vriesendorp TM, Gerdes VEA (2017) Impact of hyperglycemia on the efficacy of chemotherapy-a systematic review of preclinical studies. Crit Rev Oncol Hematol 113:235–241
Varghese S, Samuel SM, Varghese E, Kubatka P, Büsselberg D (2019) High glucose represses the anti-proliferative and pro-apoptotic effect of metformin in triple negative breast cancer cells. Biomolecules 9(1). https://doi.org/10.3390/biom9010016
Rogalska A, Forma E, Bryś M, Śliwińska A, Marczak A (2018) Hyperglycemia-associated dysregulation of O-GlcNAcylation and HIF1A reduces anticancer action of metformin in ovarian cancer cells (SKOV-3). Int J Mol Sci 19(9). https://doi.org/10.3390/ijms19092750
Litchfield LM, Mukherjee A, Eckert MA, Johnson A, Mills KA, Pan S, Shridhar V, Lengyel E, Romero IL (2015) Hyperglycemia-induced metabolic compensation inhibits metformin sensitivity in ovarian cancer. Oncotarget 6(27):23548–23560
Valaee S, Yaghoobi MM, Shamsara M (2017) Metformin inhibits gastric cancer cells metastatic traits through suppression of epithelial-mesenchymal transition in a glucose-independent manner. PLoS One 12(3):e0174486
Lv Y, Tian N, Wang J, Yang M, Kong L (2018) Metabolic switching in the hypoglycemic and antitumor effects of metformin on high glucose induced HepG2 cells. J Pharm Biomed Anal 156:153–162
Lau MF, Chua KH, Sabaratnam V, Kuppusamy UR (2020) Rosiglitazone enhances the apoptotic effect of 5-fluorouracil in colorectal cancer cells with high-glucose-induced glutathione. Sci Prog 103(1) 36850419886448. https://doi.org/10.1177/0036850419886448
Lau MF, Vellasamy S, Chua KH, Sabaratnam V, Kuppusamy UR (2018) Rosiglitazone diminishes the high-glucose-induced modulation of 5-fluorouracil cytotoxicity in colorectal cancer cells. EXCLI J 17:186–199
Vernieri C, Galli F, Ferrari L, Marchetti P, Lonardi S, Maiello E, Iaffaioli RV, Zampino MG, Zaniboni A, De Placido S, Banzi M, Damiani A, Ferrari D, Rosati G, Labianca RF, Bidoli P, Frassineti GL, Nicolini M, Pavesi L, Tronconi MC, Buonadonna A, Ferrario S, Re GL, Adamo V, Tamburini E, Clerico M, Giordani P, Leonardi F, Barni S, Ciarlo A, Cavanna L, Gori S, Cinieri S, Faedi M, Aglietta M, Antista M, Dotti KF, Galli F, Di Bartolomeo M (2019) Impact of metformin use and diabetic status during adjuvant fluoropyrimidine-oxaliplatin chemotherapy on the outcome of patients with resected colon cancer: a TOSCA study subanalysis. Oncologist 24(3):385–393
Ling S, Brown K, Miksza JK, Howells LM, Morrison A, Issa E, Yates T, Khunti K, Davies MJ, Zaccardi F (2021) Risk of cancer incidence and mortality associated with diabetes: a systematic review with trend analysis of 203 cohorts. Nutr Metab Cardiovasc Dis 31(1):14–22
Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P (2013) Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis 16(2):151–158, s151
Wang M, Yang Y, Liao Z (2020) Diabetes and cancer: epidemiological and biological links. World J Diabetes 11(6):227–238
Lega IC, Lipscombe LL (2020) Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev 41(1):33–52
Yu J, Sun R, Han X, Liu Z (2020) New-onset diabetes mellitus after distal pancreatectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 30(11):1215–1222
Shirakawa S, Matsumoto I, Toyama H, Shinzeki M, Ajiki T, Fukumoto T, Ku Y (2012) Pancreatic volumetric assessment as a predictor of new-onset diabetes following distal pancreatectomy. J Gastrointest Surg 16(12):2212–2219
Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H (2009) Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol 15(3):280–288
Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P (2006) Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 7(9):735–740
Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, Chang YJ, Choi KS, Jung SY, Woo SM, Ahn JS, Sim SH, Hong YS, Pastor-Barriuso R, Guallar E, Lee ES, Kong SY, Cho J (2018) Incidence of diabetes after cancer development: a Korean National Cohort Study. JAMA Oncol 4(8):1099–1105
Lee SY, Kurita N, Yokoyama Y, Seki M, Hasegawa Y, Okoshi Y, Chiba S (2014) Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer 22(5):1385–1390
Feng JP, Yuan XL, Li M, Fang J, Xie T, Zhou Y, Zhu YM, Luo M, Lin M, Ye DW (2013) Secondary diabetes associated with 5-fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: results from a single-centre cohort study. Color Dis 15(1):27–33
Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, Whitton JA, Stovall M, Robison LL, Oeffinger KC (2009) Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 169(15):1381–1388
Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernández D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC (2003) New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 75(10 Suppl):Ss3–S24
Honors MA, Kinzig KP (2012) The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle 3(1):5–11
Gornik I, Vujaklija-Brajkovic A, Renar IP, Gasparovic V (2010) A prospective observational study of the relationship of critical illness associated hyperglycaemia in medical ICU patients and subsequent development of type 2 diabetes. Crit Care 14(4):R130
Acknowledgements
Charupong Saengboonmee (PMAYP-10612) and Suangson Supabphol (PMAYP-10615) are awardees of Prince Mahidol Award Youth Program from Prince Mahidol Award Foundation under the Royal Patronage of HM the King of Thailand. Charupong Saengboonmee is also supported by Professor Peter Sicinski for his visiting program at Dana-Farber Cancer Institute and Harvard Medical School. We acknowledge help from Dr. Treerat Mahankasuwan, Khu Muang Hospital, Buriram, Thailand, for generating the illustrations and would like to thank Dr. Justin T. Reese for editing the manuscript via the Publication Clinic at KKU, Thailand.
Code availability
Not applicable
Author information
Authors and Affiliations
Contributions
Conceptualization: Suangson Supabphol, Wunchana Seubwai, Sopit Wongkham, and Charupong Saengboonmee. Review literature: Suangson Supabphol and Charupong Saengboonmee. Writing first draft of manuscript: Suangson Supabphol and Charupong Saengboonmee. Supervision, review, and editing of the manuscript: Wunchana Seubwai and Sopit Wongkham. All authors contribute to critically review and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Supabphol, S., Seubwai, W., Wongkham, S. et al. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med 99, 1175–1193 (2021). https://doi.org/10.1007/s00109-021-02096-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02096-w