Abstract
After the death of large numbers of cells in liver tissue is triggered by various hepatotoxic factors, intimidating and life-threatening acute liver failure (ALF) can develop with high mortality and expensive costs. Although liver transplantation and hepatocyte transplantation have become substitutes for improving liver regeneration, their applications are inhibited by scarce tissue and cell resources. Therefore, the transplantation of mesenchymal stromal cells (MSCs) and their derivatives including hepatocyte-like cells (HLCs), conditioned medium (CM), and exosomes (Ex) can help alleviate liver injury in ALF individuals or animal models via engraftment into liver tissue, hepatogenic differentiation, the promotion of host hepatocyte proliferation, the secretion of anti-inflammatory factors and antioxidants, and the enhancement of liver regeneration in vivo. In addition, biomaterial scaffolds protect MSCs against a harsh microenvironment in vitro and in vivo, in addition to providing physical and directional support for liver regeneration. In this review, we aimed to discuss the underlying mechanisms and therapeutic effects of MSCs and their derivatives on rescuing ALF animal models according to current studies. Further breakthroughs are required to establish safer, more stable, and more effective stem cell–based therapy in regenerative medicine for repairing liver injury, thus reducing the morbidity and mortality of ALF in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various hepatotoxic factors including hepatitis viruses, drugs, immunologic injury, and other factors can induce the death of a large number of cells in liver tissue, thus resulting in intimidating and life-threatening acute liver failure (ALF). The annual prevalence of ALF is approximately one to six cases per million individuals worldwide, accompanied by high mortality and expensive costs [1, 2]. Notwithstanding the multiple treatments used to prevent ALF-related complications and decelerate the rate of progression in ALF, liver transplantation serves as the most effective strategy; however, its application is restricted by scarce liver donors, high costs, and organ transplant rejection [3]. Thus, hepatocyte transplantation could become a substitute for improving liver regeneration; however, there is a lack of high-quality primary hepatocytes in vitro because they are difficult to expand and it is easy for them to lose their hepatic characteristics in vitro [4, 5].
To compensate for the shortage of liver transplantation and hepatocyte transplantation, mesenchymal stromal cell (MSC)–based therapy has emerged as a new and effective strategy in patients with ALF. In general, MSCs can be isolated and purified from various tissues, such as bone marrow, umbilical cord, adipose, umbilical cord blood, amniotic fluid, and menstrual blood [6,7,8,9,10,11]. Currently, other cell resources such as induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have become new sources of MSCs [12, 13]. MSCs are self-renewing and multipotent and can generate abundant somatic cells including adipocytes, osteocytes, chondrocytes, and hepatocyte-like cells (HLCs) [14]. After transplantation in vivo, MSCs can not only protect organisms from inflammatory injury and apoptosis but also play a critical role in immunosuppression, angiogenesis, and paracrine-mediated tissue repair [15].
MSCs respond differently according to the injured or healthy state of the liver, as shown by allogenic MSCs engrafted into injured sites significantly reducing the mortality of carbon tetrachloride (CCl4)–induced ALF mice, while fewer MSCs engraft into the normal liver [16]. Furthermore, the engrafted MSCs rarely undergo hepatogenic differentiation in injured liver sites, and these engrafted MSCs are efficiently cleared from the liver after injection for 1 month [17]. Moreover, the poor engraftment of MSCs in the liver is a consequence of the immune rejection of transplanted MSCs [18]. Baertschiger et al. [19] found that intrahepatic injection but not intrasplenic injection guarantees stable engraftment of MSCs in the liver, while these engrafted cells are mainly differentiated into myofibroblasts. Another study indicated that this obstacle should be overcome by the differentiation of MSCs into HLCs in vitro before transplantation in vivo [20]. Current evidence has shown that not only MSCs but also MSC derivatives such as HLCs and conditioned medium (CM) or exosomes (Ex) can be applied to regenerative medicine for repairing liver injuries [9, 11, 21]. In this review, we aimed to discuss the therapeutic mechanisms of MSCs and its derivatives in ALF at the molecular cell level; we then comprehensively analyzed the effects of MSCs and MSC derivative–based therapy in rescuing ALF according to recent studies.
Definition and causes of ALF
Wlodzimirow et al. [22] highlighted that there were 41 different definitions of ALF used in 87 separate studies without a definitive consensus. The definitions varied according to the grade of hepatic encephalopathy (HE), the time interval between the onset of symptoms and HE, the severity of coagulation disorders, and the type of preexisting liver disease. It is urgent to define consensus criteria to facilitate more effective management of ALF. The majority of investigators have accepted that an international normalized ratio (INR) > 1.5 and any grade of HE within 26 weeks of the onset of illness in a patient without a history of liver disease represents an incident of ALF [23]. Patients with acute-on-chronic liver failure (ACLF) include patients with chronic liver diseases that have developed into ALF. Although patients with ACLF have a lower incidence of coagulopathy and HE, it is associated with a high short-term mortality and immense health care expenditure [24]. Herein, we considered patients with ALF after the exclusion of ACLF patients with chronic liver disease but the inclusion of patients with hyperacute liver failure in which encephalopathy had an onset interval of 7 days or less [25].
The causes of ALF remain unknown, and novel viruses or toxins may induce ALF. Infections with hepatitis A and E are responsible for inducing the majority of cases of ALF in developing countries [26, 27], and hepatitis B infection is also a common cause in Asian and Mediterranean countries [28]. Other viruses including Epstein–Barr virus, cytomegalovirus, herpes simplex virus, and parvoviruses can trigger the initiation of ALF [29]. Drug-induced liver injury, particularly acetaminophen (APAP)-induced injury, is the most familiar cause of ALF in the USA [30]. Although APAP-induced hepatotoxicity typically occurs in a dose-dependent manner, some idiosyncratic individuals develop ALF independent of the dose. In addition, ischemic or hypoxic conditions induced by severe sepsis in other large organs (heart or lung) will consequently induce acute liver injury accompanied by extremely high levels of serum aminotransferases [31, 32], and the prognosis depends on both the severity of the primary disease and the subsequent severity of the liver injury. Other causes, including neoplastic infiltration, acute Budd–Chiari syndrome, heatstroke, and poisonous substance ingestion, can also induce ALF [33]. Therefore, we believe that the main causes of ALF can be divided into several categories, including infection, toxins/drugs, abnormal perfusion, metabolic disorders, autoimmune disorders, and neoplastic infiltration. All of these factors will initiate cell death in the liver tissue and cause liver injury for the generation of ALF in animals and humans.
The immunopathogenesis of ALF
Recently, multiple studies found that immune dysfunction exists in ALF, and the gradually exaggerated inflammatory response plays a key role in the pathogenesis and outcome of ALF. Patients with ALF were found to have low levels of C3 and C5 [34] and impaired neutrophil function by impairing the phagocytic capacity [35, 36]. Acute liver injury initiated the activation of Kupffer cells and natural killer T (NKT) lymphocytes [37] and then effectively recruited neutrophils, lymphocytes, and macrophages into injured sites and caused a reduction of nitric oxide (NO) production and massive liver necrosis [38, 39]. Moreover, ALF patients demonstrated higher levels of intrahepatic eosinophils, C-reactive protein, and interleukin (IL)-6 accompanied by lower levels of IL-5 in the liver and peripheral blood than healthy controls [40, 41]. Other studies indicated the number of CD8+ interferon-gamma (IFN-γ) + T lymphocytes was significantly increased [42] and a series of cytokines, such as IL-10, tumor necrosis factor alpha (TNF-α), and IL-12, were significantly upregulated in ALF [43, 44]. As a result of the immune dysregulation, patients with ALF demonstrate increased susceptibility to infection, which is associated with the development of further complications [45].
Multiple hepatotoxic factors such as concanavalin A (ConA), alpha-galactosylceramide (α-GalCer,) and lipopolysaccharide (LPS) induce immune dysfunction and result in ALF; thus, the detailed mechanisms are discussed as follows. The ConA-induced liver injury model is currently considered a model of autoimmune hepatitis, viral hepatitis, and related ALF; however, the immunology of this model is complex and only partially understood. After treatment with ConA in vivo, it is able to bind to sinusoidal endothelial cells and recruit CD4+ T lymphocytes, which thus results in the injury of endothelial cells [46]; ConA subsequently binds to Kupffer cells and promotes the release of TNF-α [47]. In ConA-induced ALF models, the activated conventional dendritic cells (DCs) are promoted to increase the expression of IL-12, and the activated NKT cells are promoted to secrete IFN-γ [48]. In addition, ConA significantly triggers neutrophil infiltration and the accumulation of macrophages in the liver, which thus lead to liver cell apoptosis and hepatocellular damage [49]. α-GalCer (a specific ligand for invariant Valpha14 NKT cells)-induced liver injury resembled acute autoimmune hepatitis and was mediated by NKT cells and autoantibody-producing B-1 cells [50]. It was reported that α-GalCer promoted the secretion of IFN-γ for the recruitment of IL-10-producing T regulatory cells (Tregs) and CXCR3+ Tregs in the liver [51]. The administration of α-GalCer also upregulated the levels of FasL and TRAIL, receptors responsible for NKT cell–mediated apoptosis and cytotoxicity, leading to liver injury [52]. Although hepatic Kupffer cells serve as a vital factor for TNF-α secretion in ConA-induced hepatitis, they are nonessential in α-GalCer-induced liver injury. In addition, α-GalCer significantly upregulated the levels of TNF-α, IFN-γ, IL-2, IL-4, and IL-6 in the liver and plasma [53]. LPS is an innate immune-activating stimulus that binds to toll-like receptor 4 (TLR4) to activate macrophages and promote the secretion of CXC chemokines and inflammatory cells [54, 55]. Evidence showed that LPS stimulated Kupffer cells to release higher levels of TNF-α, IL-1, and IL-6 [56], and the liver also responds to LPS with the production of reactive oxygen intermediates [57]. Moreover, LPS stimulation enhanced the release of NLRP3 inflammasome and TLR4 and thus enhanced the release of caspase-1 in Kupffer cells [58]. LPS-induced hepatic polymorphonuclear neutrophil (PMN) accumulation and the secretion of cytokine-induced neutrophil chemoattractant-1 thus preceded the onset of hepatic parenchymal cell injury and subsequent sinusoidal endothelial cell injury [59]. It is worth noting that other factors also trigger immune dysfunction in vivo; however, the detailed process should be further clarified after the collection of sufficient evidence.
Autophagy, apoptosis, and necrosis in the development of ALF
Programmed cell death including autophagy, apoptosis, and necrosis can lead to irreversible liver injury. The liver is a large and special organ in which autophagy often occurs, and several selective types of autophagy, including mitophagy and lipophagy, also occur in both cultured hepatocytes and liver tissue [60, 61]. Autophagy can serve as a protective pathway or a devastating pathway for the acceleration of hepatic apoptosis via the modulation of mitochondrial recycling. Apoptosis can be initiated by oxidative stress–related intrinsic pathway, including DNA damage or p53 activation, or a caspase-related extrinsic pathway that begins with the activation of TNF-α and FasL [62]. Apoptosis is a process that involves minimal inflammation because cellular shrinkage and implosion lead to silent cell death in the liver tissue, while necrosis induces cell swelling and eventual rupture, which triggers a clear inflammatory response after the depletion of adenosine triphosphate (ATP) [63]. Consequently, ATP depletion and cellular swelling lead to the formation of membrane blebs, followed by mitochondrial depolarization, lysosomal breakdown, and rapid ion changes, thus recycling the components in response to pathological changes and resulting in cell membrane rupture [64]. Membrane rupture subsequently leads to irreversible cell death and secondary inflammation, while quiescent hepatocytes can be resurrected after cellular membrane rupture in liver ischemia/reperfusion injury [62]. These molecular changes have the potential to induce a wave of systemic inflammation and the accumulation of circulating harmful cytokines, and the cell death rate becomes substantially higher than the rate of hepatocyte regeneration. In this setting, damaged liver tissue shows synthetic dysfunction for the secretion of albumin and urea and glycogen storage and impaired detoxification abilities, such as cytochrome P450 activity and metabolic disturbances (Fig. 1).
MSC transplantation and the underlying mechanisms
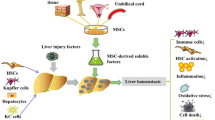
MSCs from various tissues can effectively improve the outcome of ALF animal models via paracrine pathways, immune protective effects, upregulation of hepatocyte proliferation, and maintenance of hepatic metabolic homeostasis (Table 1).
MSCs significantly reduced the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), ammonia, and inflammatory cytokines in ALF rats via the upregulation of heme oxygenase-1 (HO-1). The upregulation of HO-1 consequently reduced PMN infiltration to further promote liver regeneration [6]. To protect against the immune response in the pathogenesis of ALF, MSCs effectively inhibited cytotoxic T lymphocytes and NK cells via various intercellular contact and paracrine factors, including indoleamine 2,3-dioxygenase (IDO), transforming growth factor (TGF)-β and prostaglandin E2 (PGE2) [3]. The immune protective effect of MSCs can be achieved by enhancing the number of Treg cells and M2-type macrophages and reducing the number of Th1 and Th17 cells in ALF models [75]. MSC transplantation can effectively improve the liver functions of ALF rats via reducing the release of inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-6, and IL-10) and chemokines (CXCL1 and CXCL2) [76, 77]. In addition to paracrine mechanisms, intravenously injected MSCs can engraft into the injured sites and then attenuate lymphocyte proliferation and systemically reduce the number of activated NKT cells in vivo [78]. Moreover, MSC transplantation is also able to inhibit the activation and cytotoxicity of DCs and B cells [79, 80] and reduce the number of peripheral blood and liver neutrophils [76]. Engrafted MSCs subsequently eliminated hepatocyte necrosis, promoted liver regeneration, and prolonged the survival time of ALF models via differentiation into HLCs and the secretion of albumin, alpha fetal protein (AFP), and cytokeratin (CK)-18 after implanting into liver tissue [7, 8, 65]. In addition to maintaining normal liver enzymes and synthetic function, MSCs also participate in the promotion of revascularization via vascular endothelial growth factor (VEGF)– mediated pathways [66]. In addition, MSC transplantation significantly decreased the serum and liver levels of high mobility group box 1 protein (HMGB1), upregulated the level of epithelial cell adhesion molecule (EpCAM), and activated M2 polarization as demonstrated by the upregulation of CD163, IL-10, IL-4, and arginase-1 in ALF rats [67, 68]. However, Yuan et al. [69] argued that MSC transplantation substantially downregulated the serum and hepatic levels of CD163 and IL-10 during the early stages of MSC transplantation and determined the serum levels of CD163 and IL-10 as the prognosis indicators in the progress of ALF in animals and humans after MSC administration. Intriguingly, Shi et al. [70] showed that MSCs rescued ALF pigs and stabilized ALF within 7 days as demonstrated by the normalization of liver enzymes and inhibition of life-threatening cytokine storms in ALF pigs. The profiling analysis indicated a delta-like ligand 4 activated Notch pathway after MSC transplantation, and delta-like ligand 4 has been validated as a vital factor for improving the survival rates of ALF pigs and ALF rats. Furthermore, the metabolic trajectory, including conjugated bile acids, phosphatidylcholines, lysophosphatidylcholines, fatty acids, amino acids, and sphingomyelin, returned to the original level at week 3 after MSC transplantation [71]. APAP overdoses rapidly deplete glutathione (GSH) and cause oxidative stress-induced injury in humans and animals, and MSC transplantation increases the survival rate of APAP-induced ALF mice by upregulating the antioxidant response and weakening cytochrome P450 activity to reduce the nitrotyrosine level and upregulate the NF-E2-related factor 2 (Nrf2) level in vivo [72]. Moreover, MSCs prolong the survival rate of APAP-induced ALF mice via the upregulation of superoxide dismutase (SOD), GSH, and hepatocyte growth factor (HGF), and downregulation of the inflammatory factors TNF-α and IL-6 [73].
Yun et al. compared the safety of MSCs at a gradient concentration in vivo and found that implanted MSCs did not alter the body weight, food/water consumption, clinical symptoms, urinalysis, hematology, clinical chemistry, organ weight, or histopathology at any density, and MSCs in vivo were cleared away in mice at week 13. After long-term observation for 26 weeks, the MSCs triggered the downregulation of hepatic necrosis and lobular neutrophilic infiltration in the injured liver but did not exert tumorigenicity [74]. The long-term investigation indicated that MSC transplantation at various concentrations is safe for the acute phase of ALF and long-term survival of ALF animal models.
Choice of MSC source
It was shown that ALF did not alter the stem cell characteristics or cell activities of MSCs, and the levels of liver-specific genes and hepatogenic potency were increased in ALF-derived MSCs [81, 82]. Autologous MSCs isolated from ALF patients may highly reduce the rejection rate, while the cell preparatory period is too long for ALF patients with poor liver functions. Furthermore, MSCs have a high immune privilege and relative safety when used in allogenic hosts [83]. Although allogenic MSCs are more practical for cell transplantation in ALF patients because they can be isolated from healthy individuals and can be proliferated at any time, they also carry obstacles for safe transplantation. Allogenic MSCs are permissive for cytomegalovirus and herpes simplex virus infections in vitro and carry the risk of viral transmission to the recipient [84]. Park et al. demonstrated that autologous MSC transplantation significantly improved the outcomes of five patients with liver failure via decreasing the serum albumin levels and liver stiffness and improving the liver volume, subjective healthiness, and quality of life. Thus, they indicated that autologous MSC transplantation may serve as a bridge to liver transplantation in patients with liver failure [85]. Moreover, the outstanding therapeutic effects of allogenic MSCs for treating ALF without a clear rejection incidence indicate the promising wide application of allogenic MSCs in further studies.
As MSC transplantation has gradually replaced primary hepatocyte transplantation because of its abundance and anti-inflammatory effects, numerous studies have compared the transplantation efficacy of MSCs and cells from other sources in vivo in ALF models (Table 2). Although undifferentiated umbilical cord–derived MSCs (UCMSCs) have weaker liver-specific functions than primary hepatocytes, UCMSCs clearly improve the viability and recovery of damaged hepatocytes more than primary hepatocytes in vitro. Moreover, the transplantation of primary hepatocytes produced higher numbers of HepPar1−/albumin-positive cells than the transplantation of UCMSCs into the recipient liver, while the administration of UCMSCs more effectively rescued ALF mice and stimulated endogenous liver regeneration via the downregulation of inflammatory factors, including IL-1β, TNF-α, IL-6, IL-10, and the IL-1 receptor antagonist (IL-1Ra), rather than hepatogenic differentiation to compensate for the lost liver function [86]. Sun et al. [87] demonstrated that only the transplantation of bone marrow–derived MSCs (BMMSCs) recovered liver damage and rescued ConA-treated ALF mice via inhibiting the expression of TNF-α, IFN-γ, and FasL but increasing the IL-10 level compared with adult hepatocytes, fetal liver cells, and induced hepatic stem cells.
There are other comparisons of MSCs from different sources to guide the selection of the optimal MSC source. Umbilical cord blood–derived MSCs (UCB-MSCs) expanded weakly and thus could not be used for application in transplantation, while both adipose-derived MSCs (ADMSCs) and BMMSCs expanded in vitro can be applied to repair CCl4-induced injury of ALF mice via hepatogenic differentiation in vivo [9]. Zare et al. [88] showed that the liver functions demonstrated by ALT and AST were more significantly improved in response to ADMSCs than BMMSCs, although there were no significant differences in the survival rate and liver histopathology of ALF mice. Moreover, both iPSC-derived MSCs (iPSC-MSCs) and BMMSCs significantly decreased lipid peroxidation and increased the survival rate of ALF animals via the HGF-mediated pathway; iPSC-MSCs significantly augmented their proliferative ability and compensated for the viable cell count for transplantation [12]. In summary, other MSCs overcome the disadvantages of BMMSCs by having abundant tissue sources, enhanced proliferative capacity, and a reduced operation wound.
Transplantation routes
MSCs can be injected into organisms via an intravenous route, intrahepatic route, intraperitoneal route, hepatic artery route, and splenic route; however, the selection of the optimal transplantation route remains unclear (Table 3).
Zheng et al. [89] showed that transplantation of MSCs via the intrahepatic route and tail vein route had similar effects on improving hepatic synthesis (secretion of CK8, CK18, and AFP), decreasing liver enzymes (ALT and AST) and promoting liver repair following ALF in animal models. Transplantation via the intraperitoneal route exerted no therapeutic effect because the MSCs could not migrate into the injured liver; alternatively, the other three routes (portal vein, hepatic artery, and vena caudalis) promoted the homing of MSCs to the damaged liver tissue and decreased liver damage in ALF rats via increasing the expression of proliferating cell nuclear antigen (PCNA) and HGF, while decreasing the caspase-3 level [90]. Sun et al. concluded that the selection of blood vessels for transplantation does not affect the therapeutic outcome [90].
However, the majority of studies recommend intravenous routes for MSC transplantation in ALF animal models. Some authors recommend transplantation via the tail vein, while other authors recommend transplantation via the portal vein. Feng et al. [91] demonstrated that the administration of MSCs via the tail vein and directly into the liver lobe showed comparable efficacy in repairing liver functions and enhancing liver regeneration in ALF mice, and they considered that injection via the tail vein is more convenient than the intrahepatic route since transplantation via the hepatic artery was not more beneficial for the transdifferentiation of MSCs. Moreover, transplantation via the tail vein provided an additional survival benefit to rescue ALF than transplantation via an intrasplenic route since all of the implanted MSCs integrated into the liver parenchyma and underwent hepatogenic differentiation into HLCs at the injured site for liver regeneration [92]. Furthermore, another study highlighted that the tail vein route showed the most prominent effects on reducing the levels of biochemical parameters including ALT, AST, and ammonia in ALF mice compared with the use of the portal vein and liver parenchymal delivery [93]. In contrast, other authors report that transplanting MSCs via the portal vein can result in a large amount of engraftment of MSCs and stronger anti-inflammatory effects. For example, the transplantation of MSCs via the portal vein and tail vein both decreased the serum levels of liver enzymes and inhibited inflammation, hepatic degeneration, and necrosis in ALF rats; however, the protein levels of stromal-derived factor (SDF)-1α and VEGF were significantly higher in the portal vein group than in the tail vein group [94]. Cao et al. [95] determined that portal vein MSC transplantation enhanced hepatogenic differentiation, anti-inflammation, and liver regeneration while inhibiting hepatocyte denaturation and hepatocyte necrosis in ALF pigs; however, the transplantation of MSCs via the jugular vein did not demonstrate benefits. Li et al. [96] reported that the injection of MSCs via a peripheral vein did not rescue ALF pigs, while most of the ALF pigs survived for a long time over 6 months after transplantation of MSCs via the portal vein. Thirty percent of the hepatocytes in hepatic lobules and the liver parenchyma of the surviving pigs were derived from humans at week 10. More recently, Sang et al. [97] concluded that intraportal injection was the best route for repairing liver injury in swine with ALF compared with hepatic intra-arterial injection, peripheral intravenous injection, and intrahepatic injection, as demonstrated by the longest survival time, least liver injury, lowest histopathological score, and lowest apoptosis rate of hepatocytes via decreasing the expression of caspase-3 and elevating the expression of survivin, AKT, phospho-AKT, ERK, and phospho-ERK during the initial stage of ALF. According to the current evidence, intrahepatic injection serves as the optimal route to improve the outcome of ALF for MSC transplantation, although it is not sufficiently convenient compared with the peripheral vein route.
Modification of MSCs or recipients
To improve the transplantation efficacy, cotreatment and preconditioning of MSCs and/or the recipients have been widely applied for promoting liver regeneration in ALF models (Table 4).
Cotreatment and pretreatment/preconditioning
Jin et al. [98] demonstrated that cotreatment with SDF-1 enhanced the migrative capacity of MSCs, improved the hepatic secretion of albumin and decreased the serum aminotransferase levels in ALF mice. As the vital role of IL-10 is always highlighted in rescuing ALF animal models, Wang et al. [108] demonstrated that the administration of IL-10 and MSCs ameliorated the upregulation of ALT, AST, TBIL, ammonia, and inflammatory cytokines, while blockage of IL-10 abolished the beneficial effects of MSCs.
Preconditioning with serum from donor ALF rats clearly improved the migrative ability of MSCs into the portal area and liver parenchyma via increasing the chemokine CXC receptor 4 (CXCR4) level in ALF rats [99]. Preconditioning with zeaxanthin dipalmitate (ZD) clearly upregulated the cell survival rate and hepatocyte differentiation and abolished ROS-induced injury in MSCs treated with LPS and hydrogen dioxide (H2O2) in vitro via the activation of the PKC/Raf-1/MAPK/NF-κB pathway and upregulation of microRNA-210 (miR-210). In addition, ZD-pretreated MSCs demonstrated the best effects on improving hepatocyte proliferation and ameliorating liver injury via the acceleration of the host regenerative progress [100]. In addition, pretreatment with edaravone upregulated the ROS production, the GSH/oxidized glutathione (GSSG) ratio, and the expression of catalase (CAT) and SOD-1 in MSCs via the regulation of the MAPK-PKC-Nrf2 pathway. Transplantation of edaravone-pretreated MSCs effectively rescued the death of ALF mice via improving their homing ability, enhancing their proliferative capacity, decreasing apoptosis, and upregulating the secretion of HGF in MSCs [101].
In addition to the pretreatment of MSCs, the pretreatment of recipients will consequently activate or inhibit specific pathways for enhancing the repair capacity of MSCs in vivo. Preconditioning of recipients with anti-PMN effectively improved liver function and the survival rate of ALF rats after MSC transplantation by diminishing the number of neutrophils and decreasing the release of TNF-α, IL-1β, CXCL1, and CXCL2 while increasing the IL-10 level [76]. Preconditioning of recipients with IL-1β siRNA before CCl4 injection significantly improved the liver regeneration and survival rates of ALF mice compared with monotherapy by MSC transplantation via the downregulation of inflammatory factors, including CXCL1, IL-1β, and IL-6, and the upregulation of anti-inflammatory factors, including IL-10, VEGF, and HGF [102]. The optimal dose and safety of cotreatment and preconditioning should be further investigated to improve the MSC efficacy in vivo.
Gene modification of MSCs
In addition to external cotreatment and pretreatments with serum or pharmacokinetics, recent studies have investigated gene targeting strategies of MSCs to increase liver regeneration. Implantation of MSCs that overexpressed IL-1Ra significantly alleviated the progression of liver failure and decreased the mortality of rats with ALF more than the control MSC group as the IL-1Ra-MSCs demonstrated enhanced proliferative ability and engraftment in injured tissues [103]. The overexpression of c-Met improved the migrative capacity of MSCs in a HGF-dependent manner in vitro, and these modified MSCs showed better engraftment in the injured site accompanied by improved liver functions and higher survival rates of ALF rats [104]. Similarly, the overexpression of CXCR4 significantly increased the release of HGF and VEGF in MSCs, which thus improved the migrative capacity and colonization of MSCs, leading to a longer lifetime of ALF mice [105]. HGF-MSCs maintained redox homeostasis, reduced liver injury, and prolonged the survival of ALF mice via increments in serum GSH, γ-glutamylcysteine synthetase, SOD, and CAT. Moreover, they also inhibited hepatocyte apoptosis via the upregulation of Bcl2 and downregulation of Bax and TNF-α [106].
In addition to gene modification by the overexpression of anti-inflammatory factors, chemotactic factors and growth factors, Amiri et al. showed that the inhibition of autophagy improved the regenerative capacity of MSCs as demonstrated by reduced liver enzymes and necrosis scores in ALF rats compared with the scores in control MSC rats. Intriguingly, ALF mice that received autophagy inhibited MSCs demonstrated normal histology without necrosis, while ALF mice that received unmodified MSCs demonstrated mild necrosis [107]. Accordingly, the knockdown of inflammatory factors or apoptosis-related genes may become a hot topic to improve the therapeutic effects of MSCs in ALF animal models.
Hepatogenic differentiation and HLC transplantation
After incubation with specific combinations of growth factors in vitro, MSCs can be differentiated into HLCs with hepatocyte functions. MSCs changed the morphology and expression of hepatocyte-specific genes and acquired liver-specific functions in response to hepatogenic differentiation medium. However, HLCs typically exhibit liver-specific functions, including secretion of albumin and urea, uptake of low-density lipoprotein and indocyanine green, glycogen storage, and cytochrome P450 activity for 2–3 weeks in vitro [9, 109] but lose these beneficial functions after a prolonged culture time [110]. Multiple studies have further investigated the therapeutic effects of hepatogenic MSCs with liver-specific functions for repairing liver injury in ALF models (Table 5).
Transplantation of HLCs significantly improved the liver function of CCl4-treated mice via the secretion of TGF-β1, IL-6, and IL-10 [111]. Transplantation of HLCs before liver resection decreased the extensive lipid accumulation in hepatocytes and maintained the balance of amino acids, acylcarnitines, sphingolipids, and glycerophospholipids, thus promoting hepatocyte survival and inhibiting hepatocyte apoptosis in partial hepatectomy-induced ALF animal models [112]. Culturing on Matrigel that contained HGF and fibroblast growth factor-4 efficiently promoted the hepatogenic differentiation of MSCs, and intrasplenic injection of these HLCs prevented liver injury in 90% of hepatectomized rats [113].
An issue regarding the efficacy of HLCs compared with MSCs is that HLCs rapidly lose their liver functions and are sentenced to apoptosis after confronting a harsh environment. Transplantation of HLCs differentiated from amniotic fluid–derived MSCs (AF-MSCs) did not exert a recovery effect on ALF mice because they failed to enter the injured liver section, while transplantation of hepatic progenitor–like (HPL) cells, which are derived from AF-MSCs, underwent hepatogenesis for 1 week and showed a better effect in reducing liver injury [10]. Wang et al. [114] showed that HLCs expressed lower levels of HGF and had impaired immunosuppression compared with undifferentiated MSCs; thus, HLCs showed inferior potency to repair the injury in an ALF mouse model. However, other authors reject these points of views. Li et al. demonstrated that undifferentiated MSCs and HLCs exert similar effects on liver regeneration in ALF rats, and both groups decreased the levels of transaminases and TBIL 7 days after transplantation compared with the control group [115]. Undifferentiated MSCs and HLCs also exhibited similar abilities in homing into the injured liver tissue and rescued nearly all ALF mice after tail vein injection; while they rarely differentiated into human hepatocytes in the mouse liver, they stimulated the proliferation of mouse hepatocytes [109]. In addition, ADMSCs and BMMSCs displayed similar effects on repairing injuries compared with HLCs from both types of MSCs, although the gene expression profile of HLCs from ADMSCs was more close to a normal hepatogenic differentiation profile [9]. In our opinion, HLCs are more sensitive to harsh environments in vitro and in vivo; thus, MSCs without differentiation can benefit ALF animal models more than HLCs.
MSC-derived CM and Ex
MSC-CM and MSC-Ex, which contain many soluble factors, have been reported to exert therapeutic effects on ALF by inhibiting hepatocyte apoptosis, reducing panlobular leukocytic infiltrates and improving liver regeneration in recent years (Table 6).
MSC-CM
MSC-CM and MSC treatment comparably increased the liver function, reduced the serum levels of IFN-γ, IL-1β, and IL-6, and upregulated the serum IL-10 levels in ALF models. Moreover, IL-10 has been suggested to be the most important anti-inflammatory cytokine for the activation of signal transducer and activator of transcription 3 (STAT3) [21]. Injection of MSC-CM and MSCs clearly increased the natural killer T regulatory cell (NKTreg)/IL-17-producing natural killer T (NKT17) cell ratio in liver tissue and decreased the hepatotoxicity of NKT cells in a paracrine, indoleamine 2,3-dioxygenase-dependent manner, consequently attenuating hepatitis in vivo [116]. In addition, both MSC-CM and MSC lysates restored liver function and improved the survival rate of ALF rats via the secretion of HGF and VEGF [117].
Intriguingly, MSCs did not exhibit an additional benefit for ALF rats because of their poor engraftment and immune rejection, while MSC-CM substantially reduced hepatocellular death and bile duct duplication by promoting immune cell migration away from liver tissue and releasing chemokines [118]. Zagoura et al. [10] debated with the opinion that transplantation of CM derived from HPL cells showed a more efficient effect than AFMSC-CM via the secretion of more anti-inflammatory factors, including IL-10, IL-1Ra, IL-13, and IL-27. CM derived from MSCs cocultured with hepatocytes (MSC-H-CM) was the most effective medium for improving cell viability and total protein synthesis, decreasing the levels of lactate dehydrogenase and AST, and inhibiting the apoptosis of D-galactosamine (D-GalN)-treated LO2 cells in vitro than MSC-CM, CM derived from hepatocytes (H-CM), combinations of MSC-CM and H-CM, and nonconditioned medium (NCM). Moreover, MSC-H-CM most efficiently reduced liver injury biomarkers and enhanced the recovery of liver tissue and consequently improved the survival rate of ALF rats [119].
Although MSC and CM significantly improved the gross histopathological appearance of thioacetamide (TAA)-stimulated livers, CM did not remarkably or significantly improve the survival rate since it only enhances liver regeneration at later stages of self-recovery [75]. ESC-MSC is also an important MSC source, which has similar stemness characteristics compared with BMMSCs, but they grow faster than BMMSCs in vitro. An in vitro study showed that ESC-MSC-CM significantly improved the primary hepatocyte viability and upregulated the IL-10 levels in LPS-induced human blood mononuclear cells. However, BMMSC-CM and ESC-MSC-CM did not provide a survival benefit after 1 week of transplantation in ALF animals, although they increased the liver function after 48 h of transplantation [13]. This finding raises concerns as to whether MSC-CM always contributes to the improved outcomes of ALF.
MSC-Ex
Ex are small biological membrane vesicles from CM and contain many active substances (mRNAs and adhesion molecules) for the regulation of cellular and tissue physiology in vitro and in vivo. MSC-Ex express high levels of cytokines, such as angiopoietin-2, Axl, angiogenin, osteoprotegerin, IL-6, IL-8, insulin-like growth factor binding protein-6, and intercellular cell adhesion molecule (ICAM)-1. In vitro, MSC-Ex can be taken up by AML12 cells (a mouse hepatocyte cell line) and migrate to inhibit the apoptosis of D-GalN/LPS-induced AML12 cells [11]. Moreover, MSC-Ex in vitro inhibited APAP- and H2O2-induced hepatocyte apoptosis via the upregulation of Bcl-XL and promotion of hepatocyte proliferation but not via alleviation of oxidative stress [120].
After transplantation in vivo, MSC-Ex significantly reversed CCl4-induced ALF in mice by promoting hepatocyte proliferation and upregulation of NF-κB and STAT3 [120]. MSC-Ex significantly reduced the serum levels of ALT, AST, and inflammatory factor secretion by prohibiting the activation of macrophages, and miR-17 is an indispensable factor that targets thioredoxin-interacting protein for the suppression of NLRP3-mediated inflammation in ALF [121]. It also engrafted in the liver to serve as an antioxidant and inhibit oxidative stress–induced apoptosis via the delivery of glutathione peroxidase-1 (GPX1), upregulation of ERK1/2 and Bcl-2, and downregulation of the IKKB/NFkB/casp-9/3 pathway [122]. MSC-secreted prostaglandin (PG) E2 activated Yes-associated protein via upregulating the level of PGE4 and enhancing the phosphorylation of cAMP, and Yes-associated protein activated the mammalian target of rapamycin via suppressing phosphatase and tensin homolog for enhancing the cell proliferation of hepatocytes and promoting the recovery of ALF [123]. Moreover, pretreatment with MSC-Ex before the induction of ALF inhibited macrophage proliferation and the expression of active caspase-3 in injured livers, consequently improving liver function and enhancing survival rates in ALF mice [11]. Thus, we believe that MSC-Ex represent a highly attractive therapeutic approach compared with MSCs without the risk of iatrogenic tumor formation or pulmonary embolisms in ALF.
Biomaterials for improving MSC transplantation efficacy
Biomaterials with perfect biocompatibility, an applicable microstructure, and a proper degradation rate have gradually attracted attention for improving MSC attachment, proliferation, and secretion of beneficial cytokines via supplementing them with oxygen, nutrition, and growth factors. A nanoparticle that carries MSC-derived regenerative factors and is coated with red blood cell membranes has lower macrophagic internalization and significantly improves the proliferation of liver cells in vitro, and these coated nanoparticles can be well maintained in the injured liver of ALF mice and mitigate the liver injury after transplantation [124]. The IL-1Ra chitosan nanoparticles that have a targeting ability and controlled-release features can also improve the efficacy of MSC transplantation. Cotreatment with IL-1Ra chitosan nanoparticles and MSC transplantation significantly improved liver function and promoted hepatocyte proliferation by improving the levels of HGF and VEGF and suppressing inflammation in ALF swine [125].
The coculture of MSCs and hepatocytes in poly (lactic acid-glycolic acid) (PLGA) scaffolds at 1:5 showed a higher proliferation rate and higher hepatic synthesis function than coculture in ratios of 1:2.5 or 1:10, and this treatment could significantly decrease the levels of ALT, AST, and TBIL in mouse serum stimulated by D-GalN compared with MSC-PLGA or hepatocyte-PLGA scaffold treatments. (MSC + hepatocyte)-PLGA scaffold treatment significantly improved liver function and increased the survival rate of ALF mice via the downregulation of IL-6 and IL-1β compared with MSC-PLGA or hepatocyte-PLGA scaffold treatments. In addition, the (MSC + hepatocyte)-PLGA scaffold-treated ALF mice showed a weaker immunogenic response than the other two groups [126]. Alginate scaffold-MSCs promoted liver recovery by enhancing the secretion of albumin and glycogen, thus improving the survival rate and liver function in rats with hepatectomy-induced ALF more than alginate scaffolds after placing them onto the surface of the liver wound [127]. Furthermore, Xu et al. [128] determined that MSC-seeded regenerated silk fibroin (RSF) scaffolds that were placed onto the liver surface of ALF mice substantially improved the angiogenesis and hepatogenic differentiation of MSCs and downregulated the infiltration of inflammatory cells in vivo more than neat RSF scaffolds, attributed to their increased biocompatibility and enhancement of hepatogenic differentiation. Yagi et al. [129] highlighted the therapeutic effects of liver assist devices (LADs) that contain cocultures of MSCs and hepatocytes via decreasing inflammation and improving the survival benefit in ALF animal models compared with other coculture systems and monocellular control LADs. These effects may be attributed to the coculture system increasing the rate of engraftment and reducing the immune response of the MSCs and hepatocytes.
Therefore, biomaterial scaffolds protect MSCs against harsh microenvironments in vitro and in vivo, in addition to providing physical and directional support for liver regeneration.
Conclusion
MSC transplantation benefits liver injury in ALF models via engraftment into liver tissue, hepatogenic differentiation, immunoregulation, promotion of host hepatocyte proliferation, secretion of anti-inflammatory factors and antioxidants, and the enhancement of liver regeneration in vivo; moreover, the burgeoning application of MSC-CM and MSC-Ex mainly protect ALF animals from progressive injury via immunoregulation and paracrine effects (Fig. 2). We have previously demonstrated the optimized procedures of MSC application in vitro and in vivo in the main text; thus, we highlight several key points as follows. As gene modifications directly alter the gene phenotype of MSCs, treatment with physical or chemical factors on MSCs possesses an absolute advantage. MSCs acquire chromosomal aberrations and spontaneous malignant transformation in vitro and in vivo [130]; thus, we suggest analyzing the chromosomal integrity of MSCs before transplantation in vivo to improve the safety of the procedure. It is worth noting that rare human studies of MSC transplantation were executed to rescue ALF, and it is obligatory for us to carry out multicenter-clinical trials for MSC-based therapy in treating ALF patients. Importantly, we highlight that autologous or allogenic MSC transplantation should not be considered if the donor is bearing a genetic disease associated with a tumorigenic risk [84]. In summary, further breakthroughs are required to establish safer, more stable, and more effective stem cell–based therapy by MSCs and their derivatives in rescuing liver injury in ALF. We are looking forward to reversing acute injury before it progresses into ALF and decreasing the mortality of ALF patients worldwide via MSC-based therapy.
Abbreviations
- ALF:
-
Acute liver failure
- MSC:
-
Mesenchymal stromal cell
- iPSCs:
-
Induced pluripotent stem cells
- ESCs:
-
Embryonic stem cells
- HLCs:
-
Hepatocyte-like cells
- CCl4 :
-
Carbon tetrachloride
- CM:
-
Conditioned medium
- Ex:
-
Exosomes
- HE:
-
Hepatic encephalopathy
- INR:
-
International normalized ratio
- ACLF:
-
Acute-on-chronic liver failure
- APAP:
-
Acetaminophen
- NKT:
-
Natural killer T
- NK:
-
Natural killer
- IFN-γ:
-
Interferon-gamma
- TNF-α:
-
Tumor necrosis factor alpha
- ConA:
-
Concanavalin A
- α-GalCer:
-
Alpha-galactosylceramide
- LPS:
-
Lipopolysaccharide
- DCs:
-
Dendritic cells
- Tregs:
-
T regulatory cells
- TLR4:
-
Toll-like receptor 4
- PMNs:
-
Polymorphonuclear neutrophils
- IDO:
-
Indoleamine 2,3-dioxygenase
- TGF:
-
Transforming growth factor
- PGE2:
-
Prostaglandin E2
- ATP:
-
Adenosine triphosphate
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- TBIL:
-
Total bilirubin
- HO-1:
-
Heme oxygenase-1
- AFP:
-
Alpha fetal protein
- CK:
-
Cytokeratin
- VEGF:
-
Vascular endothelial growth factor
- HMGB1:
-
High mobility group box 1 protein
- EpCAM:
-
Epithelial cell adhesion molecule
- GSH:
-
Glutathione
- Nrf2:
-
NF-E2-related factor 2
- SOD:
-
Superoxide dismutase
- HGF:
-
Hepatocyte growth factor
- UCMSCs:
-
Umbilical cord–derived MSCs
- IL-1Ra:
-
IL-1 receptor antagonist
- BMMSCs:
-
Bone marrow–derived MSCs
- ConA:
-
Concanavalin A
- UCB-MSCs:
-
Umbilical cord blood–derived MSCs
- ADMSCs:
-
Adipose-derived MSCs
- iPSC-MSCs:
-
iPSC-derived MSCs
- PCNA:
-
Proliferating cell nuclear antigen
- SDF:
-
Stromal-derived factor
- CXCR4:
-
Chemokine CXC receptor 4
- ZD:
-
Zeaxanthin dipalmitate
- H2O2 :
-
Hydrogen dioxide
- miR-210:
-
MicroRNA-210
- CAT:
-
Catalase
- AF-MSCs:
-
Amniotic fluid–derived MSCs
- HPL:
-
Hepatic progenitor-like
- STAT3:
-
Signal transducer and activator of transcription 3
- NKTregs:
-
Natural killer T regulatory cells
- NKT17:
-
IL-17-producing natural killer T
- MSC-H-CM:
-
CM derived from MSCs cocultured with hepatocytes
- D-GalN:
-
D-galactosamine
- H-CM:
-
CM derived from hepatocytes
- NCM:
-
Nonconditioned medium
- TAA:
-
Thioacetamide
- ICAM:
-
Intercellular cell adhesion molecule
- GPX1:
-
Glutathione peroxidase-1
- PG:
-
Prostaglandin
- PLGA:
-
Poly (lactic acid-glycolic acid)
- RSF:
-
Regenerated silk fibroin
- LADs:
-
Liver assist devices
References
Saliba F, Samuel D (2013) Acute liver failure: current trends. J Hepatol 59(1):6–8
Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet (London, England) 376(9736):190–201
Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M (2014) Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells (Dayton, Ohio) 32(11):2818–2823
Huebert RC, Rakela J (2014) Cellular therapy for liver disease. Mayo Clin Proc 89(3):414–424
Ferrer JR, Chokechanachaisakul A, Wertheim JA (2015) New tools in experimental cellular therapy for the treatment of liver diseases. Curr Transplant Rep 2(2):202–210
Zhang ZH, Zhu W, Ren HZ, Zhao X, Wang S, Ma HC, Shi XL (2017) Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res Ther 8(1):70
Yang JF, Cao HC, Pan QL, Yu J, Li J, Li LJ (2015) Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatobiliary Pancreat Dis Int 14(2):186–193
Deng L, Liu G, Wu X, Wang Y, Tong M, Liu B, Wang K, Peng Y, Kong X (2014) Adipose derived mesenchymal stem cells efficiently rescue carbon tetrachloride-induced acute liver failure in mouse. ScientificWorldJournal 2014:103643
Manzini BM, da Silva Santos Duarte A, Sankaramanivel S, Ramos AL, Latuf-Filho P, Escanhoela C, Kharmandayan P, Olalla Saad ST, Boin I, Malheiros Luzo AC (2015) Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis. Cytotherapy 17(8):1052–1065
Zagoura DS, Roubelakis MG, Bitsika V, Trohatou O, Pappa KI, Kapelouzou A, Antsaklis A, Anagnou NP (2012) Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 61(6):894–906
Chen L, Xiang B, Wang X, Xiang C (2017) Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther 8(1):9
Moslem M, Valojerdi MR, Pournasr B, Muhammadnejad A, Baharvand H (2013) Therapeutic potential of human induced pluripotent stem cell-derived mesenchymal stem cells in mice with lethal fulminant hepatic failure. Cell Transplant 22(10):1785–1799
Lotfinia M, Kadivar M, Piryaei A, Pournasr B, Sardari S, Sodeifi N, Sayahpour FA, Baharvand H (2016) Effect of secreted molecules of human embryonic stem cell-derived mesenchymal stem cells on acute hepatic failure model. Stem Cells Dev 25(24):1898–1908
Hu C, Li L (2015) In vitro and in vivo hepatic differentiation of adult somatic stem cells and extraembryonic stem cells for treating end stage liver diseases. Stem Cells Int 2015:871972
Yang X, Hou J, Han Z, Wang Y, Hao C, Wei L, Shi Y (2013) One cell, multiple roles: contribution of mesenchymal stem cells to tumor development in tumor microenvironment. Cell Biosci 3(1):5
Pascual-Miguelanez I, Salinas-Gomez J, Fernandez-Luengas D, Villar-Zarra K, Clemente LV, Garcia-Arranz M, Olmo DG (2015) Systemic treatment of acute liver failure with adipose derived stem cells. J Investig Surg 28(2):120–126
di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M et al (2008) Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 57(2):223–231
Kim S, Han YS, Lee JH, Lee SH (2018) Combination of MSC spheroids wrapped within autologous composite sheet dually protects against immune rejection and enhances stem cell transplantation efficacy. Tissue Cell 53:93–103
Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clement S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C (2009) Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 4(8):e6657
Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE et al (2009) Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58(4):570–581
Ma HC, Wang X, Wu MN, Zhao X, Yuan XW, Shi XL (2016) Interleukin-10 contributes to therapeutic effect of mesenchymal stem cells for acute liver failure via signal transducer and activator of transcription 3 signaling pathway. Chin Med J 129(8):967–975
Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA (2012) Systematic review: acute liver failure - one disease, more than 40 definitions. Aliment Pharmacol Ther 35(11):1245–1256
Polson J, Lee WM (2005) AASLD position paper: the management of acute liver failure. Hepatology (Baltimore, Md) 41(5):1179–1197
Bajaj JS, Moreau R, Kamath PS, Vargas HE, Arroyo V, Reddy KR, Szabo G, Tandon P, Olson J, Karvellas C et al (2018) Acute-on-chronic liver failure: getting ready for prime time? Hepatology (Baltimore, Md) 68(4):1621–1632
O’Grady JG, Schalm SW, Williams R (1993) Acute liver failure: redefining the syndromes. Lancet (London, England) 342(8866):273–275
Kim JD, Cho EJ, Ahn C, Park SK, Choi JY, Lee HC, Kim DY, Choi MS, Wang HJ, Kim IH et al (2018) A novel model to predict 1-month risk of transplant or death in hepatitis A-related acute liver failure. Hepatology (Baltimore, Md). https://doi.org/10.1002/hep.30262
Wang L, Geng J (2017) Acute hepatitis E virus infection in patients with acute liver failure in China: not quite an uncommon cause. Hepatology (Baltimore, Md) 65(5):1769–1770
Jung DH, Hwang S, Lim YS, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Park GC, Lee SG (2018) Outcome comparison of liver transplantation for hepatitis A-related versus hepatitis B-related acute liver failure in adult recipients. Clin Transpl 32(1). https://doi.org/10.1111/ctr.13140
Gallegos-Orozco JF, Rakela-Brodner J (2010) Hepatitis viruses: not always what it seems to be. Rev Med Chil 138(10):1302–1311
Reuben A, Koch DG, Lee WM (2010) Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology (Baltimore, Md) 52(6):2065–2076
Lescot T, Karvellas C, Beaussier M, Magder S (2012) Acquired liver injury in the intensive care unit. Anesthesiology 117(4):898–904
Henrion J (2012) Hypoxic hepatitis. Liver Int 32(7):1039–1052
Ichai P, Samuel D (2008) Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl 14(Suppl 2):S67–S79
Wyke RJ, Yousif-Kadaru AG, Rajkovic IA, Eddleston AL, Williams R (1982) Serum stimulatory activity and polymorphonuclear leucocyte movement in patients with fulminant hepatic failure. Clin Exp Immunol 50(2):442–449
Clapperton M, Rolando N, Sandoval L, Davies E, Williams R (1997) Neutrophil superoxide and hydrogen peroxide production in patients with acute liver failure. Eur J Clin Investig 27(2):164–168
Manakkat Vijay GK, Ryan JM, Abeles RD, Ramage S, Patel V, Bernsmeier C, Riva A, McPhail MJ, Tranah TH, Markwick LJ et al (2016) Neutrophil toll-like receptor 9 expression and the systemic inflammatory response in acetaminophen-induced acute liver failure. Crit Care Med 44(1):43–53
Kawashima R, Mochida S, Matsui A, YouLuTu ZY, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, Ohno A et al (1999) Expression of osteopontin in Kupffer cells and hepatic macrophages and stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun 256(3):527–531
Ramaiah SK, Rittling S (2008) Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci 103(1):4–13
Chang W, Song BW, Moon JY, Cha MJ, Ham O, Lee SY, Choi E, Hwang KC (2013) Anti-death strategies against oxidative stress in grafted mesenchymal stem cells. Histol Histopathol 28(12):1529–1536
dos Santos DC, da Silva Gomes Martinho JM, Pacheco-Moreira LF, Carvalho Viana de Araujo C, Caroli-Bottino A, Pannain VL, Soares Trinta K, Gandini M, da Costa Neves PC, de Souza Matos DC et al (2009) Eosinophils involved in fulminant hepatic failure are associated with high interleukin-6 expression and absence of interleukin-5 in liver and peripheral blood. Liver Int 29(4):544–551
Izumi S, Hughes RD, Langley PG, Pernambuco JR, Williams R (1994) Extent of the acute phase response in fulminant hepatic failure. Gut 35(7):982–986
Kimura K, Ando K, Tomita E, Ohnishi H, Ishikawa T, Kakumu S, Muto Y, Moriwaki H (1999) Elevated intracellular IFN-gamma levels in circulating CD8+ lymphocytes in patients with fulminant hepatitis. J Hepatol 31(4):579–583
Nagaki M, Iwai H, Naiki T, Ohnishi H, Muto Y, Moriwaki H (2000) High levels of serum interleukin-10 and tumor necrosis factor-alpha are associated with fatality in fulminant hepatitis. J Infect Dis 182(4):1103–1108
Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U (2002) Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology (Baltimore, Md) 36(4 Pt 1):1001–1008
Leber B, Spindelboeck W, Stadlbauer V (2012) Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med 33(1):80–95
Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, Lohse AW (1996) Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology (Baltimore, Md) 24(4):824–829
Schumann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, Tiegs G (2000) Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol 157(5):1671–1683
Wang J, Cao X, Zhao J, Zhao H, Wei J, Li Q, Qi X, Yang Z, Wang L, Zhang H et al (2017) Critical roles of conventional dendritic cells in promoting T cell-dependent hepatitis through regulating natural killer T cells. Clin Exp Immunol 188(1):127–137
Tadokoro T, Morishita A, Sakamoto T, Fujihara S, Fujita K, Mimura S, Oura K, Nomura T, Tani J, Yoneyama H et al (2017) Galectin9 ameliorates fulminant liver injury. Mol Med Rep 16(1):36–42
Matsumoto H, Kawamura T, Kobayashi T, Kanda Y, Kawamura H, Abo T (2011) Coincidence of autoantibody production with the activation of natural killer T cells in alpha-galactosylceramide-mediated hepatic injury. Immunology 133(1):21–28
Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG (2009) Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology (Baltimore, Md) 49(4):1267–1276
Huang JR, Tsai YC, Chang YJ, Wu JC, Hung JT, Lin KH, Wong CH, Yu AL (2014) alpha-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3. J Immunol (Baltimore, Md : 1950) 192(4):1972–1981
Biburger M, Tiegs G (2005) Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol (Baltimore, Md : 1950) 175(3):1540–1550
Tirosh O, Artan A, Aharoni-Simon M, Ramadori G, Madar Z (2010) Impaired liver glucose production in a murine model of steatosis and endotoxemia: protection by inducible nitric oxide synthase. Antioxid Redox Signal 13(1):13–26
Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A (2014) C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun 452(1):8–13
Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M (1994) Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology (Baltimore, Md) 19(2):480–488
Arthur MJ, Kowalski-Saunders P, Wright R (1988) Effect of endotoxin on release of reactive oxygen intermediates by rat hepatic macrophages. Gastroenterology 95(6):1588–1594
Imamura M, Tsutsui H, Yasuda K, Uchiyama R, Yumikura-Futatsugi S, Mitani K, Hayashi S, Akira S, Taniguchi S, Van Rooijen N et al (2009) Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol 51(2):333–341
Yee SB, Ganey PE, Roth RA (2003) The role of Kupffer cells and TNF-alpha in monocrotaline and bacterial lipopolysaccharide-induced liver injury. Toxicol Sci 71(1):124–132
Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ (2006) Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2(1):39–46
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (2009) Autophagy regulates lipid metabolism. Nature 458(7242):1131–1135
Rutherford A, Chung RT (2008) Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis 28(2):167–174
Kaplowitz N (2000) Mechanisms of liver cell injury. J Hepatol 32(1 Suppl):39–47
Jaeschke H, Lemasters JJ (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125(4):1246–1257
Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T (2009) Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol 24(1):70–77
Yuan S, Jiang T, Sun L, Zheng R, Ahat N, Zhang Y (2013) The role of bone marrow mesenchymal stem cells in the treatment of acute liver failure. Biomed Res Int 2013:251846
Zheng S, Yang J, Tang Y, Shao Q, Guo L, Liu Q (2015) Effect of bone marrow mesenchymal stem cells transplantation on the serum and liver HMGB1 expression in rats with acute liver failure. Int J Clin Exp Pathol 8(12):15985–15992
Li YW, Zhang C, Sheng QJ, Bai H, Ding Y, Dou XG (2017) Mesenchymal stem cells rescue acute hepatic failure by polarizing M2 macrophages. World J Gastroenterol 23(45):7978–7988
Yuan S, Jiang T, Zheng R, Sun L, Cao G, Zhang Y (2014) Effect of bone marrow mesenchymal stem cell transplantation on acute hepatic failure in rats. Exp Ther Med 8(4):1150–1158
Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, Wu T, Li J, Ding W, Sun S et al (2017) Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 66(5):955–964
Li J, Xin J, Hao S, Zhang L, Jiang L, Chen D, Xie Q, Xu W, Cao H, Li L (2012) Return of the metabolic trajectory to the original area after human bone marrow mesenchymal stem cell transplantation for the treatment of fulminant hepatic failure. J Proteome Res 11(6):3414–3422
Huang YJ, Chen P, Lee CY, Yang SY, Lin MT, Lee HS, Wu YM (2016) Protection against acetaminophen-induced acute liver failure by omentum adipose tissue derived stem cells through the mediation of Nrf2 and cytochrome P450 expression. J Biomed Sci 23:5. https://doi.org/10.1186/s12929-016-0231-x
Liu Z, Meng F, Li C, Zhou X, Zeng X, He Y, Mrsny RJ, Liu M, Hu X, Hu JF et al (2014) Human umbilical cord mesenchymal stromal cells rescue mice from acetaminophen-induced acute liver failure. Cytotherapy 16(9):1207–1219
Yun JW, Ahn JH, Kwon E, Kim SH, Kim H, Jang JJ, Kim WH, Kim JH, Han SY, Kim JT et al (2016) Human umbilical cord-derived esenchymal stem cells in acute liver injury: hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul Toxicol Pharmacol 81:437–447
Huang B, Cheng X, Wang H, Huang W, la Ga Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y et al (2016) Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med 14:45. https://doi.org/10.1186/s12967-016-0792-1
Zhao X, Shi X, Zhang Z, Ma H, Yuan X, Ding Y (2016) Combined treatment with MSC transplantation and neutrophil depletion ameliorates D-GalN/LPS-induced acute liver failure in rats. Clin Res Hepatol Gastroenterol 40(6):730–738
Yoshizumi Y, Yukawa H, Iwaki R, Fujinaka S, Kanou A, Kanou Y, Yamada T, Nakagawa S, Ohara T, Nakagiri K et al (2017) Immunomodulatory effects of adipose tissue-derived stem cells on concanavalin A-induced acute liver injury in mice. Cell Med 9(1–2):21–33
Zhu X, He B, Zhou X, Ren J (2013) Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res 351(3):477–486
Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, Zhao F, Liu Q, Wei X, Jin M et al (2014) Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology (Baltimore, Md) 59(2):671–682
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107(1):367–372
Hu C, Zhou N, Li J, Shi D, Cao H, Li L (2016) Porcine adipose-derived mesenchymal stem cells retain their stem cell characteristics and cell activities while enhancing the expression of liver-specific genes after acute liver failure. Int J Mol Sci 17(1). https://doi.org/10.3390/ijms17010062
Li J, Tao R, Wu W, Cao H, Xin J, Guo J, Jiang L, Hong X, Demetriou AA, Farkas D et al (2010) Transcriptional profiling and hepatogenic potential of acute hepatic failure-derived bone marrow mesenchymal stem cells. Differ Res Biol Divers 80(2–3):166–174
Faiella W, Atoui R (2016) Immunotolerant properties of mesenchymal stem cells: updated review. Stem Cells Int 2016:1859567
Meier RP, Muller YD, Morel P, Gonelle-Gispert C, Buhler LH (2013) Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res 11(3):1348–1364
Park CH, Bae SH, Kim HY, Kim JK, Jung ES, Chun HJ, Song MJ, Lee SE, Cho SG, Lee JW et al (2013) A pilot study of autologous CD34-depleted bone marrow mononuclear cell transplantation via the hepatic artery in five patients with liver failure. Cytotherapy 15(12):1571–1579
Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y (2012) Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng A 18(13–14):1352–1364
Sun K, Xie X, Xie J, Jiao S, Chen X, Zhao X, Wang X, Wei L (2014) Cell-based therapy for acute and chronic liver failures: distinct diseases, different choices. Sci Rep 4:6494
Zare H, Jamshidi S, Dehghan MM, Saheli M, Piryaei A (2018) Bone marrow or adipose tissue mesenchymal stem cells: comparison of the therapeutic potentials in mice model of acute liver failure. J Cell Biochem 119(7):5834–5842
Zheng S, Yang J, Tang Y, Shao Q, Guo L, Liu Q (2015) Transplantation of umbilical cord mesenchymal stem cells via different routes in rats with acute liver failure. Int J Clin Exp Pathol 8(12):15854–15862
Sun L, Fan X, Zhang L, Shi G, Aili M, Lu X, Jiang T, Zhang Y (2014) Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med 34(4):987–996
Feng T, Zhang J, Zeng G, Zhou R, Tang X, Cui C, Li Y, Wang H, Li T, Zhu W et al (2015) Therapeutic potential of umbilical cord mesenchymal stem cells in mice with acute hepatic failure. Int J Artif Organs 38(5):271–276
Deng L, Kong X, Liu G, Li C, Chen H, Hong Z, Liu J, Xia J (2016) Transplantation of adipose-derived mesenchymal stem cells efficiently rescues thioacetamide-induced acute liver failure in mice. Transplant Proc 48(6):2208–2215
Kim SJ, Park KC, Lee JU, Kim KJ, Kim DG (2011) Therapeutic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes. J Korean Surg Soc 81(3):176–186
Yuan SF, Jiang T, Sun LH, Zheng RJ, Cao GQ, Ahat NZ, Zhang YX (2014) Use of bone mesenchymal stem cells to treat rats with acute liver failure. Genet Mol Res 13(3):6962–6980
Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, Li Y, Pan X, Wang Y, Li L (2012) Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med 10:56
Li J, Zhang L, Xin J, Jiang L, Zhang T, Jin L, Zhou P, Hao S, Cao H, Li L (2012) Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology (Baltimore, Md) 56(3):1044–1052
Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, Ding YT (2016) Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int 15(6):602–611
Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR (2009) Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol 15(21):2657–2664
Deng C, Qin A, Zhao W, Feng T, Shi C, Liu T (2014) Up-regulation of CXCR4 in rat umbilical mesenchymal stem cells induced by serum from rat with acute liver failure promotes stem cells migration to injured liver tissue. Mol Cell Biochem 396(1–2):107–116
Liu Y, Xiong Y, Xing F, Gao H, Wang X, He L, Ren C, Liu L, So KF, Xiao J (2017) Precise regulation of miR-210 is critical for the cellular homeostasis maintenance and transplantation efficacy enhancement of mesenchymal stem cells in acute liver failure therapy. Cell Transplant 26(5):805–820
Zeng W, Xiao J, Zheng G, Xing F, Tipoe GL, Wang X, He C, Chen ZY, Liu Y (2015) Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci Rep 5:11100
Ma H, Shi X, Yuan X, Ding Y (2016) IL-1beta siRNA adenovirus benefits liver regeneration by improving mesenchymal stem cells survival after acute liver failure. Ann Hepatol 15(2):260–270
Zheng YB, Zhang XH, Huang ZL, Lin CS, Lai J, Gu YR, Lin BL, Xie DY, Xie SB, Peng L et al (2012) Amniotic-fluid-derived mesenchymal stem cells overexpressing interleukin-1 receptor antagonist improve fulminant hepatic failure. PLoS One 7(7):e41392
Wang K, Li Y, Zhu T, Zhang Y, Li W, Lin W, Li J, Zhu C (2017) Overexpression of c-Met in bone marrow mesenchymal stem cells improves their effectiveness in homing and repair of acute liver failure. Stem Cell Res Ther 8(1):162
Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT (2014) Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol 20(40):14884–14894
Tang Y, Li Q, Meng F, Huang X, Li C, Zhou X, Zeng X, He Y, Liu J, Hu X et al (2016) Therapeutic potential of HGF-expressing human umbilical cord mesenchymal stem cells in mice with acute liver failure. Int J Hepatol 2016:5452487
Amiri F, Molaei S, Bahadori M, Nasiri F, Deyhim MR, Jalili MA, Nourani MR, Habibi Roudkenar M (2016) Autophagy-modulated human bone marrow-derived mesenchymal stem cells accelerate liver restoration in mouse models of acute liver failure. Iran Biomed J 20(3):135–144
Wang J, Ren H, Yuan X, Ma H, Shi X, Ding Y (2018) Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res 48(3):E194–E202
Zhou R, Li Z, He C, Li R, Xia H, Li C, Xiao J, Chen ZY (2014) Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model. PLoS One 9(8):e104392
Stock P, Bruckner S, Ebensing S, Hempel M, Dollinger MM, Christ B (2010) The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc 5(4):617–627
Zhang S, Zhu Z, Wang Y, Liu S, Zhao C, Guan W, Zhao Y (2018) Therapeutic potential of Bama miniature pig adipose stem cells induced hepatocytes in a mouse model with acute liver failure. Cytotechnology 70(4):1131–1141
Tautenhahn HM, Bruckner S, Baumann S, Winkler S, Otto W, von Bergen M, Bartels M, Christ B (2016) Attenuation of postoperative acute liver failure by mesenchymal stem cell treatment due to metabolic implications. Ann Surg 263(3):546–556
Miyazaki M, Hardjo M, Masaka T, Tomiyama K, Mahmut N, Medina RJ, Niida A, Sonegawa H, Du G, Yong R et al (2007) Isolation of a bone marrow-derived stem cell line with high proliferation potential and its application for preventing acute fatal liver failure. Stem Cells (Dayton, Ohio) 25(11):2855–2863
Wang H, Zhao T, Xu F, Li Y, Wu M, Zhu D, Cong X, Liu Y (2014) How important is differentiation in the therapeutic effect of mesenchymal stromal cells in liver disease? Cytotherapy 16(3):309–318
Li D, Fan J, He X, Zhang X, Zhang Z, Zeng Z, Ruan M, Cai L (2015) Therapeutic effect comparison of hepatocyte-like cells and bone marrow mesenchymal stem cells in acute liver failure of rats. Int J Clin Exp Pathol 8(1):11–24
Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, Djonov V, Lukic ML, Volarevic V (2017) Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl 23(8):1040–1050
Chen G, Jin Y, Shi X, Qiu Y, Zhang Y, Cheng M, Wang X, Chen C, Wu Y, Jiang F et al (2015) Adipose-derived stem cell-based treatment for acute liver failure. Stem Cell Res Ther 6:40. https://doi.org/10.1186/s13287-015-0040-2
Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML (2007) Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2(9):e941
Chen L, Zhang J, Yang L, Zhang G, Wang Y, Zhang S (2018) The effects of conditioned medium derived from mesenchymal stem cells cocultured with hepatocytes on damaged hepatocytes and acute liver failure in rats. Stem Cells Int 2018:9156560
Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK (2014) Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 5(3):76
Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D, Zheng M, Chen Z (2018) AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 36:140–150
Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W (2017) hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther 25(2):465–479
Liu Y, Ren H, Wang J, Yang F, Li J, Zhou Y, Yuan X, Zhu W, Shi X (2018) Prostaglandin E2 secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J 33:2514–2525
Liang H, Huang K, Su T, Li Z, Hu S, Dinh PU, Wrona EA, Shao C, Qiao L, Vandergriff AC et al (2018) Mesenchymal stem cell/red blood cell-inspired nanoparticle therapy in mice with carbon tetrachloride-induced acute liver failure. ACS Nano 12(7):6536–6544
Xiao JQ, Shi XL, Ma HC, Tan JJ, Lin z XQ, Ding YT (2013) Administration of IL-1Ra chitosan nanoparticles enhances the therapeutic efficacy of mesenchymal stem cell transplantation in acute liver failure. Arch Med Res 44(5):370–379
Liu M, Yang J, Hu W, Zhang S, Wang Y (2016) Superior performance of co-cultured mesenchymal stem cells and hepatocytes in poly(lactic acid-glycolic acid) scaffolds for the treatment of acute liver failure. Biomed Mater 11(1):015008. https://doi.org/10.1088/1748-6041/11/1/015008
Lin J, Meng L, Yao Z, Chen S, Yang J, Tang Z, Lin N, Xu R (2015) Use an alginate scaffold-bone marrow stromal cell (BMSC) complex for the treatment of acute liver failure in rats. Int J Clin Exp Med 8(8):12593–12600
Xu L, Wang S, Sui X, Wang Y, Su Y, Huang L, Zhang Y, Chen Z, Chen Q, Du H et al (2017) Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure. ACS Appl Mater Interfaces 9(17):14716–14723
Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins RG, Tilles AW, Yarmush ML (2009) Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng A 15(11):3377–3388
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM et al (2007) Sarcoma derived from cultured mesenchymal stem cells. Stem Cells (Dayton, Ohio) 25(2):371–379
Funding
This work was supported by the National Natural Science Foundation of China (No. 81700553), the China Postdoctoral Science Foundation (No. 2017 M183789), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. 81121002), and the Independent Fund of State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Zhejiang University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, C., Li, L. Improvement of mesenchymal stromal cells and their derivatives for treating acute liver failure. J Mol Med 97, 1065–1084 (2019). https://doi.org/10.1007/s00109-019-01804-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01804-x