Abstract
Low-density lipoprotein (LDL) and cholesterol homeostasis in the peripheral blood is maintained by specialized cells, such as macrophages. Macrophages express a variety of scavenger receptors (SR) that interact with lipoproteins, including SR-A1, CD36, and lectin-like oxLDL receptor-1 (LOX-1). These cells also have several cholesterol transporters, including ATP-binding cassette transporter ABCA1, ABCG1, and SR-BI, that are involved in reverse cholesterol transport. Lipids internalized by phagocytosis are transported to late endosomes/lysosomes, where lysosomal acid lipase (LAL) digests cholesteryl esters releasing free cholesterol. Free cholesterol in turn is processed by acetyl-CoA acetyltransferase (ACAT1), an enzyme that transforms cholesterol to cholesteryl esters. The endoplasmic reticulum serves as a depot for maintaining newly synthesized cholesteryl esters that can be processed by neutral cholesterol ester hydrolase (NCEH), which generates free cholesterol that can exit via cholesterol transporters. In atherosclerosis, pro-inflammatory stimuli upregulate expression of scavenger receptors, especially LOX-1, and downregulate expression of cholesterol transporters. ACAT1 is also increased, while NCEH expression is reduced. This results in deposition of free and esterified cholesterol in macrophages and generation of foam cells. Moreover, other cell types, such as endothelial (ECs) and vascular smooth muscle cells (VSMCs), can also become foam cells. In this review, we discuss known pathways of foam cell formation in atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foam cells play an important role at all stages of atherosclerotic lesion development, from initial lesions to advanced plaques. Macrophages serve as the main source of foam cells after they penetrate the endothelial barrier and accumulate in the arterial intima media in response to the pro-inflammatory activation of endothelial cells (ECs) [1]. A small part of foam cells originates from ECs and vascular smooth muscle cells (VSMCs). ECs can also differentiate to smooth muscle-like cells that can be involved in pro-atherogenic vascular remodeling. In addition, VSMCs can differentiate to macrophages that become foam cells upon lipid uptake [2]. Transformation of VSMCs to macrophage-like cells is regulated by Kruppel-like factor 4 (KLF4), a transcription factor, for which over 800 target genes were found in cholesterol-treated VSMCs [3]. Cholesterol loading of VSMC converts them to a macrophage-appearing state by downregulating the microRNA (miR)-143/145-myocardin axis, a key pathway that is essential for SMC-specific differentiation [4]. Salusin-β, a pro-atherogenic agent, induces foam formation and monocyte adhesion via inducing expression of acetyl-CoA acetyltransferase (ACAT)1 and vascular cell adhesion molecule 1 (VCAM-1) in VSMCs [5].
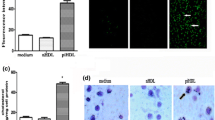
The main cause of foam cells generation is the excessive influx of modified low-density lipoproteins (LDL) and accumulation of cholesterol esters in intimal macrophages [6]. It should be noted that native (unmodified) LDL that can be found in the peripheral blood of healthy individuals, does not cause accumulation of cholesteryl esters in cultured macrophages, while modified LDL isolated from atherosclerotic patients induces a significant increase of intracellular cholesteryl esters (Fig. 1). In normal conditions, macrophages serve as a major regulator of plasma lipoprotein metabolism and content [7]. These cells express a variety of scavenger receptors (SR), such as SR-A1, CD36, and lectin-like oxLDL receptor-1 (LOX-1) with affinity to oxidized low-density lipoproteins (oxLDL). Additionally, macrophages have an advanced enzymatic machinery, such as acyl coenzyme A:cholesterol acyltransferase-1 (ACAT1), which is essential for formation of cholesterol esters [8]. Cholesteryl esters are hydrolyzed by two enzymes: neutral cholesteryl ester hydrolase 1 (NCEH1) and lysosomal acid lipase (LAL), that generate free fatty acids and cholesterol [9]. Macrophages also express a range of membrane pumps, such as ATP-binding cassette (ABC) transporters ABCA1 and ABCG1 and scavenger receptor SR-BI that are involved in reverse cholesterol transport [7]. Together, these proteins ensure an effective control of LDL and cholesterol content in the peripheral blood under normal conditions.
Effect of LDL isolated from the plasma of individuals without atherosclerosis and patients with carotid atherosclerosis on cholesterol esters in human macrophages. Human monocyte-derived macrophages were incubated in medium 199 containing 10% lipid-deficient serum and LDL for 24 h at 37 °C. Control cells were incubated in the medium without LDL. Data are presented as the mean of three repetitions ± standard deviation. The asterisk indicates significant difference from the control, p < 0.05
In atherosclerosis, macrophage-dependent cholesterol handling is deregulated. Due to increased generation of oxLDL, macrophage expression of LOX-1 is significantly upregulated by stimulation of multiple factors such as pro-inflammatory cytokines [9], oxLDL itself, lysophosphatidylcholine (a product of oxLDL degradation) [10], advanced glycation end-products (AGEs) [11], vasopressors [12], and others. Elevated expression of LOX-1 leads to increased lipid uptake by macrophages. By contrast, expression of ABCA1 and ABCG1 is decreased in atherosclerosis, further aggravating intracellular cholesterol accumulation and promoting generation foam cells formation [13].
LDL transport through the endothelial barrier
Atherosclerosis and pro-atherogenic conditions such as hypertension, smoking, and diabetes are characterized by increased vascular permeability for LDL [14, 15] and upregulated expression of LOX-1, which is associated with increased endothelial permeability for oxLDL through activation of protein kinase C (PKC) and calcium influx into ECs. In parallel, expression of desmoglein 1 (DSG1) and desmocollin 2 (DSC2) is reduced [16]. DSG1 is a component of desmosomes, which is involved in cell-cell junctional contact formation and is regulated by calcium [17]. Similarly, DSC2, a calcium-binding cadherin-type protein, is also involved in desmosomal intercellular contacts [18]. LOX-1-mediated downregulation of desmosomal cell-cell contacts weakens the endothelial junctions and increases trans-endothelial transfer of oxLDL.
Upregulation of PKC leads to activation of a RhoA/Rho kinase-dependent signaling and phosphorylation of occludin, a key structural component of tight junctions, which weakens the endothelial barrier [19]. PKC stimulation also results in activation of protein phosphatase 1 regulatory subunit 14A (PPP1R14A), an inhibitor of smooth muscle myosin phosphatase, which in turn causes cytoskeletal rearrangement, disruption of cell-cell contacts, and increased permeability [20]. Increased transfer of cholesterol-rich oxLDL into the intima media through the endothelial barrier contributes to lipid accumulation in the intimal macrophages, which is an early event in atheroma formation.
Current consensus favors the inflammatory hypothesis of atherosclerosis induction [21], according to which pro-inflammatory stimuli initiate penetration of monocytes into the intima media followed by sub-endothelial lipid accumulation in the arterial wall. In the intima media, monocytes differentiate predominantly to pro-inflammatory macrophages (the M1 phenotype) that actively take up lipids but cannot effectively empty the lipid excess due to the inhibition of efflux pumps in pro-inflammatory microenvironment [22]. Classical M1 macrophages can be induced by exposure to pro-inflammatory cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α or to pathogenic products such as lipopolysaccharide (LPS), an endotoxin of Gram-negative bacteria, and flagellin, a structural component of bacterial flagellum [23]. In infection or injury, M1 macrophages are mainly involved in inflammatory responses directed by Th1 cells. These macrophages release a variety of inflammatory cytokines and chemokines essential for propagation of inflammation. M1 macrophages also produce high amounts of nitric oxide (NO) and reactive oxygen species (ROS) to destroy a pathogen [24].
M2 (or alternatively polarized) macrophages can be induced by various stimuli and generally possess anti-inflammatory properties. A variety of M2 subtypes was characterized, with the most pro-inflammatory M2a that can be generated under exposure to Th2 cytokines, i.e., interleukin (IL)-4 and IL-13. Typically, M2 macrophages secrete significant amounts of anti-inflammatory IL-10 and transforming growth factor (TGF)-β, contribute to wound healing, phagocytosis of apoptotic cells, tissue remodeling, angiogenesis, and carcinogenesis [25].
Macrophage M2 polarization is associated with an increase of fatty acid oxidation. However, it is unclear whether this association is a simple correlation only or it directly influences M2 polarization [26]. By contrast, M1 polarization is associated with the activation of fatty acid synthesis that primarily contributes to the inflammatory response and affects cholesterol homeostasis and neutral fat accumulation [27]. Recently, Da Silva et al. (2016) performed an interesting experiment to evaluate how macrophage-derived foam cells respond to M1-polarizing stimuli [28]. Macrophage-colony stimulating factor (M-CSF)-induced macrophages were transformed into foam cells and then exposed to M1-polarizing factors (i.e., LPS + IFN-γ). While normal M-CSF-induced macrophages started to express various pro-inflammatory genes, foam cells exhibited weaker pro-inflammatory activation. In response to M2-polarizing signal (i.e., treatment with IL-4) both normal macrophages and foam cells responded by upregulation of anti-inflammatory genes with equal magnitude [28]. Indeed, in M1-polarizing microenvironments of atherosclerotic lesions, foam cell formation may locally weaken the macrophage-dependent inflammatory component of atherogenesis.
How macrophages can sense and take up lipids
Lipid internalization by macrophages has been reviewed in several previously published works [7, 8, 13, 29]. Briefly, circulating monocytes and resident macrophages can sense circulating lipids through a number of previously described receptors, including CD36, SR-A1, and LOX-1 (Fig. 2) [7].
Mechanisms of lipid handling in macrophages. Endothelial cells have a high surface expression of lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) capable to bind and transfer oxidized LDL (oxLDL) across the cell to the intima media, which is infiltrated by macrophages in atherosclerosis. Macrophages sense and bind oxLDL with several scavenger receptors (SR) such as SR-A1, CD36, and LOX-1. In late endosomes/lysosomes, lysosomal acid lipase (LAL) degrades cholesteryl esters, which are highly present in LDL particles, to free cholesterol and free fatty acids. In the endoplasmic reticulum (ER), acyl coenzyme A: cholesterol acyltransferase-1 (ACAT1) contributes to formation of cholesteryl esters from free cholesterol. Cholesteryl esters accumulate in the ER. Neutral cholesteryl ester hydrolase (NCEH) processes cholesteryl esters liberating free cholesterol that is transported outside the cells via ATP-binding cassette (ABC) transporters ABCA1 and ABCG1, as well as via SR-BI. Apolipoprotein A-1 (ApoA-1) serves as an acceptor for cholesterol carried by ABCA1. High-density lipoprotein (HDL) accepts cholesterol that is transferred by ABCG1 and SR-BI. In normal conditions, this machinery is tightly regulated ensuring cholesterol homeostasis. In atherosclerosis, the control is deregulated. Expression of scavenger receptors is increased, which leads to elevated uptake of oxLDL. By contrast, expression of cholesterol transporters ABCA1 and ABCG1 is suppressed, which diminishes cholesterol efflux and promotes cholesterol deposition in macrophages. ACAT1 is upregulated while NCEH is downregulated. This leads to accumulation of cholesteryl esters in the cell. Together, these mechanisms lead to excessive lipid deposits and transformation of macrophages to foam cells
There are three known isoforms of the SR-A1 receptor, of which two are functionally relevant and can participate in the transfer of oxLDL. The full-length isoform SR-A1 contains a large extracellular domain, the cytosolic domain, and the transmembrane domain (Fig. 3). The extracellular domain consists of α-helical coiled coils, the collagen-like domain, and the C-terminal region enriched by cysteine residues [30]. The second isoform SR-A1.1 is shorter but remains the capacity to recognize ligands due to the presence of the collagen-like domain. On the C-terminus, the third isoform SR-A1.2 contains only four cysteine residues and therefore dysfunctional for lipid transport because lacks the capability to bind any extracellular ligand [31]. This isoform serves as an inhibitor of the first two isoforms thereby downregulating lipid uptake by macrophages [32, 33]. In experimental atherosclerotic rodent models, such as Apolipoprotein E (ApoE)- and LDL receptor (LDLR)-deficient mice, knockout of SR-A1 results in anti-atherogenic effects that primarily inhibit formation of foam cells [34, 35].
Expression of SR-A1 is regulated by the nuclear transcription factor (NF)-κB, which can be stimulated by pro-inflammatory cytokines [36]. SR-A1 expression can be upregulated through stimulation of voltage-dependent K+ channel Kv1.3, which in turn leads to a higher uptake of oxLDL [37]. Furthermore, inhibition of Kv1.3 by a specific antibody leads to the downregulation of SR-A, LOX-1, and ACAT1, and increased expression of ABCA1 in cultured THP-1 macrophages and human primary macrophage cultures [38], indicative of a possible contribution of potassium influx.
Anti-atherosclerotic and antioxidant messengers, such as polyphenols and curcumin, downregulate SR-A expression. In macrophages of mice deficient for ApoE, curcumin induces ubiquitination and degradation of SR-A1 mediated by calpain, an intracellular protease [39]. Plant polyphenols suppress SR-A1 expression by inhibiting peroxisome proliferator-activated receptor γ (PPARγ), a transcriptional regulator that controls lipid uptake, fatty acid storage, and glucose metabolism [39]. Hydrogen sulfide (H2S) was shown to downregulate SR-A1 levels in macrophages, but its production is reduced in the vessels of ApoE-deficent mice [40]. In blood vessels, H2S is synthesized by cystathionine γ-lyase from an aminoacid L-cysteine, accompanied by production of pyruvate and ammonium (NH3). It has been demonstrated that H2S decreased atherosclerosis plaque size and suppressed aortic expression of intracellular adhesion molecule-1 (ICAM-1) on the endothelial surface [40]. H2S was also shown to downregulate foam cell formation by reducing SR-A1 activity through the KATP/Erk ½-dependent signaling mechanism [41].
CD36 belongs to the family B of scavenger receptors. This glycoprotein contains an extracellular domain flanked by two transmembrane domains (Fig. 3) [42, 43]. CD36 has a high affinity to oxLDL mediating its atherogenic role by internalization of the CD36-oxLDL assembly [44]. Higher blood concentrations of soluble CD36 (sCD36) were observed in monocytes of subjects affected by cardiovascular diseases [45, 46] and those who exhibit cardiometabolic risk factors [47,48,49]. Treatment with statins or suppression of CD36 with low molecular inhibitors leads to reduced uptake of lipids by monocytes/ macrophages and decreased accumulation of oxLDL in the arterial wall [50,51,52,53].
Multiple factors are able to regulate expression of CD36 in macrophages. Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) drives circumin-dependent CD36 expression [54]. In monocytes, palmitate activates expression of CD36 by newly induction of ceramide production [55], since ceramides inhibit CD36 expression and reduce oxLDL accumulation in monocytes. Astaxanthin, a plant antioxidant, was found to inhibit formation of oxLDL and to increase high-density lipoprotein (HDL)-cholesterol levels in clinical studies, thereby demonstrating atheroprotective effects [56]. Lipopolysaccharide (LPS), a toxic agent of Porphyromonas gingivalis, a main cause of gingivitis, which is associated with atherosclerosis [57], increases CD36 in macrophages through the upregulation of the c-Jun/activator protein-1 (AP-1)-mediated transcription mechanism [58].
Some compounds, like plant antioxidants, including squalene, quercitrin, and kaempferol, inhibit expression of CD36 in macrophages, thus preventing excessive lipid deposits in these cells [59,60,61]. All those are dietary components that can be considered for nutritional modulation of atherosclerotic disease.
Structurally, LOX1 receptor consists of a short N-terminal domain, transmembrane domain, coiled-coil domain, and C-type lectin-like domain (Fig. 3) [62]. In the C-type lectin-like domain of LOX1, the presence of ten C-terminal basic amino acids is essential for binding oxLDL [63].
The LOX-1 receptor seems to be strictly pro-atherogenic since its expression is very moderate in normal conditions, but becomes markedly upregulated in atherosclerosis accounting for up to 40% of oxLDL uptake by pro-inflammatory macrophages [64]. Furthermore, this receptor is not expressed in monocytes, but can be upregulated in differentiated macrophages, a fact that indirectly suggests for its pro-atherosclerotic role [65]. LOX-1 is a main receptor for binding oxLDL in ECs [66] and may also be induced in VSMCs, which indicates the possibility for conversion of VSMCs to foam cells in atherogenesis [9]. This receptor can sense moderately modified and not fully oxidized LDL, indicative of a potential contribution of LOX-1 to early atherogenic steps [67].
Inflammatory modulators, such as pro-inflammatory cytokines [9], oxLDL [10], LPS [68], AGEs [58], mitochondrial ROS [69], and others may serve as potent inductors of upregulation of LOX-1 expression in macrophages. In addition, vasopressors such as endothelin-1 and angiotensin II could also activate macrophage LOX-1 expression [12].
A pro-atherogenic role of LOX-1 is supported by the data obtained in atherosclerotic animal models. Genetic deletion or knockdown of LOX-1 in rodent atherosclerotic models led to diminished disease, less plaque progression, and decreased inflammation [67]. By contrast, hyperexpression of LOX-1 in hypercholestemic mice and rabbits caused enhanced disease, increased apoptosis of vascular cells, plaque instability, and atherothrombosis [68,69,70,71].
Finally, macrophage receptor with collagenous structure (MARCO) can also be involved in lipid uptake. Like SR-A, MARCO has internal collagen-like domains. It is expressed in macrophages and ECs and is able to interact with oxLDL [72]. It was demonstrated that MARCO is involved in lipid uptake by cultured macrophages induced by treatment with Dalcetrapib, a chemical that targets cholesteryl ester transfer protein [73]. These observations indicate a likely involvement of MARCO in handling influx of lipids by macrophages. However, further studies are needed to evaluate whether MARCO could significantly contribute to the generation of foam cells during atherogenesis.
Cholesterol-handling machinery in macrophages
The formation of cholesteryl esters is crucially involved in transformation of macrophages to foam cells (Fig. 2). Free cholesterol is a substrate for acetyl-CoA acetyltransferase (ACAT1), an enzyme that transforms cholesterol to cholesteryl esters. The newly formed cholesteryl esters reside in the endoplasmic reticulum, and their excessive intracellular accumulation drives foam cell formation. Another enzyme, neutral cholesterol ester hydrolase (NCEH) hydrolyzes cholesteryl esters liberating free cholesterol [74], which is transported outside through the system of membrane cholesterol transporters. The balance between etherification/detherification of cholesterol may therefore define whether macrophages will be converted to foam cells or not.
In ApoE-deficient mice, ACAT1 inhibition by a small inhibitory molecule F-1394 was shown to result a less advanced atherogenesis [75]. However, depletion of ACAT1 specifically in macrophages of LDL receptor-deficient mice has the pro-atherogenic role [76]. F-1394 is a non-specific inhibitor of both of the isoforms ACAT (ACAT1 and ACAT2), the second of which in predominantly expressed by parenchymal liver and intestinal cells [77]. In macrophages, excessive cholesterol uptake can cause formation of highly cytotoxic, pro-inflammatory, and pro-atherosclerotic cholesterol crystals [78].
Ghrelin, a hormone secreted by specialized intestinal cells, suppresses ACAT1 through interaction with growth hormone secretagogue receptor (GHSR) and suppressing PPARγ [79]. Protein kinase A (PKA) mediates ACAT1 suppression by incretin hormones, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [80]. Dipeptidylpeptidase 4 (DPP4) is involved in proteolysis of GLP-1 [81]. In diabetic and non-ApoE-deficient mice, vildagliptin and other DPP4 inhibitors possess anti-atherogenic properties by restoring production of both incretin hormones and repairing insulin secretion [82].
In macrophages, insulin upregulates production of ACAT1 through stimulation of CCAAT/enhancer-binding protein α (C/EBPα), a transcriptional stimulator, mediated by the extracellular signal-regulated kinase (Erk)/p38MAP kinase/Jnk mechanism [83]. Leptin, a fat tissue hormone, stimulates ACAT1 expression via Janus-activated kinase 2 (Jak2)/phosphatidylinositide 3-kinase (PI3K)-mediated signaling pathway [84].
As mentioned above, NCEH is a hormone-dependent lipase that is responsible for removal of ester group from cholesteryl and formation of free cholesterol, which is then effluxed from the cell. This enzyme exists as two isoforms, the shortest of which was found in several cell types including macrophages [85]. The longest is present in the testis and other tissues involved in the steroidal biosynthesis where NCEH activity is necessary for generation of free cholesterol followed steroid hormone synthesis [86, 87].
Suppression of NCEH causes advanced atherosclerosis [88]. Overproduction of NCEH increases degradation of cholesterol esters in lipid-overloaded macrophages [89]. However, overproduction of NCEH alone without concomitant downregulation of ACAT1 and activation of reverse cholesterol cannot protect macrophages from transformation to foam cells [88]. Mice overproducing both NCEH and ApoA4 (lipoprotein acceptor of cholesterol) develop diminished disease [90]. In LDL receptor-deficient mice, overproduction of NCEH led to reduced lesion necrotic core, thereby indicating a key role of macrophage-specific expression NCEH in manipulations with cholesterol in atherosclerotic plaque [91].
Another isoform of NCEH, NCEH1, is involved in cholesterol ester catabolism on the membrane of endoplasmic reticulum in macrophages. In ApoE-deficient mice, NCEH1 accelerates disease progression, indicative of an anti-atherosclerotic role of the enzyme [92]. Both NCEH isoforms prevent transformation of macrophages to foam cells [93, 94].
Cholesterol reverse transport is an essential stage in macrophage-mediated plasma lipoprotein metabolism. Cholesterol efflux could be performed by an intensive work of cholesterol transporters, such as ABCA1, ABCG2, and scavenger receptor SRB1 and by passive membrane diffusion (Fig. 2). Mice with deletion of ABCA1 and SR-BI had severe hypocholesterolemia mainly due to HDL atherosclerosis was absent due to the lack of the pro-atherosclerotic lipids [95]. ApoA-1, a main HDL-associated protein, accepts free cholesterol secreted by ABCA1 [96]. However, in LDL receptor-deficient mice, liver overproduction of ABCA1 caused lipid deposits and enhanced disease because of accelerated transport of HDL cholesterol and slowed degradation of LDL rich of cholesterol [95].
In macrophages, ABCA1 seems to play a central role in cholesterol efflux and therefore is regulated by various bioactive molecules. A transcription factor liver X receptor α (LXRα) primes ABCA1 expression [97]. Quercetin upregulates ABCA1 expression through activation of the PPARγ/LXRα axis [98]. Proteasome inhibitors and ApoA-1 increase cholesterol transport from foam cells by suppressing ABCA1 degradation and increasing stability [99, 100].
A number of negative regulators of ABCA1 have been described. Unsaturated free fatty acids (UFA) induce epigenetic silencing of LXR genes, ABCA1 downregulation through PKCδ-dependent phosphorylation, which in turn leads to the degradation of the transporter [101, 102]. IL-12 and IL-18 downregulate expression of ABCA1 via activation of ZNF202, a zinc finger protein and transcriptional repressor [103]. On the other hand, C-X-C motif chemokine 5 (CXCL5) positively regulates ABCA1 production and therefore limits foam cell formation [104]. Among other activators of ABCA1 expression, cAMP, sterols, PPARγ agonists, and other stimulators can be mentioned [105]. In LDL receptor-deficient mice, deletion of ABCG1 had an anti-atherogenic effect [106] or leads to moderate increase in atherosclerotic lesion size [107]. The observed discrepancy in the results may be explained by a secondary role of ABCG1 in cholesterol efflux in macrophages.
Various dietary components are involved in the regulation of ABCG1. Cineole (a eucalyptus monoterpenoid) and extra-virgin olive oil increase ABCG1 expression [108, 109]. Gut microbiota transforms cyanidin-3-O-β-glucoside (Cy-3-G), a berry anthocyanin, to protocatechuic acid (PCA). PCA can in turn stimulate ABCA1/ABCG1 production by downregulating miR-10b, which targets both cholesterol transporters [110].
SR-BI transports cholesterol to HDL. In ApoE-deficient mice, SR-BI overproduction had atheroprotective effect while depletion of SR-BI in macrophages resulted in significant plaque growth thereby indicating the anti-atherogenic role of this scavenger receptor [111]. The described properties of SR-BI could be explained by its capacity to transfer cholesterol in both directions [112]. In initial atherogenic steps, SR-BI acts like SR-A1 supporting lipid and cholesterol uptake in macrophages. In parallel, SR-BI suppresses activity of ABCA1 thereby suggesting competing role of both transporters in regulating cholesterol transfer in macrophages [113].
Multiple dietary substances were shown to influence SR-BI expression. Caffeic and ferulic acids, two main phenolic acids found in coffee, enhance cholesterol efflux in macrophages by activating ABCG1 and SR-BI, i.e., transporters that transfer cholesterol to HDL but not to ApoA-1 [114]. Resveratrol (a polyphenolic compound) and 13-hydroxy linoleic acid increase LXRα and SR-BI by stimulating PPARγ [115, 116] also stimulate the reverse cholesterol transport from macrophages.
In contrast, pappalysin-1 (PAPPA), a metalloproteinase, which hydrolyzes insulin-like growth factor-binding proteins (IGFBPs) could downregulate all three cholesterol pumps via suppression of the IGF/PI3-K/Akt-dependent stimulation of LXRα [117].
Role of miRNAs in foam cell formation
A pivotal role of aberrant expression of microRNAs (miRNAs), a class of small non-coding regulatory RNAs, in various aspects of atherogenesis including formation of foam cells is established [118, 119]. MiRNAs are involved in post-transcriptional silencing of mRNA targets (through RNA sequestration, cleavage, and decay) and inhibition of translation of mRNA targets. In macrophages, the molecular machinery responsible for cholesterol intake, storage, and efflux is targeted by multiple miRNAs since a proper regulation of this machinery is vital to maintain blood lipid homeostasis. Deregulation of this mechanism may lead to various cardiometabolic abnormalities including atherosclerosis. As formation of foam cells is pro-atherosclerotic, miRNAs whose activity leads to the generation of foam cells could be considered as pro-atherogenic. By contrast, miRNAs, which inhibit foam cell formation, are atheroprotective.
In macrophages, expression of ABCA1 and ACAT1 is regulated by multiple miRNAs, an indicator of a key role of these proteins in cholesterol and phospholipid homeostasis (Table 1). ABCA1 is a major pump involved in the reverse cholesterol transport, and inhibition of this transporter promotes foam cell formation. ACAT1 catalyzes cholesterol esterification, and downregulation of this enzyme attenuates generation of foam cells.
In addition to the direct targeting of lipid-handling machinery components, miRNAs can indirectly influence on this mechanism through the control of pathways or expression of genes involved in the regulation of cholesterol homeostasis. For example, miR-21 [122], miR-133a [126], and miR-223 [132] downregulate LPS-induced lipid accumulation and inflammation by targeting toll-like receptor 4 (TLR4)/nuclear factor (NF)-kB signaling. In human macrophages, miR-216a, which directly targets the 3′ untranslated region of cystathionine γ-lyase (CSE) mRNA, negatively influences ABCA1 expression by suppressing the CSE/H2S system [131]. miR-134 was shown to promote cholesterol deposition by suppressing angiopoietin-like 4 (Angptl4), a secreted irreversible inhibitor of lipoprotein lipase (LPL) activity [127]. Overactivation of LPL that is involved in the transformation of very light density lipoprotein (VLDL) to LDL may contribute to atherogenesis [139].
In oxLDL-treated THP-1 macrophages, miR-150-dependent inhibition of adiponectin receptor 2 (AdipoR) was observed to lead to the activation of genes responsible for cholesterol efflux and hence to the suppression of foam cell formation [129]. Increased levels of miR-155 were shown to promote conversion of macrophages to foam cells by targeting HMG-box transcription factor 1 (HBP1) [130]. In human acute monocytic leukemia macrophage-derived foam cells, Hu et al. (2015) found that the long non-coding RNA RP5-833A20.1 and miR-382-5p cooperate in the downregulation of nuclear factor 1 A-type (NFIA), a regulatory protein whose overexpression prevents intracellular lipid deposition and has anti-inflammatory and anti-atherogenic effects [135]. Finally, miR-486 controls ABCA1 expression epigenetically by targeting histone acetyltransferase 1, an epigenetic regulator that promotes ABCA1 production by acetylation of the lysines 5 and 12 of histone H4 at the promoter of the ABCA1 gene [137].
In summary, miRNAs play a significant role in the regulation of cholesterol homeostasis by promoting or inhibiting intracellular lipid deposition and formation of foam cells [140]. Thus, miRNAs may represent a promising therapeutic target to improve reverse cholesterol transport and prevent generation of foam cells in atherosclerosis.
Conclusions
Atherosclerosis is associated with profound disturbances of cholesterol metabolism. In particular, cellular cholesterol uptake is increased in atherosclerosis, while cholesterol efflux is downregulated. Increased cholesterol uptake can be explained by upregulation of oxLDL-bearing scavenger receptors expression, especially LOX-1. Moreover, the expression of cholesterol pumps that are involved in cholesterol efflux is inhibited. This may result in cholesterol deposition in macrophages and formation of foam cells. Another main imbalance observed in atherosclerosis is upregulation of ACAT1 (i.e., cholesterol esterification) and downregulation of NCEH (i.e., formation of free cholesterol), which results in the accumulation of cholesterol esters within the cell and further transformation of macrophages to foam cells.
Until recently, monocyte-derived macrophages were considered as a major source of plaque foam cells. However, Dubland and Francis (2016) found that VSMCs could substantially (up to 50% in humans and at least 1/3 in mice) contribute to a population foam cells [141]. Intracellular cholesterol accumulation leads to inhibition of SMC gene expression and induction of pro-inflammatory and macrophage markers. Foam cells originated from VSMCs have a selective loss of ABCA1. This interesting topic must be further explored to improve the understanding of new roles of VSMCs in atherosclerosis.
A standard therapy that is widely used to treat cardiovascular diseases is reducing plasma LDL cholesterol levels with lipid-lowering agents. However, over 50% of treated patients did not achieve the beneficial effects of this therapy. To prevent intracellular lipid accumulation by enhancing cholesterol efflux and targeting lipid-metabolizing enzyme is a promising approach that can significantly improve the efficiency of anti-atherosclerotic therapy. One of these strategies involves HDL-targeted therapy by optimization HDL cholesterol levels and function in the blood to promote the removal of circulating cholesterol and to prevent or mitigate atherosclerotic inflammation [142]. HDL-targeted therapy assumes implication of HDL-mimetics such as reconstituted HDL, apolipoprotein (Apo) A-IMilano, ApoA-I mimetic peptides, or full-length ApoA-I, which provide an option to enhance cholesterol efflux through the ABCA1 transporter and to act as an anti-atherosclerotic agent by enhancing the biological functions of HDL without elevating HDL cholesterol levels. HDL-mimetics were highly effective in animal models [143]. CER-001, a recombinant human ApoA-1-based HDL mimetic, developed by company Cerenis Therapeutic Holding SA (Labège, France) is evaluated in three clinical trials (NCT01515241, NCT01201837, and NCT01412034) to treat homozygous familial hypecholesteroemia, hypo-alphalipoproteinaemia, and acute coronary syndrome.
Earlier on, CER-001 showed profound anti-atherogenic effects in LDLR-deficient [144] and apoE-deficient [145] mice by promoting cholesterol efflux and inducing atherosclerosis regression. In hypercholesteroemic patients, this preparation led to the significant increase of reverse cholesterol transport and decrease in carotid mean vessel wall area and carotid artery wall thickness [146,147,148]. In atherosclerotic patients, implementation of CER-001 was well tolerated, targeted plaque regions, and caused enhancement of cholesterol efflux and increase of serum apoA-I levels [149,150,151]. Thus, preclinical and clinical testing of CER-001 showed beneficial effects of HDL-therapy on the carotid wall thickness, prevention of coronary plaque burden, and plaque size and morphology. Indeed, targeting of cholesterol efflux with apoA-I mimetics may serve as a good example of efficient anti-atherosclerotic therapy.
References
Chistiakov DA, Orekhov AN, Bobryshev YV (2015) Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 6:365
Lao KH, Zeng L, Xu Q (2015) Endothelial and smooth muscle cell transformation in atherosclerosis. Curr Opin Lipidol 26:449–456
Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC et al (2014) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21:628–637
Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM et al (2015) Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 35:535–546
Sun HJ, Zhao MX, Liu TY, Ren XS, Chen Q, Li YH, Kang YM, Zhu GQ (2016) Salusin-β induces foam cell formation and monocyte adhesion in human vascular smooth muscle cells via miR155/NOX2/NFκB pathway. Sci Rep 6:23596
Hutchins PM, Heinecke JW (2015) Cholesterol efflux capacity, macrophage reverse cholesterol transport and cardioprotective HDL. Curr Opin Lipidol 26:388–393
Chistiakov DA, Bobryshev YV, Orekhov AN (2016) Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med 20:17–28
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK (2013) Foam cells in atherosclerosis. Clin Chim Acta 424:245–252
Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, Masaki T, Kita T (2000) Inducible expression of LOX-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci 902:323–327
Pirillo A, Norata GD, Catapano AL (2013) LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm 2013:152786
Rudijanto A (2007) The expression and down stream effect of lectin like-oxidized low density lipoprotein 1 (LOX-1) in hyperglycemic state. Acta Med Indones 39:36–43
Mitra S, Goyal T, Mehta JL (2011) Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther 25:419–429
Favari E, Chroni A, Tietge UJ, Zanotti I, Escolà-Gil JC, Bernini F (2015) Cholesterol efflux and reverse cholesterol transport. Handb Exp Pharmacol 224:181–206
Nielsen LB (1996) Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis 123:1–15
Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA (2012) Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc Cell 4:12
Li YB, Zhang QH, Chen Z, He ZJ, Yi GH (2015) Oxidized low-density lipoprotein attenuated desmoglein 1 and desmocollin 2 expression via LOX-1/Ca(2+)/PKC-β signal in human umbilical vein endothelial cells. Biochem Biophys Res Commun 468:380–386
Wheeler GN, Parker AE, Thomas CL, Ataliotis P, Poynter D, Arnemann J, Rutman AJ, Pidsley SC, Watt FM, Rees DA et al (1991) Desmosomal glycoprotein DGI, a component of intercellular desmosome junctions, is related to the cadherin family of cell adhesion molecules. Proc Natl Acad Sci U S A 88:4796–4800
Syed SE, Trinnaman B, Martin S, Major S, Hutchinson J, Magee AI (2002) Molecular interactions between desmosomal cadherins. Biochem J 362:317–327
van Nieuw Amerongen GP, Vermeer MA, Nègre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VW (2000) Simvastatin improves disturbed endothelial barrier function. Circulation 102:2803–2809
Kása A, Csortos C, Verin AD (2015) Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers 3:e974448. doi:10.4161/21688370.2014.974448
Ridker PM (2009) Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 7(Suppl 1):332–339
Chistiakov DA, Bobryshev YV, Nikiforov NG, Elizova NV, Sobenin IA, Orekhov AN (2015) Macrophage phenotypic plasticity in atherosclerosis: the associated features and the peculiarities of the expression of inflammatory genes. Int J Cardiol 184:436–445
Lacavé-Lapalun JV, Benderitter M, Linard C (2013) Flagellin or lipopolysaccharide treatment modified macrophage populations after colorectal radiation of rats. J Pharmacol Exp Ther 346:75–85
Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN (2016) Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. Biomed Res Int 2016:9582430
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Namgaladze D, Brüne B (2014) Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta 1841:1329–1335
Ménégaut L, Thomas C, Lagrost L, Masson D (2017) Fatty acid metabolism in macrophages: a target in cardio-metabolic diseases. Curr Opin Lipidol 28:19–26
da Silva RF, Lappalainen J, Lee-Rueckert M, Kovanen PT (2016) Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 248:170–178
De Paoli F, Staels B, Chinetti-Gbaguidi G (2014) Macrophage phenotypes and their modulation in atherosclerosis. Circ J 78:1775–1781
Ben J, Zhu X, Zhang H, Chen Q (2015) Class A1 scavenger receptors in cardiovascular diseases. Br J Pharmacol 172:5523–5530
Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S (2005) Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 182:1–15
Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayakawa I, Kanamori H, Aburatani H, Takaku F, Suzuki H et al (1990) Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A 87:9133–9137
Dai Y, Condorelli G, Mehta JL (2016) Scavenger receptors and non-coding RNAs: relevance in atherogenesis. Cardiovasc Res 109:24–33
Mäkinen PI, Lappalainen JP, Heinonen SE, Leppänen P, Lähteenvuo MT, Aarnio JV, Heikkilä J, Turunen MP, Ylä-Herttuala S (2010) Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res 88:530–538
Dai XY, Cai Y, Mao DD, Qi YF, Tang C, Xu Q, Zhu Y, Xu MJ, Wang X (2012) Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E-deficient mice. J Mol Cell Cardiol 53:509–520
Hashizume M, Mihara M (2012) Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. Eur J Pharmacol 689:249–254
Yang XF, Yang Y, Lian YT, Wang ZH, Li XW, Cheng LX, Liu JP, Wang YF, Gao X, Liao YH et al (2012) The antibody targeting the E314 peptide of human Kv1.3 pore region serves as a novel, potent and specific channel blocker. PLoS One 7:e36379. doi:10.1371/journal.pone.0036379
Yang Y, Wang YF, Yang XF, Wang ZH, Lian YT, Yang Y, Li XW, Gao X, Chen J, Shu YW et al (2013) Specific Kv1.3 blockade modulates key cholesterol-metabolism-associated molecules in human macrophages exposed to ox-LDL. J Lipid Res 54:34–43
Zhao JF, Ching LC, Huang YC, Chen CY, Chiang AN, Kou YR, Shyue SK, Lee TS (2012) Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol Nutr Food Res 56:691–701
Wang XH, Wang F, You SJ, Cao YJ, Cao LD, Han Q, Liu CF, Hu LF (2013) Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell Signal 25:2255–2262
Zhao ZZ, Wang Z, Li GH, Wang R, Tan JM, Cao X, Suo R, Jiang ZS (2011) Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 236:169–176
Van Berkel TJ, Van Eck M, Herijgers N, Fluiter K, Nion S (2000) Scavenger receptor classes a and B. Their roles in atherogenesis and the metabolism of modified LDL and HDL. Ann N Y Acad Sci 902:113–126; discussion 126-127
Pepino MY, Kuda O, Samovski D, Abumrad NA (2014) Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr 34:281–303
Tarhda Z, Semlali O, Kettani A, Moussa A, Abumrad NA, Ibrahimi A (2013) Three dimensional structure prediction of fatty acid binding site on human transmembrane receptor CD36. Bioinform Biol Insights 7:369–373
Teupser D, Mueller MA, Koglin J, Wilfert W, Ernst J, von Scheidt W, Steinbeck G, Seidel D, Thiery J (2008) CD36 mRNA expression is increased in CD14+ monocytes of patients with coronary heart disease. Clin Exp Pharmacol Physiol 35:552–556
Piechota M, Banaszewska A, Dudziak J, Slomczynski M, Plewa R (2012) Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein J 31:511–518
Fernández-Real JM, Handberg A, Ortega F, Højlund K, Vendrell J, Ricart W (2009) Circulating soluble CD36 is a novel marker of liver injury in subjects with altered glucose tolerance. J Nutr Biochem 20:477–484
Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW (2010) Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab 95:1939–1946
Handberg A, Højlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J, Piatti P, Beck-Nielsen H, Investigators RISC (2012) Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med 271:294–304
Hrboticky N, Draude G, Hapfelmeier G, Lorenz R, Weber PC (1999) Lovastatin decreases the receptor-mediated degradation of acetylated and oxidized LDLs in human blood monocytes during the early stage of differentiation into macrophages. Arterioscler Thromb Vasc Biol 19:1267–1275
Fuhrman B, Koren L, Volkova N, Keidar S, Hayek T, Aviram M (2002) Atorvastatin therapy in hypercholesterolemic patients suppresses cellular uptake of oxidized-LDL by differentiating monocytes. Atherosclerosis 164:179–185
Geloen A, Helin L, Geeraert B, Malaud E, Holvoet P, Marguerie G (2012) CD36 inhibitors reduce postprandial hypertriglyceridemia and protect against diabetic dyslipidemia and atherosclerosis. PLoS One 7:e37633. doi:10.1371/journal.pone.0037633
Mansor LS, Sousa Fialho MDL, Yea G, Coumans WA, West JA, Kerr M, Carr CA, Luiken JJFP, Glatz JFC, Evans RD et al (2017) Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc Res doi. doi:10.1093/cvr/cvx045 [Epub ahead of print]
Mimche PN, Brady LM, Keeton S, Fenne DS, King TP, Quicke KM, Hudson LE, Lamb TJ (2015) Curcumin enhances non-opsonic phagocytosis of Plasmodium falciparum through up-regulation of CD36 surface expression on monocytes/macrophages. PLoS One 10:e0138835. doi:10.1371/journal.pone.0138835
Gao D, Pararasa C, Dunston CR, Bailey CJ, Griffiths HR (2012) Palmitate promotes monocyte atherogenicity via de novo ceramidesynthesis. Free Radic Biol Med 53:796–806
Kishimoto Y, Yoshida H, Kondo K (2016) Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Mar Drugs 14. pii: E35. doi: 10.3390/md14020035
Chistiakov DA, Orekhov AN, Bobryshev YV (2016) Links between atherosclerotic and periodontal disease. Exp Mol Pathol 100:220–235
Li L, Sawamura T, Renier G (2004) Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res 94:892–901
Choi JS, Bae JY, Kim DS, Li J, Kim JL, Lee YJ, Kang YH (2010) Dietary compound quercitrin dampens VEGF induction and PPARgamma activation in oxidized LDL-exposed murine macrophages: association with scavenger receptor CD36. J Agric Food Chem 58:1333–1341
Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J, Zhou XS, Xu SW, Liu WH, Liu PQ, Huang HQ (2011) Tanshinone IIA attenuates atherosclerosis in ApoE(-/-) mice through down-regulation of scavenger receptor expression. Eur J Pharmacol 650:275–284
Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Ochoa-Herrera J, Perez-Lopez P, Pulido-Moran M, Ramirez-Tortosa MC, Granados-Principal S, Quiles JL, Ramirez-Tortosa CL et al (2012) Squalene ameliorates atherosclerotic lesions through the reduction of CD36 scavenger receptor expression in macrophages. Mol Nutr Food Res 56:733–740
Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Yoshimoto R, Sawamura T (2009) Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J 73:1993–1999
Chen M, Inoue K, Narumiya S, Masaki T, Sawamura T (2001) Requirements of basic amino acid residues within the lectin-like domain of LOX-1 for the binding of oxidized low-density lipoprotein. FEBS Lett 499:215–219
Schaeffer DF, Riazy M, Parhar KS, Chen JH, Duronio V, Sawamura T, Steinbrecher UP (2009) LOX-1 augments oxLDL uptake by lysoPC-stimulated murine macrophages but is not required for oxLDL clearance from plasma. J Lipid Res 50:1676–1684
Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O (1998) Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J 334:9–13
Kume N, Kita T (2001) Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in atherogenesis. Trends Cardiovasc Med 11:22–25
Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T (1999) Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 99:3110–3117
Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T (2005) Overexpression of lectin-like oxidized low-density lipoprotein receptor-1induces in tramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res 97:176–184
Ding Z, Liu S, Wang X, Deng X, Fan Y, Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T et al (2015) Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res 107:556–567
Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, Minami M, Kita T, Shiomi M, Saji H (2007) Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability - analysis in hypercholesterolemic rabbits. Atherosclerosis 195:48–56
Kuge Y, Kume N, Ishino S, Takai N, Ogawa Y, Mukai T, Minami M, Shiomi M, Saji H (2008) Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull 31:1475–1482
Ohtani K, Suzuki Y, Eda S, Kawai T, Kase T, Keshi H, Sakai Y, Fukuoh A, Sakamoto T, Itabe H et al (2001) The membrane-type collectin CL-P1 is a scavenger receptor on vascular endothelial cells. J Biol Chem 276:44222–44228
Perez A, Wright MB, Maugeais C, Braendli-Baiocco A, Okamoto H, Takahashi A, Singer T, Mueller L, Niesor EJ (2010) MARCO, a macrophage scavenger receptor highly expressed in rodents, mediates dalcetrapib-induced uptake of lipids by rat and mouse macrophages. Toxicol in Vitro 24:745–750
Ghosh S (2012) Early steps in reverse cholesterol transport: cholesteryl ester hydrolase and other hydrolases. Curr Opin Endocrinol Diabetes Obes 19:136–141
Kusunoki J, Hansoty DK, Aragane K, Fallon JT, Badimon JJ, Fisher EA (2001) Acyl-CoA: cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 103:2604–2609
Fazio S, Major AS, Swift LL, Gleaves LA, Accad M, Linton MF, Farese RV Jr (2001) Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J Clin Invest 107:163–171
Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R et al (2000) Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 275:28083–28092
Corr EM, Cunningham CC, Dunne A (2016) Cholesterol crystals activate Syk and PI3 kinase in human macrophages and dendritic cells. Atherosclerosis 251:197–205
Cheng B, Wan J, Wang Y, Mei C, Liu W, Ke L, He P (2010) Ghrelin inhibits foam cell formation via simultaneously down-regulating the expression of acyl-coenzyme A:cholesterol acyltransferase 1 and up-regulating adenosine triphosphate-binding cassette transporter A1. Cardiovasc Pathol 19:e159–e166
Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, Miyazaki A, Hirano T (2011) Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54:2649–2659
Darsalia V, Larsson M, Nathanson D, Klein T, Nyström T, Patrone C (2015) Glucagon-like receptor 1 agonists and DPP-4 inhibitors: potential therapies for the treatment of stroke. J Cereb Blood Flow Metab 35:718–723
Terasaki M, Nagashima M, Nohtomi K, Kohashi K, Tomoyasu M, Sinmura K, Nogi Y, Katayama Y, Sato K, Itoh F et al (2013) Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin’s actions in nondiabetic and diabeticapolipoprotein E-null mice. PLoS One 8:e70933. doi:10.1371/journal.pone.0070933
Ce J, Zhai W, Cheng B, He P, Qi B, Lu H, Zeng Y, Chen X (2013) Insulin induces human acyl-coenzyme A: cholesterol acyltransferase1 gene expression via MAP kinases and CCAAT/enhancer-binding protein α. J Cell Biochem 114:2188–2198
Hongo S, Watanabe T, Arita S, Kanome T, Kageyama H, Shioda S, Miyazaki A (2009) Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am J Physiol Endocrinol Metab 297:E474–E482
Okazaki H, Igarashi M, Nishi M, Sekiya M, Tajima M, Takase S, Takanashi M, Ohta K, Tamura Y, Okazaki S et al (2008) Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J Biol Chem 283:33357–33364
Johnson WJ, Jang SY, Bernard DW (2000) Hormone sensitive lipase mRNA in both monocyte and macrophage forms of the human THP-1 cell line. Comp Biochem Physiol B Biochem Mol Biol 126:543–552
Yeaman SJ (2004) Hormone-sensitive lipase—new roles for an old enzyme. Biochem J 379:11–22
Igarashi M, Osuga J, Isshiki M, Sekiya M, Okazaki H, Takase S, Takanashi M, Ohta K, Kumagai M, Nishi M et al (2010) Targeting of neutral cholesterol ester hydrolase to the endoplasmic reticulum via its N-terminal sequence. J Lipid Res 51:274–285
Igarashi M, Osuga J, Uozaki H, Sekiya M, Nagashima S, Takahashi M, Takase S, Takanashi M, Li Y, Ohta K et al (2010) The critical role of neutral cholesterol ester hydrolase 1 in cholesterol removal from human macrophages. Circ Res 107:1387–1395
Choy HA, Wang XP, Schotz MC (2003) Reduced atherosclerosis in hormone-sensitive lipase transgenic mice overexpressing cholesterol acceptors. Biochim Biophys Acta 1634:76–85
Zhao B, Song J, Chow WN, St Clair RW, Rudel LL, Ghosh S (2007) Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J Clin Invest 117:2983–2992
Sekiya M, Osuga J, Nagashima S, Ohshiro T, Igarashi M, Okazaki H, Takahashi M, Tazoe F, Wada T, Ohta K et al (2009) Ablation of neutral cholesterol ester hydrolase 1 accelerates atherosclerosis. Cell Metab 10:219–228
Sekiya M, Osuga J, Igarashi M, Okazaki H, Ishibashi S (2011) The role of neutral cholesterol ester hydrolysis in macrophage foam cells. J Atheroscler Thromb 18:359–364
Sakai K, Igarashi M, Yamamuro D, Ohshiro T, Nagashima S, Takahashi M, Enkhtuvshin B, Sekiya M, Okazaki H, Osuga J et al (2014) Critical role of neutral cholesteryl ester hydrolase 1 in cholesteryl ester hydrolysis in murine macrophages. J Lipid Res 55:2033–2040
Joyce CW, Wagner EM, Basso F, Amar MJ, Freeman LA, Shamburek RD, Knapper CL, Syed J, Wu J, Vaisman BL et al (2006) ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro atherogenic lipoproteins and enhanced atherosclerosis. J Biol Chem 281:33053–33065
Lorenzi I, von Eckardstein A, Cavelier C, Radosavljevic S, Rohrer L (2008) Apolipoprotein A-I but not high-density lipoproteins are internalised by RAW macrophages: roles of ATP-binding cassette transporter A1 and scavenger receptor BI. J Mol Med (Berl) 86:171–183
Bennett DJ, Cooke AJ, Edwards AS (2006) Non-steroidal LXR agonists; an emerging therapeutic strategy for the treatment of atherosclerosis. Recent Pat Cardiovasc Drug Discov 1:21–46
Lee SM, Moon J, Cho Y, Chung JH, Shin MJ (2013) Quercetin up-regulates expressions of peroxisome proliferator-activated receptor γ, liver X receptor α, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr Res 33:136–143
Tang CK, Tang GH, Yi GH, Wang Z, Liu LS, Wan S, Yuan ZH, He XS, Yang JH, Ruan CG et al (2004) Effect of apolipoprotein A-I on ATP binding cassette transporter A1 degradation and cholesterol efflux in THP-1 macrophage-derived foam cells. Acta Biochim Biophys Sin Shanghai 36:218–226
Ogura M, Ayaori M, Terao Y, Hisada T, Iizuka M, Takiguchi S, Uto-Kondo H, Yakushiji E, Nakaya K, Sasaki M et al (2011) Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler Thromb Vasc Biol 31:1980–1987
Wang Y, Oram JF (2007) Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J Lipid Res 48:1062–1068
Ku CS, Park Y, Coleman SL, Lee J (2012) Unsaturated fatty acids repress expression of ATP binding cassette transporter A1 and G1 in RAW 264.7 macrophages. J Nutr Biochem 23:1271–1276
Yu XH, Jiang HL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Ouyang XP, Lv YC, Jiang ZS, Zhang DW et al (2012) Interleukin-18 and interleukin-12 together downregulate ATP-binding cassette transporter A1 expression through the interleukin-18R/nuclear factor-κB signaling pathway in THP-1 macrophage-derived foam cells. Circ J 76:1780–1791
Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, Bader M, Ahluwalia A, Duchene J (2013) CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest 123:1343–1347
Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB Jr (2001) Regulation and intracellular trafficking of the ABCA1 transporter. J Lipid Res 42:1339–1345
Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA (2006) Impaired development of atherosclerosis in hyperlipidemic Ldlr-/- and ApoE-/- mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol 26:2301–2307
Meurs I, Lammers B, Zhao Y, Out R, Hildebrand RB, Hoekstra M, Van Berkel TJ, Van Eck M (2012) The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis 221:41–47
Helal O, Berrougui H, Loued S, Khalil A (2013) Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br J Nutr 109:1844–1855
Jun HJ, Hoang MH, Yeo SK, Jia Y, Lee SJ (2013) Induction of ABCA1 and ABCG1 expression by the liver X receptor modulator cineole in macrophages. Bioorg Med Chem Lett 23:579–583
Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W (2012) Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res 111:967–981
Zhang W, Yancey PG, Su YR, Babaev VR, Zhang Y, Fazio S, Linton MF (2003) Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 108:2258–2263
Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH (2000) High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem 275:36596–36604
Chen W, Silver DL, Smith JD, Tall AR (2000) Scavenger receptor-BI inhibits ATP-binding cassette transporter 1-mediated cholesterol efflux in macrophages. J Biol Chem 275:30794–30800
Uto-Kondo H, Ayaori M, Ogura M, Nakaya K, Ito M, Suzuki A, Takiguchi S, Yakushiji E, Terao Y, Ozasa H et al (2010) Coffee consumption enhances high-density lipoprotein-mediated cholesterol efflux in macrophages. Circ Res 106:779–787
Kämmerer I, Ringseis R, Biemann R, Wen G, Eder K (2011) 13-hydroxy linoleic acid increases expression of the cholesterol transporters ABCA1, ABCG1 and SR-BI and stimulates apoA-I-dependent cholesterol efflux in RAW264.7 macrophages. Lipids Health Dis 10:222
Voloshyna I, Hai O, Littlefield MJ, Carsons S, Reiss AB (2013) Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur J Pharmacol 698:299–309
Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS et al (2012) PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis 222:344–354
Najafi-Shoushtari SH (2011) MicroRNAs in cardiometabolic disease. Curr Atheroscler Rep 13:202–207
Araldi E, Chamorro-Jorganes A, van Solingen C, Fernandez-Hernando C, Suarez Y (2015) Therapeutic potential of modulating microRNAs in atherosclerotic vascular disease. Curr Vasc Pharmacol 13:291–304
Xu J, Hu G, Lu M, Xiong Y, Li Q, Chang CC, Song B, Chang T, Li B (2013) MiR-9 reduces human acyl-coenzyme A:cholesterol acyltransferase-1 to decrease THP-1 macrophage-derived foam cell formation. Acta Biochim Biophys Sin Shanghai 45:953–962
Lv YC, Tang YY, Peng J, Zhao GJ, Yang J, Yao F, Ouyang XP, He PP, Xie W, Tan YL et al (2014) MicroRNA-19b promotes macrophage cholesterol accumulation and aortic atherosclerosis by targeting ATP-binding cassette transporter A1. Atherosclerosis:215–226. doi:10.1016/j.atherosclerosis.2014.07.005
Feng J, Li A, Deng J, Yang Y, Dang L, Ye Y, Li Y, Zhang W (2014) miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response: potential role in cerebrovascular disease. Lipids Health Dis 13:27. doi: 10.1186/1476-511X-13-27
Zhao GJ, Mo ZC, Tang SL, Ouyang XP, He PP, Lv YC, Yao F, Tan YL, Xie W, Shi JF et al (2014) Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-nuclear factor-kappa B and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis 235:519–525
Zhang M, Wu JF, Chen WJ, Tang SL, Mo ZC, Tang YY, Li Y, Wang JL, Liu XY, Peng J et al (2014) MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 234:54–64
Zhang N, Lei J, Lei H, Ruan X, Liu Q, Chen Y, Huang W (2015) MicroRNA-101 overexpression by IL-6 and TNF-α inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression. Exp Cell Res 336:33–42
Peng XP, Huang L, Liu ZH (2016) miRNA-133a attenuates lipid accumulation via TR4-CD36 pathway in macrophages. Biochimie 127:79–85
Lan G, Xie W, Li L, Zhang M, Liu D, Tan YL, Cheng HP, Gong D, Huang C, Zheng XL et al (2016) MicroRNA-134 actives lipoprotein lipase-mediated lipid accumulation and inflammatory response by targeting angiopoietin-like 4 in THP-1 macrophages. Biochem Biophys Res Commun 472:410–417
Hu YW, Hu YR, Zhao JY, Li SF, Ma X, Wu SG, Lu JB, Qiu YR, Sha YH, Wang YC et al (2014) An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS One 9:e94997. doi:10.1371/journal.pone.0094997
Li J, Zhang S (2016) microRNA-150 inhibits the formation of macrophage foam cells through targeting adiponectin receptor 2. Biochem Biophys Res Commun 476:218–224
Tian FJ, An LN, Wang GK, Zhu JQ, Li Q, Zhang YY, Zeng A, Zou J, Zhu RF, Han XS et al (2014) Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res 103:100–110
Gong D, Cheng HP, Xie W, Zhang M, Liu D, Lan G, Huang C, Zhao ZW, Chen LY, Yao F et al (2016) Cystathionine γ-lyase(CSE)/hydrogen sulfide system is regulated by miR-216a and influences cholesterol efflux in macrophages via the PI3K/AKT/ABCA1 pathway. Biochem Biophys Res Commun 470:107–116
Wang J, Bai X, Song Q, Fan F, Hu Z, Cheng G, Zhang Y (2015) miR-223 inhibits lipid deposition and inflammation by suppressing toll-like receptor 4 signaling in macrophages. Int J Mol Sci 16:24965–24982
Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA (2015) MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol 35:323–331
Wang D, Yan X, Xia M, Yang Y, Li D, Li X, Song F, Ling W (2014) Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol 34(9):1860–1870
Hu YW, Zhao JY, Li SF, Huang JL, Qiu YR, Ma X, Wu SG, Chen ZP, Hu YR, Yang JY et al (2015) RP5-833A20.1/miR-382-5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol 35:87–101
Wang B, He PP, Zeng GF, Zhang T, Ou Yang XP (2017) miR-467b regulates the cholesterol ester formation via targeting ACAT1 gene in RAW 264.7 macrophages. Biochimie 132:38–44
Liu D, Zhang M, Xie W, Lan G, Cheng HP, Gong D, Huang C, Lv YC, Yao F, Tan YL et al (2016) MiR-486 regulates cholesterol efflux by targeting HAT1. Biochem Biophys Res Commun 472:418–424
He PP, Ouyang XP, Tang YY, Liao L, Wang ZB, Lv YC, Tian GP, Zhao GJ, Huang L, Yao F et al (2014) MicroRNA-590 attenuates lipid accumulation and pro-inflammatory cytokine secretion by targeting lipoprotein lipase gene in human THP-1 macrophages. Biochimie 106:81–90
Li Y, He PP, Zhang DW, Zheng XL, Cayabyab FS, Yin WD, Tang CK (2014) Lipoprotein lipase: from gene to atherosclerosis. Atherosclerosis 237:597–608
Rotllan N, Price N, Pati P, Goedeke L, Fernández-Hernando C (2016) microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis 246:352–360
Dubland JA, Francis GA (2016) So much cholesterol: the unrecognized importance of smooth muscle cells in atherosclerotic foam cell formation. Curr Opin Lipidol 27:155–161
Ikenaga M, Higaki Y, Saku K, Uehara Y (2016) High-density lipoprotein mimetics: a therapeutic tool for atherosclerotic diseases. J Atheroscler Thromb 23:385–394
Uehara Y, Chiesa G, Saku K (2015) High-density lipoprotein-targeted therapy and apolipoprotein A-I mimetic peptides. Circ J 79:2523–2528
Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, Cholez G, Keyserling C, Lalwani N, Paolini JF et al (2014) CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 232:110–118
Tardy C, Goffinet M, Boubekeur N, Cholez G, Ackermann R, Sy G, Keyserling C, Lalwani N, Paolini JF, Dasseux JL et al (2015) HDL and CER-001 inverse-dose dependent inhibition of atherosclerotic plaque formation in apoE-/- mice: evidence of ABCA1 down-regulation. PLoS One 10:e0137584. doi:10.1371/journal.pone.0137584
Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, Kastelein JJ, Keyserling C, Klepp H, Koenig W et al (2014) Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 35:3277–3286
Kootte RS, Smits LP, van der Valk FM, Dasseux JL, Keyserling CH, Barbaras R, Paolini JF, Santos RD, van Dijk TH, Dallinga-van Thie GM et al (2015) Effect of open-label infusion of an apoA-I-containing particle (CER-001) on RCT and artery wall thickness in patients with FHA. J Lipid Res 56:703–712
Hovingh GK, Smits LP, Stefanutti C, Soran H, Kwok S, de Graaf J, Gaudet D, Keyserling CH, Klepp H, Frick J et al (2015) The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am Heart J 169:736–742.e1. doi:
Zheng KH, van der Valk FM, Smits LP, Sandberg M, Dasseux JL, Baron R, Barbaras R, Keyserling C, Coolen BF, Nederveen AJ et al (2016) HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 251:381–388
Andrews J, Janssan A, Nguyen T, Pisaniello AD, Scherer DJ, Kastelein JJ, Merkely B, Nissen SE, Ray K, Schwartz GG et al (2017) Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: rationale and design of the CARAT study. Cardiovasc Diagn Ther 7:45–51
Keyserling CH, Barbaras R, Benghozi R, Dasseux JL (2017) Development of CER-001: preclinical dose selection through to phase I clinical findings. Clin Drug Investig 37:483–491
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Russian Science Foundation (Grant no. 14-15-00112).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chistiakov, D.A., Melnichenko, A.A., Myasoedova, V.A. et al. Mechanisms of foam cell formation in atherosclerosis. J Mol Med 95, 1153–1165 (2017). https://doi.org/10.1007/s00109-017-1575-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1575-8