Abstract
The mucopolysaccharidoses (MPS) are a subgroup of lysosomal storage disorders that are caused by mutations in the genes involved in glycosaminoglycan breakdown. Multiple organs and tissues are affected, including the central nervous system. At present, hematopoietic stem cell transplantation and enzyme replacement therapies are approved for some of the (non-neurological) MPS. Treatments that effectively ameliorate the neurological aspects of the disease are being assessed in clinical trials. This review will focus on the recent outcomes and planned viral vector-mediated gene therapy clinical trials, and the pre-clinical data that supported these studies, for MPS-I (Hurler/Scheie syndrome), MPS-II (Hunter syndrome), and MPS-IIIA and -IIIB (Sanfilippo syndrome).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lysosomal storage disorders (LSD) are a heterogenous group of >50 monogenetic diseases caused by mutations in genes encoding proteins required for the breakdown of substrates within the endosomal-lysosomal pathway. Consequently, partially metabolized substrates accumulate initiating a progressive pathogenic cascade [1]. While individual LSD are relatively rare (collective incidence of 1:4000 to 1:7700 births [2,3,4]), the prevalence of LSDs increases when late-onset, “non-classical” forms are considered [5, 6]. For example, the prevalence of the LSD Fabry disease was reported as 1:117,000 based on patient referrals and prenatal diagnoses [3], but when large cohorts of newborn dried blood spots were screened for α-galactosidase A activity, the incidence is estimated at 1:1250 to 1:3100 [5, 6].
Mucopolysaccharidoses (MPS) are a subgroup of LSD representing approximately one-third of diagnosed patients [3], with a collective prevalence of 1:22,500 [3]–1:52,000 [7]. They are caused by a deficiency of a specific exoenzyme resulting in accumulation of incompletely degraded sulfated glycosaminoglycans. The age of symptom onset and the rate of disease progression, together with the organs and tissues affected, varies widely between MPS subtypes. For example, MPS-I, -II, -III, and -VII patients with a classical phenotype experience progressive neurological decline while those with MPS-IV and -VI demonstrate predominantly skeletal abnormalities.
This review will discuss the progress and findings of viral gene therapy clinical trials for neurological MPS that are in progress, or have received regulatory approval, with specific reference to MPS-I, -II, -IIIA and -IIIB. MPS-I (Hurler-Scheie syndrome) is caused by mutations in the IDUA gene [8] resulting in a loss of functional α-l-iduronidase activity (EC 3.2.1.76) and improper cleavage of iduronic acid residues at the non-reducing terminus of heparan sulfate and dermatan sulfate glycosaminoglycans. Heparan sulfate and dermatan sulfate oligosaccharides also accumulate in MPS-II (Hunter syndrome) due to insufficient iduronate-2-sulfatase activity (EC 3.1.6.13) as a result of IDS mutations [9]. MPS-IIIA and -IIIB (as well as -IIIC to -IIIE subtypes) collectively result in Sanfilippo syndrome and are caused by non-functional copies of SGSH or NAGLU genes, respectively [10,11,12]. The lysosomal enzymes that are encoded by these genes, N-sulfoglucosamine sulfohydrolase (EC 3.10.1.1) and N-acetyl-α-glucosaminidase (EC 3.2.1.50), are involved in the catabolism of heparan sulfate glycosaminoglycans. These are hereditary diseases, with MPS-I, -IIIA and -IIIB inherited in an autosomal recessive manner, while MPS-II has an X-linked mode of inheritance. All are considered rare, with an estimated incidence of 1:88,000 (MPS-I), 1:136,000 (MPS-II), 1:114,000 (MPS-IIIA) and 1:211,000 (MPS-IIIB) live births in Australia [3]. While the clinical presentation varies within each disorder, all generally feature rapid, progressive cognitive decline, with MPS-I and -II patients also exhibiting skeletal abnormalities [13], and greatly shortened life span.
Trafficking of lysosomal enzymes
Newly synthesized lysosomal enzymes are glycosylated at selected asparagine residues in the rough endoplasmic reticulum (Fig. 1; [14]). The polypeptide signal sequence is cleaved and enzymes undergo vesicular transport to the endoplasmic reticulum and trans-Golgi where enzymes are modified [15]. Terminal mannose residues are phosphorylated and, at physiological pH, these moieties have a high affinity for the mannose-6-phosphate/insulin-like growth factor 2 (M6P/IGF2) receptors primarily localized within intracellular compartments such as the clathrin-coated pits of the trans-Golgi network [16]. The enzyme-receptor complex is then trafficked through the endosomal system and the acidic environment in the late endosome dissociates the lysosomal enzyme from the receptor, which then recycles to the trans-Golgi network or the plasma membrane [17].
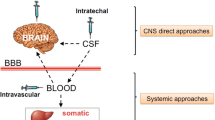
Gene delivery routes and mechanism of cross-correction is some MPS disorders. a Viral vectors can be introduced into the central nervous system via injection into the brain tissue or cerebrospinal fluid (CSF). While these modes of delivery are more invasive, they can directly access the primary site of pathology, the central nervous system. Targeted delivery to specific brain regions is also possible via stereotaxic delivery to the brain parenchyma. However, the volume of vector that can be administered may be limited. Some vectors, e.g., AAV serotype 9, can transduce neural cells following delivery to the vascular system. This affords a less invasive mode of delivery and can simultaneously treat peripheral pathology. However, specific brain regions cannot be targeted and the vector must cross the blood-brain barrier to reach the central nervous system. b (i) AAV vector particles dock and bind to serotype-specific, cell surface receptors. (ii) The viral particles then undergo receptor-mediated endocytosis and are eventually transported to the nucleus via the endosomal system. (iii) The viral capsids are uncoated and the single-stranded viral genome is converted to double-stranded DNA allowing the expression of the therapeutic lysosomal enzymes (filled circles). Nascent lysosomal enzymes are glycosylated in the rough endoplasmic reticulum and are trafficked to, and are posttranslationally modified in the endoplasmic reticulum (ER) and trans-Golgi network. The addition of the mannose-6-phosphate (M6P) tag, allows the selective trafficking of the enzymes to the lysosomes via binding to the M6P receptor (M6PR) which dissociates upon reaching the acidic environment of the lysosome. The M6PR can recycle to the trans-Golgi compartment or plasma membrane. (iv) The accumulated substrates within the lysosome are then degraded by the lysosomal enzymes. (v) For majority of the MPS disorders, a small fraction of M6P-tagged lysosomal enzymes are secreted into the extracellular space where they can be recaptured by cell surface cation-independent M6PR of nearby cells in a process referred to as “cross-correction,” thus enhancing the spread of treatment. (vi) The phosphorylated lysosomal enzymes are then targeted to the lysosome of the enzyme-deficient, MPS cell, via the endosome where further degradation of stored substrates can occur.
Newly synthesized lysosomal enzymes are shuttled to the lysosome by vesicular transport but a small fraction is secreted into the extracellular milieu. Once in the extracellular space, neighboring cells expressing the cation-independent M6P/IGF2 receptor at the cell surface recapture phosphorylated lysosomal enzymes and selectively target them towards the lysosome. Exogenously supplied mannose-6-phosphorylated lysosomal enzymes are similarly endocytosed, “cross-correcting” substrate-storing cells [18]. Cell-type dependent, M6P-independent targeting of lysosomal enzymes to the lysosome can also occur [19].
Approved treatments for central nervous system disease in the MPS
Treatment for neurological forms of MPS is currently limited to hematopoietic stem cell transplantation, which is only effective in some sub-types. Enzyme secreted by transplanted cells is taken up by host cells via M6P-mediated endocytosis and trafficked to the lysosome. Hematopoietic stem cells differentiate into lymphoid/myeloid cells [20] and are distributed throughout the body via the circulatory system. Where this treatment approach has been shown to be effective, timely identification of a compatible donor (ideally expressing normal enzyme levels) is required and the transplantation procedure entails a significant risk of morbidity and mortality. The patient also requires lifelong immunosuppression.
Transplantation of bone marrow as a source of hematopoietic stem cells was first conducted in MPS-I [21]. Within weeks of transplant, α-l-iduronidase leukocyte activity reached heterozygote levels and reversed hepatosplenomegaly and corneal clouding. Better outcomes are evident in those transplanted at a younger age, using non-carrier donors and where complete donor chimerism is obtained [22]. Bone marrow transplantation in MPS-VII patients has a favorable neurological response, albeit there are fewer patients in which long-term outcomes can be assessed due to the ultra-rarity of this condition [23,24,25]. Neuropsychological measures are not improved in transplanted MPS-II patients with an early-onset phenotype [26, 27], or in transplanted MPS-III patients who show a continued deterioration of cognitive function [28, 29].
Evaluation of intravenous and cerebrospinal fluid-directed enzyme replacement therapy for neurological MPS
Intravenous enzyme replacement therapy is only approved for use in non-neuropathic forms of MPS-I and -II, and also in MPS-IVA and -VI. Clinical trials assessing dose, safety, and efficacy of intravenous infusion of recombinant human β-glucuronidase in MPS-VII patients are in the recruitment phase or underway (clinicaltrials.gov identifiers NCT01856218, NCT02418455, NCT02230566), following compassionate use of this treatment approach in a single advanced stage-disease patient [30]. Intravenous infusion of recombinant proteins at conventional doses is thought to not treat neurological complications due to the inability of the enzyme to efficiently penetrate the blood-brain barrier.
Recombinant human α-l-iduronidase has been administered into cerebrospinal fluid (CSF) in MPS-I patients to treat spinal cord compression caused in part by thickened spinal meninges [31]. A further trial assessing cognition in nine patients has recently been completed (NCT00852358). This treatment is also under assessment for treating MPS-II (NCT00920647, NCT01506141, NCT02055118, NCT02412787; NCT01506141; NCT02055118, EudraCT 2013-002885-38; [32]) and MPS-IIIA patients (NCT01155778, NCT01299727, NCT02060526, NCT02350816, EudraCT 2009-015984-15, 2010-021348-16, 2013-003450-24). While reduced substrate levels in MPS-IIIA patient CSF were observed [33], these trials have recently been halted due to a lack of efficacy at the frequency and enzyme doses applied.
Circumventing the blood-brain barrier by directly and repeatedly delivering recombinant enzyme into CSF is associated with significant risk. This treatment is also costly [34]. Further, as companies are given exclusive rights for 7 years following FDA approval under the U.S. Orphan Drug Act of 1983, there is also a risk to the supply of the enzyme if the manufacturing process becomes contaminated [35]. Consequently, alternative treatments are required.
Gene therapy for neurological MPS
Gene therapy refers to the transfer of nucleic acids into cells for a therapeutic purpose. The genes can be introduced using viral or non-viral modalities either ex vivo, where a patient’s cells are removed, genetically modified and then returned, or in vivo, where gene transfer occurs directly within the patient. LSD are ideal candidates for gene transfer for several reasons. Firstly, they are monogenic disorders and the molecular basis of each disease is well understood. Also, the pathophysiology has been extensively characterized in animal models, enabling assessment of pre-clinical safety and efficacy. In addition, the ability of lysosomal enzymes to undergo anterograde [36] and retrograde axonal transport [37], trans-synaptic transfer [38] and diffusion from the site of injection may mean that a small number of transduced cells could distribute enzyme to widespread areas in the central nervous system. There is also the potential that the transgene could be expressed for several years after a single intervention.
Various viruses have been assessed in preclinical trials including retrovirus, lentivirus, adenovirus, and adeno-associated virus (AAV) [39, 40]. Non-viral approaches have also been evaluated, e.g., Sleeping Beauty-mediated transposon [41]. This review will outline the preclinical animal model data supporting translation of gene therapy and will describe the outcomes from recent human trials. Future/planned human studies will also be discussed.
AAV vectors under assessment in MPS clinical trials
AAV are non-enveloped, single-stranded DNA viruses containing a 4.7-kb genome flanked by two inverted terminal repeat regions that belong to the genus Dependoparvovirus (family Parvoviridae). AAV do not appear to cause pathogenic disease in humans and only replicate in the presence of a helper virus [42]. AAV vectors, except those in which the rep genes are deleted, persist episomally, and are also capable of semi-random integration in the host genome at specific sites, such as in human chromosome 19 [43], at an incidence of ~0.1% per infectious genome [44]. This feature, combined with a low inflammatory potential due to the absence of all viral proteins except the inverted terminal repeats, results in long-term transgene expression (at least 1-year postinjection) [45]. AAV vectors have also been readministered without inducing humoral immune responses in rodents [46]. Their greatest limitation is the high incidence (96%) of preexisting AAV-specific antibodies in humans, with 32% of the population harboring AAV-neutralizing antibodies [47].
At least 13 AAV serotypes have been developed as vectors, which display serotype-dependent transduction efficiencies and cellular tropism [48]. Early gene therapy studies commonly focussed on AAV2 vectors, given it was the most extensively characterized serotype following its discovery as a contaminant in an adenoviral stock [49] and subsequent cloning into bacterial plasmids in 1982 [50]. The utility of recombinant AAV vectors as a gene therapy tool was significantly advanced following the discovery that the AAV2 genome could be cross-packaged into the capsids of other AAV isolates [51], thus altering the level of expression and cellular tropism patterns following transduction to allow for the optimal selection of cell specificity/expression for the target organ of interest.
AAV5, AAV9, and AAVrh10 serotypes have been assessed in human clinical trials for the neurodegenerative MPS. Compared to AAV2, AAV1 and AAV5 vectors display higher transduction efficiencies and retrograde transport properties, allowing for wider reporter transgene expression following injection into various regions of the rat brain parenchyma [52]. Later studies in primate brains comparing serotypes AAV1–AAV6 confirmed that AAV5 had the widest distribution of transgene expression following delivery to the substantia nigra and striatum, with transduction of both neuronal and glial cells [53]. Of the newer AAV serotypes, AAVrh10 mediated the most widespread distribution of the lysosomal enzyme tripeptidyl peptidase I in a mouse model of late infantile neuronal ceroid lipofuscinosis following intracerebral delivery, when compared to AAV2, AAV5, and AAV8 [54]. A comparison of AAV7–AAVrh10 vectors unilaterally injected into various brain regions of the MPS-VII mouse model showed that AAV9 and AAVrh10 vectors produced the greatest rostral-caudal spread throughout the brain and spinal cord of the transgene, β-glucuronidase, including the contralateral brain hemisphere [55].
A further advantage of AAV9 and AAVrh10 serotypes is the potential for less immunogenicity against the vectors. The seroprevalence of neutralizing IgG antibodies in human sera is considerably reduced for AAV9 (18%) and AAVrh10 (21%) compared to AAV2 (71%) [56]. AAV5 has a reported seroprevalence of 40% compared to AAV2 (97%) [57]. AAVrh10 is also less immunogenic than AAV9 in rodent studies, with fewer total IgG anti-AAV raised postimmunization and reduced neutralizing ability [56].
AAV9 has the additional benefit that following intravenous delivery, the vector can cross the neonatal and adult blood-brain barrier to target the brain [58, 59]. This affords a noninvasive approach for gene delivery to the central nervous system in patients. However, local delivery of single-stranded AAV9 via the intrathecal route to the cerebrospinal fluid mediates a higher transduction efficiency to the central nervous system than when an equivalent dose of the same vector is delivered intravenously [60]. This may potentially be overcome by using self-complementary AAV9 vectors, that result in greater than 10-fold more transgene expression in the brain than single-stranded AAV9 vectors, after intravascular administration [61]. However, one of the major caveats of using self-complementary AAV9 vectors is the packaging capacity of this vector, ~2.2 kb, which limits the transgene size that can be delivered [61].
AAVrh10-based treatment of MPS-IIIA
A preclinical study of an AAV2 genome carrying human SGSH and SUMF1 cDNAs under the control of the phosphoglycerate kinase promoter, packaged into a serotype rh10 capsid was undertaken in presymptomatic, 5-week-old MPS-IIIA mice [62]. SUMF1 encodes the sulfatase-modifying factor 1 which is required for the posttranslational activation of all sulfatase enzymes, including SGSH. The vector was delivered unilaterally to the striatum (7.5 × 109 viral genome (vg) copies/mouse). Widespread expression of SGSH (up to 337-fold normal) significantly reduced brain heparan sulfate levels. Regional improvements in a number of secondary pathological changes were also noted. Antibody responses to SGSH were not detected. Given the localized expression of SGSH activity, vector targeting of multiple intra-parenchymal injection sites in subsequent studies was recommended for global correction.
The safety and efficacy of this vector (SAF-301), was subsequently evaluated in an open-label, single-arm, monocentric phase-I/II clinical trial (NCT01474343). The vector (7.2 × 1011 vg in total) was delivered to four MPS-IIIA patients via bilateral, stereotaxic injection using six tracks, with two deposits per track [63]. At enrolment, patients aged 32 months to 6 years were symptomatic (cognitive decline), but retained the ability to walk. One year safety data showed that both the vector and procedure were well tolerated. Although changes in CSF heparan sulfate levels were not evident, brain atrophy, evaluated by magnetic resonance imaging, stabilized in some patients. Follow-up studies are underway (NCT02053064).
AAV5-based treatment of MPS-IIIB
Studies in MPS-IIIB mice compared the transduction efficiency and distribution of AAV2 and AAV5 vectors expressing human NAGLU under the control of the mouse phosphoglycerate kinase-1 promotor [64]. Mice were treated at 6 weeks of age by unilateral, intracerebral injection into the striatum. Home cage activity was normalized in MPS-IIIB mice 12-weeks posttreatment. While the AAV5 genome was predominantly detected in the ipsilateral brain hemisphere, active, supra-physiological levels of NAGLU were detected in both hemispheres. GM2 and GM3 ganglioside storage was reduced [64].
Further evaluation of AAV5 was carried out in MPS-IIIB Schipperke dogs. Nine animals were treated by stereotaxic injection of 5 × 1011 vg targeting the putamen and the centrum semi-ovale [65]. Dogs were presymptomatic at the time of treatment (8 to 14 months old) and some were immunosuppressed. In the absence of immunosuppression, NAGLU activity was undetectable after ~4 months. In contrast, widespread NAGLU was detected in the brains of immunosuppressed dogs (>70% of regions assessed in 4/5 animals), although levels were low or undetectable in some of the rostral and more caudal regions [65]. AAV5 administration reduced, but did not normalize, glycosaminoglycan and GM2/GM3 ganglioside levels. Similar outcomes were observed when the vector was produced in insect or mammalian cells.
This vector (AMT-110) has now been administered to four MPS-IIIB patients aged 20 to 53 months at the start of treatment (ISRCTN19853672; [66, 67]). Participants received vector (AAV2 genome encoding the human NAGLU cDNA packaged in a serotype 5 capsid) via intracerebral injection targeting white matter over 16 injection sites, combined with immunosuppression [66, 67]. One year follow-up data indicated that NAGLU enzyme activity increased from 0 to 14–17% 3-months postinjection, and was detectable after 1 year [67]. There were no serious safety concerns and “incremental cognitive development was maintained”.
AAV9–based treatment of MPS-I
AAV9-based gene therapy has been assessed in two large MPS-I animal models and in nonhuman primates. In the first study, five MPS-I cats aged 4 to 7 months received CSF injections of vector (1012 vg/kg of a vector containing codon-optimized feline IDUA driven by either the chicken beta-actin or cytomegalovirus promoter) [68]. IDUA activity in sera and CSF peaked at ~21-days postinjection, eventually approaching wild-type levels in three animals. The decline in CSF enzyme activity in the other two cats may have been associated with an antibody response to the IDUA protein. GM3 ganglioside and cholesterol levels, LIMP-II expansion and secondary elevations in lysosomal enzymes were all improved [68]. Amelioration of glycosaminoglycan storage in peripheral tissues (liver, spleen) was also demonstrated, with the greatest correction observed in the cats that exhibited the lowest antibody titers.
Intravenous delivery of AAV9 vectors expressing canine IDUA in MPS-I mice or dogs has also been investigated [69]. Animals received 5 × 1013 (mice) or 4 × 1013 (dogs) vg/kg. Treated mice showed stable transgene expression and normalization of disease lesions systemically and in brain. In contrast, MPS-I dogs treated at ~3 months and monitored for a further 4 months, showed a transient increase in IDUA activity (~2-weeks posttreatment), then loss of transgene expression. IDUA activity remained higher than in untreated controls and reduced brain glycosaminoglycan storage by ~30%. GM3 ganglioside and cholesterol levels were not improved. Intrathecal administration of AAV9 expressing canine IDUA under the control of the chicken beta-actin promoter in three younger (1-month–old) MPS-I dogs showed a similar pattern, with widespread transduction of brain and spinal cord resulting in supra-physiological expression of IDUA [70]. While stable transgene expression was obtained, IDUA expression dropped below wild-type levels after ~100–150 days, attributed to the development of anti-IDUA antibodies [70].
In contrast, MPS-I dogs treated intravenously within the first week of life with 3-5 × 1012 vg/kg of an AAV8 vector expressing canine IDUA driven by a liver-specific TBG promoter, followed by intrathecal delivery of AAV9-CB-cIDUA (1012 vg/kg) at 1 month of age, did not develop anti-IDUA antibodies [70]. All five dogs exhibited >30-fold normal CSF IDUA activity at peak transgene expression and reduced CSF glycosaminoglycan and GM3 ganglioside, cholesterol, and brain LIMP-II levels [70]. Tolerance to IDUA has also been demonstrated in rhesus monkeys treated systemically with AAV8-TBG-human IDUA at birth, followed by AAV9–CB-human IDUA at 1 month of age [70]. CSF IDUA activity increased up to 60-days post-intrathecal AAV9, but only reached wild-type levels in animals previously tolerized with a mock AAV8 vector.
AAV9-IDUA (RGX-111) has received orphan drug designation, an investigational new drug (IND) application will be sought in 2017 and a phase-I/II dose escalation clinical trial in MPS-I patients initiated [71].
AAV9-based treatment of MPS-II
Preclinical studies of intra-CSF delivery of AAV9 encoding murine Ids under the control of the CAG promoter (5 × 1012 vg) has been assessed in male MPS-II mice [72]. Vector delivered at 2 months of age normalized open-field behavior 4-months postinjection. IDS activity rose to ~40% of wild-type glycosaminoglycan levels were normalized and improvements in LAMP-2 expression and neuroinflammation were evident. The vector also mediated improvements in glycosaminoglycan storage in somatic tissues and significantly extended life span [72].
AAV9 expressing human IDS has also been assessed in MPS-II mice. The vectors, containing either the ubiquitously active mouse U1a promoter or a miniaturized promoter derived from CMV, were delivered intravenously (5 × 1012 vg/kg) [73]. Reductions in glycosaminoglycan levels were seen in central and peripheral nervous system and somatic tissues, leading to improved cognitive and motor function and extended life span.
The European Medicines Agency and the FDA have granted Orphan Drug Designation for EGT-301, an AAV9 vector encoding human I2S [74]. RGX-121, an AAV9-IDS vector, has also been granted Orphan Drug Product and Rare Pediatric Disease designation [75]. No information regarding these clinical trials is presently available.
AAV9-based treatment of MPS-IIIA
At least two groups have undertaken preclinical assessment of AAV9-based therapies in MPS-IIIA mice. AAV9-human SGSH controlled by the U1a promoter (5 × 1012 vg/kg) was injected intravenously in mice <6 months of age, leading to elevated SGSH activity, normalized glycosaminoglycan levels in both brain and somatic organs and improved cognition [76]. Behavioral changes did not occur in MPS-IIIA mice treated at 9 months of age, despite correction of glycosaminoglycan levels. Earlier initiation of treatment also improved longevity.
Systemic delivery of AAV9-murine Sgsh under the control of a chicken beta-actin promoter and CMV enhancer (1 × 1012 vg intravenously) to 2-month-old MPS IIIA mice resulted in gender-based outcomes [77]. Higher enzyme activity was observed in sera and liver lysates taken from male mice, with five- to sixfold normal SGSH activity seen after 8 months (cf. wild-type levels in females). Amelioration of brain glycosaminoglycan storage, astrocytosis and microgliosis occurred, glycosaminoglycan levels in peripheral organs was normalized and life span increased.
The effect of 5 × 109 or 5 × 1010 vg of AAV9-murine Sgsh under the control of the ubiquitous CAG promoter (hybrid of the chicken β-actin promoter and CMV enhancer), injected into the CSF of 8-week-old MPS-IIIA mice was subsequently evaluated [78]. Brain SGSH activity increased up to 39% of wild type at the higher dose resulting in brain-region dependent correction of stored glycosaminoglycans 4-months posttreatment. By 10- to 12-months postinjection, brain glycosaminoglycan content in other brain regions was also normalized. Glycosaminoglycan levels in all peripheral organs assessed (except for the kidney) were reduced by at least 70%, and mice that received the higher dose lived significantly longer than untreated, affected mice, approaching the life span of unaffected mice and exhibiting normalization of motor activity. This serotype (encoding either canine or human SGSH) also mediated increased SGSH activity in unaffected beagles [78]. Dogs received 2 × 1013 vg via the cisterna magna and while detectable activity of the human protein peaked at 2 weeks followed by low transgene levels due to an immune response against the non-species-specific transgene, canine SGSH activity was maintained at higher levels for the duration of the study (3 months).
The FDA granted Orphan Drug Designation, Rare Pediatric Disease Designation and IND status to ABO-102, a self-complementary AAV9-SGSH vector [79, 80]. To date, six patients have received intravenous infusion of ABO-102 in a phase-I/II dose escalation trial [81]. In a press release by the study sponsor, three patients received the low dose (5 × 1012 vg/kg) and two patient received the high dose (1 × 1013 vg/kg; NCT02716246), with all patients tolerating the treatment well through 1100 cumulative days without any serious adverse events [81]. Reductions in glycosaminoglycan levels in CSF (by ~61%) and decreased liver volumes (−15%) were observed in the two patients receiving the higher dose after 30 days of treatment [81, 82]. In patients receiving 1 × 1013 vg/kg, cognitive assessments (Leiter-R non-verbal IQ and Vineland (adaptive behavior) scales) demonstrated evidence of stabilized or improved scores at 6-months posttreatment [81]. A second, unrelated phase-I/II clinical trial will assess the safety and efficacy of EGT-101, also an AAV9-SGSH vector, which received orphan drug designation from the FDA and EMA [74].
AAV9-based treatment of MPS-IIIB
Systemic delivery of AAV9–CMV–NAGLU (5 × 1012 or 1.5 × 1013 vg/kg) improved the performance of MPS-IIIB mice in the accelerating rotarod and increased life span [83]. Dose-dependent transgene expression was evident with normal or supra-physiological NAGLU activity measured in the brains of mice treated with low or high doses, respectively. A separate cohort of mice was treated with 2 × 1013 vg/kg, either with or without mannitol pretreatment to open the blood-brain barrier, but no statistically significant increases in NAGLU brain activity were found in the mannitol-treated mice. Despite this, all AAV9-treated mice regardless of dose or pretreatment status showed striking reductions in glycosaminoglycan content, with normalization achieved in brain and some, but not all, somatic tissues. Treated mice exhibited reduced astrocytosis in brain and Purkinje neuron preservation.
A subsequent GLP-compliant toxicology study (1 or 2 × 1014 vg/kg administered to wild-type mice) reported no adverse events; however, dose-, sex- and genotype-specific acute liver toxicity were observed at days 6–8 postinjection in 5/15 male mice receiving the higher dose [70]. Blood alanine and aspartate aminotransferase levels were elevated in these mice, particularly at earlier time points, suggesting acute liver toxicity. Transgene overexpression rather than an inflammatory response towards vector/NAGLU is believed to be the cause, given the absence of white cell infiltration into liver. A non-GLP compliant study saw MPS-IIIB mice systemically injected with 2 × 1014 vg/kg AAV9-NAGLU [84]. No adverse events were detected, although one mouse that underwent necropsy 5 days postinjection showed liver pallor. While anti-AAV9 and anti-NAGLU antibodies were detected in sera, they did not impair NAGLU activity. Transient T cell responses were observed against the vector and transgene.
Safety and toxicity has been assessed after systemic delivery in cynomolgus monkeys with/without immunosuppression [85]. There were no adverse events, detectable toxicity or specific changes in blood chemistry or cell counts noted in vector-treated animals (1 × 1013 or 2 × 1013 vg/kg). Increased NAGLU activity was evident in brain and somatic tissues, with higher expression noted in the kidney and less in the heart and skeletal muscles (cf. mouse studies), potentially suggesting species-specific tropism. Immunostaining revealed neuronal and endothelial NAGLU expression in the brain and spinal cord. There was no correlation between enzyme activity levels, anti-AAV9 antibody titers and immunosuppression status. Serum NAGLU activity peaked 2-weeks posttreatment, declining to baseline after 2 months and did not correlate with tissue NAGLU activity.
One non-human primate in the study had high serum anti-AAV9 antibodies before treatment. In this animal, serum NAGLU remained less than threefold baseline levels, compared to six to ninefold seen in equivalently treated animals without preexisting antibodies. However, it had the second highest brain NAGLU activity levels of six animals receiving this dose. Strong anti-AAV9, in addition to modest humoral responses to NAGLU developed in all vector-treated animals with one animal exhibiting helper T cell activation and concomitant loss of serum NAGLU activity.
AAV9 vectors (3 × 1012 vg) encoding a codon-optimized version of murine Naglu under the control of the CAG ubiquitous promoter have also been administered to the CSF of 8-week-old MPS-IIIB mice [86]. NAGLU activity in brain reached 400–800% of normal and led to complete normalization of glycosaminoglycan content. Gene therapy also improved secondary neuropathological changes after 3 months, mediated significant systemic improvements, normalized locomotor activity, and extended life span [86].
To evaluate the effect of preexisting immunity, two healthy Beagles were immunized with 1 × 1012 vg of AAV9-null vector and 6 weeks later received 6.5 × 1012 vg of AAV9 expressing canine NAGLU via cisternal CSF [86]. Controls received intra-CSF-delivered vector only. Serum neutralizing antibodies rose quickly following the first exposure to the NAGLU vector, but were undetectable in the CSF. The presence of circulating neutralizing antibodies did not influence NAGLU transgene expression in CSF.
A phase-I/II dose escalation clinical trial of ABO-101 (AAV9-NAGLU) has been approved [80] and Orphan Drug Designation has been obtained [74].
Concluding remarks
A growing body of pre-clinical study data indicates that AAV-based gene therapy may provide a clinically relevant treatment for MPS with neurological manifestations. While there are risks associated with immune tolerance towards the vector and the neurosurgical procedures, AAV gene therapy is a promising platform for MPS patients and their families. Early intervention is likely to be critical for the successful application of this and other therapeutic strategies, and consequently, implementation of diagnostic newborn screening programs will be required to identify affected children in a timely manner. Outcomes in both animal models and humans have only been reported following short-term treatment; however, so capacity for life-long disease prevention/stabilization remains to be determined.
References
Vitner EB, Platt FM, Futerman AH (2010) Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem 285:20423–20427
Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcao A et al (2004) Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet 12:87–92
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281:249–254
Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP (1999) The frequency of lysosomal storage diseases in The Netherlands. Hum Genet 105:151–156
Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T et al (2009) Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A). Hum Mutat 30:1397–1405
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ (2006) High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet 79:31–40
Applegarth DA, Toone JR, Lowry RB (2000) Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatrics 105:e10
Scott HS, Anson DS, Orsborn AM, Nelson PV, Clements PR, Morris CP, Hopwood JJ (1991) Human alpha-L-iduronidase: cDNA isolation and expression. Proc Natl Acad Sci U S A 88:9695–9699
Wilson PJ, Morris CP, Anson DS, Occhiodoro T, Bielicki J, Clements PR, Hopwood JJ (1990) Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc Natl Acad Sci U S A 87:8531–8535
Scott HS, Blanch L, Guo X-H, Freeman VC, Orsborn A, Baker E, Sutherland GR, Morris P, Hopwood JJ (1995) Cloning of the sulphamidase gene and identification of mutations in Sanfilippo A syndrome. Nat Genet 11:465–467
Weber B, Blanch L, Clements PR, Scott HS, Hopwood JJ (1996) Cloning and expression of the gene involved in Sanfilippo B syndrome (Mucopolysaccharidosis III B). Hum Mol Genet 5:771–777
Zhao HG, Li HH, Bach G, Schmidtchen A, Neufeld EF (1996) The molecular basis of Sanfilippo syndrome type B. Proc Natl Acad Sci U S A 93:6101–6105
Hopwood JJ, Morris CP (1990) The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med 7:381–404
Dahms NM, Lobel P, Kornfeld S (1989) Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem 264:12115–12118
Hopwood JJ, Brooks DA (1997) An introduction to the basic science and biology of the lysosome and storage disorders. In: Appelgarth DA (ed) organelle diseases chapman and hall Medical, London, pp. 7-35
Kornfeld S (1992) Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 61:307–330
Brown WJ, Goodhouse J, Farquhar MG (1986) Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol 103:1235–1247
Willingham MC, Pastan IH, Sahagian GG, Jourdian GW, Neufeld EF (1981) Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A 78:6967–6971
Hasilik A (1992) The early and late processing of lysosomal enzymes: proteolysis and compartmentation. Experientia 48:130–151
Smith LG, Weissman IL, Heimfeld S (1991) Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A 88:2788–2792
Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC, Lucas CF, Rogers TR, Benson PF et al (1981) Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 2:709–712
Aldenhoven M, Wynn RF, Orchard PJ, O'Meara A, Veys P, Fischer A, Valayannopoulos V, Neven B, Rovelli A, Prasad VK et al (2015) Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood 125:2164–2172
Krivit W (2004) Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immun 26:119–132
Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N et al (1998) Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619v mutation. Bone Marrow Transplant 21:629–634
Montano AM, Lock-Hock N, Steiner RD, Graham BH, Szlago M, Greenstein R, Pineda M, Gonzalez-Meneses A, Coker M, Bartholomew D et al (2016) Clinical course of sly syndrome (mucopolysaccharidosis type VII). J Med Genet. doi:10.1136/jmedgenet-2015-103322
Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R (2009) Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J Pediatr 154:733–737
Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith JE (1999) Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis 22:638–648
Hoogerbrugge PM, Brouwer OF, Bordigoni P, Ringden O, Kapaun P, Ortega JJ, O'Meara A, Cornu G, Souillet G, Frappaz D et al (1995) Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation Lancet 345:1398–1402
Shapiro EG, Lockman LA, Balthazor M, Krivit W (1995) Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis 18:413–429
Fox JE, Volpe L, Bullaro J, Kakkis ED, Sly WS (2015) First human treatment with investigational rhGUS enzyme replacement therapy in an advanced stage MPS VII patient. Mol Genet Metab 114:203–208
Munoz-Rojas MV, Vieira T, Costa R, Fagondes S, John A, Jardim LB, Vedolin LM, Raymundo M, Dickson PI, Kakkis E et al (2008) Intrathecal enzyme replacement therapy in a patient with mucopolysaccharidosis type I and symptomatic spinal cord compression. Am J Med Genet A 146A:2538–2544
Muenzer J, Hendriksz CJ, Fan Z, Vijayaraghavan S, Perry V, Santra S, Solanki GA, Mascelli MA, Pan L, Wang N et al (2016) A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med 18:73–81
Jones SA, Breen C, Heap F, Rust S, de Ruijter J, Tump E, Marchal JP, Pan L, Qiu Y, Chung JK et al (2016) A phase 1/2 study of intrathecal heparan-N-sulfatase in patients with mucopolysaccharidosis IIIA. Mol Genet Metab 118:198–205
Beutler E (2006) Lysosomal storage diseases: natural history and ethical and economic aspects. Mol Genet Metab 88:208–215
Goldblatt J, Fletcher JM, McGill J, Szer J, Wilson M (2011) Enzyme replacement therapy "drug holiday": results from an unexpected shortage of an orphan drug supply in Australia. Blood Cells Mol Dis 46:107–110
Chen F, Vitry S, Hocquemiller M, Desmaris N, Ausseil J, Heard JM (2006) alpha-L-Iduronidase transport in neurites. Mol Genet Metab 87:349–358
Passini MA, Lee EB, Heuer GG, Wolfe JH (2002) Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J Neurosci 22:6437–6446
Hennig AK, Ogilvie JM, Ohlemiller KK, Timmers AM, Hauswirth WW, Sands MS (2004) AAV-mediated intravitreal gene therapy reduces lysosomal storage in the retinal pigmented epithelium and improves retinal function in adult MPS VII mice. Mol Ther 10:106–116
Sands MS, Davidson BL (2006) Gene therapy for lysosomal storage diseases. Mol Ther 13:839–849
Byrne BJ, Falk DJ, Clement N, Mah CS (2012) Gene therapy approaches for lysosomal storage disease: next-generation treatment. Hum Gene Ther 23:808–815
Aronovich EL, Bell JB, Belur LR, Gunther R, Koniar B, Erickson DC, Schachern PA, Matise I, McIvor RS, Whitley CB et al (2007) Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med 9:403–415
Zolotukhin S (2005) Production of recombinant adeno-associated virus vectors. Hum Gene Ther 16:551–557
Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, Hunter LA (1991) Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J 10:3941–3950
Baum C, Kustikova O, Modlich U, Li Z, Fehse B (2006) Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther 17:253–263
McCown TJ (2005) Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther 5:333–338
Mastakov MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ (2002) Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol 76:8446–8454
Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J (1999) Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 6:1574–1583
Srivastava A (2016) In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol 21:75–80
Hoggan MD, Blacklow NR, Rowe WP (1966) Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A 55:1467–1474
Samulski RJ, Berns KI, Tan M, Muzyczka N (1982) Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci U S A 79:2077–2081
Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ (2002) Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 76:791–801
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10:302–317
Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, Elsworth JD, Roth RH, Samulski RJ, Redmond DE Jr (2010) Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain. Mol Ther 18:588–593
Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG (2007) Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther 15:481–491
Cearley CN, Wolfe JH (2006) Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther 13:528–537
Thwaite R, Pages G, Chillon M, Bosch A (2015) AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Ther 22:196–201
Liu Q, Huang W, Zhang H, Wang Y, Zhao J, Song A, Xie H, Zhao C, Gao D, Wang Y (2014) Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther 21:732–738
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65
Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P, Colle MA, Barkats M (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 17:1187–1196
Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, Elde RP, Fairbanks CA, Vulchanova L (2014) Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 8:42
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ (2011) Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19:1058–1069
Winner LK, Beard H, Hassiotis S, Lau AA, Luck AJ, Hopwood JJ, Hemsley KM (2016) A pre-clinical study evaluating AAVrh10-based gene therapy for Sanfilippo syndrome. Hum Gene Ther 27:363–375
Tardieu M, Zerah M, Husson B, de Bournonville S, Deiva K, Adamsbaum C, Vincent F, Hocquemiller M, Broissand C, Furlan V et al (2014) Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Hum Gene Ther 25:506–516
Cressant A, Desmaris N, Verot L, Brejot T, Froissart R, Vanier MT, Maire I, Heard JM (2004) Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum. J Neurosci 24:10229–10239
Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK, Snella EM, Mohammed EE, Thomson CB, Raoul S et al (2011) Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther 19:251–259
Unknown (2016) Intra-cerebral gene therapy for Sanfilippo type B syndrome.
Unknown (2015) Positive Topline Results Announced from Phase I/II Trial in Sanfilippo B Syndrome Patients Using uniQure’s Novel AAV5-Based Gene Therapy, Globe Newswire, Amsterdam, the Netherlands
Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin JP, Zhu Y, Bagel J, O'Donnell P, Sikora T, Ruane T et al (2014) Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther 22:2018–2027
Bell P, Gurda B, Zhu Y, Sikora T, Ruane T, O'Donnell P, Haskins ME, Wilson JM (2015) Evaluating the impact of systemic AAV9.cIDUA administration on brain pathology in MPS I dogs. Mol Genet Metab 114:S25
Hinderer C, Bell P, Louboutin JP, Zhu Y, Yu H, Lin G, Choa R, Gurda BL, Bagel J, O'Donnell P et al (2015) Neonatal systemic AAV induces tolerance to CNS gene therapy in MPS I dogs and nonhuman primates. Mol Ther 23:1298–1307
Unknown (2015) ReGenXBio licenses key technologies from University of Pennsylvania and University of Minnesota for the development of treatments for MPS I and MPS II, REGENXBIO, Washington, DC
Motas S, Haurigot V, Garcia M, Marco S, Ribera A, Roca C, Sanchez X, Sanchez V, Molas M, Bertolin J et al (2016) CNS-directed gene therapy for the treatment of neurologic and somatic mucopolysaccharidosis type II (Hunter syndrome). JCI Insight 1:e86696
Fu H, Zaraspe K, Meadows A, Camboni M, McCarty DM (2016) Functional benefits of systemic rAAV9-hids gene delivery in MPS II mouse model. Mol Genet Metab 117:S47
Unknown (2016) Esteve and UAB expand their research to two new gene therapies for Sanfilippo B syndrome and Hunter syndrome, PRNewswire, Barcelona.
Unknown (2016) FDA grants rare pediatric disease designation to REGENXBIO’s RGX-121 gene therapy for the treatment of mucopolysaccharidosis type II (MPS II), Globe Newswire, Rockville, MD.
McCarty DM, Zaraspea K, Murreya D, Warea T, Fu H (2014) Functional correction and reversal of MPS IIIA in a mouse model by an intravenous hSGSH gene delivery using a self-complementary AAV9 vector. Mol Genet Metab 111:S75
Ruzo A, Marco S, Garcia M, Villacampa P, Ribera A, Ayuso E, Maggioni L, Mingozzi F, Haurigot V, Bosch F (2012) Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther 23:1237–1246
Haurigot V, Marco S, Ribera A, Garcia M, Ruzo A, Villacampa P, Ayuso E, Anor S, Andaluz A, Pineda M et al (2013) Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 123:3254–3271
Unknown (2016) Abeona therapeutics announces FDA allowance of investigational new drug (IND) for systemic AAV phase 1/2 clinical study with ABO-102 gene therapy for patients with Sanfilippo syndrome type A (MPS IIIA), Marketwired, New York, NY and Cleveland, OH
Unknown (2016) Abeona therapeutics announces initial European regulatory approvals for phase 1/2 gene therapy clinical studies for patients with Sanfilippo syndromes type A (MPS IIIA) and type B (MPS IIIB), Marketwired New York, NY and Cleveland, OH
Unknown (2017) Abeona therapeutics announces top-line data for ABO-102 phase 1/2 MPS IIIA gene therapy trial at ASGCT, Globe Newswire, New York, NY and Cleveland, OH
Unknown (2017) Abeona therapeutics provides update from ABO-102 phase 1/2 MPS IIIA clinical trial at the 13th Annual WORLDSymposium™ 2017, Globe Newswire New York, NY and Cleveland, OH
Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM (2011) Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther 19:1025–1033
Meadows AS, Duncan FJ, Camboni M, Waligura K, Montgomery CL, Zaraspe K, Naughton BJ, Bremer WG, Shilling C, Walker C et al (2015) A GLP-compliant toxicology and biodistribution study: systemic delivery of a rAAV9 vector for the treatment of mucopolysaccharidosis IIIB. Hum Gene Ther Clin Dev 26:228–242
Murrey DA, Naughton BJ, Duncan FJ, Meadows AS, Ware TA, Campbell KJ, Bremer WG, Walker CM, Goodchild L, Bolon B et al (2014) Feasibility and safety of systemic rAAV9-hNAGLU delivery for treating mucopolysaccharidosis IIIB: toxicology, biodistribution, and immunological assessments in primates. Hum Gene Ther Clin Dev 25:72–84
Ribera A, Haurigot V, Garcia M, Marco S, Motas S, Villacampa P, Maggioni L, Leon X, Molas M, Sanchez V et al (2015) Biochemical, histological and functional correction of mucopolysaccharidosis type IIIB by intra-cerebrospinal fluid gene therapy. Hum Mol Genet 24:2078–2095
Acknowledgements
The authors have received funding for their research from the Australian National Health and Medical Research Council of Australia, the Women’s and Children’s Hospital Foundation (Australia), the Sanfilippo Children’s Research Foundation (Canada), Swiss Sanfilippo Foundation, Alliance Sanfilippo, the National MPS Society (USA), the Isaac Foundation, Lysogene, and Shire Human Genetic Therapies. The funding sources did not have any role in the writing of this review or in the decision to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, A.A., Hemsley, K.M. Adeno-associated viral gene therapy for mucopolysaccharidoses exhibiting neurodegeneration. J Mol Med 95, 1043–1052 (2017). https://doi.org/10.1007/s00109-017-1562-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1562-0