Abstract
Psoriasis is considered as a model for chronic immune-mediated disorders. Th17-cells are pivotal players in those diseases. Recently, we demonstrated that Th17-cells produce interleukin (IL)-29 and that IL-29 is highly present in psoriatic lesions. Whether IL-29, with its action on epithelial cells and melanocytes, contributes to psoriasis pathogenesis, was unknown so far. Analysis of IL-29-treated human keratinocytes revealed induction of the chemokines CXCL10, CXCL11, and, to a much lesser extent, CXCL9. Unlike these CXCR3A ligands, known to attract Th1-, CD8+, NK-, and Th1/Th17 transient cells, no influence was found on chemokines attracting other immune cell populations or on molecules modulating the CXCR3A/CXCR3A ligand interaction. CXCR3A ligand expression was also induced by IL-29 in melanocytes and in epidermis models and explanted skin. Regarding other psoriasis-relevant cytokines, interferon-γ and, less potently, tumor necrosis factor-α and IL-1β shared and strengthened IL-29’s capacity. Murine IL-29 counterpart injected into mouse skin provoked local CXCL10 and CXCL11 expression, T-cell infiltration, and, in consequence, skin swelling. The elevated IL-29 expression in psoriatic lesions was associated with upregulation of CXCR3A ligands compared to non-lesional skin of these patients and to the skin of healthy donors and atopic dermatitis patients, which lack IL-29 production. Importantly, neutralization of IL-29 reduced CXCR3A ligand levels in explant cultures of psoriatic lesions. Finally, elevated blood CXCL11 levels were found in psoriasis that might be useful for monitoring lesional activity of the IL-29 axis. In summary, the Th17-cytokine IL-29 induces specific chemokines and, in consequence, provokes skin infiltration of potentially pathogenic T-cells.
Key messages
-
IL-29 selectively induces CXCR3A-binding chemokines (CXCL9, CXCL10, CXCL11) in skin cells.
-
Murine IL-29 counterpart induces skin T-cell infiltration and inflammation in mice.
-
CXCR3A ligands are IL-29-dependently increased in lesional skin of psoriasis patients.
-
CXCR3A ligand levels in psoriatic skin correlate with epidermal T-cell numbers.
-
Increased blood CXCL11 levels in psoriasis may be a biomarker for local IL-29 action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Affecting more than 2 % of the western population, psoriasis is one of the most common skin diseases [1]. Emerged as an easy-access model for immune-mediated disorders, pathogenetic principles learnt from psoriasis may help to understand a range of other important conditions.
Skin lesions in psoriasis are characterized by the presence of different immune cell populations in both the epidermis and dermis, by hyperproliferation of keratinocyte stem/progenitor cells, disturbed differentiation of suprabasal keratinocytes, increased angiogenesis of dermal blood capillaries, and high epidermal, both antibacterial and antiviral, resistance [1]. For some of these events, the underlying mechanisms are being progressively clarified. In fact, different mediators including epidermal growth factor receptor ligands [2] and the antimicrobial/regenerative protein regenerating islet-derived protein 3 alpha (REG3A, acting via exostosin-like 3) [3] contribute to the keratinocyte hyperproliferation in psoriasis. IL-22/IL-20 family members mediate the altered keratinocyte differentiation. This disturbed keratinocyte proliferation and differentiation leads to typical changes of the epidermis including thickening and hypogranularity of its living part and thickening of its upper cornified layer with appearance of cell nucleus remnants [4–10]. The strengthened angiogenesis in psoriatic plaques, which is responsible for the pronounced capillary vessel extension in the papillary dermis, seems to mainly depend on the action of vascular endothelial growth factor [11]. The antibacterial and antiviral resistance in psoriatic plaques highly compensates for the disturbed epidermal barrier function. At this, the antibacterial competence is mediated by massive production of antibacterial proteins (ABPs) including β-defensin 2 and 3, S100A7, S100A8/9 and S100A12, lipocalin-2, RNase-7, and cathelicidin/LL37, which are mainly upregulated by the synergistic action of two T-cell/innate lymphoid cell cytokines: IL-22 and IL-17 [12–15]. We identified the mechanisms underlying the high antiviral resistance in psoriatic epidermis by demonstrating overexpression of antiviral proteins (AVPs) such as myxovirus resistance protein 1, bone marrow stromal antigen 2, interferon (IFN)-stimulated gene 15, and 2′-5′-oligoadenylate synthetase [16]. This high AVP expression is induced by IL-29, which, in that situation, is mainly produced by skin-infiltrated Th17-cells and, to a smaller extent, also Th1-cells [16]. IL-29, together with IL-28A and IL-28B (also designated as IFN-λ1, -2, and -3, respectively) are members of the IL-10—IFN cytokine family, which act via a transmembrane heterodimeric receptor complex consisting of IL-28R1 and ubiquitous IL-10R2, and have a restricted target cell range [17–19]. Interestingly, IL-29 is the only IFN-λ present in psoriatic skin [16]. While they do not seem to efficiently influence immune cells, target cells of IL-29/28 in the skin are clearly keratinocytes and melanocytes [20]. In contrast to the keratinocyte AVP expression, IL-29 influenced neither the ABP production nor the differentiation of these cells, and it did not increase but rather modestly inhibited their proliferation [16, 21]. So far, the role of this cytokine in the infiltration of immune cells into psoriatic plaques remains undefined. We therefore set out the current study to investigate the role of IL-29 in the cutaneous chemokine expression and immune cell infiltration.
Materials and methods
Cell, tissue, and explant culture
Primary human epidermal keratinocytes were cultured in KGM-Gold medium (both from Lonza) and stimulated or not (control) with 40 ng/ml IL-29 for 24 h, for 72 h (CD26 activity study), or for 6 to 72 h (kinetic study). In some settings, one of the following cytokines was present during this stimulation period: 10 ng/ml IL-17A, 20 ng/ml IL-22, 20 ng/ml IL-21, 10 ng/ml IFN-γ, 10 ng/ml IL-1β, 40 ng/ml IL-19, 10 ng/ml tumor necrosis factor (TNF)-α. Primary human epidermal melanocytes were cultured in recommended medium (Life Technologies) and stimulated or not (control) with 40 ng/ml IL-29 for 24 h. Primary murine keratinocytes (CELLnTEC) were cultured in Dermalife medium with 0.03 to 0.06 mM calcium (Lifeline Cell technology) and stimulated or not (control) with 40 ng/ml murine IL-28A for 24 h. Underdeveloped EpiDerm-201™ reconstituted human epidermis tissues (MatTek Corp.) were cultured as described previously [8] in the absence (control) or presence of 20 ng/ml IL-29 for 72 h. Paired 3-mm punch skin biopsies were either obtained from healthy participants and cultured in KGM-Gold medium in the presence and absence of 10 ng/ml IL-29 for 24 h or were obtained from lesional and non-lesional areas of psoriatic patients and cultured in KGM-Gold medium in the presence of 5 μg/ml of neutralizing anti-IL-29 polyclonal antibodies or control goat immunoglobulin (Ig)G for 72 h. All cytokines and antibodies mentioned above were purchased from R&D Systems with the exception of IL-21, which was purchased from Miltenyi.
Mice
Depilated 9–11-week-old male 129/SvJ mice were sensitized with 2,4-dinitro-1-fluorobenzene (DNFB, Sigma-Aldrich) [0.5 %, dissolved in acetone/olive oil (4:1); application on the ventral skin] at day 5. At day 0, 2, and 4, 20 μl of phosphate-buffered saline was intradermally injected into the right ear and the right back side. Furthermore, phosphate-buffered saline, 500 ng mIL-28A, 500 ng mIFN-γ, or both (R&D Systems) in a total volume of 20 μl were injected into the left ear and the left back side. Ear thickness was measured at day 0, 4 (both before injection), and 5. At day 5, mice were sacrificed and ear and back skin punch biopsies were taken. All experimental protocols were conducted according to the German animal protection laws and approved by the Berlin Landesamt für Gesundheit und Soziales.
Patients
Skin biopsies for polymerase chain reaction on reverse transcribed RNA (RT-qPCR) and explant cultures as well as blood for plasma recovery were obtained from adult control participants [21 to 63 years old (mean ± SD: 40.5 ± 9.6)], patients with plaque psoriasis [19 to 67 years old (mean ± SD: 43.7 ± 13.3), 48.7 % moderate to severe disease (psoriasis area and severity index >10)], and patients with atopic dermatitis (AD) [20 to 55 years old (mean ± SD: 29.4 ± 11.1), 90 % moderate to severe disease]. The study protocol was approved by the clinical institutional review board of the Charité University Medicine, Berlin. The study was conducted according to the principles expressed in the Declaration of Helsinki.
RT-qPCR
Tissue homogenization, isolation of total cellular RNA, mRNA reverse transcription, and qPCR analysis were done as described previously [22]. Assays using fluorescent probes were purchased from Applied Biosystems. Analysis of the house-keeping gene human hypoxanthine-guanine phosphoribosyltransferase (HPRT) and mHPRT was included to normalize expressions.
ELISA
CXCL10 and CXCL11 detection in cell culture supernatants was performed using Duoset systems from R&D Systems. CXCL9, CXCL10, and CXCL11 detection in heparinized blood plasma was performed using Quantikine systems from R&D Systems.
CD26 enzyme activity assay
Enzymatic activity was analyzed through the ability of cell culture lysate to convert H-Gly-Pro-7-amino-4-methyl coumarin into fluorescent 7-amino-4-methyl coumarin using the fluorometric DPP4 activity assay from Sigma-Aldrich according to the manufacturer’s instructions. Units of proteolytic activity are expressed as the amount of enzyme to yield 1 μmol of product per minute at 37 °C.
Immunohistochemistry
Mouse skin biopsies were stained with anti-CD3 antibody (CD3-12; Acris) or matching isotype control antibody (rat IgG, Biolegend). Detection was performed using biotinylated polyclonal rabbit anti-rat IgG, streptavidin horseradish peroxidase, and AEC+ substrate (all from DAKO). Counter-staining was done with hematoxylin.
Statistical analysis
SPSS 19.0 software (SPSS Inc.) was used. Results of independent samples were analyzed using the Mann-Whitney U test (two-tailed). Results of paired samples were analyzed applying the Wilcoxon matched-pairs signed-rank test (two-tailed).
Results
IL-29 induces a selective release of CXCR3A-binding chemokines in skin cells
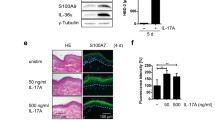
To investigate the role of IL-29 in the cutaneous chemokine network, we first treated primary human keratinocytes with the recombinant cytokine for 24 h and screened for the expression of chemokines using RT-qPCR. Among those chemokines attracting neutrophilic granulocytes (CXCL1, CXCL8), eosinophilic granulocytes/Th2-cells (CCL11, CCL26), Th1-cells/CD8+ T-cells/NK-cells (CXCL11), Th-cells/basophilic granulocytes (CCL17, CCL22), Th17-cells/myeloid dendritic cells (CCL20), Th22-cells (CCL27), and mixed mononuclear leukocytes including monocytes (CCL2, CCL4), only CXCL11 was found to be significantly induced by IL-29 (Fig. 1). Together with its siblings CXCL9 and CXCL10, CXCL11 is known to attract Th1-cells, CD8+ T-cells, NK-cells, and Th1/Th17 “transient” cells via the cellular Gαi-protein-coupled receptor CXCR3A [23–32]. Investigating whether IL-29 modulates expression of CXCR3A ligands in general, we found a significant albeit less pronounced (CXCL10) or minimal (CXCL9) upregulation of the CXCL11 siblings in the IL-29-stimulated keratinocytes (Fig. 2a). CXCR3A ligand expression was also upregulated in human primary melanocytes in response to IL-29, reaching levels that were 75- (CXCL11) or 10-fold (CXCL10) lower or were comparable (CXCL9) to those in keratinocytes (Fig. S1 and data not shown). The interaction between CXCR3A ligands and CXCR3A can be prevented by molecules including CD26, whose dipeptidyl peptidase activity transforms CXCL10 and CXCL11 into CXCR3A antagonists [33] and by the atypical chemokine receptor ACKR3, which may scavenge CXCL11 [34]. Our investigation revealed clear expression of CD26 and ACKR3 in keratinocytes but did not show any transcriptional influence of IL-29 on these molecules (Fig. 2b). Moreover, IL-29 did not modulate the catalytic activity of CD26 (Fig. S2).
IL-29 provokes a selective release of CXCR3A-binding chemokines in keratinocytes. Primary human keratinocytes were treated with IL-29 or were left without stimulation (control) for 24 h. Expressions were analyzed by RT-qPCR. Data from 5 to 6 experiments are shown (mean ± SEM). Significance of differences was analyzed using the Wilcoxon matched-pairs signed-rank test (two-tailed; *P < 0.05; n.s. not significant)
IL-29 induces further CXCR3A ligands without affecting modulators of the CXCR3A–CXCR3A ligand system. Primary human keratinocytes were treated with IL-29 or were left without stimulation (control) for 24 h. a, b Expressions were analyzed by RT-qPCR. Data from 5 to 6 experiments are shown (mean ± SEM). Significance of differences was analyzed using the Wilcoxon matched-pairs signed-rank test (two-tailed; *P < 0.05; n.s. not significant)
These data therefore suggest that IL-29, by specifically attracting CXCR3A-bearing cells, plays a proinflammatory role in the skin. A sustained IL-29 effect may be deduced from our observation that elevated CXCR3A ligand levels were detectable over a considerable period of time (Fig. 3a) and were not accompanied by negative feedback modulation as previously shown in Fig. 2b. In order to complement our results with in vivo-like systems, we first treated three-dimensional human epidermis models composed of stratified keratinocytes with IL-29 for 72 h. As demonstrated in Fig. 3b, IL-29 provoked a clear transcriptional increase of CXCR3A ligands but not of CCL17 or CCL22 in this system. Second, paired biopsies of total skin were obtained from healthy donors and were cultured in the presence and absence of IL-29 for 24 h. Again, CXCL11 and CXCL10 expression was reliably increased in response to IL-29 treatment (Fig. 3c).
The CXCR3A ligand-inducing effect of IL-29 is long-lived and recapitulated in complex stratified keratinocyte systems. a Primary human keratinocytes were treated with IL-29 or were left without stimulation (control) for 6 to 72 h. Expressions were analyzed by RT-qPCR. Data from 2 experiments are shown (mean ± range). b Human epidermis models were treated with IL-29 or were left unstimulated (control) for 72 h. Expressions were analyzed by RT-qPCR. Data from 3 experiments are shown (mean ± SEM). c Skin biopsies obtained from healthy donors were cultured in the presence or absence (control) of IL-29 for 24 h. Individual data from 5 experiments are shown. Significance of differences was analyzed using the Wilcoxon matched-pairs signed-rank test (two-tailed; *P < 0.05; n.s. not significant)
IL-29 cooperates with IFN-γ in the induction of CXCR3A ligands
During skin inflammation, a wide range of different cytokines is usually present in situ [35]. Consequently, we investigated the interaction of relevant mediators with IL-29 regarding its CXCR3A ligand-inducing ability. To this aim, primary keratinocytes were cultured with a range of keratinocyte-targeting cytokines including IL-17A, IL-22, IL-21, IFN-γ, IL-1β, IL-19, and TNF-α, each in the presence and absence of IL-29 for 24 h. IFN-γ induced CXCL10 (Fig. S3) and CXCL11 (Fig. 4a) mRNA even more strongly than IL-29, and some synergy could be suggested with both cytokines. Assessing a higher number of experiments for secreted CXCL10 and CXCL11 after 24 and 48 h, a stronger capacity of IFN-γ and cooperation of both inducers could be confirmed [fold increase over summed levels induced by the single cytokines: 1.89 ± 0.49 (CXCL10, 24 h); 2.24 ± 0.36 (CXCL11, 24 h); 3.78 ± 1.43 (CXCL11, 48 h), P < 0.05] (Fig. 4b and Fig. S4). Apart from IFN-γ, also TNF-α and IL-1β (slightly) induced CXCL10 and CXCL11 expressions and strengthen the IL-29-induced increase (Fig. 4b and Fig. S4).
IFN-γ amplifies the chemokine-inducing effects of IL-29. a Primary human keratinocytes were treated with IL-29 in the presence and absence of psoriasis-relevant cytokines or were left unstimulated (control) for 24 h. Expression was analyzed by RT-qPCR. Data from 3 experiments are shown (mean ± SEM). b Primary human keratinocytes were treated with IL-29 in the presence and absence of IFN-γ or were left unstimulated (control) for 24 h. Chemokine concentration in supernatant was analyzed by ELISA. Data from 6 to 9 experiments are shown (mean ± SEM). Significance of differences between IL-29/IFN-γ group and all other groups as well as between control and IL-29 group was analyzed using the Wilcoxon matched-pairs signed-rank test (two-tailed; *P < 0.05)
Murine IL-29 counterpart induces cutaneous T-cell infiltration in vivo
In the next step, we aimed to investigate the consequence of the IL-29 effect and of IL-29’s cooperative action with IFN-γ in vivo. Interestingly, while in humans, the IL-28/29 cytokine subfamily consists of IL-29, IL-28A, and IL-28B, in the mouse, only IL-28A and IL-28B exist [36]. We therefore compared the effects of the murine (m)IL-28A (on murine keratinocytes) with the effects of human IL-29 (on human keratinocytes). As demonstrated in Fig. 5a, b, both cytokines induced a similar pattern and strength of effects, including the upregulation of CXCL10, the antiviral protein MX1, and the signaling molecule signal transducers and activators of transcription(STAT)1, while having no effect on the differentiation marker keratin (K)10 and the ABP S100A9. We then injected mIL-28A in the presence and absence of mIFN-γ into the skin of mice that had been topically sensitized to enable immune cell diapedesis. In line with our in vitro results obtained with the conventional and more complex human systems, mIL-28A induced cutaneous expression of mCXCL10 and mCXCL11 and amplified the effect of mIFN-γ in this regard [fold increase over summed levels induced by the single cytokines: 2.21 ± 0.38 (CXCL10); 2.07 ± 0.32 (CXCL11), P < 0.05] (Fig. 6a and data not shown). Moreover, mIL-28A and mIFN-γ cooperatively provoked cutaneous T-cell infiltration (Fig. 6b, c) and, in consequence, cytokine expression (Fig. 6d) and skin swelling (Fig. 6e).
IL-29 and its murine counterpart IL-28A show similar effects. Primary human (a) and murine (b) keratinocytes were treated with IL-29 and mIL-28A, respectively, or were left unstimulated (controls) for 24 h. Expressions were analyzed by RT-qPCR. Data from 5 (mean ± SEM) experiments are shown. Significance of differences was analyzed using the Wilcoxon matched-pairs signed-rank test (two-tailed; *P < 0.05; n.s. not significant)
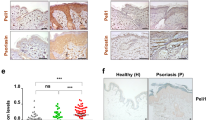
Murine IL-28A induces skin T-cell infiltration in mice, which is further increased by murine IFN-γ. Mice were sensitized by cutaneous application of DNFB. After 5 days (day 0), 7 days (day 2), and 9 days (day 4), PBS, murine IL-28A, murine IFN-γ, or a mixture of both cytokines were intradermally injected into one ear or one side of the back, and PBS (control) was injected into the other ear or side of the back. At day 5, mice were sacrificed and back skin samples were taken. (a, d) Day 5 skin samples were analyzed for expression by qRT-PCR. Mean ± SEM data from 4 mice are shown. (b, c) Day 5 skin samples were analyzed for epidermal CD3+ cell numbers by immunohistochemistry. Mean (±SEM) data (per millimeter skin section length) from 8 mice per group (*P < 0.05; Wilcoxon matched-pairs signed-rank test) (b) or a representative picture set of pictures (magnification 1:50) (c) are presented. Staining with respective isotype control Ab did not give any signal. (e) Ear thickness was measured at day 0, 4 (both before injection), and 5 is shown as mean data (±SEM) from 3 to 4 mice
In psoriasis, elevated IL-29 expression in lesional skin is associated with high CXCR3A ligand levels
Finally, we sought to translate our findings into human disease. First, we investigated whether the known elevated IL-29 expression in psoriasis would be associated with elevated CXCR3A ligand expression. Skin biopsies were taken from chronic lesions of respective patients and, for comparison, from chronic lesions of AD patients as well as from healthy skin of control donors, both of which lack IL-29 expression [16]. Actually, the expression of all three chemokines was found to be elevated in psoriasis compared to healthy and AD skin, with CXCL11 showing the greatest increase compared to healthy skin (Fig. 7a and Fig. S5a). In line with the differential IL-29 expression in lesional versus non-lesional psoriatic skin [16], cutaneous CXCL10 and CXCL11 levels were significantly higher in lesional compared to paired non-lesional psoriatic skin (Fig. 7b). Correlation analysis revealed a clear association between elevated lesional CXCL10 and CXCL11 levels with epidermal T-cell infiltration in the patients (Table S1).
CXCR3A ligand expression is elevated in lesional skin of psoriatic patients in an IL-29-dependent manner. a Chemokine expression was analyzed in biopsies from skin of 10 healthy control donors and lesional skin of 15 psoriasis and 10 AD patients by RT-qPCR. Mean data ± SEM are shown. b Chemokine expression in paired non-lesional (nl) and lesional (l) skin biopsies of 8 psoriasis patients was analyzed by RT-qPCR and is given as individual values. c Biopsies obtained from lesional skin of psoriasis patient were cultured in the presence of neutralizing anti-IL-29 or control antibodies for 72 h. Chemokine expression was analyzed by RT-qPCR and is given as percent of the IgG treated group. d Chemokine blood plasma levels of 20 healthy control donors and 20 psoriasis patients were analyzed by ELISA. Mean data ± SEM are shown. Significance of differences was analyzed using either the Mann-Whitney U test (two-tailed; **P < 0.01, ***P < 0.001; n.s. not significant) (a, d) or the Wilcoxon matched-pairs signed-rank test (two-tailed; **P < 0.01) (b). AD atopic dermatitis
To further support the role of IL-29 expression for the elevated cutaneous CXCR3A ligand expression in psoriasis, we secondly investigated the effect of IL-29 neutralization in skin biopsies obtained from these patients. Two biopsies obtained from the same psoriatic lesion were cultured in the presence of anti-IL-29 or control antibodies for 72 h. As demonstrated in Fig. 7c, anti-IL-29 antibody actually decreased the expression of CXCL10 and CXCL11 but did not influence CCL20 expression. These results clearly demonstrate that IL-29 is an important mediator of the elevated CXCR3A ligand levels in psoriatic skin. Third, we asked whether elevated lesional CXCRA3 ligand levels would reach the circulation and measured blood plasma concentrations of these chemokines in psoriasis patients versus healthy donors. Indeed, patients’ levels were significantly increased for CXCL11 (Fig. 7d) and may therefore reflect both the lesional IL-29 production and the lesional CXCR3A+ cell infiltration in this situation. In contrast, blood levels of CXCL9 and CXCL10 were not significantly increased (Fig. 7d and Fig. S5b).
Discussion
Our study provides evidence that IL-29 contributes to the infiltration of CXCR3A-bearing immune cells into the psoriatic skin. First, IL-29 induced the expression of CXCR3A ligands CXCL9, CXCL10, and CXCL11 in its target cells in the human skin: in keratinocytes and, with a much less potency, in melanocytes. Interestingly, we observed a clear grading of the IL-29 effect with respect to the individual ligands. Whereas the CXCL9 induction was rather weak, followed by the level of CXCL10 induction, strong upregulation of CXCL11 was observed in IL-29-treated keratinocytes. CXCR3A ligand induction was also seen in complex systems like three-dimensional epidermis models, whole human skin explants, and in mouse skin in vivo. Second, IL-29 production in lesional psoriatic skin was associated with elevated local CXCR3A ligand expression, and neutralization of IL-29 in explanted skin from psoriatic lesions markedly reduced abundance of these chemokine transcripts. Third, when injected into mouse skin, murine IL-29 counterpart provoked local infiltration of T-cells, T-cell cytokine expression and, as a consequence of induced inflammation, skin thickening.
CXCR3A is one variant expressed from the CXCR3 gene, which has been redesignated from the formerly simple “CXCR3” upon knowledge of an alternatively spliced transcript (CXCR3B), which additionally binds CXCL4 and mediates the antiangiogenic activity of CXCL4, CXCL9, CXCL10, and CXCL11. The expression of CXCR3A is controlled by the transcription factor T-bet. Coupled to Gαi, CXCR3A mediates pro-migratory signaling [32, 37, 38]. CXCR3A-bearing cells include Th1-cells, CD8+ cells, NK-cells, and Th1/Th17 “transient” cells [23–32]. The latter express both, Th1-cell features (CXCR3A, T-bet, high IFN-γ production capacity) and Th17-cell markers (CCR6, RORγt) ([25, 27, 39] and own unpublished data). All of these cells are present in the psoriatic skin [1], and especially the Th1/Th17 transient cells might be highly pathogenic in human chronic inflammatory diseases [39–41]. Thus, although IL-29 does not seem to efficiently influence immune cells directly [20, 42], it may contribute indirectly to the pathogenesis of such disorders.
IL-29 (and its siblings IL-28A and IL-28B) is known to bind to the IL-28R1/IL-10R2 receptor complex, which initiates signal transduction mainly via the phosphorylation of STAT1 and STAT2. Activated STATs form either homodimers or, involving IRF9, the ISGF3 transcription factor, both of which translocate in the cell nucleus, where they bind to the GAS and ISRE sites within the promoters of target genes. IL-28R1/IL-10R2 receptor complex engagement was additionally described to activate the NFκB pathway [19]. Indeed, the promoter regions of CXCL10 and CXCL11 contain multiple GAS and ISRE sites and NFκB response elements [43, 44]. Interestingly, the chemokine induction by IL-29 was quite selective, with no induction of other chemokines or modulation of molecules interfering with the CXCR3A/CXCR3A ligand interaction in keratinocytes.
Regarding the CXCR3A ligand induction in keratinocytes, IL-29 demonstrated cooperative action with IFN-γ and, in a much less pronounced manner, with IL-1β and TNF-α. Correspondingly, co-administration of murine IL-29 and IFN-γ counterparts into mouse skin led to amplified T-cell skin infiltration and inflammatory response.
Although IL-29 production can be induced by viral and bacterial stimuli via pattern recognition receptors mainly in plasmacytoid dendritic cells (pDCs) and myeloid DCs (mDCs) (mainly those mDCs with high CD141 expression) [45, 46] and it has become famous for its antiviral role [19, 42], the main IL-29 producers in the psoriatic skin seem to be skin-infiltrating Th17-cells, Th1-cells, and perhaps Th17/Th1 transient cells or RORγt-expressing innate lymphoid cells [16]. The support of cutaneous CXCR3A-based cell infiltration by IL-29 might therefore represent an important positive feedback regulation in the psoriatic skin. This in turn may contribute to the maintenance of lesions and is in line with the elevated IL-29 expression particularly in chronic lesions.
Interestingly, CXCR3A ligand expression in psoriatic skin was associated with significantly increased CXCL11 blood plasma levels and a trend to increased CXCL10 levels in these patients, while the blood concentrations of the ligand with the lowest induction by IL-29 (CXCL9) were not altered. CXCL11 blood levels may therefore be useful as a biomarker for the local action of IL-29 (and IFN-γ). Increased circulating CXCR3A ligand concentration (CXCL10 and CXCL11) was also found after single application of pegylated IL-29 into humans [47].
Besides psoriasis, IL-29-induced CXCR3A ligand-dependent immune cell infiltration might play a pathogenetic role in cutaneous lupus erythematosus, a disease with superior cutaneous IL-29 production, as recently demonstrated by the Wenzel group [48].
Based on the data of this manuscript, we suggest that the excellent clinical effectiveness of pegylated IL-29, which is currently under development for the therapy of hepatitis C infection [49], may be due not only to the direct antiviral effect (by inducing AVPs and virus-recognizing pattern recognition receptors) on liver cells but also on its contribution regarding the infiltration of virus-combating immune cells (CD8+ T-cells, NK-cells, Th1-cells).
In summary, our data demonstrate that the Th17-cell cytokine IL-29, by specifically inducing the release of CXCR3A ligands in skin tissue cells, triggers cutaneous immune cell infiltration and might play a pathogenetic role in chronic inflammatory diseases.
References
Sabat R, Wolk K (2014) Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S (eds) Psoriasis: diagnosis and management. Wiley-Blackwell, pp. 28–48
Schneider MR, Werner S, Paus R, Wolf E (2008) Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol 173:14–24
Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y et al (2012) The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37:74–84
Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB et al (2006) IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 203:2577–2587
He M, Liang P (2010) IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J Immunol 184:1793–1798
Kumari S, Bonnet MC, Ulvmar MH, Wolk K, Karagianni N, Witte E, Uthoff-Hachenberg C, Renauld JC, Kollias G, Toftgard R et al (2013) Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 39:899–911
Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM et al (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178:2229–2240
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S et al (2009) IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 87:523–536
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36:1309–1323
Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Docke WD, Asadullah K, Volk HD et al (2009) The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol 39:3570–3581
Li W, Man XY, Chen JQ, Zhou J, Cai SQ, Zheng M (2014) Targeting VEGF/VEGFR in the treatment of psoriasis. Discov Med 18:97–104
Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM (2005) Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 6:57–64
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203:2271–2279
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21:241–254
Wolk K, Warszawska K, Hoeflich C, Witte E, Schneider-Burrus S, Witte K, Kunz S, Buss A, Roewert HJ, Krause M et al (2011) Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol 186:1228–1239
Wolk K, Witte K, Witte E, Raftery M, Kokolakis G, Philipp S, Schonrich G, Warszawska K, Kirsch S, Prosch S et al (2013) IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci Transl Med 5:204ra129
Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP (2003) IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4:69–77
Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J et al (2003) IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4:63–68
Witte K, Witte E, Sabat R, Wolk K (2010) IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev 21:237–251
Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K (2009) Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun 10:702–714
Wolk K, Witte K, Sabat R (2010) Interleukin-28 and interleukin-29: novel regulators of skin biology. J Interferon Cytokine Res 30:617–628
Wolk K, Mitsui H, Witte K, Gellrich S, Gulati N, Humme D, Witte E, Gonsior M, Beyer M, Kadin ME et al (2014) Deficient cutaneous antibacterial competence in cutaneous T-cell lymphomas: role of Th2-mediated biased Th17 function. Clin Cancer Res 20:5507–5516
Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE et al (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med 187:2009–2021
Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B (1996) Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med 184:963–969
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646
Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A et al (1998) Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 187:129–134
Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N et al (2010) Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467:967–971
Hickman HD, Reynoso GV, Ngudiankama BF, Cush SS, Gibbs J, Bennink JR, Yewdell JW (2015) CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity 42:524–537
Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR (1998) The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101:746–754
Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM (1999) Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol 162:3840–3850
Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, Galli G, Francalanci M, Manetti R, Marra F et al (2001) Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood 97:601–607
Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S (2015) CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev 26:311–327
Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, Detheux M, Parmentier M, Durinx C, Lambeir AM et al (2001) Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98:3554–3561
Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ et al (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203:2201–2213
Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, Ding J, Stuart PE, Xing X, Kochkodan JJ et al (2014) Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol 134:1828–1838
Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP et al (2006) Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res 66:4468–4477
Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E et al (2003) An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197:1537–1549
Taqueti VR, Grabie N, Colvin R, Pang H, Jarolim P, Luster AD, Glimcher LH, Lichtman AH (2006) T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol 177:5890–5901
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F et al (2007) Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861
Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A (2009) Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol 66:390–402
Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, Reynolds GM, Lee-Turner L, Kalia N, Hubscher SG et al (2012) CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol 57:1044–1051
Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, Virgin HW (2015) Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347:269–273
Casrouge A, Bisiaux A, Stephen L, Schmolz M, Mapes J, Pfister C, Pol S, Mallet V, Albert ML (2012) Discrimination of agonist and antagonist forms of CXCL10 in biological samples. Clin Exp Immunol 167:137–148
Tensen CP, Flier J, Rampersad SS, Sampat-Sardjoepersad S, Scheper RJ, Boorsma DM, Willemze R (1999) Genomic organization, sequence and transcriptional regulation of the human CXCL 11(1) gene. Biochim Biophys Acta 1446:167–172
Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G (2004) Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol 34:796–805
Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A et al (2010) Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med 207:2703–2717
Freeman J, Baglino S, Friborg J, Kraft Z, Gray T, Hill M, McPhee F, Hillson J, Lopez-Talavera JC, Wind-Rotolo M (2014) Pegylated interferons Lambda-1a and alfa-2a display different gene induction and cytokine and chemokine release profiles in whole blood, human hepatocytes and peripheral blood mononuclear cells. J Viral Hepat 21:e1–e9
Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, Wenzel J (2011) Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol 131:133–140
Muir AJ, Arora S, Everson G, Flisiak R, George J, Ghalib R, Gordon SC, Gray T, Greenbloom S, Hassanein T et al (2014) A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J Hepatol 61:1238–1246
Acknowledgments
The authors would like to acknowledge Annette Buss and Brigitte Ketel for excellent technical assistance. This study was supported by research grant WO 1567/1-1/2 (to Kerstin Wolk) and SA1868/2 (to Robert Sabat) from the German Research Foundation (Deutsche Forschungsgemeinschaft, http://www.dfg.de/) and by a research grant 01ZX1312A from the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, http://www.bmbf.de/) (to Kerstin Wolk and Robert Sabat).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 91 kb)
Rights and permissions
About this article
Cite this article
Witte, E., Kokolakis, G., Witte, K. et al. Interleukin-29 induces epithelial production of CXCR3A ligands and T-cell infiltration. J Mol Med 94, 391–400 (2016). https://doi.org/10.1007/s00109-015-1367-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1367-y