Abstract
In this review, we provide recommendations as well as summarize available data on the optimal time to initiate venous thromboembolism chemoprophylaxis after severe trauma. A general approach to the severe polytrauma patient is provided as well as in-depth reviews of three high-risk injury subgroups: patients with traumatic brain injury, solid organ injury, and pelvic fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) has long been recognized as a serious and potentially life-threatening complication following major trauma. Paul von Bruns reported the occurrence of pulmonary embolism (PE) among 30 patients with fractures, many of which were fatal, in 1885 [1]. In the 1960s, Sevitt and Gallagher identified deep vein thromboses (DVT) in 81% of trauma decedents who underwent autopsy [2], while Freeark et al. diagnosed DVT in 35% of immobilized trauma patients using venography [3]. These early investigations contributed to the finding by a 1986 National Institutes of Health Consensus Panel on VTE prevention that the risks of VTE had “not been defined for the general trauma population” and that “efficacy and safety of various forms of prophylaxis have not been evaluated in patients with multisystem trauma” [4].
Recognizing the need for high-quality evidence, Geerts et al. published their landmark prospective study using venography in 349 consecutive trauma patients with severe injuries [5]. No mechanical or pharmacologic prophylaxis was used during the study period. Venography identified lower extremity DVT in 58% of patients, of which one in five (18%) were proximal vein DVT. This study emphasized the urgent need to establish effective strategies to prevent DVT in trauma patients. Foremost has been the use of pharmacologic agents for VTE prophylaxis (chemoprophylaxis), typically in the form of low-dose unfractionated heparin (UH) or low molecular weight heparin (LMWH). Geerts et al. compared the effectiveness of LMWH to UH in a randomized controlled trial (RCT) of severely injured patients [6]. While DVT were identified in 29%, LMWH was associated with 30% lower risk of DVT overall, and 58% lower risk of proximal vein DVT. Major bleeding complications occurred in only 6 of 344 patients (< 2%).
These early studies provided foundational evidence to the research of VTE after trauma: VTE are common, and chemoprophylaxis is a highly effective risk reduction strategy. Therefore, chemoprophylaxis should be initiated as early as feasible in patients with serious injuries. However, trauma is a heterogenous disease and the risks for both thrombosis and bleeding vary widely between patients. For this reason, the study of optimal strategies for chemoprophylaxis has been at the center of this field of trauma research for three decades. In this paper, we summarize the current evidence for the optimal timing and type of chemoprophylaxis in patients with severe polytrauma and discuss the nuances of these strategies in two specific subpopulations, those with traumatic brain injury (TBI), those with solid organ injury, and patients with pelvic fractures.
VTE chemoprophylaxis after severe polytrauma: general principles
The risks for both VTE and bleeding complications vary widely among patients with multiple severe injuries. Geerts et al. identified specific patterns of injury that were independent risk factors for VTE, a list which has been built upon in subsequent studies [5, 7,8,9]. Such injuries include TBI, orthopedic injuries such as pelvic and long bone fractures, spinal cord injury, and major venous injury. Conceptually, this heightened risk of thrombosis is related to a constellation of factors known as Virchow’s triad [10], where patients experience inflammation, immobility, and hypercoagulability as the result of their injuries. This increased risk of VTE is associated with significant morbidity and mortality [11, 12]. The most feared complication, PE, is associated with an attributable mortality of 10–50% [7, 13]. Nonetheless, the burden of morbidity resultant from DVTs themselves should not be minimized. This morbidity includes post-thrombotic (or post-phlebitic) syndrome, a condition in which the limb develops symptoms of chronic venous insufficiency in the wake of an acute DVT [14]. These symptoms include heaviness, edema, and discoloration of the involved extremity. The pathophysiology remains incompletely understood, but a negative impact on patient quality of life is evident. Phlegmasia cerulea dolens may also occur, in which the DVT-induced edema becomes limb-threatening due to its extent and can lead to compartment syndrome and/or ischemia [15]. DVTs can also cause skin necrosis and ulceration [16]. Fortunately, the timely administration of chemoprophylaxis with UH or LMWH is associated with a dramatic reduction in VTE risk [11].
With scientific evaluation over the past three decades, we have begun to appreciate several nuances with chemoprophylaxis against VTE in trauma. These include practical details about the optimal delivery of chemoprophylaxis and which patient populations warrant special consideration. Practical details of chemoprophylaxis include the selection of pharmacologic agent, dosing, potential utility of monitoring for therapeutic efficacy, and timing of initiation. In general, LMWH is preferred over UH. This is based on literature demonstrating greater reduction in risk of VTE with LMWH [17,18,19], lower risk of heparin-induced thrombocytopenia [20], and the potential for improved neurologic outcomes among patients with TBI [21].
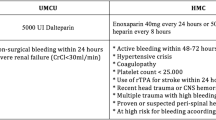
In contemporary trauma practice, the use of UH as chemoprophylaxis is reserved for patients with renal insufficiency. Even among these patients, some clinicians favor reduced dose LMWH due to the superior effectiveness of this agent to prevent VTE after major trauma. Enoxaparin is the most common form of LMWH used for chemoprophylaxis. Its optimal dosing in this context is controversial. Broadly, 30 mg Q12H has been considered standard, but this is evolving with evidence that many patients may be underdosed [22]. Recently published guidelines by the Western Trauma Association and American Association for the Surgery of Trauma recommend the use of 40 mg Q12H, except in the setting of actively bleeding, reduced creatinine clearance, TBI, or a spinal cord injury [23, 24]. Monitoring for effective dosing of chemoprophylactic agents is possible via measurement anti-Xa levels [25,26,27], although the implementation and cost-effectiveness of this approach remains controversial.
The optimal time to initiate chemoprophylaxis has also been the subject of controversy. In brief, chemoprophylaxis should be initiated as soon as it is safe. This can be a complex decision in clinical practice as one weighs the risk of VTE against the possibility of propagating hemorrhage. As the nonoperative management of certain injury types, such as solid organ injuries, becomes more common this balance becomes harder to strike. Historically, chemoprophylaxis was deferred until several days after injury, until mounting evidence showed the intuitive association of prophylaxis delay with increased risk of VTE [11, 17, 28]. For example, Nathens et al. demonstrated a three-fold increase in VTE risk when chemoprophylaxis was delayed longer than 4 days after injury [11]. Byrne et al. showed an 8% increase in odds of VTE for each day that chemoprophylaxis was delayed in patients with severe TBI [28]. Contemporarily, most trauma patients should receive their first dose of chemoprophylaxis within 24 h of admission [23]. Patients at high-risk for bleeding, or in whom bleeding might have catastrophic consequences, warrant special consideration and a nuanced approach to safe initiation. We discuss these nuances further in two specific patient populations, those with TBI and those with solid organ injuries, in the remainder of this review.
VTE chemoprophylaxis after severe polytrauma: traumatic brain injury
Traumatic brain injury and coma have been identified as independent predictors of VTE [7, 8, 29]. This risk is even higher among patients with multiple high-risk injuries, including spinal column and orthopedic injuries, which are common [5, 7,8,9, 29]. Therefore, pharmacologic VTE prophylaxis should be commenced as early as feasible in this population. However, this imperative must be balanced against the risk of precipitating expansion of intracranial hemorrhage (ICH). The fear of provoking life-threatening bleeding has historically led physicians to defer chemoprophylaxis, often indefinitely. This clinical challenge has been further compounded by a lack of evidence, as such patients have been routinely excluded from studies evaluating pharmacologic VTE prophylaxis in trauma [6].
Recent studies have sought to clarify when it is safe to initiate chemoprophylaxis in patients with TBI and ICH. One great contribution has been the classification of patients into categories of risk for worsening of ICH. Phelan et al. recognized that smaller injury patterns would likely stabilize more rapidly and therefore tolerate chemoprophylaxis earlier [30]. This was the basis for the Modified Berne-Norwood Criteria, which grouped patients into low, moderate, or high-risk of ICH progression based on number and size of intraparenchymal and extra-axial hemorrhage [31]. The Criteria proposed initiation of chemoprophylaxis when stability of ICH is demonstrated on repeated head computed tomography (CT), at 24 h in low-risk patients and at 72 h in moderate-risk patients. High-risk patients, those who undergo neurosurgical interventions or have expansion after 72 h, are recommended to receive an inferior vena cava filter in lieu of chemoprophylaxis. In the DEEP I RCT, Phelan et al. found that early chemoprophylaxis with LMWH in low-risk patients with stable head computed tomography (CT) at 24 h was safe [32]. Specifically, those study authors conducted a dual institution study in which patients with minor TBI who had a stable CT head 24 h after injury were then randomized to either BID enoxaparin or placebo. A routine CT head was then conducted 24 h after initiation of enoxaparin or placebo to investigate for radiographic progression of TBI, the primary outcome of the study. The primary finding from this study was that TBIs progressed at similar rates following enoxaparin vs. placebo initiation (5.9% vs. 3.6%), a difference which was subclinical.
There remains a lack of high-quality clinical evidence to support the practices recommended for moderate and high-risk patients. Nonetheless, the Modified Berne-Norwood Criteria is used by the American College of Surgeons Trauma Quality Improvement Program (TQIP) in their Traumatic Brain Injury Best Practices Guideline [33] and recent society guidelines [24].
A series of retrospective observational studies have provided further evidence that timely chemoprophylaxis is safe in patients with TBI. A study of TQIP data from 3,634 patients with severe TBI treated at 186 trauma centers found that chemoprophylaxis initiated within 72 h was associated with a significant reduction in odds of both PE and DVT, but no increased risk of late neurosurgical intervention [34]. Patients treated at hospitals favoring early prophylaxis benefited from significantly fewer VTE. These observations have been replicated in subsequent observational studies and meta-analyses [35,36,37,38]. The safety of chemoprophylaxis remains less clear among patients that undergo early neurosurgical interventions (deemed high-risk by the Modified Berne-Norwood Criteria). In a recent retrospective cohort study of patients with severe TBI who received early craniotomy/craniectomy or intracranial monitor/drain insertion, earlier chemoprophylaxis was associated with lower odds of VTE, but also higher risk of repeated neurosurgical intervention [28]. Taken together, the timely initiation of chemoprophylaxis after stable head CT is likely safe in most patients. However, variation in practice persists and further blinded randomized trials are justified [39–40].
Controversy formerly surrounded the selection of pharmacologic agent for patients with TBI. Historically, UH was preferred because its lower half-life was felt to confer a lower risk of hemorrhagic complication. However, severe studies have demonstrated the superiority of LMWH over UH to prevent VTE following major trauma and TBI [6, 17, 28, 34]. Among patients with isolated severe TBI, LMWH is associated with 40–50% lower odds of PE compared with UH [17, 34], but no difference in occurrence of ICH-related complications [28, 34]. The risk of heparin-induced thrombocytopenia is dramatically lower with use of prophylactic dose LMWH (0.2% vs. 2.6%) [20]. There is also preliminary evidence that LMWH may confer a survival benefit compared to UH in patients with severe TBI [21, 28, 34], possibly owing to reduced post-injury neuroinflammation as demonstrated in animal studies [41, 42]. Such studies postulate empirically that enoxaparin may interfere with neurotransmitter signaling and thereby lead to reduced cerebral edema and neurologic recovery.
Despite these benefits of LMWH over UH, UH remains in common usage for chemoprophylaxis in patients with TBI, likely due to concerns of expansion of intracranial bleeding with the use of LMWH and historical comfort with UH as a chemoprophylactic agent for VTE. Foremost, a collaborative multidisciplinary effort between trauma, neurosurgery, critical care, pharmacy, and other supporting services is needed to achieve the delivery of chemoprophylaxis as early as safely feasible in this patient population.
VTE chemoprophylaxis after severe polytrauma: solid organ injury
The optimal approach for chemoprophylaxis among patients with solid organ injuries has also been a subject of controversy. It is a challenging topic to study due to the many possible permutations of injuries in such patients, including blunt versus penetrating mechanism, severity of the solid organ injury (typically quantified as AAST injury grade), therapeutic modality including operative or angioembolization strategies, and the presence of associated injuries that influence both VTE and bleeding risk (such as TBI or pelvic fractures) [28, 34, 43].
The literature guiding chemoprophylaxis strategies among patients with solid organ injuries are largely based on blunt trauma patients managed nonoperatively. These data are extrapolated, whether appropriate or not, to all subsets of patients with solid organ injury. Conceptually, prophylaxis should be started as early as feasible, when the patient is no longer bleeding, and the risk of provoking hemorrhage is no longer clinically meaningful. Studies of patients with blunt solid organ injuries managed nonoperatively suggest that chemoprophylaxis may be initiated within 48 h of admission, without increasing the risk of bleeding or failure of nonoperative management. This practice has been evaluated in several single center cohort studies and meta-analyses [44,45,46,47]. Most recently, a large prospective multicenter study found that early chemoprophylaxis, begun within 48 h of injury, was associated with significantly lower risk of VTE and no increased risk of bleeding complication [48]. Therefore, while no randomized study has been performed on this subject, initiation of chemoprophylaxis within 48 h of solid organ injury is believed to be safe.
Anecdotally, many clinicians believe that 48 h is too broad a time frame and that chemoprophylaxis should be initiated earlier within that window, even within the first 12 h. Clinical practice and experience have evolved dramatically, with trauma surgeons and intensivists now more aggressive in the goal of initiating chemoprophylaxis early. Some institutions even strive to start chemoprophylaxis in the emergency department among patients without clear contraindications. This emphasis on the importance of early prophylaxis has been captured in recent society guidelines published by the Western Trauma Association and American Association for the Surgery of Trauma [23–24]. Even in solid organ injury, these guidelines prioritize initiation of chemoprophylaxis within 24 h in low to moderate grade injuries, or within 48 h for high grade injuries when hemoglobin is stable.
Considerations for the selection of pharmacologic agent, dosing, and utility of monitoring are the same in patients with solid organ injury as they are among the general major trauma population. There is no evidence that the approach to these strategies should be tailored to the type or severity of solid organ injury. Society guidelines recommend either weight-based or uniform Enoxaparin 30 mg or 40 mg dosing Q12H LMWH for these patients [23–24].
Limitations to our current understanding of optimal chemoprophylaxis strategies in patients with solid organ injury stem from a lack of high-quality randomized studies, as is common in many domains of trauma research. The generalizability of existing evidence to specific subgroups of patients with solid organ injury is unclear, such as in those managed operatively or those with high grade injuries. The optimal strategies in these patients, who are at greatest risk for bleeding complications, require further study.
VTE chemoprophylaxis after severe polytrauma: pelvic fractures
The ideal management of VTEp after pelvic fractures, based on available data, can generally be considered to mirror the data regarding solid organ injury. VTEp is critical in these patients and ought to be initiated within 48 h of pelvic fractures [43], although in the absence of ongoing clinical significant bleeding, earlier may be prudent. The need for a particularly aggressive approach to VTEp initiation after pelvic fractures is suggested by a number of studies indicating that patients with pelvic fractures, particularly those who have undergone pelvic angioembolization or pre-peritoneal packing, are at especially heightened risk for VTE [49–50].
Conclusions
The body of evidence for optimal delivery of chemoprophylaxis to prevent VTE after major trauma has progressed immensely over the past three decades. Chemoprophylaxis should be initiated as early as possible, when clinically meaningful bleeding has stopped. For most patients, initiation within the first 24 h is safe. LMWH is the pharmacologic agent of choice. Specific subgroups of patients with major injury require special consideration, including those with TBI and solid organ injury. While early evidence and society guidelines advocate for early initiation of LMWH for chemoprophylaxis in these populations, future prospective and randomized studies are needed to elucidate the optimal strategies. A collaborative multidisciplinary approach should be taken to implementing durable guidelines for chemoprophylaxis at any trauma center.
Data availability
No datasets were generated or analysed during the current study.
References
Bruns P. Ueber Plotzliche Todesfalle Nach Knochenbrüchen in Folge Von Venenthrombose Und Embolie. Klin Chir 1886;2:1–18.
Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg. 1961;48:475–89. https://doi.org/10.1002/bjs.18004821103.
Freeark RJ, Boswick J, Fardin R. Posttraumatic venous thrombosis. Arch Surg. 1967;95(4):567–75. https://doi.org/10.1001/archsurg.1967.01330160037005.
NIH Consensus Panel. Prevention of venous thrombosis and pulmonary embolism. JAMA. 1986;256(6):744–9.
Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6. https://doi.org/10.1056/nejm199412153312401.
Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, Hamilton PA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7. https://doi.org/10.1056/nejm199609053351003.
Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625–32. https://doi.org/10.1097/SLA.0b013e3182300209.
Knudson MM, Lewis FR, Clinton A, Atkinson K, Megerman J. Prevention of venous thromboembolism in trauma patients. J Trauma. 1994;37(3):480–7. https://doi.org/10.1097/00005373-199409000-00025.
Haut ER, Chang DC, Pierce CA, Colantuoni E, Efron DT, Haider AH et al. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors-an analysis of the National Trauma Data Bank (NTDB). J Trauma. 2009;66(4):994-9; discussion 9-1001. https://doi.org/10.1097/TA.0b013e3181991adc
Kushner A, West WP, Khan Suheb MZ, Pillarisetty LS, Virchow Triad. 2022 Dec 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK539697/. Accessed: May 1, 2024.
Nathens AB, McMurray MK, Cuschieri J, Durr EA, Moore EE, Bankey PE et al. The practice of venous thromboembolism prophylaxis in the major trauma patient. J Trauma. 2007;62(3):557– 62; discussion 62– 3. https://doi.org/10.1097/TA.0b013e318031b5f5
Hecht JP, Han EJ, Cain-Nielsen AH, Scott JW, Hemmila MR, Wahl WL. Association of timing of initiation of pharmacologic venous thromboembolism prophylaxis with outcomes in trauma patients. J Trauma Acute Care Surg. 2021;90(1):54–63. https://doi.org/10.1097/ta.0000000000002912.
Ho KM, Burrell M, Rao S, Baker R. Incidence and risk factors for fatal pulmonary embolism after major trauma: a nested cohort study. Br J Anaesth. 2010;105(5):596–602. https://doi.org/10.1093/bja/aeq254.
Calderon Martinez E, Garza Morales R, Postthrombotic Syndrome. [Updated 2024 Jun 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK604213/
Ibrahim B, Kattimani R. Phlegmasia Cerulea Dolens, a Deadly complication of deep vein thrombosis: Case Report and Review of Literature. Cureus. 2021;13(11):e19927. https://doi.org/10.7759/cureus.19927.
Walker N, Rodgers A, Birchall N, Norton R, MacMahon S. Leg ulceration as a long-term complication of deep vein thrombosis. J Vasc Surg. 2003;38(6):1331–5. https://doi.org/10.1016/s0741-5214(03)00917-0.
Byrne JP, Geerts W, Mason SA, Gomez D, Hoeft C, Murphy R, et al. Effectiveness of low-molecular-weight heparin versus unfractionated heparin to prevent pulmonary embolism following major trauma: a propensity-matched analysis. J Trauma Acute Care Surg. 2017;82(2):252–62. https://doi.org/10.1097/ta.0000000000001321.
Gritsiouk Y, Hegsted DA, Schlesinger P, Gardiner SK, Gubler KD. A retrospective analysis of the effectiveness of low molecular weight heparin for venous thromboembolism prophylaxis in trauma patients. Am J Surg. 2014;207(5):648–51. https://doi.org/10.1016/j.amjsurg.2013.12.010. discussion 51– 2.
Singer GA, Riggi G, Karcutskie CA, Vaghaiwalla TM, Lieberman HM, Ginzburg E, et al. Anti-xa-guided enoxaparin thromboprophylaxis reduces rate of deep venous thromboembolism in high-risk trauma patients. J Trauma Acute Care Surg. 2016;81(6):1101–8. https://doi.org/10.1097/ta.0000000000001193.
Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710–5. https://doi.org/10.1182/blood-2005-04-1546.
Benjamin E, Recinos G, Aiolfi A, Inaba K, Demetriades D. Pharmacological thromboembolic prophylaxis in traumatic brain injuries: low Molecular Weight Heparin is Superior to Unfractionated Heparin. Ann Surg. 2017;266(3):463–9. https://doi.org/10.1097/sla.0000000000002359.
Robinson S, Zincuk A, Larsen UL, Ekstrøm C, Nybo M, Rasmussen B, Toft P. A comparative study of varying doses of Enoxaparin for Thromboprophylaxis in critically ill patients: a Double-Blinded, Randomised Controlled Trial. Crit Care. 2013;17(2):R75. https://doi.org/10.1186/cc12684.
Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: a Western Trauma Association critical decisions algorithm. J Trauma Acute Care Surg. 2020;89(5):971–81. https://doi.org/10.1097/ta.0000000000002830.
Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, et al. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg. 2022;92(3):597–604. https://doi.org/10.1097/ta.0000000000003475.
Hashim YM, Dhillon NK, Veatch JM, Barmparas G, Ley EJ. Clinical characteristics Associated with higher enoxaparin dosing requirements for venous thromboembolism Prophylaxis in Trauma patients. Am Surg. 2021;87(7):1177–81. https://doi.org/10.1177/0003134820979574.
Ko A, Harada MY, Barmparas G, Chung K, Mason R, Yim DA, et al. Association between Enoxaparin Dosage adjusted by Anti-factor Xa Trough Level and clinically evident venous thromboembolism after trauma. JAMA Surg. 2016;151(11):1006–13. https://doi.org/10.1001/jamasurg.2016.1662.
Teichman AL, Cotton BA, Byrne J, Dhillon NK, Berndtson AE, Price MA et al. Approaches for optimizing venous thromboembolism prevention in injured patients: Findings from the consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2023;94(3):469– 78. https://doi.org/10.1097/ta.0000000000003854
Byrne JP, Witiw CD, Schuster JM, Pascual JL, Cannon JW, Martin ND, et al. Association of venous thromboembolism Prophylaxis after neurosurgical intervention for traumatic brain Injury with Thromboembolic complications, repeated neurosurgery, and Mortality. JAMA Surg. 2022;157(3):e215794. https://doi.org/10.1001/jamasurg.2021.5794.
Reiff DA, Haricharan RN, Bullington NM, Griffin RL, McGwin G Jr., Rue LW 3. Traumatic brain injury is associated with the development of deep vein thrombosis independent of pharmacological prophylaxis. J Trauma. 2009;66(5):1436–40. https://doi.org/10.1097/TA.0b013e31817fdf1c.
Phelan HA. Pharmacologic venous thromboembolism prophylaxis after traumatic brain injury: a critical literature review. J Neurotrauma. 2012;29(10):1821–8. https://doi.org/10.1089/neu.2012.2459.
Phelan HA, Eastman AL, Madden CJ, Aldy K, Berne JD, Norwood SH, et al. TBI risk stratification at presentation: a prospective study of the incidence and timing of radiographic worsening in the Parkland Protocol. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S122–7. https://doi.org/10.1097/TA.0b013e3182606327.
Phelan HA, Wolf SE, Norwood SH, Aldy K, Brakenridge SC, Eastman AL, et al. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk traumatic brain injury: the delayed Versus Early Enoxaparin Prophylaxis I (DEEP I) study. J Trauma Acute Care Surg. 2012;73(6):1434–41. https://doi.org/10.1097/TA.0b013e31825ac49e.
American College of Surgeons Trauma Quality Improvement Program. Best Practices in Management of Traumatic Brain Injury. January 2015. https://www.facs.org/media/mkej5u3b/tbi_guidelines.pdf. Accessed: May 1, 2024.
Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain Injury: a propensity-matched cohort study. J Am Coll Surg. 2016;223(4):621–e315. https://doi.org/10.1016/j.jamcollsurg.2016.06.382.
Rivas L, Vella M, Ju T, Fernandez-Moure JS, Sparks A, Seamon MJ, Sarani B. Early chemoprophylaxis against venous thromboembolism in patients with traumatic brain Injury. Am Surg. 2022;88(2):187–93. https://doi.org/10.1177/0003134820983171.
Wu YT, Chien CY, Matsushima K, Schellenberg M, Inaba K, Moore EE, et al. Early venous thromboembolism prophylaxis in patients with trauma intracranial hemorrhage: analysis of the prospective multicenter Consortium of leaders in traumatic thromboembolism study. J Trauma Acute Care Surg. 2023;95(5):649–56. https://doi.org/10.1097/ta.0000000000004007.
Lu VM, Alvi MA, Rovin RA, Kasper EM. Clinical outcomes following early versus late pharmacologic thromboprophylaxis in patients with traumatic intracranial hemorrhage: a systematic review and meta-analysis. Neurosurg Rev. 2020;43(3):861–72. https://doi.org/10.1007/s10143-018-1045-y.
Störmann P, Osinloye W, Freiman TM, Seifert V, Marzi I, Lustenberger T. Early Chemical Thromboprophylaxis does not increase the risk of intracranial hematoma progression in patients with isolated severe traumatic brain Injury. World J Surg. 2019;43(11):2804–11. https://doi.org/10.1007/s00268-019-05072-1.
Hachem LD, Mansouri A, Scales DC, Geerts W, Pirouzmand F. Anticoagulant prophylaxis against venous thromboembolism following severe traumatic brain injury: a prospective observational study and systematic review of the literature. Clin Neurol Neurosurg. 2018;175:68–73. https://doi.org/10.1016/j.clineuro.2018.09.032.
Huijben JA, Pisica D, Ceyisakar I, Stocchetti N, Citerio G, Maas AIR, et al. Pharmaceutical venous thrombosis Prophylaxis in critically ill traumatic brain Injury patients. Neurotrauma Rep. 2022;2(1):4–14. https://doi.org/10.1089/neur.2021.0037.
Li S, Eisenstadt R, Kumasaka K, Johnson VE, Marks J, Nagata K et al. Does enoxaparin interfere with HMGB1 signaling after TBI? A potential mechanism for reduced cerebral edema and neurologic recovery. J Trauma Acute Care Surg. 2016;80(3):381-7; discussion 7–9. https://doi.org/10.1097/ta.0000000000000935
Wahl F, Grosjean-Piot O, Bareyre F, Uzan A, Stutzmann JM. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma. 2000;17(11):1055–65. https://doi.org/10.1089/neu.2000.17.1055.
Schellenberg M, Benjamin E, Inaba K, Heindel P, Biswas S, Mooney JL, Demetriades D. When is it safe to start pharmacologic venous thromboembolism Prophylaxis after pelvic fractures? A prospective study from a level I trauma Center. J Surg Res. 2021;258:272–7. https://doi.org/10.1016/j.jss.2020.08.077.
Murphy PB, Sothilingam N, Charyk Stewart T, Batey B, Moffat B, Gray DK, et al. Very early initiation of chemical venous thromboembolism prophylaxis after blunt solid organ injury is safe. Can J Surg. 2016;59(2):118–22. https://doi.org/10.1503/cjs.010815.
Joseph B, Pandit V, Harrison C, Lubin D, Kulvatunyou N, Zangbar B, et al. Early thromboembolic prophylaxis in patients with blunt solid abdominal organ injuries undergoing nonoperative management: is it safe? Am J Surg. 2015;209(1):194–8. https://doi.org/10.1503/cjs.010815.
Schellenberg M, Inaba K, Biswas S, Heindel P, Benjamin E, Strumwasser A, et al. When is it safe to start VTE Prophylaxis after Blunt Solid Organ Injury? A prospective study from a level I trauma Center. World J Surg. 2019;43(11):2797–803. https://doi.org/10.1007/s00268-019-05096-7.
Murphy PB, de Moya M, Karam B, Menard L, Holder E, Inaba K, Schellenberg M. Optimal timing of venous thromboembolic chemoprophylaxis initiation following blunt solid organ injury: meta-analysis and systematic review. Eur J Trauma Emerg Surg. 2022;48(3):2039–46. https://doi.org/10.1007/s00068-021-01783-0.
Schellenberg M, Owattanapanich N, Emigh B, Van Gent JM, Egodage T, Murphy PB, et al. When is it safe to start venous thromboembolism prophylaxis after blunt solid organ injury? A prospective American Association for the surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2024;96(2):209–15. https://doi.org/10.1097/ta.0000000000004163.
Bokenkamp M, Dorken Gallastegi A, Brown T, Hwabejire JO, Fawley J, Mendoza AE, Saillant NN, Fagenholz PJ, Kaafarani HMA, Velmahos GC, Parks JJ. Angioembolization in severe pelvic trauma is Associated with venous thromboembolism. J Surg Res. 2023;283:540–9. https://doi.org/10.1016/j.jss.2022.10.054.
Kolitsas A, Williams EC, Lewis MR, Benjamin ER, Demetriades D. Preperitoneal pelvic packing in isolated severe pelvic fractures is associated with higher mortality and venous thromboembolism: a matched-cohort study. Am J Surg. 2024;236:115828. https://doi.org/10.1016/j.amjsurg.2024.115828.
Author information
Authors and Affiliations
Contributions
Both authors wrote the main manuscript text, reviewed the final manuscript, and approved the manuscript for submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Byrne, J.P., Schellenberg, M. Venous thromboembolism chemoprophylaxis after severe polytrauma: timing and type of prophylaxis matter. Eur J Trauma Emerg Surg (2024). https://doi.org/10.1007/s00068-024-02651-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00068-024-02651-3