Abstract
Purpose
There are several hints that bacterial colonization might be an often overseen cause of non-union. Modern procedures like PCR have been reported to diagnose bacterial colonization with a high degree of accuracy. While PCR is not ubiquitously available, we hypothesize that biopsies from the non-union site are comparable to PCR results reported in the literature.
Methods
Retrospective analysis of microbiological results of biopsies from non-unions (femoral or tibial, history of revision surgery, and/or open fracture) with stable osteosynthesis, no clinical signs of local infection were analysed. CRP and leucocyte count were taken on admission. Multiple tissue samples (soft tissue and bone) were from the non-union (1–4 cm incision). Samples were cultivated for 2 weeks and tested following EUCAST protocols using VITEK® 2.
Results
11 tibia- and 7 femur non-union (44 ± 23.9 years), 11 open fractures (1 I°, 6 II°, 4 III° Gustillo Anderson), 0–5 revisions, and 4.1 (± 1.8) tissue samples were taken 8.5 (± 1.7) months after trauma. Cultures were positive in 8/18 (44,4%) (3/18 Propionibacterium acnes, 1/18 S. capitis, and 4/18 S. epidermidis). There was neither a correlation between number of biopsies taken and positive culture results (Pearson R: − 0.0503, R2 0.0025), nor between positive culture results and leucocytes counts (Pearson R: − 0.0245, R2 0.0006) or CRP concentration (Pearson R: 0.2823, R2 0.0797).
Conclusion
The results confirm that the presence of bacteria in cases with no clinical signs of infection is a relevant issue. The prevalence of bacteria reported here is comparable that reported from cohorts tested with PCR or sonication. In most cases, there was only one positive biopsy, raising the question whether a contamination has been detected. Thus, to better understand the problem, it is necessary to gather more knowledge regarding the sensitivities and specificities of the different diagnostic procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fracture non-union occurs, depending on fracture localization and trauma mechanism, in 5–40% [1] of fractures. Even today, the classification of non-unions remains coarse grained and is based mainly on morphological patters distinguishing atrophic, hypertrophic, and oligotrophic types of non-union. Based on these observations, the therapeutic strategy aimed to optimize either the biological or the mechanical environment rendering treatment long lasting and costly [2, 3]. However, a thorough analysis of the cause for a non-union is the key to successful treatment. The “Diamond Concept” [4, 5] introduced by Giannoudis et al. provides a more sophisticated framework to analyze cases of disturbed bone healing. However, the presence of bacteria as a possible cause of non-union is not included in the diamond concept. Lately, the relevance of bacterial contamination of the non-union site and the possible impact of bacteria on osteoblasts has been proposed as an additional cause of non-union [3, 6, 7]. Particularly, open fractures and fractures that required multiple surgical procedures are considered at risk [8, 9]. While overt infection resulting from highly invasive bacterial species is easily diagnosed, clinically, the question to which extend clinically unapparent non-unions after open fractures might be caused by low-grade pathogens remains unanswered to date. Currently, the diagnostic golden standard is cultivation of bacteria from biopsies of the non-union site [3]. In comparison with other diagnostic procedures like histopathological analysis [10] and imaging studies [11], only microbiological work up can identify the pathogen and its pattern of resistance, which is the basis for an effective antibiotic therapy. While low-grade infection after prothetic joint replacement is a well-known entity with established diagnostic and therapeutic strategies, there is only sparse evidence even regarding the prevalence of bacteria in fracture non-unions.
It is well known that the sensitivity of bacterial swaps from the non-union site, though still used as primary diagnostic modality in many places, is too low to be used as a routine diagnostic procedure. As PCR-based techniques are not available comprehensively, and there are still several unanswered equations regarding their application in bone and non-union infection diagnosis, we hypothesize that in cases of fracture non-union after open fractures and/or multiple revision surgeries without clinical signs of infection the prevalence of bacteria as detected by open biopsies from the non-union site is at least equivalent to the prevalence reported from PCR-based infection diagnosis in the medical literature.
Materials and methods
We retrospectively analyzed the microbiological results of tissue samples collected from femoral and tibial non-union sites. The study was approved by the local ethics committee.
Non-union was defined as bony fusion of less than than ¾ of circumference on CT scan and pain at non-union site during weigh bearing at least 6 months after trauma, without progression of bony healing for at least 2 months on CT scan or plain radiograph. Patients who were admitted to our limb reconstruction unit between March 2012 and June 2013 with a femoral or tibial non-union and a history of revision surgery and/or open fracture were included. Further inclusion criteria were stable osteosynthesis on CT scan and absence of clinical signs of infection (local hyperaemia, warmth, swelling, and pain on palpation). In addition, C-reactive protein and leucocyte count were taken on admission.
Multiple tissue samples (soft tissue and bone) were obtained from the non-union site over an open incision of 1–4 cm depending on the anatomical location under aseptic conditions in the operating theatre. Before incision the skin was disinfected three times using SkinSept Color (Fa. Henkel, Germany), which contains chlorhexidine and alcohol. Patients did not receive antibiotic prophylaxis perioperatively. Radiological guidance was used to ensure that samples were taken from different locations of the non-union. Care was taken that the instruments did not get into contact with the skin during excision of the biopsies. The same surgical instrument was used for all biopsies.
Samples were cultivated for 2 weeks and tested following EUCAST protocols using VITEK® 2 (Fa. bioMérieux, France). Open fractures were classified following the Gustilo Anderson system. Values in brackets are standard deviations. Correlations were calculated as Pearson R.
Results
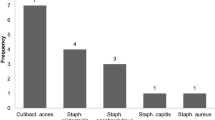
During the study period, 22 patients with tibial and 20 patients with femoral non-unions were admitted to our trauma centre. Eleven patients with tibia- and seven patients with femur-non-union met the inclusion criteria. Mean age of those 18 patients was 44 (± 23.9) years. Eleven had a history of open fractures (one I°, six II°, and four III° open fractures) and zero-to-five revisions. Seven patients had a history of one-to-three revision surgeries. From each patient, 4.1 (± 1.8) tissue samples were taken 8.5 (± 1.7) months after trauma. Bacteria were detected in 8 of the 18 non-unions (44%). In five cases, coagulase negative Staphylococci (COST) and, in three cases, Propionibacterium acnes (P. acnes) were cultivated (Fig. 1). More precisely, in six non-unions, bacteria were detected in one of the samples; in two non-unions, the same bacterium (genus, species, and antibiogram) was detected in two different samples.
There was no correlation between the number of biopsies and the number of biopsies with positive culture results (Pearsons R: − 0.0503, R2 0.0025).
Twelve patients had no elevation of CRP and leucocyte levels. Bacteria were cultivated from five non-unions in that group. Three patients had an elevation of the CRP level only with bacterial growth in one non-union. Two patients showed an elevation in both, CRP and leucocyte levels and from both non-unions bacteria were cultivated. One patient had an elevation of the leucocyte, but not of the CRP level. There was no correlation between the number of positive biopsies and the leucocyte counts or the CRP level, respectively (Pearsons R: − 0.0245, R2 0.0006, Pearsons R: 0.2823, R2 0.0797).
Discussion
In the presented cohort, pathogens were detected in 8 of 18 non-unions (44%) despite the absence of clinical signs of infection. All identified bacteria are constituents of the skin flora. These species are well known as causative agents of low-grade infections in prosthetic joint infection (PJI) [12,13,14]. In general, this observation is in accordance with the pathomechanics of open fractures, where the bone gets into contact with the skin. A similar mechanism might be considered in cases presenting with low-grade infection after multiple revision surgeries. Thus, the qualitative observation, i.e., the type of bacteria identified in the specimen, appears comprehensible. However, to become either a valid diagnostic or prognostic parameter, the quantitative component of the measurement is at least as important.
In PJI, it is assumed that two positive periprosthetic (tissue or fluid) cultures with matching organisms following the CDC definition [15] are sufficient to establish the diagnosis; otherwise, the risk of false-positive results due to contamination is considered too great. Applying this criterion to the reported population results in 2/18 (11%) non-unions (Table 1), which might be diagnosed as infected non-union. However, PJI is a distinct entity. While there is no CDC definition for infected non-unions, in cases of osteomyelitis and disc space infections, one positive culture, in combination with additional diagnostic items, is considered sufficient to establish the diagnosis. Thus, while in PJI, the diagnosis can be made based on tissue biopsies alone, in entities like osteomyelitis or disc space infection, the biopsy is only considered valid when additional parameters are positive as well. From a practical point of view, this appears reasonable, given the fact that in PJI, the tissue volumes available for culture are larger than in osteomyelitis, disc space infection, or non-union.
The observation that biopsies alone are not sufficient for the diagnosis of infected non-union is supported by the fact that there is no positive correlation between the number of biopsies and the resulting number of biopsies positive for bacteria. In general, evidence on the diagnosis of sub-clinically infected non-union is sparse. The microbiological data on 23 patients with tibia non-unions presented by Gille et al. [16] are comparable with our observations. In their cohort, 35% of the subjects had open fractures and 78% had 1–8 surgical revisions. With routine culturing methods, no bacteria were found, while 16 S ribosomal RNA pathogens were detected in two patients (1 × Methylobacterium species, 1 × Staphylococcus species) (8.7%) using a polymerase chain reaction (PCR) assay. In their setting, perioperative antibiotic prophylaxis was administered before biopsies were obtained, which might have decreased sensitivity and given advantage to detection with the PCR method [17].
Palmer et al. analyzed tissue samples of 34 patients with non-unions using standard culture analysis, Ibis’s second generation molecular diagnostics (Ibis Biosystems), and bacterial 16S rRNA-based fluorescence in situ hybridization (FISH) [18, 19]. In eight subjects, positive intraoperative culture results were found (25.5%). Ibis and FISH confirmed the presence of bacteria in all eight samples. Both methods identified bacteria in a total of 30 of 34 encounters (88.2%).
Szczesny et al. analyzed deep tissue samples of 43 patients with delayed fracture healing and unstable union of femur and tibia after closed fractures [20]. Bacteria were found in 35% of the cases by conventional culturing methods. 16S rRNA PCR examination was also positive in all of those cases and detected bacterial rRNA in three more cases (42% of patients), which is very close to our results. As in our work, COST were the predominantly isolated organisms.
Dapunt et al. analyzed tissue samples, swabs, and sonication fluid of removed metal implants gained from 49 patients with atrophic non-union [21]. Swabs were cultured, and tissue samples were cultured and analyzed by histopathology. Sonication fluid was cultured as well and was analyzed by 16S rRNA PCR. Samples of 45 patients undergoing routine implant removal served as control. In the study group, cultures of tissue samples revealed positive results in 10.2% of cases. The most sensitive method was sonication fluid culture with 57.1% positive results. Again, COST were the predominantly cultivated pathogens. Interestingly, cultures of sonication fluid in the control group were positive in 40% of cases, which raises the question of the contaminations’ clinical relevance. The authors assume that the variance of bacterial virulence and the variance of the immune response might be responsible for the divergent results.
So far, the available evidence strongly suggests that low-grade infection is a relevant issue in fracture non-union (Table 2). The results reported here unequivocally support this notion. However, there remains a highly unsolved problem regarding the quantitative aspect of the diagnosis. The quantitative aspect of the diagnosis naturally collides with the question of sensitivity of the different diagnostic techniques. While PCR-based methods appear to be over sensitive, our results suggest that biopsies alone are insufficient to establish the diagnosis of infected non-union, as it remains unclear whether a single positive biopsy needs to be considered as a contamination, especially in patients with a large number of biopsies and also regarding the findings of Dapunt et al., i.e., a high number of PCR positive cultures in the control group. Thus, given the current sensitivities of culture and biopsy technique, biopsy alone is not sufficient to establish the diagnosis of an infected non-union.
Prior to the definition of a diagnostic standard comparable to PJI, more evidence needs to be gathered regarding the sensitivities of emerging techniques like sonication.
The major short coming of this study is the retrospective design and the limited size of the cohort. Besides, as in other studies, here too, the technical and strategical choice of biopsy sites has not been defined a priori resulting in an additional source of bias.
Although a high rate of positive cultures has been obtained in this cohort, this finding has to be interpreted carefully. Despite the wide spread availability of biopsy-based diagnostics, and the apparent superiority of biopsies over classical swap-based analyses, the role of contamination remains unclear. This is partly due to unknown sensitivity and specificity of the different analysis techniques and partly due to a method inherent danger of over sensitivity, e.g., PCR. Thus, further research should focus to gain a deeper understanding of the diagnostic tools with respect to their sensitivity and specificity before further attempts are made to define a diagnostic standard for infected non-unions in clinically unapparent cases.
References
Steinhausen E, Glombitza M, Bohm HJ, et al. [Non-unions. From diagnosis to healing]. Unfallchirurg. 2013;116(7):633–47 (quiz 48–9).
Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38(Suppl 2):77–84.
Kanakaris NK, Tosounidis TH, Giannoudis PV. Surgical management of infected non-unions: an update. Injury. 2015;46(Suppl 5):25–32.
Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):3–6.
Calori GM, Giannoudis PV. Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury. 2011;42(11):1191–3.
Wright JA, Nair SP. Interaction of staphylococci with bone. Int J Med Microbiol. 2010;300(2–3):193–204.
Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol. 2015;5:85.
Ryan SP, Pugliano V. Controversies in initial management of open fractures. Scand J Surg. 2014;103(2):132–37.
Suzuki T, Minehara A, Matsuura T, et al. Negative-pressure wound therapy over surgically closed wounds in open fractures. J Orthop Surg (Hong Kong). 2014;22(1):30–4.
Simpson AH, Wood MK, Athanasou NA. Histological assessment of the presence or absence of infection in fracture non-union. Injury. 2002;33(2):151–5.
Pugmire BS, Shailam R, Gee MS. Role of MRI in the diagnosis and treatment of osteomyelitis in pediatric patients. World J Radiol. 2014;6(8):530–7.
Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–54.
Trampuz A, Zimmerli W. New strategies for the treatment of infections associated with prosthetic joints. Curr Opin Investig Drugs. 2005;6(2):185–90.
Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10(5):394–403.
Centers for Disease Control and Prevention (CDC). CDC/NHSN surveillance definitions for specific types of infections. 2018. CDC website: https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf. Accessed 25 Sept 2018
Gille J, Wallstabe S, Schulz AP, et al. Is non-union of tibial shaft fractures due to nonculturable bacterial pathogens? A clinical investigation using PCR and culture techniques. J Orthop Surg Res. 2012;7:20.
Achermann Y, Vogt M, Leunig M, et al. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48(4):1208–14.
Palmer M, Costerton W, Sewecke J, et al. Molecular techniques to detect biofilm bacteria in long bone nonunion: a case report. Clin Orthop Relat Res. 2011;469(11):3037–42.
Palmer MP, Altman DT, Altman GT, et al. Can we trust intraoperative culture results in nonunions? J Orthop Trauma. 2014;28(7):384–90.
Szczesny G, Interewicz B, Swoboda-Kopec E, et al. Bacteriology of callus of closed fractures of tibia and femur. J Trauma. 2008;65(4):837–42.
Dapunt U, Spranger O, Gantz S, et al. Are atrophic long-bone nonunions associated with low-grade infections? Ther Clin Risk Manag. 2015;11:1843–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Otchwemah R, Moczko T, Marche B, Mattner F, Probst C, and Tjardes T declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Otchwemah, R., Moczko, T., Marche, B. et al. High prevalence of bacteria in clinically aseptic non-unions of the tibia and the femur in tissue biopsies. Eur J Trauma Emerg Surg 46, 1093–1097 (2020). https://doi.org/10.1007/s00068-018-1010-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-018-1010-z