Abstract
Purpose
Stereotactic body radiotherapy (SBRT) has been firmly established as a treatment choice for patients with oligometastases, as it has demonstrated both safety and efficacy by consistently achieving high rates of local control. Moreover, it offers potential survival benefits for carefully selected patients in real-world clinical settings.
Methods
Between January 2008 and May 2020, a total of 149 patients (with 414 liver metastases) received treatment. The Active Breathing Coordinator device was used for 68 patients, while respiratory gating was used for 65 and abdominal compression was used for 16 patients. The most common histological finding was colorectal adenocarcinoma, with 37.6% of patients having three or more metastases, and 18% having two metastases. The prescribed dose ranged from 36 to 60 Gy, delivered in 3–5 fractions.
Results
Local control rates at 2 and 3 years were 76.1% and 61.2%, respectively, with no instances of local recurrence after 3 years. Factors negatively impacting local control included colorectal histology, lower prescribed dose, and the occurrence of new liver metastases. The median overall survival from SBRT was 32 months, with the presence of metastases outside the liver and the development of new liver metastases after SBRT affecting survival. The median disease-free survival was 10 months. No substantial differences in both local control and survival were observed between the respiratory motion control techniques employed. Treatment tolerance was excellent, with only one patient experiencing acute grade IV thrombocytopenia and two patients suffering from ≥ grade II chronic toxicity.
Conclusion
For radical management of single or multiple liver metastases, SBRT is an effective and well-tolerated treatment option. Regardless of the technology employed, experienced physicians can achieve similarly positive outcomes. However, additional studies are required to elucidate prognostic factors that can facilitate improved patient selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the recognition of a new type of metastatic cancer patient by Hellman and Weichselbaum in 1995 [1], numerous treatment modalities have been developed to eradicate oligometastases, as this local control could be related to an increase in the patient’s survival, as previous studies have speculated [2] and recent phase II trials have supported [3,4,5].
Stereotactic body radiotherapy (SBRT) is a significant advancement in liver metastases therapies, wherein a noninvasive radiotherapy technique is employed to deliver high radiation doses in a limited number of fractions to effectively ablate the metastases [6]. Previous evidence showed the harmful potential of conventional radiation techniques to the liver [7, 8], but, with SBRT, numerous studies have demonstrated its safety and efficacy [9,10,11,12,13,14,15].
Several studies have consistently supported the safety and demonstrated promising local control and survival rates for liver oligometastatic patients undergoing SBRT. These findings remain significant despite variations in dose, fractionation, and patient selection across the studies [16,17,18,19,20,21,22,23,24,25,26].
While there is no precise definition for the total number of metastases that fall within the oligometastatic scenario, most studies adopt a definition of one to five metastases [27]. Ongoing studies such as the SABR-COMET-10 are evaluating the possibility of treating up to 10 metastases with radical intention.
Liver metastases are subjected to physiological liver movements caused by diaphragm displacement during the respiratory cycle. Since SBRT involves administering high doses, it is crucial to control target motion during treatment. Various commercially available options exist for motion control, such as abdominal compression, which restricts respiratory motion by exerting pressure on the upper abdomen, thus limiting diaphragm displacement. Another option is respiratory gating, where treatment is delivered within a specific window of the respiratory cycle, achieved either in free-breathing or through deep inspiration breath-holding.
The objective of this study was to analyze the long-term outcomes (based on a 12-year experience) of liver SBRT in a real-world setting with patients with oligometastases, identify prognostic factors, and compare the results among different motion-control management and treatment platforms utilized. The results obtained were compared with existing data from the literature.
Methods and materials
The study of unresectable liver metastases in patients with oligometastases received approval from the internal ethics committee at our institution. All patients who were included underwent radical-intention liver SBRT treatment. The treatments were conducted using both the classic Novalis (Brainlab-Varian™, Munich, Germany; Palo Alto, California, US) and the Versa HD (Elekta™, Stockholm, Sweden) systems. The treatment decision was made during a multidisciplinary meeting attended by surgeons, medical and radiation oncologists, gastroenterologists, radiologists, and pathologists.
Three distinct tumor motion control techniques were employed: respiratory gating (RG), Active Breathing Coordinator device (ABC), and abdominal compression (AC). Treatments were carried out using internal-fiducial-based RG on a classic Novalis LINAC (Brainlab-Varian™) and on a Versa HD LINAC (Elekta™), primarily using the ABC technique, with AC utilized only for patients unable to undergo breath-holding for ABC. The favored options were RG and ABC as they did not require additional margins to account for residual movement. In AC, an internal target volume (ITV) was generated considering residual movements of the treated metastases as measured in simulation 4D-CT and verified on treatment days using 4D-CBCT (Synergy, Elekta™). A planning target volume (PTV) was created by adding a 5-mm isotropic margin.
The criteria for SBRT encompassed patients with liver metastases who were deemed unsuitable for surgery or had refused it, age 18 years or older, had a Karnofsky Performance Scale score of ≥ 70, showed no evidence of untreated or progressive gross disease outside the liver, and had adequate liver function: total bilirubin ≤ 2.5 g/dL, normal prothrombin time (PT)/partial thromboplastin time (PTT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) less than three times the upper limit of normal. Chemotherapy was permissible within 14 days before or after SBRT, previous local treatment for the targeted metastases was strictly prohibited, except in cases where salvage treatment of relapse to the previous therapy was necessary. All patients provided written informed consent before treatment.
The prescribed doses for SBRT varied based on the size, location, and total number of treated liver metastases. Options included 36, 45, or 60 Gy administered in three fractions with a minimum interval of 48 h between fractions, or 50 or 60 Gy delivered in five daily fractions. The prescribed dose aimed to cover 90–95% of the target volume, with a PTV isotropic expansion of 5 mm added to the gross tumor volume/ITV (GTV/ITV) for planning purposes.
Patients were treated either in a Novalis (Brainlab-Varian™) LINAC with internal fiducial-based respiratory gating technique (RG; [28]) or in a Versa HD (Elekta™) LINAC, using both AC, 4D-CT simulation and 4D-CBCT IGRT (Image-Guided Radiation Therapy), or Elekta’s ABC techniques [29].
The patient monitoring consisted of a physical examination and a complete blood test before and after SBRT. Following the completion of SBRT, the patient’s follow-up involved an interview, a physical examination, and a blood test 1 month after treatment, followed by subsequent tests every three months. Tumor response assessment was conducted using contrast-enhanced computed tomography (CT) scans or positron emission tomography (PET)-CT scans every 3 months, utilizing the EORTC-RECIST 1.1 [29] and PERCIST [30] criteria for image reporting.
For statistical purposes, local control, disease-free survival, and overall survival were measured from the conclusion of SBRT treatment.
Statistical analyses were performed with the Statistical Package for Social Science (v.20; SPSS Inc. Chicago, IL, USA). Local control and survival were measured through the Kaplan–Meier method, the log-rank test was used for univariate analyses, while a Cox regression model was used for multivariate comparisons. Group comparisons were performed with analyses of variance (ANOVA) for numeric variables, and contingency tables for ordinal and nominal variables. Statistical significance was considered for all values of p < 0.05.
Toxicity was determined according to the CTCAE V 5.0 guidelines. The evaluation of toxicity was conducted independently for each SBRT treatment course, considering that certain patients underwent multiple treatments over the years. Liver function was assessed by measuring the blood levels of alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, and gamma-glutamyltransferase (GGT).
Results
A total of 149 patients with 414 liver metastases were treated from January 2008 to May 2020. Baseline patient and treatment characteristics are summarized in Tables 1 and 2.
The most frequently used motion control techniques were ABC and RG: ABC 68, RG 65, AC 16 patients; 53%, 39.7%, and 7.3% respectively. Differences between patients in each respiratory motion control group are represented in Table 3. The number of metastases per patient treated with SBRT was significantly lower in the AC group (p = 0.033): The PTV volume was larger in the AC group (p = 0.01) and colorectal primaries were less frequent in the AC group (p = 0.009), while the prescribed dose under BED10 < 100 Gy was more frequent in the RG group.
Colorectal cancer was the primary tumor with the highest frequency, and the occurrence of two or more metastases was more prevalent than single lesions (55.7% vs- 44.3%, respectively). The majority of patients did not have metastases in other locations apart from the liver, and most had only undergone first-line chemotherapy prior to SBRT treatment.
Acute toxicity was observed in 26.2% of the treatments, with grade I/II asthenia and abdominal pain being the most common occurrences (14% and 5.2%, respectively). Among the patients, one individual experienced grade IV thrombocytopenia following SBRT treatment for four liver metastases. Chronic toxicity was detected in 2.7% of the patients, including grade I transaminitis in one patient, grade I rib pain in one patient, grade II muscular pain in one patient, and grade III rib pain in one patient. Notably, all these cases involved individuals who had received treatment for multiple liver metastases.
With an average follow-up of 24 months (range 1–138), actuarial local control of the liver metastases at 2 and 3 years was 76.1% and 61.2% respectively, no local relapse was observed beyond the third year of local control for the treated lesions, resulting in the median local control not being reached.
Several factors influenced local control in this study. The primary tumor histology was found to be significant, with colorectal cancer associated with poorer local control compared to other histologies (p = 0.002). When assessing local control based on the number of treated metastases (single, two, or three or more), excellent local control rates were observed for single metastasis in both adenocarcinoma (80%) and other histologies (90%), with no significant differences noted. For two metastases, the mean local control for colorectal adenocarcinoma was 33 months, significantly lower than in other histologies where the mean was not reached. In the case of three or more metastases, colorectal adenocarcinoma exhibited significantly lower local control (mean 28 months) compared to other histologies (mean not reached).
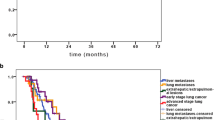
Prescribed dose was related to local control, with a median local control of 30 months for metastases receiving ≤ BED10 (biologically effective dose with an α/β ratio of 10) = 100 Gy, and thus median local control was not reached for those receiving more than BED10 = 100 Gy (p = 0.000; Fig. 1). This dose dependence is significant for colorectal metastases, but not for other histology. The emergence of new metastases had a detrimental effect on the local control of previously treated lesions in the liver (p = 0.009), whereas the presence or absence of new metastases outside the liver had no impact. Additionally, patients with synchronous oligometastatic disease experienced lower rates of local control compared to those with metachronous metastases (p = 0.033). The number of treated lesions, the treating center, the patient’s age, previous chemotherapy, previous local treatment, respiratory motion control technique, and liver segment of the treated metastases were not related to local control.
All significant variables were selected for multivariate analyses, and only colorectal histology (p = 0.036), prescription dose BED (p = 0.005), and presence of new liver metastases (p = 0.009) remained significant.
Median overall survival measured from SBRT was 32 months (95% CI = 27.6–36.3; Fig. 2). Primary histology was found to be a significant predictor of survival, with colorectal and breast cancer associated with longer survival, while other histologies such as NSCLC or pancreas exhibited poorer outcomes (p = 0.009). Patients with synchronous metastases outside the liver at the time of liver SBRT demonstrated worse overall survival, with a median survival of 36 months compared to 31 months for those without synchronous metastases (p = 0.018). Furthermore, patients who developed new metastases after SBRT experienced lower overall survival (p = 0.000), with a median survival of 33 months for patients with new metastases compared to those without new lesions, where median survival was not reached. The prescribed dose of SBRT, previous chemotherapy, the presence of local relapse after SBRT, patient age or sex, and the synchronicity of the primary tumor and metastases were not found to be associated with overall survival.
In multivariate analyses, only the presence of synchronous metastases outside the liver at the time of SBRT remained significant for overall survival (p = 0.035), with a trend toward the development of new metastases after SBRT (p = 0.053). Table 4 shows the univariate and multivariate analysis for local control and overall survival.
The median overall survival from diagnosis was 70 months (95% CI = 61.2–78.7). Histology, the development of new metastases, and the metachronal appearance of metastatic disease were found to be strong predictors of survival (p = 0.000, p = 0.001, and p = 0.001, respectively). In multivariate analyses, survival from the time of diagnosis was significantly influenced by the development of new metastases and the metachronous appearance of metastatic disease (p = 0.01, p = 0.001, respectively).
The median disease-free survival was 10 months (95% CI = 7.7–12.2). Although the presence of metastases outside the liver at the time of treatment was associated with disease-free survival, this relationship did not reach statistical significance (p = 0.054). The use of respiratory gating techniques was correlated with prolonged disease-free survival (p = 0.02). The number of treated lesions, patient age or sex, histology, previous chemotherapy, previous local treatment, prescribed dose, liver segment of the metastases, and the synchronicity of the primary tumor and metastases were not found to be associated with disease-free survival.
A secondary analysis was performed, specifically targeting patients who received a BED10 greater than 100 Gy, with the aim of identifying potential differences in terms of local control, disease-free survival, and overall survival. No significant differences were found.
Discussion
Over the past decade, the use of SBRT has been established as a viable treatment option for liver metastases, supported by both prospective studies [10,11,12, 20, 22,23,24,25] and retrospective studies [2, 13,14,15, 17, 18, 21]. The ESMO guidelines [30] have recognized SBRT as a treatment option for liver metastases since 2016. Alongside other techniques such as radiofrequency ablation or microwaves, SBRT is considered a viable option for treating liver metastases. Several studies have indirectly compared these techniques and have found that SBRT offers improved local control and less restriction in terms of metastasis size and location [31, 32]. Table 5 presents a summary of the main findings from selected studies of liver SBRT, along with the findings from the present study.
In this study, we presented our real-world experience of 12 years in the treatment of liver metastases using SBRT. Among the different respiratory management systems employed, the most utilized technique was ABC, which was used for 219 metastases (53%). This was followed by RG, which was employed for 164 metastases (39.7%). Our primary aim was to minimize the use of AC and ITV due to its potential impact on meeting dose constraints for the liver and other organs at risk, especially in cases involving multiple metastases or sequential treatments within a single patient. Therefore, only 30 metastases (7.3%) were treated using the AC technique. No significant differences were observed in terms of local recurrence, survival, or toxicity among the various respiratory management systems. Additionally, patients treated with AC had a lower number of metastases, fewer cases of colorectal primaries, and received higher doses; all these factors may introduce confounding variables that potentially contribute to more favorable overall outcomes associated with this technique. However, it is important to note that patients treated with this technique had larger PTVs, which is an inherent characteristic of the technique itself and does not necessarily indicate the presence of larger tumors (Table 3).
It is important to note that in the RG group, patients more frequently received BED10 below 100 Gy compared to the other respiratory control groups, to comprehensively investigate these differences and thoroughly examine the impact of respiratory motion techniques on local control, disease-free survival, and overall survival, an additional analysis was conducted. This analysis involved the exclusion of patients who received a BED10 below 100 Gy. As most of these patients belonged to the RG group, and lower doses were associated with poorer outcomes, this modification aimed to achieve better balance between the groups. However, despite these adjustments, the results once again demonstrated no significant differences. We observed comparable results in the existing literature: Esposito et al. conducted an analysis investigating the impact of treatment planning methodologies employed by 14 centers, which included 3‑D conformal-RT, IMRT, VMAT, CyberKnife, and TomoTherapy. Their findings indicated nonsignificant differences, leading them to conclude that the human factor holds more significance than technological aspects in SBRT treatments [33]. Moreover, the existing literature provides evidence in favor of advanced motion management techniques such as RG and ABC. In a pooled analysis conducted by Andratschke on behalf of the German Society of Radiation Oncology (DEGRO), it was observed that patients treated with advanced respiratory control methods after 2003 achieved better local control compared to methods relying on target localization during free breathing. However, the analysis also highlighted the shift in treatment doses around 2003 toward modern high-dose schemes [34]. Van der Being et al. also reported differences in local control related to respiratory motion management. In their study, they treated 105 metastases and observed that liver and lung lesions, affected by diaphragm respiratory displacements, achieved lower local control rates compared to non-moving lymph nodes (53% vs. 79% at 1 year, p = 0.01). Since the treatments were performed without specific respiratory motion management, the authors concluded the necessity of implementing individual respiratory motion management techniques for the treatment of moving tumors [35]. In our study, the lack of disparities in terms of local control, survival, and toxicity between the RG, ABC, and AC techniques could be attributed to the technique selection, as all eligible patients were selected for RG or ABC, leaving AC to those patients for whom the previous techniques could not be implemented, and also, to the consistent involvement of a highly experienced team of physicians who performed the respiratory control and treatment procedures.
No significant differences were identified in terms of toxicity between different respiratory motion control techniques. Again, the authors believe that the patient selection for the treatment technique also plays a significant role in influencing these results. The authors speculate that if AC had been utilized for the treatment of multiple metastases, it may have led to different outcomes and variations in local control and toxicity might have been observed. Local control could have been compromised if the total dose was reduced to meet organ-at-risk constraints, while toxicity could have increased if the total dose was maintained, and healthy tissues received higher doses. However, due to the specific nature of the patient selection, these potential differences remained undetectable.
Colorectal adenocarcinoma was the most common histology observed, accounting for 64% of cases. Pancreatic adenocarcinoma was the second most frequent histology. There is some controversy regarding the treatment of pancreatic liver metastases, with previous studies reporting poor local and distant control [36]. However, in our series, we did not observe any significant differences in terms of local or distant recurrence among selected patients with oligometastases and pancreatic primaries.
Actuarial local control at 2 and 3 years was 76.1% and 61.2% and median local control was not reached. Other studies found similar results with local control rates ranging between 67–96% at 1 year and 55–84% at 5 years. Rusthoven reported 2‑year local control of 92% in the phase I/II trial [10], related to the careful selection of patients with a low burden of disease, treating only up to three metastases, which has been the main criterion in most of the studies. The Dutch–Belgian registry, which provides real-world data, reports local control rates of 87%, 75%, and 68% at 1, 2, and 3 years, respectively [26]. The local control achieved in our study is consistent with the findings reported in the existing literature. We found that patients who remain relapse-free for 3 years following SBRT are less likely to experience subsequent relapses. These findings align with similar results reported in the literature, supporting the hypothesis that the probability of local relapse tends to decrease over time in patients with controlled metastases during the follow-up period [12, 22, 24, 34, 36,37,38,39,40,41].
Despite not imposing specific limits on the number and size of metastases in our selection criteria, as long as all the disease could be safely treated with a radical intention and meet dosimetric constraints, our approach has demonstrated excellent outcomes in terms of local control and survival rates, while maintaining low toxicity levels, as previously reported [42] and maintained in the present study.
Colorectal histology was found to have a negative impact on local control in multivariate analyses (p = 0.036). This finding has been previously addressed in the literature: As demonstrated by Andratschke’s pooled analysis, metastases originating from colorectal cancer had significantly lower control rates at 1 year (67%) compared to breast cancer, non-small-cell lung cancer, and other histologies (91%, 88%, and 80% respectively; [34]). The authors acknowledge that they cannot solely attribute the differences in local control to colorectal histology, considering the presence of two or more liver metastases. It is possible that these observations are merely incidental statistical findings and may not have a causal relationship.
Furthermore, multivariate analysis indicated that prescription dose BED10 < 100 Gy (p = 0.005) was associated with lower control of the treated metastases. McPartlin conducted a study involving 51 patients with 93 liver metastases and found that lesions receiving higher doses (GTVmin ≥ 37.6 Gy in six fractions, BED10 = 61 Gy) achieved higher local control rates, with 41% at 4 years compared to 0% for the remaining lesions [24]. Similarly, Méndez Romero observed differences in local control between two groups of 40 patients with 55 liver metastases, where one group received 3 × 12.5 Gy (BED10 = 84.4 Gy) and the other received 3 × 16.75 Gy (BED10 > 130 Gy). The low-dose group exhibited local control rates of 74% and 76% at 2 and 3 years, respectively, while the high-dose group showed rates of 90% and 81% at the same time points [37]. Additionally, Fode’s study also found a positive association between BED10 > 100 Gy and local control [40].
In our study, we observed a significant negative impact on the local control of previously treated liver lesions in the presence of new liver metastases (p = 0.009). Interestingly, the presence or absence of new metastases outside the liver did not show any influence on local control. Previous studies have focused on investigating the influence of new metastatic foci on previously treated tumors, supporting the self-seeding theory. According to this theory, the intercommunication between tumors can reactivate dormant treatment-resistant cells from the previously treated lesions [43,44,45]. While these theories provide an explanation for the increased recurrence rates when new metastases emerge, the authors acknowledge that the reasons behind the specific influence of new liver metastases and the lack of impact from new metastases originating outside the liver remain unclear and warrant further investigation.
The median overall survival from SBRT treatment was 32 months. The presence of two or more metastases, whether in the liver or elsewhere, at the time of SBRT had a significant association with poorer survival (p = 0.035). Additionally, the development of new metastases exhibited a trend toward significance (p = 0.053). The overall survival from the time of initial diagnosis was 70 months, and it was influenced by multiple factors including histology, the development of new metastases, and the metachronous appearance of the metastases (p = 0.000, p = 0.001, and p = 0.001, respectively). Significantly, these last two factors are consistent with previous findings reported in the literature. In an analysis of survival and prognostic factors involving 321 patients with oligometastatic disease, the presence of two or more metastases and synchronous appearance, along with performance status, size of the largest metastases, and pre-SBRT chemotherapy, were identified as predictors of worse survival [40]. With a follow-up period of over 5 years, Scorsetti et al. reported that histology was significantly associated with overall survival. Specifically, colorectal, breast, and gynecological primaries were identified as favorable histologies in terms of survival outcomes [22]. A multicenter phase I/II trial showed that patients with colorectal, breast, sarcomas, and renal cancer treated with SBRT of liver metastases achieved higher 2‑year overall survival compared to less favorable tumor types (non-colorectal gastrointestinal malignancies, lung and ovarian cancer; [10]). In alignment with the studies conducted by Scorsetti et al. and Schefter, our own study revealed similar findings regarding the influence of histology on overall survival. We observed that patients with colorectal and breast cancer exhibited the longest survival rates, which is consistent with these histologies being associated with favorable outcomes in terms of overall survival. These findings reinforce the importance of considering histology as a crucial factor in patient selection to optimize the outcomes of liver SBRT.
The median disease-free survival was 10 months. The only factor significantly associated with improved disease-free survival was the RG technique (p = 0.02). However, we could not provide a definitive explanation for this finding, and it may be attributed to chance, as the RG and ABC groups were well-balanced and showed no differences (Table 3). The presence of metastases outside the liver showed a trend toward poorer disease-free survival (p = 0.054), indicating a potential association between the presence of metastases at other sites and earlier relapse. This finding suggests that more aggressive tumor characteristics may contribute to the occurrence of metastases in multiple locations, leading to shorter disease-free intervals.
The incidence of treatment-related toxicity was low, with 26.2% experiencing grade I/II acute toxicity, with asthenia being the most common symptom. Additionally, we identified one case of grade IV acute thrombocytopenia, which resolved after 4 weeks following SBRT. Chronic toxicity was observed in a small proportion of patients, affecting 2.7% of the study population. These toxicities included one case of grade I rib pain, one case of grade III rib pain, grade II muscular pain, and grade I persistent elevation of liver enzymes (transaminitis). In our study, the occurrence of transient low-grade transaminase increase, muscular pain, and rib pain was relatively low, despite the treatment of multiple liver lesions. These findings align with previous reports in the literature, which have associated these toxicities with specific dose thresholds to subcutaneous tissue and ribs [10, 20, 46]. Notably, all cases of pain in our study received more than a single course of SBRT to the liver, suggesting that the utilization of respiratory gating techniques, which spare healthy tissues, may be crucial to maintain the low rates of toxicity when treating multiple liver targets in the same patient.
Importantly, no patient experienced radiation-induced liver disease (RILD) following single or multiple treatments. This is consistent with the findings of a phase I trial, which included 68 patients and did not report any cases of RILD despite a median liver dose of 16.9 Gy (range 3–22) delivered in six fractions [20]. Similarly, the Rusthoven phase I/II trial also demonstrated the absence of RILD when a constraint of at least 700 mL of healthy liver receiving 15 Gy or greater in three fractions was applied [10]. In our own study, we adhered to this constraint and adapted it to different fractionation schemes based on our experience. These findings further reinforce the safety and tolerability of liver SBRT, even in cases involving the treatment of multiple liver metastases. Our study demonstrated low rates of chronic toxicity and a minimal risk of RILD when appropriate dose constraints were followed.
Previous reports have documented cases of central biliary tract toxicity, such as a grade V biliary stenosis reported in the Dutch–Belgian registry involving a patient treated for two centrally located metastases [26]. However, in our experience of treating multiple metastases, we have not encountered any biliary tract toxicity thus far.
Our findings with respect to toxicity indicate that liver SBRT can be safely and effectively utilized in the management of multiple liver metastases, providing reassurance regarding its feasibility and favorable toxicity profile.
Limitations
The authors acknowledge several limitations of this study, including its retrospective nature, the extended data collection period, and the heterogeneity of systemic treatments. These factors have the potential to introduce biases in the findings and should be taken into consideration when interpreting the results. However, this real-world experience in treating liver metastases, particularly in patients with multiple metastases, employing modern respiratory motion control techniques, contributes to a better understanding of the complex and evolving landscape of patients with oligometastatic disease and their selection.
Conclusion
The present study offers compelling evidence supporting the use of modern respiratory motion control in stereotactic body radiotherapy (SBRT) of the liver, demonstrating its favorable tolerability and efficacy even in patients with multiple liver metastases. The findings indicate excellent local control and favorable long-term survival outcomes. Additionally, prognostic factors have been identified, which can assist in the selection of patients who are most likely to benefit from SBRT. Further studies are necessary to confirm and validate the results obtained in this study.
References
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13(1):8–10
Klement RJ, Abbasi-Senger N, Adebahr S, Alheid H, Allgaeuer M, Becker G et al (2019) The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: A combined analysis of 388 patients with 500 metastases. BMC Cancer 19(1):173
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C et al (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393(10185):2051–2058
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES et al (2020) Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol 6(5):650–659
Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR et al (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 17(12):1672–1682
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B et al (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37(8):4078–4101
Ingold JA, Reed GB, Kaplan HS, Bagshaw MA (1965) Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 93:200–208
Dawson LA, Ten Haken RK (2005) Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 15(4):279–283
Blomgren H, Lax I, Näslund I, Svanström R (1995) Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: clinical experience of the first thirty-one patients. Acta Oncol 34(6):861–870
Schefter TE, Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH et al (2009) Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 27(10):1572–1578
Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ et al (2006) Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i–ii study. Acta Oncol 45(7):831–837
Hoyer M, Roed H, Hansen AT, Ohlhuis L, Petersen J, Nellemann H et al (2006) Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 45(7):823–830
Herfarth KK, Debus J, Lohr F, Bahner ML, Wannenmacher M (2001) Stereotactic irradiation of liver metastases. Radiologe 41(1):64–68
Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K et al (2006) Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol 45(7):838–847
Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P (2007) Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 67(3):793–798
Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J et al (2011) Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117(17):4060–4069
Vautravers-Dewas C, Dewas S, Bonodeau F, Adenis A, Lacornerie T, Penel N et al (2011) Image-guided robotic stereotactic body radiation therapy for liver metastases: Is there a dose response relationship? Int J Radiat Oncol Biol Phys 81(3):e39–e47
Lanciano R, Lamond J, Yang J, Feng J, Arrigo S, Good M et al (2012) Stereotactic body radiation therapy for patients with heavily pretreated liver metastases and liver tumors. Front Oncol 2:23
Habermehl D, Herfarth KK, Bermejo JL, Hof H, Rieken S, Kuhn S et al (2013) Single-dose radiosurgical treatment for hepatic metastases—therapeutic outcome of 138 treated lesions from a single institution. Radiat Oncol 8:175
Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R et al (2009) Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 27(10):1585–1591
Feng M, Suresh K, Schipper MJ, Bazzi L, Ben-Josef E, Matuszak MM et al (2018) Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage a phase 2 clinical trial. JAMA Oncol 4(1):40–47
Scorsetti M, Comito T, Clerici E, Franzese C, Tozzi A, Iftode C et al (2018) Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol 13(1):234
Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G et al (2010) Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 78(2):486–493
McPartlin A, Swaminath A, Wang R, Pintilie M, Brierley J, Kim J et al (2017) Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys 99(2):388–395
Folkert MR, Meyer JJ, Aguilera TA, Yokoo T, Sanford NN, Rule WG et al (2021) Long-term results of a phase 1 dose-escalation trial and subsequent institutional experience of single-fraction stereotactic ablative radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys 109(5):1387–1395
Méndez Romero A, Schillemans W, van Os R, Koppe F, Haasbeek CJ, Hendriksen EM et al (2021) The Dutch-Belgian registry of Stereotactic body radiation therapy for liver metastases: clinical outcomes of 515 patients and 668 metastases. Int J Radiat Oncol Biol Phys 109(5):1377
Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R et al (2014) The oligometastatic state-separating truth from wishful thinking. Nat Rev Clin Oncol 11(9):549–557
Hernando-Requejo O, Sánchez E, Fernández P, Zucca D, Pérez JM, García-Aranda M et al (2011) Institutional experience on the treatment of lung and liver lesions with stereotactic body radiotherapy and Novalis Exactrac Adaptive Gating technique. J Radiosurg SBRT 1(3):231–236
Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA (2006) Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys 64(3):751–759
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422
Jackson WC, Tao Y, Mendiratta-Lala M, Bazzi L, Wahl DR, Schipper MJ et al (2018) Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of Intrahepatic metastases. Int J Radiat Oncol Biol Phys 100(4):950–958
Loi M, Desideri I, Dominici L, Francolini G, Garlatti P, Ciccone LP et al (2020) Thermal ablation versus SBRT in liver tumours: pros and cons. Med Oncol 37(6):52
Esposito M, Maggi G, Marino C, Bottalico L, Cagni E, Carbonini C et al (2016) Multicentre treatment planning inter-comparison in a national context: the liver stereotactic ablative radiotherapy case. Phys Med 32(1):277–283
Andratschke N, Alheid H, Allgäuer M, Becker G, Blanck O, Boda-Heggemann J et al (2018) The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer 18(1):283
Van Den Begin R, Engels B, Gevaert T, Duchateau M, Tournel K, Verellen D et al (2014) Impact of inadequate respiratory motion management in SBRT for oligometastatic colorectal cancer. Radiother Oncol 113(2):235
Berber B, Ibarra R, Snyder L, Yao M, Fabien J, Milano MT et al (2013) Multicentre results of stereotactic body radiotherapy for secondary liver tumours. HPB 15(11):851–857
Méndez Romero A, Keskin-Cambay F, van Os RM, Nuyttens JJ, Heijmen BJM et al (2017) Institutional experience in the treatment of colorectal liver metastases with stereotactic body radiation therapy. Oncol Radiother 22(2):126–131
Joo JH, Park JH, Kim JC, Yu CS, Lim SB et al (2017) Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys 99(4):876–883
Goodman BD, Mannina EM, Althouse SK, Maluccio MA, Cárdenes HR (2016) Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol 6(2):86–95
Fode MM, Høyer M (2015) Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol 114(2):155–160
Clerici E, Comito T, Franzese C, Di Brina L, Tozzi A et al (2020) Role of stereotactic body radiation therapy in the treatment of liver metastases: clinical results and prognostic factors. Strahlenther Onkol 196(4):325–333
Rubio C, Hernando-Requejo O, Zucca Aparicio D, ALlona Krauel M, López Gonzalez M, Pérez JM et al (2017) Image guided SBRT for multiple liver metastases with ExacTrac® Adaptive Gating. Rep Pract Oncol Radiother 22(2):150–157
Comen E, Norton L, Massagué J (2011) Clinical implications of cancer self-seeding. Nat Rev Clin Oncol 8(6):369–377
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L et al (2009) Tumor self-seeding by circulating cancer cells. Cell 139(7):1315–1326
Aguirre-Ghiso JA (2010) On the theory of tumor self-seeding: Implications for metastasis progression in humans. Breast Cancer Res 12(2):304
Méndez Romero A, Wunderink W, van Os RM, Nowak PJCM, Heijmen BJM, Nuyttens JJ et al (2008) Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys 70(5):1447–1452
Acknowledgements
We thank Daniel García de Quinto, for reviewing, revising, and editing this manuscript for English language grammar and syntax.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O. Hernando-Requejo, X. Chen, M. López, E. Sánchez, J. García, P. García, R. Alonso, A. Montero, R. Ciervide, B. Álvarez, D. Zucca, M. García Aranda, J. Valero, P. Fernández Letón and C. Rubio declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernando-Requejo, O., Chen, X., López, M. et al. Real-world effectiveness and safety of stereotactic body radiotherapy for liver metastases with different respiratory motion management techniques. Strahlenther Onkol 199, 1000–1010 (2023). https://doi.org/10.1007/s00066-023-02147-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02147-w