Abstract
Purpose
We retrospectively evaluated the patterns of failure for robotic guided real-time breathing-motion-compensated (BMC) stereotactic body radiation therapy (SBRT) in the treatment of tumors in moving organs.
Patients and methods
Between 2011 and 2016, a total of 198 patients with 280 lung, liver, and abdominal tumors were treated with BMC-SBRT. The median gross tumor volume (GTV) was 12.3 cc (0.1–372.0 cc). Medians of mean GTV BEDα/β = 10 Gy (BED = biological effective dose) was 148.5 Gy10 (31.5–233.3 Gy10) and prescribed planning target volume (PTV) BEDα/β = 10 Gy was 89.7 Gy10 (28.8–151.2 Gy10), respectively. We analyzed overall survival (OS) and local control (LC) based on various factors, including BEDs with α/β ratios of 15 Gy (lung metastases), 21 Gy (primary lung tumors), and 27 Gy (liver metastases).
Results
Median follow-up was 10.4 months (2.0–59.0 months). The 2‑year actuarial LC was 100 and 86.4% for primary early and advanced stage lung tumors, respectively, 100% for lung metastases, 82.2% for liver metastases, and 90% for extrapulmonary extrahepatic metastases. The 2‑year OS rate was 47.9% for all patients. In uni- and multivariate analysis, comparatively lower PTV prescription dose (equivalence of 3 × 12–13 Gy) and higher average GTV dose (equivalence of 3 × 18 Gy) to current practice were significantly associated with LC. For OS, Karnofsky performance score (100%), gender (female), and SBRT without simultaneous chemotherapy were significant prognostic factors. Grade 3 side effects were rare (0.5%).
Conclusions
Robotic guided BMC-SBRT can be considered a safe and effective treatment for solid tumors in moving organs. To reach sufficient local control rates, high average GTV doses are necessary. Further prospective studies are warranted to evaluate these points.
Zusammenfassung
Zweck
Wir führten eine retrospektive Untersuchung der Rezidivmuster bei der Behandlung von Tumoren in bewegten Organen mittels robotergeführter in Echtzeit atembewegungskompensierter (EAK) stereotaktischer Körperstammstrahlentherapie (SBRT) durch.
Patienten und Methoden
Zwischen 2011 und 2016 wurden insgesamt 198 Patienten mit 280 Lungen‑, Leber- und Abdominaltumoren mit EAK-SBRT behandelt. Das mediane makroskopische Tumorvolumen (GTV) lag bei 12,3 cm3 (0,1–372,0 cm3). Die mediane mittlere GTV-BEDα / β = 10 Gy lag bei 148,5 Gy10 (31,5–233,3 Gy10; BED = biologisch effektive Dosis) und die verschriebene PTV-BEDα / β = 10 Gy bei 89,7 Gy10 (28,8–151,2 Gy10; PTV = Planungszielvolumen). Wir analysierten das Gesamtüberleben (GÜ) und die lokale Kontrolle (LK) basierend auf verschiedenen Faktoren, einschließlich BED mit α/β-Verhältnissen von 15 Gy (Lungenmetastasen), 21 Gy (primäre Lungentumoren) und 27 Gy (Lebermetastasen).
Ergebnisse
Die mediane Nachbeobachtungszeit betrug 10,4 Monate (2,0–59,0 Monate). Die 2‑Jahres-LK betrug 100 und 86,4 % für primäre Lungentumoren im Früh- bzw. fortgeschrittenen Stadium, 100 % für Lungenmetastasen, 82,2 % für Lebermetastasen und 90 % für extrapulmonale, extrahepatische Metastasen. Die 2‑Jahres-GÜ-Rate über alle Patienten betrug 47,9 %. In der uni- und multivariaten Analyse wurde die LK vor allem durch eine zur üblichen Praxis vergleichsweise niedrige PTV-Verschreibungsdosis (äquivalent zu 3‑mal 12–13 Gy) sowie durch höhere mittlere GTV-Dosen (äquivalent zu 3‑mal 18 Gy) beeinflusst. Für ein hohes GÜ waren ein hoher Karnofsky-Index (100 %), das Geschlecht (weiblich) und die SBRT ohne gleichzeitige Chemotherapie prognostisch signifikant. Grad-3-Nebenwirkungen waren selten (0,5 %).
Schlussfolgerungen
Die robotergeführte EAK-SBRT kann als eine sichere und wirksame Behandlung für solide Tumoren in beweglichen Organen angesehen werden. Für eine ausreichend hohe lokale Kontrollrate sind hohe mittlere GTV-Dosen erforderlich. Weitere prospektive Studien sind nötig, um diese Punkte zu evaluieren.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Stereotactic body radiation therapy (SBRT) is frequently used for treating primary lung tumors [1,2,3,4], lung metastases [4,5,6,7], liver metastases [8,9,10,11], and lately for extrapulmonary extrahepatic oligometastatic disease [12,13,14]. For most indications, well-defined dose–response relationships for local control have been established based on planning target volume (PTV) prescription dose and/or based on maximum gross tumor volume (GTV) and isocenter dose, respectively [1, 3,4,5, 7, 10, 11, 15, 16]. However, even though these data were generated with large patient and lesion numbers, the platforms used were heterogeneous and there remains doubt if the reported tumor control probabilities (TCP) can be fully applied to all treatment systems and techniques and all lesion sites.
For example, the CyberKnife system (Accuray Inc., Sunnyvale, CA, USA) [17] makes use of small noncoplanar, nonisocentric cylindrical beams which can in turn generate highly modulated dose distributions with geometrically variable maximum dose regions. The CyberKnife is also capable of real-time breathing-motion-compensation (BMC) [18] which allows the application of high radiation doses with minimal safety margins [19] resulting in very small clinical dosimetry errors [20, 21]. Therefore, robotic BMC-SBRT may not perfectly fit into current TCP modeling paradigms mostly derived from gantry-based coplanar isocentric SBRT with integrated tumor volume (ITV) concepts.
Furthermore, current developments in dose calculation accuracy (i. e., Monte Carlo simulations) and their implementation into clinical routine highlight the need for different dose prescriptions in the lung (i. e., the prescription based on GTV mean dose) [22]. Such concepts may also be warranted for larger tumors in the abdomen and may especially be important for CyberKnife BMC-SBRT [10].
Finally, advanced TCP modeling of historical data recently suggested different radiation sensitivity for various tumor histology and lesion sites (i. e., higher alpha/beta (α/β) ratios than 10 Gy were proposed for primary lung tumors and lung and liver metastases) [4, 11, 15, 16]. Hence, different dose concepts may be required for separate treatment systems or even for institutional techniques for each different lesion site.
The aim of this retrospective pattern-of-failure analysis was to investigate local control (LC), overall survival (OS), and toxicity for our dedicated GTV-optimized treatment approach [10] for moving lesions using robotic BMC-SBRT and to compare our results to previously published studies and common clinical practice.
Patients and methods
This retrospective study was approved by the local ethics committees of the universities of Frankfurt (477/15), Rostock (A2016-0008), and Lübeck (13–218A).
Patient characteristics
Between March 2011 and September 2016, a total of 198 patients (118 men, 80 women) with 280 lesions were treated with robotic BMC-SBRT at two treatment centers with homogenized treatment planning and treatment delivery protocols. All patients were considered early stage or oligometastatic having ≤4 lesions in a single organ per treatment. Most of the patients were treated for a single lesion (n = 156) and the rest of the patients for two (n = 24), three (n = 6) or four and more (n = 12) lesions, repeat procedures of new lesions included. The median age of the patients was 68 years (range 34–89 years) and the median baseline Karnofsky performance score was 90% (range 60–100%). Of all treated patients, 12.6% did not stop chemotherapy during the time of SBRT delivery.
Out of the 280 lesions, 131 liver metastasis were treated, the largest number originating from colorectal cancer (n = 62). Furthermore, 132 lung lesions were treated, of which 70 lesions were primary lung tumors (42 early stage, 28 advanced stage) and 62 lesions were lung metastases, mostly originating from colorectal cancer (n = 22) or lung cancer (n = 18). All other lesions (n = 17) were either lymph node metastases or soft tissue metastases in the upper abdomen. The median GTV was 12.3 cc (range 0.1–372.0 cc) and the corresponding maximum GTV dimensions ranged from 0.6 to 8.9 cm (median 2.9 cm). A detailed description of the patient characteristics can be found in Table 1.
Treatment planning and delivery
For all extrapulmonary and for 9/132 (6.8%) intrapulmonary lesions, either GoldAnchor™ (Naslund Medical AB, Huddinge, Sweden) or solid gold fiducial markers (IZI Medical Products, Owings Mills, MD, USA) were implanted prior to treatment as close to the lesions as possible. Treatment planning was performed on standard computer tomography (CT) at regular end expiration breath hold with 1.5 mm slice thickness. The planning CT was fused with magnetic resonance images (MRI) for extrapulmonary or with positron emission tomography (PET) for intrapulmonary lesions, whenever available. The GTV was defined according to common standards and guidelines [2, 8, 10]. The clinical target volume (CTV) consisted of the GTV with an expansion of 5 mm for intrahepatic and 2 mm for extrahepatic lesions. The PTV included the CTV and an expansion of 3 mm in all directions to encompass the targeting uncertainties for the CyberKnife system [19,20,21].
Plan optimization was done with the CyberKnife MultiPlan® software (Accuray, versions 3.5 and 4.5) utilizing Sequential Multi-Objective Optimization [23] and the consensus guidelines for robotic radiosurgery treatment planning [24]. The main objective for optimization was to maximize the GTV mean dose to more than 3 × 18 Gy for extrapulmonary [10], 3 × 20 Gy for lung metastases, and 3 × 21.5 Gy for primary lung tumors [22], while for all intrapulmonary lesions the Monte Carlo algorithm was used for dose calculation [25]. The secondary objective was to minimize the dose to all critical structures according to the ALARA (As Low As Reasonably Achievable) principle. Standard PTV prescription dose was sought to reach 3 × 15 Gy according to standard practice. However, if this was not possible due to critical organ constraints [26], either the fractions were increased or the prescription dose was lowered while for both we tried to maintain high GTV mean doses [10]. Rarely, very small lung lesions were treated in a single fraction (6.8%) [27]. The final biological effective doses (BEDα/β = 10 Gy) surrounding 95% of the PTV ranged between 28.8 and 151.2 Gy10 (median 89.7 Gy10) prescribed to the 40–85% isodose (median 71%). and the mean GTV BEDα/β = 10 Gy ranged between 31.5 and 233.3 Gy10 (median 148.5 Gy10). We also calculated the BEDs with variable α/β ratios for 2‑year TCP based on recent studies [15, 16] resulting in mean GTV BEDα/β = 15 Gy of median 122.2 Gy15 for lung metastases (range 44.3–178.9 Gy15), BEDα/β = 21 Gy of median 108.2 Gy21 for primary lung tumors (range 35.5–147.8 Gy21), and BEDα/β = 27 Gy of median 85.4 Gy27 for liver metastases (range 22.4–99.5 Gy27).
For all lesions, SBRT was delivered using the CyberKnife Synchrony® real-time BMC tracking system (Accuray, versions 8.5 and 9.5) [17, 18]. A custom-made vacuum mattress (HEK Medical, Germany) was used for immobilization and all patients were initially aligned using the spinal vertebra. The breathing-induced motion of the lesions was compensated for by the robot during beam-on time [17, 18] using the prediction of chest markers [28] which were correlated to either the previously implanted fiducial markers (56.1%) or to the lung lesion directly (43.9%), both detected on the orthogonal x‑ray imaging [29, 30]. BMC tracking accuracy performance was kept according to best practice guidelines [20, 21, 31]. The median fraction treatment time was 45 min (range 15–110 min), excluding setup time, and mostly depended on treatment complexity and number of treated lesions.
Follow-up and statistical analysis
All patients were observed in time intervals of 3 months in the first year and then 6 months thereafter. We estimated LC and OS as the clinical end points with Kaplan–Meier survival curves. These endpoints were calculated from the end of treatment to the date of last contact or local progression or death, respectively. LC was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST). Suspected local progression was confirmed with biopsy or functional imaging (i. e., PET/CT). Whenever possible, we also registered the follow-up imaging to the treatment plan for determination on in-field or field-border recurrence. Patients that were still alive and had no local failure were censored at last follow-up.
To identify prognostic factors for the respective clinical endpoints, we conducted univariate analysis using the log-rank method taking into account different clinical variables—PTV prescription, GTV mean, and plan maximum dose—all three expressed as BEDα/β = xGy with different histology-dependent α/β ratios, lesion volume and diameter, and histology of the primary tumor as independent variables. For analyzing overall survival we used the Karnofsky performance score, age, gender, lesion site, and simultaneous chemotherapy. For the subsequent multivariate Cox regression analysis, we included all factors found to be significant in the univariate analysis. For the statistical analysis we used SPSS (Version 21.0, IBM, Amonk, NY, USA) and we considered a p-value of ≤0.05 as the limit for statistical significance.
Results
At the time of analysis, the median follow-up for all patients was 10.4 months (range 2.0–59.0 months).
Local control
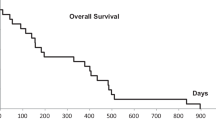
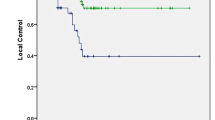
The crude LC for all lesions (n = 280) was 92.9% at the time of analysis. The 2‑year actuarial LC was 100% for early stage primary lung tumors, 86.4% for advanced stage primary lung tumors (including re-irradiation of previously treated lesions), 100% for lung metastases, 82.2% for liver metastases, and 90.0% for extrapulmonary extrahepatic metastases, respectively (Fig. 1).
In the univariate analysis for all primary lung tumors, we found a significant correlation between no prior lung tumor therapy (operation, chemotherapy, radiotherapy, etc.) and better LC (hazard ratio [HR] 9.386, 95% confidence interval [CI] 0.911–96.655, p = 0.027). For the lung metastases group, we had only one local failure in the third year after treatment; hence, we combined all lung lesions for statistical analysis for volume, dimension, number of fractions and dose (BED10 and combined BED15 for metastases with BED21 for primary tumors, respectively). In univariate analysis for all lung lesions, a PTV prescription dose ≥89.7 Gy10 (HR 0.077, 95% CI 0.012–0.503, p = 0.001) and ≥70.0 Gy15/21 (HR 0.128, 95% CI 0.021–0.801, p = 0.011) was associated with better LC, but PTV prescription doses above 3 × 15 Gy (112.5 Gy10 and 82.5 Gy15/21) were not (p = 0.176 and p = 0.114, respectively). For the GTV mean dose, a dose level ≥151.2 Gy10 (HR 0.172, 95% CI 0.028–1.074, p = 0.035) and ≥100.0 Gy15/21 (HR 0.132, 95% CI 0.021–0.823, p = 0.012) was associated with better LC (Fig. 2), while a maximum plan dose above 3 × 21.3 Gy presented only a trend for LC (p = 0.070). Interestingly, univariate analysis also showed a significantly better LC with multiple fraction compared to single fraction radiosurgery (p = 0.042), but this result has to be considered with caution as for single fraction, the PTV prescription was generally lower (by approximately 25%) and rarely used. Hence, in the multivariate analysis only the PTV prescription BED10 (p = 0.037 for 89.7 Gy10) reached statistical significance for LC.
For liver metastases, a clear correlation between LC and histology (p < 0.001) could be shown in the univariate analysis, as all local recurrences were found to have colorectal cancer as primary tumor origin. Furthermore, a PTV prescription dose ≥79.2 Gy10 (HR 0.329, 95% CI 0.102–1.588, p = 0.047) and ≥60.0 Gy27 (HR 0.240, 95% CI 0.053–1.077, p = 0.041) was associated with better LC, but PTV prescription doses above 3 × 14 Gy (100.8 Gy10 and 63.8 Gy27) were not (both p = 0.089). For the GTV mean dose, a dose level ≥151.2 Gy10 (HR 0.122, 95% CI 0.016–0.933, p = 0.014) and ≥90.0 Gy27 (HR 0.166, 95% CI 0.050–0.551, p = 0.001) was associated with better LC (Fig. 2), while a maximum plan dose above 3 × 20 Gy was not (p = 0.552). In the multivariate analysis only the GTV mean doses (p = 0.037 for 151.2 Gy10 and p = 0.049 for 90.0 Gy27) reached statistical significance for LC. However, the results of the multivariate analysis should be interpreted with caution due to all events occurring in colorectal cancer patients harboring the risk of over-fitting. Noteworthy was also the fact that GTV and maximum GTV dimension for both lung and liver lesions did not affect LC in our patient cohort.
Due to the low number of cases, the extrahepatic extrapulmonary lesions were not subjected to formal statistical analysis for LC. All treated lymph node metastases were locally controlled at the time of analysis and one of four treated adrenal gland metastases had a local recurrence after delivering a low dose of 4 × 5 Gy, restricted due to previous local radiotherapy (20 × 2 Gy) in this case. The complete statistical analysis for LC is presented in Table 2, detailed descriptions of the local recurrences can be found in Table 3, and examples of local and regional failure in Fig. 3.
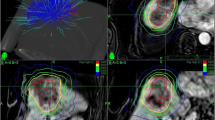
Examples for in-field, field border, and regional failure after robotic breathing-motion-compensated stereotactic body radiation therapy (BMC-SBRT). a Treatment plan (1 × 23 Gy, Monte Carlo) and isodose lines for a lung metastasis from primary non-small cell lung cancer (NSCLC). b In-field recurrence after 26.1 months: 2‑deoxy-2-[F-19]fluoro-D-glucose positron emission tomography (19FDG-PET) overlay (green) to the treatment plan (23 Gy in red). c Treatment plan (3 × 15 Gy, Ray Trace) and isodose lines for a liver metastasis from primary colorectal cancer (CRC). d Field border recurrence after 14.2 months: 19FDG-PET overlay (green) to the treatment plan (45 Gy in red). e Treatment plan (3 × 16 Gy, Monte Carlo) and isodose lines for a primary NSCLC. f New regional tumor after 8.7 months: 19FDG-PET overlay (green) to the treatment plan (48 Gy in red)
Overall survival
The median OS at the time of analysis was 11.2 months. The 1‑, 2‑, and 3‑year OS of all patients were 72.4, 47.9, and 34.0%, respectively (Fig. 1). Worse OS was associated with a Karnofsky performance score less than 100% (HR 2.279, 95% CI 0.997–5.210, p = 0.045), the administration of continuous chemotherapy during SBRT (HR 1.793, 95% CI 1.004–3.203, p = 0.045), and interestingly a male gender (HR 1.657, 95% CI 1.071–2.566, p = 0.022) in univariate analysis. Those parameters remained significant in multivariate analysis. Furthermore, a GTV of ≥35 cc was significantly associated with worse OS in univariate analysis (HR 1.548, 95% CI 1.021–2.346, p = 0.038), but the lesion size was not significant in multivariate analysis (p = 0.085). Not predictive for OS was the age of the patient at the time of first SBRT treatment (p = 0.135). Patients with early stage lung cancer showed clear trend (p = 0.067) for better OS (1-year OS = 100%) and patients with advanced stage lung cancer showed clear trend (p = 0.080) for worse OS (1-year OS = 51.7%), as expected. For metastatic patients only, the primary tumor histology was not a predictive factor for OS. The detailed results of the OS analysis can be found in Table 4.
Toxicity
For the gold marker implantation, one extrapulmonary patient (1.1%) developed an infected encapsulated hematoma in the liver requiring antibiotics and drainage therapy and 4/9 intrapulmonary patients (44.4%) developed a self-resolving minor pneumothorax requiring observation only. Radiation treatment-related side-effects of grade III were identified only in a single patient (0.5%), requiring a stent implantation for a hepatic vein occlusion as previously reported [10].
Discussion
We are presenting one of the largest patterns of failure studies with a single dedicated treatment planning and delivery technique for robotic real-time BMC-SBRT. Our main findings were high LC rates (82.2–100% for various tumor sites) with overall low risks of grade III side effects (0.5%) and a clear dose–response relationship for PTV prescription and GTV mean doses up to certain dose levels.
For primary non-small cell lung cancer (NSCLC), we further found that local control is significantly dependent upon whether the patient had prior lung tumor therapy (e. g., operation, chemotherapy, or radiation therapy) or not (p = 0.027). For the retreatment of local recurrences after prior local lung radiotherapy, this may seem obvious due to dose limitations [32], but those patients were a minority in the presented cohort (4.6%) and more importantly did not show any local failure. This was also recently found in similar studies with BMC-SBRT [33]. Three of the four lung tumors that showed local failure during follow-up were classified as regional recurrent NSCLC after curative radiochemotherapy or resection with adjuvant chemotherapy before SBRT (Table 3). It may be speculated that recurrent NSCLC especially after prior chemotherapy have a tendency to be more radioresistant and require higher doses [34], but the overall data for this hypothesis remains small. Furthermore, in comparison to local (in-field or field border) recurrences after SBRT, regional recurrences may also occur due to the small and focused treatment fields [35], but those were rare in our patient cohort (2.9%, compare Fig. 1 for one of the two cases). The treatment of local recurrences after SBRT of NSCLC is also controversial and very individualized (compare Table 3), but a recent recommendation for such specific situations [36] may help to generate guidelines in the future.
For all lung tumors combined, our LC rates were high (i. e., reaching 100%) and well in agreement with previously published large multicenter studies [1, 3,4,5, 7]. However, we found that the PTV prescription doses required for high LC (>90%) were noticeably lower with our method compared to those previously presented. Arguably, we routinely use the Monte Carlo dose calculation algorithm and some of those studies used older pencil beam algorithms, which may significantly overestimate the dose [22, 37,38,39,40] and hence are difficult to compare [40]. But also in contrast to CyberKnife Monte Carlo data, our PTV prescription dose for high LC seem lower [37,38,39]. Allowance for larger inhomogeneity within the PTV and the optimization of the GTV mean dose per se, which appears to be a necessary task for the small CyberKnife beams [10], rather than only prescribing to the GTV mean dose [22] may have significantly contributed to this phenomenon. Yet of course, the evidence for this hypothesis is immature.
Nevertheless, the results concerning the liver metastases in our study also point in this direction, confirming our previous results with a larger dual-center patient cohort [10]. While again our LC rates were similar compared to other studies [41, 42], the needed PTV prescription doses for high LC (>90%) were definitively lower in our study (3 × 12–13 Gy compared to 3 × 15 Gy or more), but of course, due to the difference in treatment planning techniques, the results remain complex to compare. However, regardless of the lack of similar studies, we did not find better LC for PTV prescription doses above 112.5 Gy10 and 100.8 Gy10 for lung and liver lesions, respectively. This may, different to previous publications [3, 7, 9, 37,38,39, 41, 42], suggest an efficacy plateau for our technique concerning the PTV prescription dose, while at the same time higher GTV mean doses above 151.2 Gy10 still increase the likelihood of local control significantly.
A similar finding, i. e., better correlation of maximum dose to LC compared to prescription dose, was noticed recently in the working group Stereotactic Radiotherapy of the Deutsche Gesellschaft für Radioonkologie (DEGRO) evaluation of lung and liver SBRT [4, 11]. However, most of the data originated from static field isocenter treatments which naturally links maximum dose with GTV mean dose (i. e., the maximum dose is centrally located and directly influences the GTV mean dose), whereas the maximum dose for robotic SBRT is geometrically flexible (e. g., it can even easily be located outside the GTV for larger tumors [10]) due to the small nonisocentric noncoplanar beam arrangement. Therefore, it is no surprise that the maximum dose in our analysis did not significantly impact LC, though there was a small trend identified in the lung lesion group likely explained due to the build-up effects in the lung which naturally moves the maximum dose further into the GTV center.
Another finding of our study was a significant correlation between histology (i. e., primary colorectal cancer) and LC for liver metastases, whereas for lung metastases no correlation between histology and LC was found. While for the lung metastases group, the statistical analysis has to be taken with caution due to the minimal number of events (n = 1), both findings concerning histology are well in agreement with published multicenter studies [4, 5, 11]. Looking more closely at the local failures in the liver metastases group (compare Table 3), we found that all patients received prior chemotherapy which could point to a decrease in radiosensitivity, hence, requiring higher SBRT doses for those lesions, as very recently demonstrated in the aforementioned multicenter study [11]. Interestingly, we also found no correlation between tumor volumes or tumor dimensions with LC seemingly overcoming the limitations of ablation techniques as recently published for larger liver tumors [43]. The literature remains very controversial on this topic [3, 5, 7, 11, 41, 43,44,45], as on one hand, patients with larger tumor volumes have a tendency for lower overall survival and likely do not reach the time point for local recurrences and, on the other hand, are more often treated with lower doses due to critical organ limitations. With our GTV-optimized PTV dose-reduction technique in larger volume SBRT (5–9 cm), we however believe that at least the dose limitation effects can be overcome to achieve high local tumor control within 3–5 years. Whether or not the BMC technology also had an impact on LC remains to be seen and may likely be only relevant in heavily moving tumors in the lower lung and abdomen [11, 46], but regardless, further studies including advanced biological modeling approaches [15, 16, 47, 48] are clearly needed to investigate the GTV dose–volume effects in greater detail.
Overall survival of 60.2, 41.3, 50.0, and 50.2% at 2 years for early and late stage NSCLC and for lung and liver metastases was comparable to published literature [1, 3, 5,6,7, 32, 44]. As expected, patients with early stage NSCLC had a trend for better OS compared to patients with advanced stage NSCLC, although the result was not statistically significant (Table 3). For metastatic patients only, primary tumor histology was not a predictive factor for OS in our patient cohort. Histology may be a predictive factor for patients with lung metastases [7, 45], but for liver metastases it remains unclear [9, 10, 42] and our patient numbers are too small to render any final conclusion in this regard. Furthermore, patients with a high Karnofsky performance score had a better OS, as previously noted for lung metastases [45]. A Karnofsky performance score of 90% is still quite high but there was only a relatively low number of patients with a Karnofsky score lower than 90% in our cohort (the median score was 90%). Even so we think that this statistically significant difference shows the impact of performance score for oncological outcomes and should be always taken into account also in trials examining SBRT. Interestingly, the simultaneous combination of chemotherapy and BMC-SBRT had a significant negative influence on OS in both uni- and multivariate analysis, which may likely point to the fact that those patients had further underlying systemic disease spread as compared to the typical early stage or oligometastatic cancer patient where chemotherapy is either paused or not administered at all.
The limitations to our study are inherent to its retrospective nature with inhomogeneous patients and lesions, even though we treated all patients according to strict study-like institutional guidelines with a single dedicated treatment technique. Furthermore, we identified a low number of local recurrences which may not allow drawing any definitive conclusions from the multivariate analysis. Larger, prospective, multi-institutional patient cohorts with unified techniques or quality, longer follow-up periods, and detailed patient and dosimetry information are needed in order to validate our hypotheses further.
Conclusions
Overall, robotic real-time breathing-motion-compensated SBRT can be an effective and safe treatment modality for solid tumors in moving organs allowing for high local tumor control rates with minimal toxicities. To reach sufficient local control rates (>90%), high mean GTV doses (>151.2 Gy10, >90 Gy15/21, and >90 Gy27 for lung and liver lesions, respectively) appear to be necessary. On the other hand, if the standard practice PTV prescription dose of 3 × 15 Gy cannot be reached due to critical organ limitations, the prescription dose may be reduced to certain dose levels below the current standard with only minimal reduction in tumor control probability if a high mean GTV dose is maintained. Further studies regarding this topic are highly warranted.
References
Guckenberger M, Allgäuer M, Appold S et al (2013) Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 8(8):1050–1058
Guckenberger M, Andratschke N, Alheit H et al (2014) Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 190(1):26–33
Davis JN, Medbery C 3rd, Sharma S et al (2015) Stereotactic body radiotherapy for early-stage non-small cell lung cancer: clinical outcomes from a National Patient Registry. J Radiat Oncol 4(1):55–63
Guckenberger M, Klement RJ, Allgäuer M et al (2015) Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy. Radiother Oncol. https://doi.org/10.1016/j.radonc.2015.09.008
Rieber J, Streblow J, Uhlmann L et al (2016) Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases – A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer 97:51–58
Tanadini-Lang S, Rieber J, Filippi AR et al (2017) Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.003
Ricco A, Davis J, Rate W et al (2017) Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry’s experience. Radiat Oncol 12(1):35–31
Sterzing F, Brunner TB, Ernst I et al (2014) Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 190(10):872–881
Vautravers-Dewas C, Dewas S, Bonodeau F et al (2011) Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys 81(3):e39–e47
Andratschke N, Parys A, Stadtfeld S et al (2016) Clinical results of mean GTV dose optimized robotic guided SBRT for liver metastases. Radiat Oncol 11:74
Klement RJ, Guckenberger M, Alheid H et al (2017) Stereotactic body radiotherapy for oligo-metastatic liver disease – Influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.013
Nuyttens JJ, Prevost JB, Van der Voort van Zijp NC et al (2007) Curative stereotactic robotic radiotherapy treatment for extracranial, extrapulmonary, extrahepatic, and extraspinal tumors: technique, early results, and toxicity. Technol Cancer Res Treat 6(6):605–610
Casamassima F, Livi L, Masciullo S et al (2012) Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys 1;82(2):919–923
Jereczek-Fossa BA, Fanetti G, Fodor C et al (2017) Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2017.01.004
Liu F, Tai A, Lee P et al (2017) Tumor control probability modeling for stereotactic body radiation therapy of early-stage lung cancer using multiple bio-physical models. Radiother Oncol 122(2):286–294
Klement RJ (2017) Radiobiological parameters of liver and lung metastases derived from tumor control data of 3719 metastases. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.03.014
Kilby W, Dooley JR, Kuduvalli G et al (2010) The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat 9(5):433–452
Schweikard A, Shiomi H, Adler J (2004) Respiration tracking in radiosurgery. Med Phys 31(10):2738–2741
Pepin EW, Wu H, Zhang Y et al (2011) Correlation and prediction uncertainties in the cyberknife synchrony respiratory tracking system. Med Phys 38(7):4036–4044
Chan MK, Werner R, Ayadi M et al (2015) Comparison of 3D and 4D Monte Carlo optimization in robotic tracking stereotactic body radiotherapy of lung cancer. Strahlenther Onkol 191(2):161–171
Chan M, Grehn M, Cremers F et al (2017) Dosimetric Implications of Residual Tracking Errors During Robotic SBRT of Liver Metastases. Int J Radiat Oncol Biol Phys 97(4):839–848
Lacornerie T, Lisbona A, Mirabel X et al (2014) GTV-based prescription in SBRT for lung lesions using advanced dose calculation algorithms. Radiat Oncol 9(16):223
Schlaefer A, Schweikard A (2008) Stepwise multi-criteria optimization for robotic radiosurgery. Med Phys 35(5):2094–2103
Blanck O, Wang L, Baus W et al (2016) Inverse Treatment Planning for Spinal Robotic Radiosurgery: An International Multi-Institutional Benchmark Trial. J Appl Clin Med Phys 17(3):313–330
Deng J, Guerrero T, Ma CM et al (2004) Modelling 6 MV photon beams of a stereotactic radiosurgery system for Monte Carlo treatment planning. Phys Med Biol 7;49(9):1689–1704 (May)
Grimm J, LaCouture T, Croce R et al (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):3368–3368 (Review)
Trakul N, Chang CN, Harris J et al (2012) Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys 1;84(1):231–237 (Sep)
Ernst F, Dürichen R, Schlaefer A, Schweikard A (2013) Evaluating and comparing algorithms for respiratory motion prediction. Phys Med Biol 58(11):3911–3929
Malinowski KT, McAvoy TJ, George R et al (2012) Mitigating errors in external respiratory surrogate-based models of tumor position. Int J Radiat Oncol Biol Phys 82(5):e709–e716
Bibault JE, Prevost B, Dansin E et al (2012) Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol 7(24):102
Malinowski K, McAvoy TJ, George R et al (2013) Maintaining tumor targeting accuracy in real-time motion compensation systems for respiration-induced tumor motion. Med Phys 40(7):71709
Mantel F, Flentje M, Guckenberger M (2013) Stereotactic body radiation therapy in the re-irradiation situation – a review. Radiat Oncol 8(5):7
Ceylan C, Hamacı A, Ayata H et al (2017) Re-Irradiation of Locoregional NSCLC Recurrence Using Robotic Stereotactic Body Radiotherapy. Oncol Res Treat 40(4):207–214
Trovo M, Minatel E, Durofil E et al (2014) Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88(5):1114–1119
Giuliani ME, Hope A, Mangona V et al (2017) Predictors and Patterns of Regional Recurrence Following Lung SBRT: A Report From the Elekta Lung Research Group. Clin Lung Cancer 18(2):162–168
Nieder C, De Ruysscher D, Gaspar LE et al (2017) Reirradiation of recurrent node-positive non-small cell lung cancer after previous stereotactic radiotherapy for stage I disease : A multi-institutional treatment recommendation. Strahlenther Onkol. https://doi.org/10.1007/s00066-017-1130-0
Bibault JE, Mirabel X, Lacornerie T et al (2015) Adapted Prescription Dose for Monte Carlo Algorithm in Lung SBRT: Clinical Outcome on 205 Patients. PLOS ONE 10(7):e133617
van der Voort van Zyp NC, Hoogeman MS, van de Water S et al (2010) Clinical introduction of Monte Carlo treatment planning: a different prescription dose for non-small cell lung cancer according to tumor location and size. Radiother Oncol 96(1):55–60
Iwata H, Ishikura S, Murai T et al (2017) A phase I/II study on stereotactic body radiotherapy with real-time tumor tracking using CyberKnife based on the Monte Carlo algorithm for lung tumors. Int J Clin Oncol. https://doi.org/10.1007/s10147-017-1123-0
Zheng D, Zhu X, Zhang Q et al (2016) Target dose conversion modeling from pencil beam (PB) to Monte Carlo (MC) for lung SBRT. Radiat Oncol 11:83
Dewas S, Bibault JE, Mirabel X et al (2012) Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol 7:166
Chang DT, Swaminath A, Kozak M et al (2011) Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117(17):4060–4069
Wahl DR, Stenmark MH, Tao Y et al (2016) Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 34(5):452–459
Peterson J, Niles C, Patel A et al (2016) Stereotactic Body Radiotherapy for Large (〉 5 cm) Non-Small-Cell Lung Cancer. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2016.11.020
Tanadini-Lang S, Rieber J, Filippi AR et al (2017) Nomogram based overall survival prediction in stereotactic body radiotherapy for oligo-metastatic lung disease. Radiother Oncol. https://doi.org/10.1016/j.radonc.2017.01.003
Rieber J, Abbassi-Senger N, Adebahr S et al (2016) Influence of Institutional Experience and Technological Advances on Outcome of Stereotactic Body Radiation Therapy for Oligometastatic Lung Disease. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2016.09.026
Klement RJ, Allgäuer M, Appold S et al (2014) Support vector machine-based prediction of local tumor control after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 88(3):732–738
Klement RJ, Allgäuer M, Andratschke N et al (2016) Bayesian Cure Rate Modeling of Local Tumor Control: Evaluation in Stereotactic Body Radiation Therapy for Pulmonary Metastases. Int J Radiat Oncol Biol Phys 94(4):841–849
Acknowledgements
The authors would kindly thank Rainer Klement (Schweinfurt, Germany) for his helpful comments and discussions on biological effective dose calculation. The authors would also like to thank Prof. Dr. Jost Philipp Schäfer (Kiel, Germany), PD Dr. Peter Hunold (Lübeck, Germany), Dr. Gunnar Gaffke (Güstrow, Germany), Dr. Klaus-Rainer Bogun (Rostock, Germany), Prof. Dr. Norbert Hosten (Greifswald), PD Dr. Nikolaos Tselis (Frankfurt, Germany) and Prof. Thomas Vogl (Frankfurt, Germany) for implanting the fiducials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Stera, P. Balermpas, M.K.H. Chan, S. Huttenlocher, S. Wurster, C. Keller, D. Imhoff, D. Rades, J. Dunst, C. Rödel, G. Hildebrandt and O. Blanck declare that they have no competing interests.
Ethical standards
This retrospective analysis was approved by the local ethics committee of the medical faculty of the university Frankfurt (477/15), Rostock (A2016-0008) and Lübeck (13–218 A).
Rights and permissions
About this article
Cite this article
Stera, S., Balermpas, P., Chan, M.K.H. et al. Breathing-motion-compensated robotic guided stereotactic body radiation therapy. Strahlenther Onkol 194, 143–155 (2018). https://doi.org/10.1007/s00066-017-1204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1204-z