Abstract
Purpose
To assess oncological outcomes of patients receiving neoadjuvant radiochemotherapy (RCT) for soft tissue sarcoma (STS) of the extremities.
Methods
Patients who were treated with preoperative radiotherapy and concomitant chemotherapy—3 cycles of mitomycin/doxorubicin/cisplatin (MAP) or 2–4 cycles of doxorubicin/cisplatin (AP)—followed by surgery were analyzed retrospectively. Survival rates were estimated, and prognostic factors were identified.
Results
Between 1994 and 2017, a total of 108 patients were included. Median ages were 43 years and 51 years for patients receiving MAP and AP, respectively. The 5‑year local relapse-free survival (LRFS), disease-free survival (DFS), disease-specific survival (DSS), and overall survival (OS) were 94.1, 63.6, 75.3, and 71.9%, respectively. In the multivariate analysis, significant predictors were identified as follows: de novo or R1/R2 resected tumor on admission before RCT (p = 0.017; hazard ratio [HR] 0.112, 95% confidence interval [CI] 0.019–0.675) and R0 resection after RCT (p = 0.010; HR 0.121, 95% CI 0.024–0.598) for LRFS; female gender (p = 0.042; HR 0.569, 95% CI 0.330–0.979) and liposarcoma histology (p = 0.014; HR 0.436, 95% CI 0.224–0.845) for DFS; liposarcoma histology (p = 0.003; HR 0.114, 95% CI 0.027–0.478) and AP regimen (p = 0.017; HR 0.371, 95% CI 0.165–0.836) for DSS; age ≤ 45 years (p = 0.043; HR 0.537, 95% CI 0.294–0.980) and liposarcoma histology (p = 0.006; HR 0.318, 95% CI 0.141–0.716) for OS, respectively.
Conclusion
An increased risk for local failure seems to exist for patients with relapsed tumor on admission and having positive surgical margins after neoadjuvant RCT. Intensity of chemotherapy influenced DSS but not OS, which could be due to younger patients receiving MAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) are uncommon tumors having more than 50 histologically subtypes [1]. Approximately half of the STS are located in the extremities [2]. Leiomyosarcoma, undifferentiated pleomorphic sarcoma, and liposarcoma are the most common forms of STS in adults [3]. Tumor histologic grade was shown to be the most important prognostic factor and to be the best indicator of metastatic risk [4].
Mainstay of the STS treatment is surgery. Historically amputation was the standard surgery of STS located at the extremities; however, this brutal approach was replaced by limb-sparing surgery followed by postoperative radiotherapy (XRT) in the 1980s. Although postoperative XRT prevents loss of the limb, it requires irradiation of a large volume of the extremity up to 60–70 Gy and may potentially cause serious permanent late toxicity such as fibrosis [5, 6]. In the last two decades, preoperative XRT was used more commonly than postoperative XRT in the management of extremity STS. Preoperative XRT requires lower doses around 44–50 Gy to a smaller volume [5,6,7]. A phase III trial comparing preoperative vs postoperative XRT showed that both methods provide similar tumor control; however, although the postoperative complication rate was higher with preoperative XRT, serious late effects were much lower compared to postoperative XRT [8].
Surgery and XRT provide good local control in high-grade STS; however, distant metastases to lungs, brain, and bones are common, and determine the survival. A trend towards improved overall survival (OS) was shown following the use of adjuvant chemotherapy (CHT) [9]. However, more improved survival data come from the phase 2 studies where preoperative XRT was combined with neoadjuvant CHT, but these were not randomized or multi-institutional studies [10, 11].
This study presents the retrospective data of a single center’s patients with the diagnosis of extremity STS, who had received neoadjuvant radiochemotherapy (RCT) before surgery. Our institute is the reference center for sarcoma management and all sarcoma patients are evaluated by the weekly multidisciplinary sarcoma board which was shown to have a significant impact on treatment success [3].
Materials and methods
This study was approved by the institutional ethics committee.
Study population
Patients with biopsy-proven, nonmetastatic high-grade or large STS of the extremities who were treated with neoadjuvant RCT followed by limb-sparing surgery from 1994–2017 were retrospectively analyzed. Patient and tumor characteristics including age, date of diagnosis, imaging studies, histology, tumor size and location as well as treatment parameters including surgery, XRT and CHT details were extracted from the patient charts. Treatment response, tumor progression, and follow-up notes were carefully recorded.
Neoadjuvant treatment protocol
The treatment schedule for each patient was decided at the weekly multidisciplinary bone and soft tissue sarcoma meetings. We recommended neoadjuvant RCT for most patients with high-grade STS and a few patients with low-grade STS. Indications were deep-seated grade 2–3 soft tissue sarcoma of the extremities which were larger than 5 cm. Principally all patients fulfilling these criteria were considered for neoadjuvant RCT; however, exclusions included superficial tumors which were amenable to safe resection with wide margins, patients medically unfit for CHT, and patients unsuitable for long treatment period. Although high-grade histology was the main selection criteria, there were few selected patients with low-grade STS in whom we decided to use RCT. These were the cases for whom obtaining negative surgical margin or preserving extremity function could be difficult without RCT due to the size and location of the tumor.
Radiotherapy
XRT was administered to 50.4 Gy in 28 fractions. The tumor-bearing extremity was immobilized using vacuum bags, and proper patient positioning was provided. Magnetic resonance imaging (MRI) was ordered to define the size, exact location, and borders of the tumor prior to XRT planning. Until 2004, two-dimensional XRT planning was used—usually two oblique portals with wedges, to include macroscopic tumor plus 5 cm margins longitudinally and 2 cm margins radially. Extra care was given for not to irradiate the whole circumference of the limb. From 2004, a three-dimensional conformal XRT technique was used in all patients. Clinical target volume (CTV) included gross tumor volume plus 3–4 cm margins longitudinally and 1–1.5 cm margins radially. These margins were modified to allow lymphatic flow and not to cross intact bone and fascial barriers. Planning target volume was created by adding 0.5–1 cm uniform expansion margin to the CTV.
An additional dose of 18 Gy was given after surgery to those patients with R1 resection, or those with very close surgical margins (1–2 mm) with the decision of the multidisciplinary tumor board if postoperative wound healing was achieved.
Chemotherapy
Doxorubicin-based CHT was administered concomitantly with XRT, at the first irradiation day (D1), and 3rd week of XRT (D22). The third cycle was given at D43 prior to surgery. From 1995–2007, 3 cycles of MAP (mitomycin 8 mg/m2, D1-22; doxorubicin 40 mg/m2, D1-22-43; cisplatin 60 mg/m2, D1-22-43), which was inspired by the phase 3 trial, were used [12]. From 2007–2017, mitomycin was not used anymore and patients received 4 cycles of AP (doxorubicin 60–75 mg/m2; cisplatin 60–75 mg/m2). The first CHT cycle was 3 weeks before the first day of XRT and the other 3 cycles were given at D1-22-43 (cisplatin was only given at D1 and D22). Later, intensity of this schedule was reduced to 2 cycles (D1-22) due to high toxicity observed with 4 cycles of AP. Overall 63 patients received MAP and 45 received AP. The total median drug doses were as follows: mitomycin 16 mg/m2, doxorubicin 120 mg/m2 and cisplatin 180 mg/m2 for MAP regimen, and doxorubicin 225 mg/m2 and cisplatin 150 mg/m2 for patients for AP regimen.
Additional CHT cycles were given to 25 patients postoperatively who had large and/or high-grade tumors at presentation.
Surgery
One month after the completion of XRT, tumor response was evaluated with dynamic contrast-enhanced MRI scan, and limb-sparing surgery was performed in 2–3 weeks. The goal of the surgery was to remove the tumor completely with wide margins to provide R0 resection without compromising the extremity function.
Follow-up procedure
During follow-up, physical examination, MRI of the treated extremity, and chest X‑ray or chest computed tomography were performed. Follow-up was scheduled every 3 months for the first 2 years, every 6 months between the third and fifth year, and then annually.
Outcomes
Outcome measures were as follows: local relapse-free survival (LRFS), metastasis-free survival (MFS), disease-free survival (DFS), disease-specific survival (DSS), and OS. All times to event were measured from the first day of the treatment and patients without an event were censored at the last contact date known alive for all survival metrics. Event was defined for each survival metric as follows: LRFS, recurrence at local site confirmed by either pathology or imaging; MFS, distant metastasis, or death from any cause, whichever occurred first; DFS, local recurrence, regional lymph node recurrence, distant metastasis, second malignancy, or death from any cause, whichever occurred first; DSS, dying of cause related to sarcoma; OS, death from any cause. Patients who died of unrelated causes were censored for DSS. Patients were also censored at the date of death if no prior local recurrence was observed when calculating LRFS.
Toxicities were scored using the common toxicity criteria for adverse events (CTCAE) version 5.0 [13].
Statistics
The data processing and statistical analysis were performed with statistical software package SPSS Statistics for Windows (version 22.0, IBM Corp., Armonk, NY, USA). Patient or treatment related characteristics including age (≤ 45 years vs > 45 years), gender, status on admission (de novo or R1/R2 resected tumor vs relapsed tumor after previous surgery), size of tumor (T1&T2 vs T3&T4), histology (liposarcoma vs non-liposarcoma), tumor location (upper extremity vs lower extremity), neoadjuvant CHT (MAP vs AP), surgical resection after neoadjuvant RCT (R0 vs R1), adjuvant XRT (present vs not present), and adjuvant CHT (present vs not present) were evaluated as categorical data. Time to event data was calculated using the Kaplan–Meier method. For time-to-event endpoints, a univariate test comparing categorical variables was conducted using a log-rank test along with Kaplan–Meier estimates. Variables statistically significant on the log rank method were subsequently entered into Cox proportional hazards regression model for multivariate analysis. Hazard ratio (HR) and 95% confidence interval (CI) were calculated.
Toxicities were compared among MAP and AP applied groups with the use of Fisher’s exact test and Yate’s continuity correction chi-square (χ2) test.
Two-sided p-values were reported, and p < 0.05 was considered statistically significant.
Results
Patient and treatment characteristics
A total of 108 patients were included in the study. Median age was 45 years (43 years for patients receiving MAP and 51 years for patients receiving AP), and 58 patients (54%) were male and 50 (46%) were female. Tumor was located at lower extremity in 81% of patients. Median size of the tumors was 10.3 cm. Undifferentiated pleomorphic sarcoma (n = 41, 38%) and liposarcoma (n = 31, 29%) were the most common histologies. Most patients (76%) had no previous surgery, whereas one out of every four patients had previous resections with positive margins (R1–R2), or recurrence after previous surgery. Data regarding patient and treatment-related characteristics are presented in Table 1.
Tumor control and limb preservation
Limb preservation was possible in 103 patients (95.4%). Primary closure or skin grafts were mostly sufficient for wound closure; however, pedicled flaps and rarely free flaps were required. Five (4.6%) patients were amputated due to insufficient tumor shrinkage not allowing R0 or R1 resection despite neoadjuvant RCT. Margins were clear (R0) in 91 (88.4%) of the resections and microscopic residue left (R1) in 12 (11.6%). There were no patients with gross tumor left (R2) after surgery. In addition, 2 patients underwent amputation during the follow-up period: one was due to postoperative complication and the other was due to local recurrence. Overall extremity preservation rate was 93.5%.
Recurrence and survival
The mean follow-up time was 114 months (range 5–311 months). Eleven of 108 (10.2%) patients were lost to follow-up; however, we were able to follow these 11 patients at least 3 years (median 102 months, range 35–206 months).
Eight (7.4%) patients developed local recurrence with a median time of 16 months (range 8–87 months). LRFS was 94.1% at 5 years and 91.2% at 10 years (Fig. 1a). Distant metastases occurred in 38 (35.2%) patients with a median time of 19 months (range 5–155 months). Lung metastases were observed in 31 patients. Other metastatic sites were soft tissue in 7 patients, bones in 4 patients, brain in 3 patients, and adrenal gland in 1 patient. MFS was 67.1% and DFS was 63.6% at 5 years; 58.3% and 51.4% at 10 years, respectively (Fig. 1b,c). In all, 11 (10%) patients developed 13 second primary cancers during follow-up (Table 2).
A total of 32 (29.6%) patients died due to tumor progression and 12 (11.1%) patients died of other causes and 2 (1.9%) patients died of second primary cancer. DSS and OS were 75.3–71.9% at 5 years and 70.8–64.2% at 10 years, respectively (Fig. 1d,e).
The univariate and multivariate associations of patient and tumor characteristics with LRFS, MFS, DFS, DSS, and OS are listed in Tables 3 and 4, respectively.
LRFS was improved for patients whom were treated for de novo or R1/2 resected tumor (tumor status on admission) compared to patients whom were treated for a relapsed tumor following a previous surgery (p = 0.030 [5-year LRFS 95.7% vs 75.0%]) and for patients applied R0 resection compared to patients applied R1 resection after neoadjuvant RCT (p = 0.010 [5-year LRFS 96.6% vs 74.1%]). Also in the multivariable analysis, de novo or R1/R2 resected tumor on admission (p = 0.017; HR 0.112, 95% CI 0.019–0.675) and R0 resection after neoadjuvant RCT (p = 0.010; HR 0.121, 95% CI 0.024–0.598) were found as the significant predictors of improved LRFS.
Only liposarcoma histology compared to non-liposarcoma histologies was associated with improved MFS (p = 0.006 [5-year MFS 90.2% vs 57.7%]) on univariate analysis; therefore, no multivariate analysis was performed. On univariate analysis, female gender compared to male gender (p = 0.022 [5-year DFS 69.8% vs 58.3%]) and liposarcoma histology compared to non-liposarcoma histologies (p = 0.007 [5-year DFS 90.2% vs 53%]) were associated with improved DFS. In the multivariable analysis; female gender (p = 0.042; HR 0.569, 95% CI 0.330–0.979) and liposarcoma histology (p = 0.014; HR 0.436, 95% CI 0.224–0.845) remained to be the significant predictors of improved DFS.
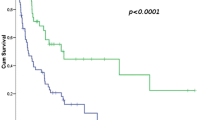
Patients with liposarcoma histology compared to patients with non-liposarcoma histologies (p = 0.001 [5-year DSS 100.0% vs 65.5%]), and patients receiving AP compared to patients receiving MAP (p = 0.039 [5-year DSS 86.6% vs 67.0%]) had improved DSS. These results were also confirmed by multivariate analysis that patients with liposarcoma histology performed better than patients with other histologic subtypes (p = 0.003; HR 0.114, 95% CI 0.027–0.478), and patients applied AP performed better than patients applied MAP (p = 0.017; HR 0.371, 95% CI 0.165–0.836).
On univariate analysis, age ≤ 45 years compared to > 45 years (p = 0.027 [5-year OS 76.6% vs 66.7%]), female gender compared to male gender (p = 0.036 [5-year OS 77.6% vs 66.9%]), and liposarcoma histology compared to non-liposarcoma histologies (p = 0.003 [5-year OS 96.8% vs 61.8%]) were associated with improved OS. Multivariate analysis revealed that age ≤ 45 years (p = 0.043; HR 0.537, 95% CI 0.294–0.980) and liposarcoma histology (p = 0.006; HR 0.318, 95% CI 0.141–0.716) were significant predictors of increased OS.
Adverse events
Treatment-related toxicities were compared between patients receiving MAP regimen and those receiving AP regimen (Table 5). Thrombocytopenia of grade 3, grade 4 neutropenia, and febrile neutropenia were significantly higher in patients who received AP regimen compared to those received MAP regimen (p = 0.029, p = 0.035, and p = 0.040, respectively). Other adverse events were not statistically different.
Discussion
Five-year LRFS of entire patient cohort was 94.1% which was similar with the phase 3 randomized trial that compared preoperative vs postoperative XRT [8]. At 10 years, it was 91.2%, demonstrating that high local control benefit with XRT persists over time. We were able to preserve the extremity in 93.5% of the patients, which was very satisfactory.
As stated in the previous studies, tumor positivity at the surgical margins after neoadjuvant RCT was also an independent predictor of decreased local control in our study [14, 15]. Although local recurrence rate significantly decreased after the utilization of XRT in extremity STS, adequate surgical margin still remains a predictor of local control. Another subgroup of patients with inferior local control rate included patients irradiated for a local relapse after previous surgery. Although at first sight a relapsed tumor may exhibit more aggressive clinical behavior, survival of this group was similar with others and increase in local relapse rate did not translate into decrease of survival.
The 5‑year OS was 71.9% in our entire patient cohort. It was 67% in the EORTC 62931 phase 3 randomized trial involving high-risk STS patients [16]. In that trial, surgery was compared with surgery plus CHT and 73% of all patients received XRT. In the two notable phase 2 studies for neoadjuvant RCT, 5‑year OS was 71.2% in the RTOG 9514 trial and 80% in the study conducted by Edmonson et al. [7, 10]. Our result appears consistent with the literature. Multivariate analysis revealed that younger age (≤ 45 years) and liposarcoma histology were associated with improved OS, as expected.
Patients with a diagnosis of liposarcoma histology fared better compared to patients with non-liposarcoma histologies in all survival metrics except LRFS (which was also improved for non-liposarcoma histologies) and 5‑year DSS was 100% for liposarcoma histology in our study. In a similar study conducted by Fiore et al., 5‑year DSS was 83% for all patient cohort consisting of primary and recurrent liposarcoma. However, although limited patients received XRT (39%) and CHT (18%), DSS was 90% for those presenting with primary tumor [17]. The much better DSS in our group may be due to the relatively high ratio of myxoid liposarcoma cases.
The role of CHT in the treatment of non-metastatic STS of the extremities has not been clarified exclusively. Furthermore, very limited data exist for neoadjuvant CHT compared to adjuvant CHT. Although no randomized trials addressing the benefit of neoadjuvant CHT given concomitantly with XRT are available, various doxorubicin-based CHT regimens were frequently used with preoperative XRT for the treatment of locally advanced STS [10, 11, 18,19,20]. Eilber et al. showed that the tumor necrosis rate increased from 60 to 70% when cisplatin was added to doxorubicin in concomitant use of CHT with XRT [21]. Following two cycles of ifosfamide, mitomycin, and cisplatin, XRT and three concomitant cycles of MAP were given preoperatively to the patients with limb or pelvic girdle high-grade soft tissue sarcoma in the phase 2 trial conducted by Edmonson et al., and 5‑year survival rate was 80% [10]. In the RTOG 9514 phase‑2 trial, patients having a high-grade STS larger than 8 cm were treated preoperatively with three cycles of the modified mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) regimen and XRT, and additional three cycles of MAID were given postoperatively. The results showed 5‑year OS rate of 71.2% albeit with significant toxicity as grade 3 or higher in 97% and death due to toxicity in 5% of patients [7]. Our study was not a randomized trial; however, DSS was better with the AP regimen although XRT dose schedule and approach of target volume delineation were similar in all patients. We think that despite increased treatment-related toxicities, the intense doxorubicin dose might play an important role for improved DSS. However, although patients receiving AP had improved OS compared to patients receiving MAP (81.9% vs 64.7% at 5 years), the difference did not reach statistical significance. We suppose this statistically nonsignificant result for OS could be related to the fact that patients receiving MAP were younger than those receiving AP (median age 43 years vs 51 years). Nonetheless, one needs to be careful when interpreting this result, since our patient cohort comprises several tumor types and are thereby not appropriate for subgroup estimation. Thus, further research on the role of concomitant CHT and XRT for specific tumor types is required.
We are aware of the limitations of this study which were retrospective design and consisting of heterogeneous tumor types; however, prospective trials for STS are quite limited. Nonetheless, apart from survival outcomes, prognostic factors, and treatment-related toxicities, we also highlighted the details of second primary cancers occurring during follow-up. We believe in that experience of our center will contribute to the sarcoma literature.
Conclusion
Neoadjuvant RCT followed by surgery provides long-term and very high local control in high-grade or large STS of the extremities. Treating a relapsed tumor and surgical margin positivity after neoadjuvant RCT pose an increased risk for local failure. Finally, apart from relatively innocent histological subtype such as liposarcoma, CHT intensity also seems to significantly influence DSS. However, CHT intensity did not have a statistically significant impact on OS. Nonetheless, this could be due to younger patients receiving MAP.
References
The WHO Classification of Tumours Editorial Board (2020) WHO classification of tumours soft tissue and bone tumours, 5th edn. IARC, Lyon
Gamboa AC, Gronchi A, Cardona K (2020) Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 70(3):200–229
Blay JY, Honore C, Stoeckle E, Meeus P, Jafari M, Gouin F et al (2019) Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol 30(7):1143–1153
Coindre JM (2006) Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med 130(10):1448–1453
Kaushal A, Citrin D (2008) The role of radiation therapy in the management of sarcomas. Surg Clin North Am 88(3):629–646
Spalek MJ, Kozak K, Czarnecka AM, Bartnik E, Borkowska A, Rutkowski P (2020) Neoadjuvant treatment options in soft tissue sarcomas. Cancers (Basel) 12(8):2061
Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH et al (2010) Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer 116(19):4613–4621
O’Sullivan B, Davis A, Turcotte R, Bell R, Wunder J, Catton C et al (2004) Five-year results of a randomized phase III trial of pre-operative vs post-operative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol 22(14_suppl):9007
Sarcoma Meta-analysis Collaboration (SMAC) (2000) Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev 2:CD1419
Edmonson JH, Petersen IA, Shives TC, Mahoney MR, Rock MG, Haddock MG et al (2002) Chemotherapy, irradiation, and surgery for function-preserving therapy of primary extremity soft tissue sarcomas: initial treatment with ifosfamide, mitomycin, doxorubicin, and cisplatin plus granulocyte macrophage-colony-stimulating factor. Cancer 94(3):786–792
DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ et al (2003) Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 56(4):1117–1127
Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S et al (1993) Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11(7):1269–1275
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 17 July 2022
Endo M, Lin PP (2018) Surgical margins in the management of extremity soft tissue sarcoma. Chin Clin Oncol 7(4):4
Sambri A, Caldari E, Fiore M, Zucchini R, Giannini C, Pirini MG et al (2021) Margin assessment in soft tissue sarcomas: review of the literature. Cancers (Basel) 13(7):1687
Woll PJ, Reichardt P, Le Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ et al (2012) Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13(10):1045–1054
Fiore M, Grosso F, Lo Vullo S, Pennacchioli E, Stacchiotti S, Ferrari A et al (2007) Myxoid/round cell and pleomorphic liposarcomas: prognostic factors and survival in a series of patients treated at a single institution. Cancer 109(12):2522–2531
Temple WJ, Temple CL, Arthur K, Schachar NS, Paterson AH, Crabtree TS (1997) Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol 4(7):586–590
Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH et al (2006) Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 24(4):619–625
Spałek M, Koseła-Paterczyk H, Borkowska A, Wągrodzki M, Szumera-Ciećkiewicz A, Cieszanowski A et al (2019) OC-0069 5x5 Gy with chemotherapy in borderline resectable soft tissue sarcomas: early results of a trial. Radiother Oncol 133:S31–S32
Eilber FR, Eckardt JJ, Rosen G, Fu YS, Seeger LL, Selch MT (1993) Neoadjuvant chemotherapy and radiotherapy in the multidisciplinary management of soft tissue sarcomas of the extremity. Surg Oncol Clin N Am 2(4):611–620
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ugur Yilmaz, Serra Kamer, Yavuz Anacak; Methodology: Ugur Yilmaz, Serra Kamer, Yavuz Anacak; Formal analysis and investigation: Ugur Yilmaz, Serra Kamer, Yavuz Anacak; Writing—original draft preparation: Ugur Yilmaz, Serra Kamer; Writing—review and editing: Yavuz Anaca; Resources: Ugur Yilmaz, Serra Kamer, Huseyin Kaya, Dundar Sabah, Ulus Ali Sanli, Ipek Tamsel, Banu Yaman, Taner Akalin, Yavuz Anacak; Supervision: Serra Kamer, Yavuz Anacak.
Corresponding author
Ethics declarations
Conflict of interest
U. Yilmaz, S. Kamer, H. Kaya, D. Sabah, U.A. Sanli, I. Tamsel, B. Yaman, T. Akalin and Y. Anacak declare that they have no competing interests.
Ethical standards
This study was approved by the institutional ethics committee (21-8T/56).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yilmaz, U., Kamer, S., Kaya, H. et al. Preoperative radiotherapy with concomitant chemotherapy in extremity soft tissue sarcomas: long-term results of a single center. Strahlenther Onkol 199, 585–594 (2023). https://doi.org/10.1007/s00066-022-02041-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-02041-x