Abstract
Purpose
The purpose of this study was to evaluate acute skin toxicity in early breast cancer patients treated with hypofractionated radiotherapy (HFRT) after breast-conserving surgery and to identify factors predictive for grade ≥ 2 acute skin toxicity.
Materials and methods
A monocentric retrospective study was carried out using cases treated between December 2017 and November 2020. We analyzed data from 202 patients with early breast cancer treated with 3D hypofractionated RT (40.05 Gy in 15 fractions) to the whole breast with or without regional lymph nodes, followed by 13.35 Gy in 5 fractions to the tumor bed. Acute skin toxicity was monitored during RT according to CTCAE (common toxicity criteria for adverse events) scale. Univariate and multivariate analyses were performed to assess predictive factors of acute skin toxicity.
Results
Overall, there was no erythema in 9%, grade 1 erythema in 64.5%, grade 2 in 24%, and grade 3 in 2.5%. No grade 4 erythema was seen. Median delay between RT initiating and maximum skin reaction was 22 days (range 4–44 days). No patient interrupted treatment. In univariate analysis, the rate of acute skin toxicity grade 2--–3 (G2-3) was significantly higher for patients with larger tumor size (p = 0.02), body mass index > 27 (p = 0.04), and time between chemotherapy (CT) and RT less than 20 days (p = 0.01). Dosimetric risk factors for acute skin toxicity G2‑3 were breast volume > 800 cc (p = 0.000), boost volume > 18 cc (p = 0.002), V105% > 40 cc (p = 0.03), and Dmax > 56 Gy (p = 0.007). CT, trastuzumab, regional lymph node radiation, and age were not correlated with increased skin toxicity. In multivariate analysis, acute skin toxicity correlated with T stage (p = 0.032), breast volume > 800 cc (p = 0.012), boost volume > 18 cc (p = 0.04), and Dmax > 56 Gy (p = 0.035).
Conclusion
Our results confirm that whole breast with or without lymph nodes hypofractionated RT is safe and well tolerated. The factors strongly associated with a decreased risk of G2‑3 skin toxicity are T1, breast volume < 800 c, boost volume < 18 cc, and Dmax < 56 Gy. Long-term follow-up is needed to evaluate late toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The benefits of adjuvant radiotherapy (RT) following breast-conserving surgery (BCS) for early-stage breast cancer are well established [1]. The most widely used schedule is 50 Gy, 2 Gy/fraction (Fr) of whole-breast irradiation. In recent years, there has been a growing trend towards hypofractionated radiotherapy regimens [2]. Large multicenter randomized trials of hypofractionated radiotherapy (HFRT) with more than 10-year follow-up data have shown efficacy and safety in terms of local control and cosmetic outcome [2, 3].
The main intent of HFRT was to reduce treatment time by reducing the number of individual fractions, creating capacity to treat additional patients without compromising oncologic outcomes.
The reasons of slow adoption of HFRT in some countries are likely to be multifactorial and reflect both scientific and pragmatic concerns. The main arguments against routine adoption of HFRT include the concern that HFRT may increase toxicity due to higher doses per fraction, particularly in patients with a high body mass index (BMI) [4] and the lack of data in the case of associated chemotherapy or regional lymph node radiation (RLNR). Only a few studies have investigated cosmetics and acute toxicities after HFRT [5].
The aim of this study was to evaluate acute skin toxicity in early breast cancer patients treated with HFRT with or without regional lymph node radiation after breast-conserving surgery and to analyze correlations with clinical and dosimetric characteristics.
Methods
Study design
A retrospective study including patients treated with 3D HFRT after breast-conserving surgery was performed. Patients were treated from December 2017 to November 2020.
The inclusion criteria were age ≥ 18 years, histological proven breast cancer, prior conservative surgery (lumpectomy or quadrantectomy), pathological stage pT1-pT2, pN0-pN+, M0 according to American Joint Committee–International Union against Cancer staging system (AJCC-UICC, 6th edition) with negative surgical margins.
Exclusion criteria were previous RT to the same breast, or synchronous bilateral breast cancers requiring adjuvant RT.
The study was approved by the institution’s ethics committee.
Data collection
Data including history of allergies, diabetes, arterial hypertension (AHT), age, BMI, stage, pathology, and treatment details (neoadjuvant/adjuvant chemotherapy [CT], RT, RLNR, hormone therapy [HT], targeted therapy) were retrospectively evaluated.
Weekly monitoring was carried out during the RT treatment and one month after treatment completion. The maximum acute skin toxicity during RT was recorded, according to CTCAE (Common Toxicity Criteria for Adverse Events) scale, version 4 [6].
The dosimetric parameters included the following: breast volume (the volume corresponding to the target that was contoured), boost volume, volume of the breast receiving 95, 105, and 107% of the prescribed dose (V95%, V105%, and V107%), the maximum dose (Dmax), and beam energies.
Radiation treatment
CT planning scans were performed with 3 mm slice thickness, in the supine position on an angled board, from the level of larynx to upper abdomen, including bilateral breasts and lungs. Clinical target volumes (CTV) breast, the supraclavicular, and internal mammary nodes were delineated according to ESTRO consensus guidelines for RT in patients with early breast cancer [7].
CTV breast includes the entire mammary gland materialized by radiopaque landmarks on computed tomography scan, including the soft tissues from 5 mm below the skin surface to the deep fascia, excluding underlying muscle and rib cage. The tumor bed (boost volume) was delineated based on surgical clips, preoperative mammography, and postoperative breast tissue remodeling and changes. In our current practices, PTV breast corresponds to CTV breast due to the tangential fields’ large margins compensating for positioning uncertainties and modification during treatment. Organs at risk, lungs and heart, were delineated.
The prescribed dose was 40.05 Gy in 15 fractions to the whole breast and to the supraclavicular and internal mammary nodes if indicated, with sequential boost to the tumor bed (13.35 Gy in 5 fractions). Patients were treated 5 days a week. RT was delivered using a monoisocentric technique, with opposed tangential fields with or without a direct supraclavicular field using 6–18 MV photons. Boost was delivered by two tangential fields or by direct electron field. Field-in-field technique was used to achieve a homogeneous dose distribution between 95 and 107% of the prescribed dose in the target volumes. OAR constraints used for planning were the following: for lung V17 (the lung volume percentage receiving > 17 Gy) < 30% and V25.8 (the lung volume percentage receiving > 25.8 Gy) < 20%. For the heart, the mean dose was < 4.3 Gy. Treatment planning was carried out with Monaco treatment planning system version 5.1 using a collapsed cone algorithm (CCC). All patients were treated using a linear accelerator.
Statistical analysis
The data were analyzed on the Statistical Package for the Social Sciences (SPSS) version 20 (IBM SPSS, Armonk, NY, USA). Results were expressed as mean ± standard deviation (SD). Two groups were identified: patients with grade 0–1 (G0-1) acute skin toxicity and patients with grade 2–3 (G2-3). The χ2 and Fisher exact tests or t‑test for independent samples were used to compare the two groups and to analyze associations between acute skin toxicity and patient characteristics (age, BMI, diabetes, AHT, allergy, T stage), regional lymph node radiation, treatments given in addition to RT (CT, HT, trastuzumab) and dosimetric parameters (breast volume, boost volume, V95%, V105%, V107%, Dmax, beam energy).
Multivariate analysis to identify independent risk factors predictive for higher grade skin toxicity was performed using binary logistic regression. P < 0.05 was considered significant.
Results
Data from 202 breast cancer patients were analyzed in this study. Patient characteristics and details of treatment are shown in Tables 1 and 2.
The median age was 53 years (25–82 years). The tumors were classified as T1 in 55% (n = 111) and T2 in 45% (n = 91) of cases. CT was given in 84% of patients, HT in 83%, and trastuzumab in 24%.
The median interval between CT and RT was 60 days (12–228 days) and the median overall radiation treatment duration was 29 days (28–43 days). No patient interrupted treatment due to toxicity.
Regional lymph node radiation was performed in 41.6% of patients (n = 84). The median breast volume was 693.80 cc (100–2777 cc). The median boost volume was 14.65 cc (1.86–201 cc).
The median volume of tissue receiving 95, 105, and 107% of prescribed dose was 683 cc (99.8–2664 cc), 48 cc (0–1140 cc), and 0.017 cc (0–732 cc), respectively.
Acute skin toxicity
There was no erythema in 9%, grade 1 erythema in 64.5%, grade 2 in 24%, and grade 3 in 2.5%. No grade 4 erythema was observed (Table 3).
The median time to appearance of maximum skin toxicity was 22 days (4–44 days). This maximum reaction was observed at a dose of 37 Gy (10.6–53.35 Gy), corresponding to the 14th session.
Radiation dermatitis was localized in the whole breast in 65.2% (n = 120), in the submammary region in 27.7% (n = 51), and in the axillary area in 10.3% (n = 19). Among 54 patients with grade 2 and 3 skin toxicity, dermatitis was localized in the submammary region in 71% (n = 39).
Patient and treatment related factors have been evaluated in a univariate analysis (Table 4).
The rate of acute G2‑3 skin toxicity was significantly higher in patients with a BMI > 27 than those with a BMI ≤ 27 (37% vs 15%, p = 0.04), for patients with T2 rather than a T1 tumor (35% vs. 21%, p = 0.02), and for patients treated with a gap between CT and RT less than 20 days compared with > 20 days (60% vs 25% p = 0.01).
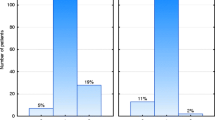
There was no significant difference in G2‑3 skin toxicity between patients taking hormone therapy or not (27% vs 28%, p = 0.8). However, among patients taking HT, those on letrozole developed more significant G2‑3 skin toxicity than those on tamoxifen or anastrozole (37% vs 28% vs 11%, respectively, p = 0.02) (Fig. 1).
Dosimetric risk factors were identified for acute skin toxicity. The mean breast volume, boost volume, and V95% were significantly higher in patients with acute skin toxicity G2‑3 than in patients with skin toxicity G0‑1 (Table 5). A positive correlation was found between G2‑3 skin and breast volume > 800 cc (p = 0.000), boost volume > 18 cc (p = 0.002), V105% > 40 cc (p = 0.03), and Dmax > 56 Gy (p = 0.007) (Table 6).
There was no significant difference in G2‑3 skin toxicity between patients receiving or not receiving regional lymph node radiation (28% vs 25%, p = 0.33).
Other factors such as age, AH, allergy, chemotherapy, trastuzumab, and beam energy did not correlate with the severity of acute skin reaction.
The results of multivariate analysis are summarized in Table 7. These confirm the significant impact of T stage (p = 0.026), breast volume > 800 cc (p = 0.012), boost volume > 18 cc (p = 0.02), and Dmax > 56 Gy (p = 0.034).
Discussion
Our study confirmed that HFRT was well tolerated after breast-conserving surgery (BCS) in 202 patients. G2 and 3 erythema were observed in 24 and 2.5%, respectively. No grade 4 erythema was seen. In univariate analysis, the rate of G2‑3 acute skin toxicity was significantly higher for patients with larger tumor size, BMI > 27 and time between CT and RT under 20 days. Dosimetric factors for G2‑3 acute skin toxicity were the following: breast volume, boost volume, V105%, and Dmax > 56 Gy (105% of prescribed dose).
In multivariate analysis, acute skin toxicity was correlated to tumor size, breast volume > 800 cc, boost volume > 18 cc, and Dmax > 56 Gy. These results are consistent with published data [8, 9]. The UK FAST-Forward trial group reported an acute skin toxicity substudy, although only 43 patients were evaluable in the 40 Gy/15 fr group. G2 and 3 CTCAE toxicity were reported in 22 (51%) and 0 patients [10]. However, it should be noted that only patients requiring radiotherapy to the breast alone were recruited in the FAST-Forward trial; regional lymph node irradiation was not permitted and only 25% of patients were boosted. In our study, all patients received a boost to the tumor bed and 84 patients (41%) had locoregional irradiation.
In a Cochrane review, Hickey et al. reviewed randomized, controlled trials of HFRT vs conventional fractionated radiotherapy (CF-RT) with early breast cancer after BCS and reported that acute radiation skin toxicity was significantly decreased in HFRT, with a risk ratio of 0.32 (95% confidence interval [CI] 0.22–0.45). No analysis of predictive factors was reported [11].
In addition, in a prospective Japanese single-arm trial of 306 patients, grade 2 acute skin toxicity was found in 38 patients (12.4%); none had grade 3 or 4 toxicity [12]. Based on a low α/β ratio (3–4), acute skin toxicity could be affected more by treatment time and total dose of radiation than by the fraction dose [10, 11, 13].
This is consistent with our study showing a maximum skin reaction on the third week (14th fraction) without progressing over time.
Some patient-related factors have been shown to increase acute skin toxicity. High BMI and large tumor size both increased acute skin toxicity [7, 9, 11, 12, 14]. We noticed the same correlation with G2‑3 for patients with BMI > 27 (37% vs 15% for BMI < 27 p = 0.04) and for patients with T2 (35% vs 21% for T1, p = 0.02). Age and comorbidities were not correlated with the severity of acute skin reactions.
Breast volume has frequently been reported as predictive for enhanced skin toxicity with different cut offs, probably due to interoperator contouring variability and volume definition. Dorn et al. studied skin toxicity in women with large breasts treated with a hypofractionated schedule of 42.56 Gy in 2.66 Gy per fraction [15]. A total of 80 patients were included with median breast volume of 1351 cc. On univariate and multivariable analysis, breast volume > 2500 cc was the only patient-related factor significantly associated with moist desquamation [16]. Harsolia et al. found no RTOG grade 3 acute toxicity when the breast volume was below 975 cc. Patients with breast volumes > 1600 cc developed G2 and G3 skin reactions in 59 and 3% of cases, respectively [17]. The median breast volume treated in our study was smaller (893.8 cc, range 100–2777 cc) than those reported in these studies, but we confirmed the correlation of breast volumes > 800 cc with increased acute skin toxicity.
There is no clear evidence that chemotherapy has an impact on acute skin toxicity when a HFRT schedule is used [18]. Kouloulias et al. analyzed 116 patients treated with HFRT, of which 33 underwent adjuvant CT. In a univariate analysis, CT was associated with acute skin toxicity with critical value of p = 0.05 [19]. Our results, including 169 patients (84%) receiving CT, did not show increase in acute skin toxicity in univariate and multivariate analysis (p = 0.9).
The acute G2‑3 skin toxicity was significantly higher when the time between CT and RT was less than 20 days compared with a longer interval. The same results were reported by Zygogianni et al., with reference to 44 patients with early stage breast cancer treated with adjuvant CT and HFRT (p < 0.05) [20].
To best of our knowledge, there are no studies that have investigated the impact of HT on acute skin toxicity after HFRT. This correlation has been studied only with conventional fractionation (CF-RT). In our study, two types of HT, tamoxifen or aromatase-inhibitors were administered to hormone receptor positive breast cancer patients. There was no significant difference between G2–3 skin toxicity in patients taking HT or not (p = 0.7). However, G2–3 erythema was significantly higher in the subgroup of patients taking concurrent letrozole compared to tamoxifen or anastrozole, 42.5% vs 28% vs 12.5%, respectively (p = 0.01).
De Langhe et al. reported that concomitant conventional fractionated RT and HT was associated with an increased risk of acute dermatitis (p = 0.041), with no difference regarding the type of HT [21]. On the contrary, the COHORT randomized trial, showed no difference between concurrent and sequential administration of letrozole [22].
There are few data on the predictive value of clinically applicable dosimetric factors on acute skin toxicity after HFRT. On multivariate analysis, Keenan et al., proved that the V105% > 30 cc (p = 0.013) and the use of CF-RT (p = 0.001) increased acute skin toxicity [23]. On literature review, current quantitative dosimetric parameters that have shown significant impact on skin toxicity include a V107% > 3 cc in hypofractionation [24].In analysis of 537 breast cancer, De santis et al. found that administration of a boost, the boost volume, V105% ≥ 92.5 cc, V107% ≥ 34.4 cc, and V110% ≥ 4.8 cc were statistically significant as factors predictive of more severe acute skin reaction on univariate analysis, but only boost administration (p = 0.02) on multivariate analysis [25].
In our local protocol validation process, hotspots should be < 107% of the prescribed dose and “field-in-field” and monoisocentric techniques are used to decrease dose distribution inhomogeneities. It is likely that this favorable toxicity profile reflected the treatment planning accuracy achieved by this requirement, which resulted in particularly low dose distribution inhomogeneities. The median V105% and V107% were respectively 48 cc and 0.017 cc. In multivariate analysis, boost volume > 18 cc and Dmax > 56 Gy were significantly correlated with a higher acute skin toxicity. This is in line with ASTRO recommendations to minimize V105% using 3D conformal treatment planning with a “field-in-field” technique, and the Fast Forward protocol which recommended that less than 5% of PTV should receive 105% or more, less than 2% of PTV 107% or more, and a global maximum dose below 110% [5].
Conclusion
Our study confirmed that HFRT is safe and well tolerated. The rate of acute skin toxicity was low, 24% G2, 2.5% G3, and no G4. We identified Dmax < 56 Gy (105% of the prescribed dose) and V105% ≤ 40 cc as dosimetric parameters to be used to optimize treatment planning to minimize acute skin toxicity. Care should be taken in patients with breast volume > 800 cc and boost volume > 18 cc. For these patients, strict application of defined dosimetric parameters and close monitoring during RT are encouraged to further reduce the risk of acute toxicity. Concurrent letrozole or a short interval time between CT and RT may increase acute skin toxicity. However, chemotherapy or regional lymph node radiation should not be considered as contraindication to hypofractionated radiotherapy. Longer follow-up is needed to evaluate late skin reactions.
References
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135
START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EGA, Barrett JM, Barrett-Lee PJ et al (2008) The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371(9618):1098–1107
Whelan TJ, Kim D‑H, Sussman J (2008) Clinical experience using hypofractionated radiation schedules in breast cancer. Semin Radiat Oncol 18(4):257–264
Shaitelman SF, Schlembach PJ, Arzu I, et al (2015) Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol 1(7):931–941. https://doi.org/10.1001/jamaoncol.2015.2666
Murray Brunt A, Haviland JS, Wheatley DA, et al (2020) Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 395(10237):1613–1626. https://doi.org/10.1016/S0140-6736(20)30932-6
CTEP Common terminology criteria for adverse events (CTCAE) | protocol development. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed 12.2021
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A et al (2015) ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 114(1):3–10
Sanz J, Rodríguez N, Foro P, et al (2017) Hypofractionated boost after whole breast irradiation in breast carcinoma: chronic toxicity results and cosmesis. Clin Transl Oncol 19(4):464–469. https://doi.org/10.1007/s12094-016-1548-3
Tortorelli G, Di Murro L, Barbarino R, Cicchetti S, di Cristino D, Falco MD et al (2013) Standard or hypofractionated radiotherapy in the postoperative treatment of breast cancer: a retrospective analysis of acute skin toxicity and dose inhomogeneities. Bmc Cancer 13(1):230. https://doi.org/10.1186/1471-2407-13-230
Brunt AM, Wheatley D, Yarnold J, Somaiah N, Kelly S, Harnett A et al (2016) Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3‑week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 120(1):114–118
Hickey BE, James ML, Lehman M, Hider PN, Jeffery M, Francis DP et al (2016) Fraction size in radiation therapy for breast conservation in early breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003860.pub4
Nozaki M, Kagami Y, Shibata T, Nakamura K, Ito Y, Nishimura Y et al (2019) A primary analysis of a multicenter, prospective, single-arm, confirmatory trial of hypofractionated whole breast irradiation after breast-conserving surgery in Japan: JCOG0906. Jpn J Clin Oncol 49(1):57–62. https://doi.org/10.1093/jjco/hyy160
Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J et al (2005) Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 75(1):9–17
Holloway CL, Panet-Raymond V, Olivotto I (2010) Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast 19(3):163–167
Dorn PL, Corbin KS, Al-Hallaq H, Hasan Y, Chmura SJ (2012) Feasibility and acute toxicity of Hypofractionated radiation in large-breasted patients. Int J Radiat Oncol Biol Phys 83(1):79–83
Ciammella P, Podgornii A, Galeandro M, Micera R, Ramundo D, Palmieri T et al (2014) Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol 9(1):97. https://doi.org/10.1186/1748-717X-9-97
Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C et al (2007) Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys 68(5):1375–1380
Hijal T, Al Hamad AA, Niazi T, Sultanem K, Bahoric B, Vuong T et al (2010) Hypofractionated radiotherapy and adjuvant chemotherapy do not increase radiation-induced dermatitis in breast cancer patients. Curr Oncol 17(5):22–27
Kouloulias V, Zygogianni A, Kypraiou E, Georgakopoulos J, Thrapsanioti Z, Beli I et al (2014) Adjuvant chemotherapy and acute toxicity in hypofractionated radiotherapy for early breast cancer. WJCC 2(11):705–710
Zygogianni A, Kouloulias V, Antypas C, Armpilia C, Kyrgias G, Kouvaris J (2014) The impact of intermediate time between chemotherapy and hypofractionated radiotherapy to the radiation induced skin toxicity for breast adjuvant treatment. Breast J 20(1):74–78. https://doi.org/10.1111/tbj.12206
De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M et al (2014) Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. Bmc Cancer 14(1):711. https://doi.org/10.1186/1471-2407-14-711
Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K et al (2010) Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol 11(3):258–265
Keenan LG, Lavan N, Dunne M, McArdle O (2019) Modifiable risk factors for acute skin toxicity in adjuvant breast radiotherapy: dosimetric analysis and review of the literature. Med Dosim 44(1):51–55
Taher AN, El-Baradie MM, Essa H, Zaki O, Ezzat S (2004) Hypofractionation versus conventional fractionation radiotherapy after conservative treatment of breast cancer: early skin reactions and cosmetic results. J Egypt Natl Canc Inst 16(3):178–187
De Santis MC, Bonfantini F, Di Salvo F, Dispinzieri M, Mantero E, Soncini F et al (2016) Factors influencing acute and late toxicity in the era of adjuvant hypofractionated breast radiotherapy. Breast 29:90–95
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Ben Amor, M. Bohli, Z. Naimi, D. Aissaoui, N. Mejri, J. Yahyaoui, A. Hamdoun, and L. Kochbati certify that they have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Ethical standards
The study has been approved by the institution’s ethics committee. Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent to publish: Patients signed informed consent regarding publishing their data.
Additional information
Data Availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Amor, R., Bohli, M., Naimi, Z. et al. Hypofractionated radiotherapy after breast-conserving surgery: Clinical and dosimetric factors predictive of acute skin toxicity. Strahlenther Onkol 199, 48–54 (2023). https://doi.org/10.1007/s00066-022-01985-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-022-01985-4