Abstract
Object
The anterior choroidal artery (AChoA) is a rare location for intracranial aneurysms. The treatment of these aneurysms may be challenging due to the risk of occlusion of such a small and eloquent artery as the AChoA. We aimed to evaluate the risk factors for complications in AChoA aneurysm treatment.
Methods
We retrospectively analyzed 47 consecutive AChoA aneurysms in 40 patients treated in our institution from 1999 and 2014 by endovascular means (87%) or surgical clipping (13%). Minor (transient or minor neurological deficits) and major complications (severe permanent neurological deficits or death) were systematically recorded. The influence of patient age, sex, aneurysm size, neck size, shape, dome-to-neck ratio and treatment technique on the occurrence of procedure-related complications was evaluated.
Results
Of the patients 11 experienced procedure-related complications (5 major, 6 minor). Aneurysms with multilobed shape were significantly associated with a higher procedure-related complication rate. There was a tendency for higher major procedure-related complication rate in small volume aneurysms. We did not find any association between the other factors analyzed and occurrence of procedure-related complications.
Conclusion
Treatment of AChoA aneurysms has an acceptable complication risk. We did not find any significant differences between surgical and endovascular treatment in terms of procedure-related complication rates. Multilobed aneurysms were significantly associated with a higher procedure-related complication rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anterior choroidal artery (AChoA) aneurysms are rare vascular malformations (2–5% of all intracranial aneurysms [1]) that can be challenging to treat. The treatment difficulties of AChoA aneurysms may be related to the particular conformation of the parent artery, its small size and the eloquence of the territory it supplies. Potential treatment options to secure the aneurysm include either endovascular techniques, including regular coiling, remodeling techniques and stenting [2, 3] or surgical procedures with a clip [4].
The objective of this study was to evaluate the safety of aneurysmal repair techniques (microsurgical or endovascular) for AChoA aneurysms and identify risk factors for procedure-related complications.
Materials and Methods
Study Design
We conducted a retrospective single center observational study. The inclusion criteria were as follows: 1) patients admitted in our institution from January 1999 and December 2014 for the treatment of an AChoA aneurysm (ruptured and unruptured; recanalized or not, single or multiple), 2) age of 15 years and 3 months or more and 3) at least 3 months of clinical follow-up. The exclusion criteria were as follows: 1) children under 15 years and 3 months, 2) aneurysm in location other than the AChoA origin, particularly aneurysms purely emerging from the internal carotid artery (ICA) or its choroidal segment after the origin of the posterior communicating artery (PCom), 3) major surgery contraindications (organ failure, moribund status), 4) mycotic, posttraumatic or tumor-induced aneurysms and 5) incomplete clinical records.
Patient Demographics
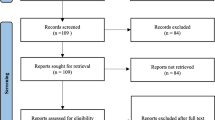
Patient demographics are summarized in the flow chart (Fig. 1) and in Table 1.
From 1999 to 2014, 49 patients (34 females, 15 males; median age = 50 years; IQR = 11 years; range 17–62 years) attended our Institution for the management of 56 AChoA aneurysms. Among these patients, 8 patients with 8 AChoA received conservative treatment due to the aneurysms characteristics (unruptured/small size/stable on imaging follow-up), 1 patient died from subarachnoid hemorrhage before any therapeutic measures could be performed. A total of 47 AChoA aneurysms in 40 patients were treated by therapeutic repair (either surgery or endovascular treatment).
Aneurysm Characteristics
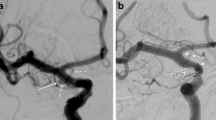
The following data were collected on angiographic explorations (DSA and/or CTA) (Table 2): orientation, sac size (i. e. aneurysm sac maximum diameter), neck size and dome-to-neck ratio. In cases of dome-to-neck ratio lower than 2, the neck was considered large. The volume of each aneurysm was estimated using the Angiocalc software (http://www.angiocalc.com). The shape of the aneurysm sac was also systematically assessed as saccular (spherical, ellipsoid or multilobed) or fusiform. Multilobed shape was defined by the presence of one or more daughter sac(s) arising from the main aneurysmal sac (Fig. 2).
Example of an unruptured multilobed aneurysm. a Right internal carotid artery (ICA) DSA in lateral projection showing a multilobed AChoA aneurysm. b Right ICA DSA in working projection demonstrating more precisely the multilobed shape of the aneurysm with two daughter sacs. Note the stenosis of the distal aspect of the ICA. c 3D rotational angiography (RA); volume rendering reconstruction showing more precisely the blebs as well as the origin of the AChoA
Technical Considerations
The choice of the therapeutic option was made by a multidisciplinary team, including neurosurgeons, interventional neuroradiologists and anesthesiologists, based on patient clinical characteristics (e.g. age, comorbidities, neurological status), presentation of the aneurysm (ruptured aneurysm, associated hematoma, morphology of the sac and neck size) and the best technique available at the time of the procedure.
Endovascular Treatment
All endovascular procedures were performed under general anesthesia via a right femoral approach. Patients were treated either by regular coiling; remodeling technique due to the presence of a wide neck or stents (Flow diverter stents: Pipeline stents [ev3/Covidien, Irvine, CA] and Silk stent [Balt Montmorency, France]; open cell stent [Neuroform 3, Stryker, Fremont, CA]). Coiling procedures and remodeling were carried out under effective heparin therapy, 50 UI/kg bolus of heparin was given at the start of the procedure for non-ruptured aneurysms, and then 15 UI/kg/h of heparin to maintain Activated Clotting Time (ACT) 2 to 3 fold the baseline. The first bolus of heparin was given after deployment of the first coil for ruptured aneurysms. Patients treated with stent were loaded with clopidogrel (75 mg/day) and aspirin (75 mg/day) 5 days before procedure. After the procedure, the dual antiplatelet therapy was pursued for 3–6 months; then, only aspirin was kept for a total of 1 year (Fig. 3).
Unruptured left AChoA aneurysm, revealed by non-specific headache. a Left ICA DSA in lateral projection showing a large neck aneurysm. The AChoA seems to arise from the dome of the aneurysm. b CT plat panel acquisition with 20% diluted contrast media performed after treatment by flow diverter stent (Pipeline Embolization Device, eV3/Covidien, Irvine, CA) showing the satisfactory deployment of the stent, covering the aneurysm neck. c,d Control DSA at 6 months; c left ICA DSA in lateral projection; d left vertebral DSA in lateral projection. Complete exclusion of the aneurysm is demonstrated; the proximal segment of the AChoA is opacified via the ICA (c, arrow); its distal segment is supplied by anastomosis with the posterolateral choroidal artery (d, arrow)
Surgical Treatment
In all surgical procedures, a regular pterional approach was performed. A curvilinear frontotemporal incision and a careful subfrontal dissection were done until visualization of the optic nerve. If necessary, the lamina terminalis was opened in order to get a flow of cerebrospinal fluid (CSF) from the third ventricle to the basal cisterns, lowering intracranial pressure. The opening of the Sylvian fissure in its proximal part could be useful for better exposure. Supraclinoid ICA was exposed by first ensuring control of its proximal portion and its distal portion at the MCA-ACA junction. The ICA was then exposed around its choroidal portion to visualize the aneurysm. The AChoA origin was then separated from the aneurysm, dissecting arachnoid ties, in order to assess its shape and volume, and to ensure optimum control of its neck. The most appropriate clip was chosen and placed at the neck, making sure to exclude AChoA itself from the clip and to avoid stenosis the adjacent wall of the ICA. Intraoperative visual checking as well as postoperative control DSA were routinely performed to ensure the satisfactory exclusion of the aneurysm (Fig. 4).
Surgical treatment of a right ruptured AChoA aneurysm. a Pretreatment right ICA DSA in right anterior oblique (RAO) projection; b right ICA 3D RA acquisition in working projection. The multilobed AChoA is clearly seen with the AChoA arising from the neck. d,e Control DSA in RAO projection (d) and 3D RA in left anterior oblique projection (e). f Satisfactory exclusion of the aneurysm is seen, as well as the patency of the AChoA
Complications/Procedure-Related Morbidity and Mortality
Procedure-related morbidity and mortality were defined as the occurrence of adverse events during the procedure or within the post-procedural period (≤7 days). Procedure-related complications (clipping or coiling) were divided into ischemic and hemorrhagic ones. Ischemic complications were defined as follows: the onset of a new neurological deficits in the postoperative 48 h and/or CT hypodensity in the postoperative 48 h and/or presence of a hyperintense signal associated with a significant apparent diffusion coefficient (ADC) restriction on MRI diffusion-weighed images (DWI). Hemorrhagic complications were defined as follows: intraoperative rupture or occurrence of postoperative hematoma depicted on CT and/or MRI. For endovascular procedure, a contrast extravasation on CT control was considered as a procedure-related hemorrhagic complication. Procedure-related complications were divided into: 1) major complications including fatal complications (immediate/delayed death or limitation of aggressive care) or persistent and debilitating neurological deficit and 2) minor complications: transient or non-disabling neurological deficit.
Complications related to the SHA itself were excluded from complications attributable to the therapeutic procedure. Thus, any ischemic injury secondary to vasospasm, any neurological deterioration due to hydrocephalus, or any other specific complication, such as adrenergic myocarditis and ventilation assisted acquired pneumopathy were recorded separately.
Clinical and Angiographic Follow-Ups
Clinical evaluations were performed in post-procedure (immediately and at 12 h post-procedure), at discharge, at early follow-up at 2 months and at late follow-up. Late follow-up consisted of a telephone call. Mean delay for late clinical follow-up was 66 ± 50 months (range 3–188 months). Assessment of the modified Rankin score (mRS) was performed as well as an overall evaluation of the quality of life in terms of major sequelae, and minor dependency [5].
Angiographic follow-up evaluation was performed in consensus by a junior neurosurgeon and a senior interventional neuroradiologist on the PACS (Picture Archiving Communicating System) workstation (Agfa, Mortsel, Belgium). Aneurysm exclusion was assessed on post-procedure DSA and on DSA (and/or MRA for aneurysms treated endovascularly) on long-term angiographic follow-up. Mean delay for the angiographic follow-up was 32 ± 30 months (range 0–106 months). The exclusion of the aneurysm sac was evaluated using the Roy-Raymond classification [6].
The presence of a recanalization or aneurysmal recurrence was defined as a degree of change in the Roy-Raymond classification as follows: evolution from grade I to II or III, from grade II to III or worsening of the circulating portion in a recanalized aneurysm previously graded III.
Statistical Analysis
Statistical tests used for non-continuous variables were the χ2 or the Fisher’s exact tests depending on the population size. Regarding the continuous variables, we used Student’s t‑test. A multivariate analysis was conducted using a logistic regression taking into account dichotomized data for age, sex, rupture status, aneurysm size, volume and multilobed shape. The statistical tests were performed using the MedCalc software (MedCalc for Windows, Version 9.3.2.0, MedCalc Software, Mariakerke, Belgium). A p-value <0.05 was accepted as statistically significant.
Results
Patient Demographics/Aneurysm Characteristics
Among the 40 patients who met our inclusion criteria (AChoA aneurysm treated by repair technique, surgery or embolization) there were 27 females and 13 males (sex ratio M/F = 0.48). The median age was 50 years (IQR: 53.42 years; range: 17–62 years). Of the patients, 20 (42.5%) had multiple aneurysms (at any location), 26 aneurysms (55%) were located on the left AChoA, 21 (45%) on the right AChoA, 5 patients (12.5%) had a retreatment of the same aneurysm during the follow-up period and 2 patients (5%) had mirror aneurysms. These 47 AChoA aneurysms were finally analyzed for assessing the safety and effectiveness of aneurysmal exclusion techniques. The average size of aneurysms (maximum diameter) was 4.6 ± 2.9 mm (1–15 mm). The average neck size was 2.9 ± 1.5 mm (0.8 to 6.4 mm). The average volume of aneurysmal lesions was 112 ± 358 mm3 (1–1767 mm3), 28 aneurysms (60%) had a wide neck (dome-to-neck ratio <2) and 7 aneurysms (15%) had a multilobed shape. Finally, two patients (4%) had duplicated AChoA. Among the 47 patients, 31 (66%) had a ruptured aneurysm, 11 patients (35.5%) had a World Federation of Neurological Surgeons (WFNS) grade I, 11 (35.5%) a grade II or III, and 9 (29%) a grade IV or V. Three patients (10%) had a Fisher 1 grade on CT, 7 (22.5%) Fisher 2, 11 (35.5%) were Fisher 3 and 10 (32%) were Fisher 4.
Treatment Options
Endovascular Procedure
Of the patients 42 (87%) were treated by endovascular means, 33 patients were treated by regular coiling, 5 patients were treated by remodeling technique due to the presence of a wide neck and the remaining 4 patients with stents (3 flow diverters stents: 2 Pipeline stents and 1 Silk stent; 1 open cell stent). Aneurysms treated by stents were either unruptured aneurysms (n = 2), or previously coiled and recanalized ruptured aneurysms (n = 2).
Surgical Treatment
Of the patients 5 (13%) underwent surgical treatment by clipping.
Procedure-Related Complications
A total of 11 (24.3%) procedure-related complications were recorded. Their characteristics and statistical comparisons are listed in Tables 3 and 4. Among these 11 patients, only 5 (10.6% of all procedures) had major complications (mRS 3–6), including 2 deaths (4.3%), 4 out of these 5 patients (80%) were treated by embolization, 1 patient (20%) by clipping (9.7% major complications in the embolization group vs. 16.7% in the surgery group, p = 0.5). Among the 4 embolization patients, 2 presented cerebral ischemia secondary to multiple emboli and 1 an acute ischemic stroke in the AChoA territory treated by antiplatelet therapy (i.v. aspirin 250 mg as bolus and i.v. reopro as an infusion for 12 h), responsible for a fatal hemorrhage in the following days (brain stem hematoma). The remaining patient died from an internal carotid artery dissection that occurred during endovascular treatment of cerebral vasospasm few days after the aneurysm coiling. In the surgical group, one major complication was recorded that consisted of a postoperative extensive ischemia along the frontal access to the aneurysm and an ipsilateral third cranial nerve palsy. The remaining 6 patients (12.8% of all procedures) who experienced minor procedure-related complications showed transient ischemia or minor sequel (mRS 0–2). In three procedures (6.4%), the patient developed transient occlusion of the AChoA. In one case, the AChoA occlusion was immediately depicted after the deployment of the first coil, before detachment; the coil was recaptured and redeployed more accurately and the AChoA recanalized after an i.v. bolus of aspirin (Fig. 5). One patient presented a post-embolization hemiparesis related to a DWI-proven acute ischemic stroke in the AChoA territory. This hemiparesis progressively improved over in few days under both antiplatelet (aspirin) and full heparin. Another patient had a delayed (20 months) acute ischemic stroke in the AChoA territory with hemiplegia and hemianopia. This patient had been treated of a recanalized ruptured AChoA aneurysm by means of flow diverter stent (PED). He partially recovered from his deficit after intravenous thrombolysis (rt-PA). On control DSA, the flow diverter stent was patent without any stenosis or in-stent thrombus, but the AChoA was occluded. This may be due to an endothelial recovering of the stent at the AChoA origin. One patient had a periprocedural aneurysm perforation that was immediately and efficiently managed by further coiling at the rupture point, without any clinical consequence. Finally, 2 patients presented small distal emboli on DWI, with no long-term sequelae.
Example of the management of per-procedure AChoA occlusion in an irregular 2 mm ruptured AChoA aneurysm. a Left ICA DSA in working projection showing the AChoA aneurysm with the AChoA arising from the inferior aspect of the neck. Note the presence of an ipsilateral saccular PCom aneurysm. b Left ICA DSA after the deployment of the first coil under temporary balloon inflation (remodeling technique), before detachment. There is no obvious contrast filing of the aneurysm; the AChoA is patent. However, a loop of the coil is protruding at the AChoA origin. c Control DSA 10 min latter: occlusion of the AChoA is seen in its cisternal segment. The non-detached coil is thus recaptured and repositioned. d DSA after repositioning of the coil showing a better conformation of the coil mesh. Intravenous aspirin (250 mg) is injected. e Control DSA 10 min after aspirin injection showing a complete occlusion of the aneurysm and AChoA’s patency. The coil is thus detached. f Final left ICA control DSA in lateral projection after additional coiling of the PCom aneurysm showing satisfactory occlusion of both aneurysms and patency of the AChoA
The aneurysm shape significantly impacted the procedure-related complication rate. Indeed, among the 11 patients who experienced a procedure-related complication (either major or minor), 4 (37% of all procedures) had a multilobed shape aneurysm; on the other hand, only 3 (8%) of the aneurysms were multilobed in the complication-free population (p = 0.04). On multivariate analysis, the only factor associated with a procedure-related complication was the multilobed shape (p = 0.03).
We saw an increased rate of severe procedure-related complications in patients with small volume aneurysms, however, this did not reach a statistical significance (mean aneurysm volume = 24.2 mm3 in patients with severe complications vs. 112 mm3 for the rest of the population, p = 0.1).
There was no significant difference in terms of age, sex, aneurysm size, neck width, dome-to-neck ratio, aneurysm orientation, ruptured status or not, treatment modality, between groups treatment-related complications and no treatment-related complication. There was also no statistically significant difference for the abovementioned criteria between the patients who presented severe procedure-related complications and the rest of the population either in univariate or multivariate analysis.
Interestingly, 3 patients of our cohort (6.4% of all procedures) were treated by means of flow diverter stents (a recanalized ruptured coiled aneurysm, an unruptured aneurysm associated with ipsilateral posterior communicating artery aneurysm and an unruptured aneurysm revealed by headache). In one case, the patient experienced a very late occlusion of the AChoA revealed by an acute ischemic stroke that partially resolved after i.v. thrombolysis. In another case, the AChoA was arising from the dome of the aneurysm; the AChoA remained patent at late follow-up but decreased in size with its distal segment being supplied by the posterolateral choroidal artery, via intra-choroidal plexus anastomosis (Fig. 3).
Non-Treatment-Related Complications
Of the patients, 14 (30% of all procedures) had non-treatment-related complications. All of them presented a ruptured aneurysm. Among the 31 patients with a ruptured aneurysm, 10 patients (32.3%) had cerebral vasospasm, 4 (12.9%) with ischemic sequel, 7 patients (22.5%) developed hydrocephalus requiring ventricle external drainage; among them 1 patient had a ventriculitis. Two patients developed an adrenergic myocarditis after the subarachnoid hemorrhage. One death occurred because of cardiac arrest.
Recurrence and Recanalization
In our cohort 5 patients (12.5%) had an aneurysm recurrence during the follow-up period, among them 4 (10%) patients underwent a new treatment (all by endovascular means), the remaining patient presented a slight coil compaction and imaging follow-up was considered. Recanalized aneurysms had a tendency to present larger volume than stable ones (310 mm3 vs. 112 mm3, p = 0.07). No other factor (such as age, sex, ruptured status, maximum diameter, neck size) was found to be predictive for delayed recanalization/recurrence.
Untreated Aneurysms
Of the patients 8 (16%) had conservative management with imaging follow-up. AChoA aneurysms were sharing the same characteristics: unruptured and regular in shape. Their average size was 1.9 mm and average volume was 5.3 mm3. None of these aneurysms showed any bleeding event during the clinical follow-up period (33 months on average).
Discussion
Several studies have focused on the treatment of anterior choroidal location of aneurysms (Table 5). Some series have studied only surgical treatment [4, 7–14], others exclusively endovascular techniques [15–17], and rarely both treatments [18, 19]. To the best of our knowledge, only one series has compared the 2 techniques [19]. In the literature, treatment-related complication rates varied from 4.5% to 50% [4, 7–22]. This wide range of complication rates may be explained by the heterogeneity of these series, some of them mixing true AChoA aneurysms and ICA aneurysms in the vicinity of the AChoA origin [9, 13, 14, 16, 21, 22]. In our series, we report an overall morbidity mortality rate of 24% (minor morbidity 13.5%, major morbidity 6.5% and 4% mortality). These results are within the ranges of the complication rates reported in the literature (see Table 3). However, this complication rate remains higher compared to the ones reported for other intracranial locations: a meta-analysis carried out in 2010 of 71 studies combining a total of 5044 patients found an overall morbidity and mortality rate of 4.8% and a mortality of 1.2% [23].
Among the 6 patients with post-procedural ischemia in our series, 3 (50%) were located in the AChoA territory (proven on DWI MRI and/or late CT scan). One patient had a complete AChoA syndrome with hemiplegia, hemianopia and hemi sensory deficit, from which he recovered after intravenous thrombolysis. One patient presented a complete right hemiplegia associated with aphasia. He did not recover until his early death related to brainstem hematoma precipitated by dual antiplatelet therapy. Finally, one patient had a left regressive hemiparesis.
Location
In the literature, the nomenclature of AChoA aneurysms is often confusing, sometimes including aneurysms arising from the choroidal segment of the ICA, and not from the AChoA origin itself. Thus, the rate of true AChoA aneurysm varies from 32 to 61% in several studies presenting such heterogeneous population [13, 14, 16, 21]. The largest series in the literature describing aneurysms exclusively arising from the AChoA exclusively reports 127 cases over a 26-year period [18]. In our study, we strictly selected aneurysms emerging from the AChoA itself, or from the ICA-AChoA junction. Specifically, in order to obtain a homogeneous series, we systematically excluded aneurysms not clearly separate from the PCom or emerging from the posterior aspect of the ICA in its cisternal segment, with a gap between the neck and the AChoA origin. Consequently, we found a treatment-related complication rate (24% in overall) that may seem higher compared to other series, but which is actually in accordance with the literature for this specific AChoA location.
This issue concerning the relationship between the aneurysm and the origin of the AChoA is frequently found in the literature, and partly explains the complication rate variability. Thus, several authors report a significant decrease of the complication rate when the AChoA does not arise from the neck of the aneurysm [9, 16, 21, 24]. Indeed, in this configuration the aneurysms arise from the posterior aspect of the internal carotid artery, distant from the AChoA origin. Moreover, some authors have stressed the importance of the orientation of the aneurysms compared to the origin of the AChoA [11]. However, we did not find in our series any significant association between the orientation of the aneurysm’s sac and procedure-related complications.
Size, Volume and Shape
AChoA aneurysms are usually small in comparison with other locations. In the literature, the reported mean aneurysms’ maximum diameter varies from 5.6 to 6.8 mm [9, 14, 24, 25]. The proportion of small AChoA aneurysms (i. e. less than 10 mm) varies from 81.6% [24] to 92% [21]. In our series, the mean aneurysm maximum diameter was smaller than the ones of the previous series (4.6 mm) and 95% of the aneurysms in our series were less than 10 mm. In the study of Cho et al. [21], very small aneurysms (less than 5 mm) had a tendency to be associated with an increased ischemic complication rate (p = 0.235). In our study, despite a significant proportion (8/11; 72%) of small aneurysms in the population with severe complications, we did not show a statistically significant influence of the aneurysm maximum diameter on the occurrence of severe complications. Conversely, patients who experienced a severe procedure-related complicated tended to have lower aneurysm volume than the rest of the population (24.2 mm3 vs. 112 mm3, p = 0.103). This result reflects the intuitive feeling shared by both neurosurgeons and interventional neuroradiologists that aneurysms with a smaller volume are technically more difficult to treat, regarding the risks of perforation or of compromising the AChoA patency.
In our study, we report a higher risk of complications (minor or major) when the aneurysm had a multilobed shape (37% vs 8%, p = 0.04). This is, to our knowledge, the first morphological risk factor reported do date linked with procedure-related complications in AChoA aneurysms. The multilobed aspect may carry some technical issues, like higher difficulties to implant coils in the aneurysm sac for embolization or increased difficulties in the aneurysm dissection for microsurgery.
Clinical Presentation
We did not show any relationship between ruptured status and the occurrence of treatment-related complications, despite some controversial, while non-significant, observations in the literature [18]. Similarly, the severity of the SAH did not appear to affect the procedure-related complication risk in our study.
In 3 cases, the patients had preoperative focal neurological deficit, without consciousness disorder. In 2 cases the patients had contralateral hemiparesis, in 1 case a dysarthria; all them were patients with ruptured aneurysms at the acute phase before any treatment and without associated hematoma. After treatment, all patients recovered from their deficit. These reversible neurological deficits observed at the acute phase of the rupture may be due to local hemodynamic changes (“vasospasm-like”) on such a small caliber artery as AChoA [26].
Treatment Modality
Some series in the literature report a lower complication rate among cases treated by embolization. Kim et al. [19] report the only study retrospectively comparing the two techniques in 73 patients. Despite a comparable overall complication rate, there was significantly more ischemia in the AChoA territory in the clipping group. However, it should be mentioned that there was no randomization in this study concerning the allocation for the treatment modality. In our study, no difference was found between the two methods (9.7% vs. 16.7%, p = 0.5).
Endovascular Treatment
Kim et al. have described the possibility to assess the AChoA permeability during the endovascular procedure as an advantage, compared to surgery [19]. We report such a situation where an inadvertent occlusion was observed during the procedure before the first coil detachment and a rescue withdrawal of the coil was immediately performed (Fig. 5).
In our study, 5 patients (10.6%) were treated by remodeling technique. The complication rate was not higher in the group “remodelling” than in the “simple coiling” group. These results are consistent with numerous series published in the literature on this topic [27]. The use of an additional protective balloon in AChoA endovascular treatment seems relevant for two reasons. First, a moderate over-inflation of the balloon helps protect the origin of the AChoA close to the aneurysmal neck. Second, in case of ruptured aneurysm, especially small ones, the protective balloon can effectively stop acute bleeding during endovascular procedure, while the coiling in pursued.
Surgical Technique
Surgery for AChoA aneurysms, although presented with no particular difficulty by some authors [7, 18], seems actually to require a rigorous technique [4], regarding higher complication rates in comparison with other locations [11]. Thus, if the pterional approach, with its sub-frontal [7] or supra-orbital [28] variants, is classical, the artery and aneurysm are often superimposed and adherent to each other [8] and require a delicate dissection. Moreover, a posterolateral-oriented AChoA aneurysm may be adherent to the temporo-mesial structures such as the uncus, or the PCom artery. A thorough pre-surgical planning is essential. Careful dissection is essential to individualize the sac while minimizing manipulation of the artery. Indeed, AChoA is particularly sensitive to hemodynamic changes as well as micro-thrombotic phenomena. The choice of the final clip must be a compromise between the widest coverage of the aneurysm neck on one hand, and the respect of the ICA caliber and the origin of the AChoA on the other hand. It may be useful to place a pilot clip perpendicularly to the aneurysm dome, to partially collapse it and facilitate dissection and then place a clip parallel to the neck, and possibly replace the first clip by a smaller one.
Potential Strategies to Reduce Complications
Recently, flow-diverter stents have offered an interesting alternative to surgery or coiling/balloon assisted coiling for very wide necked aneurysm. Indeed, these devices induce a progressive thrombosis of the aneurysm sac and a scaffold for endothelial regrowth, without theoretically compromising covered branches ([29–31]; Fig. 3). In addition, flow-diverter stents are also convenient for the treatment of very small aneurysms, avoiding a challenging micro-catheterization at risk of perforation. Three recent studies have shown the interest of these stents in AChoA preservation during ICA aneurysms treatment [3, 32, 33]. Nevertheless, the use of stents has some limitations, especially for ruptured aneurysms, because of the need for antiplatelet treatment. Additionally, long-term behavior of these new devices is still unknown. Indeed, we observed in our series a case of very delayed ischemic complication (20 months) in a patient treated with a flow-diverter stent for recanalization, with occlusion of the AChoA at late angiographic follow-up.
Concerning surgical treatment, some authors mention technical possibilities to monitor the patency of the AChoA intraoperatively during clipping procedures using devices such as micro-probe Doppler [12] or motor evoked potentials [22]. Finally, the fluorescence visualization technique using indocyanine green, enable intraoperative angiographic vision of circulating vessels before and after the clip installation. It appears to be a significant advance in aneurysm surgery [34]. Although its benefit has never been specifically studied in AChoA aneurysms, it could have a substantial interest in the periprocedural control of the clip positioning.
Limitations
Our study presents some limitations. First this is a retrospective monocenter observational study. Second, there was no randomization for the repair technique (i. e. endovascular or surgical), which weakens a potential comparison. Then, due to the low number of cases, statistical significance should be interpreted with caution. Additionally, the assessment of the aneurysm shape could be controversial. Indeed, one can argue that lobe(s) in multilobed aneurysm may correspond to false sac(s) related to the rupture. However, we also observed multilobed shape in unruptured aneurysms and we stress the fact that the multilobed shape was purely descriptive, without prejudging of the underlying pathomechanisms of these daughter sacs. Finally, we included in our series a relatively low volume of aneurysms. However, one should keep in mind that AChoA is a rare location for intracranial aneurysms. Additionally, we only included in our series “pure” AChoA aneurysms, and excluded those arising from the posterior aspect of the ICA, in the vicinity of AChoA origin.
Conclusion
The treatment of AChoA aneurysms has acceptable morbidity and mortality rates, that still remains higher compared to the ones reported for other intracranial locations. In our series, there was no difference in terms of procedure-related complication rates between surgery and embolization. Multilobed aneurysms were associated with an excess risk of procedure-related complications. There was a tendency, despite non-significant, for higher major complication rate in aneurysms with small volume. These morphological features should therefore be carefully investigated before the treatment of AChoA aneurysms.
Abbreviations
- ACA:
-
Anterior cerebral artery
- AChoA:
-
Anterior choroidal artery
- CT:
-
Computed tomography
- DSA:
-
Digital subtraction angiography
- ICA:
-
Internal carotid artery
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin scale
- PCom:
-
Posterior communicating artery
- SAH:
-
Subarachnoid hemorrhage
References
Locksley HB, Sahs AL, Sandler R. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. 3. Subarachnoid hemorrhage unrelated to intracranial aneurysm and A‑V malformation. A study of associated diseases and prognosis. J Neurosurg. 1966;24(6):1034–56.
Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30(2):470–6.
Rangel-Castilla L, Munich SA, Jaleel N, Cress MC, Krishna C, Sonig A, Snyder KV, Siddiqui AH, Levy EI. Patency of anterior circulation branch vessels after pipeline embolization: longer-term results from 82 aneurysm cases. J Neurosurg. 2016 Jun 10:1–6. [Epub ahead of print]
Yaşargil MG, Yonas H, Gasser JC. Anterior choroidal artery aneurysms: their anatomy and surgical significance. Surg Neurol. 1978;9(2):129–38.
Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–6.
Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998–2004.
Drake CG, Vanderlinden RG, Amacher AL. Carotid-choroidal aneurysms. J Neurosurg. 1968;29(1):32–6.
Perria L, Viale GL, Rivano C. Aneurysms of the junction internal carotid artery and anterior choroid artery. Acta Neurochir (Wien). 1969;21(2):153–66.
Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, Hansen KK. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001;94(4):565–72.
Furtado SV, Venkatesh PK, Hegde AS. Neurological complications and surgical outcome in patients with anterior choroidal segment aneurysms. Int J Neurosci. 2010;120(4):291–7.
Lehecka M, Dashti R, Laakso A, van Popta JS, Romani R, Navratil O, Kivipelto L, Kivisaari R, Foroughi M, Kokuzawa J, Lehto H, Niemelä M, Rinne J, Ronkainen A, Koivisto T, Jääskelainen JE, Hernesniemi J. Microneurosurgical management of anterior choroid artery aneurysms. World Neurosurg. 2010;73(5):486–99.
Shibata Y, Fujita S, Kawaguchi T, Hosoda K, Komatsu H, Tamaki N. Use of microvascular Doppler sonography in aneurysm surgery on the anterior choroidal artery. Neurol Med Chir (Tokyo). 2000;40(1):30–5, discussion 35–7.
Li J, Mukherjee R, Lan Z, Liu Y, He M. Microneurosurgical management of anterior choroidal artery aneurysms: a 16-year institutional experience of 102 patients. Neurol Res. 2012;34(3):272–80.
Lee YS, Park J. Anterior choroidal artery aneurysm surgery: ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013;54(2):86–92.
Piotin M, Mounayer C, Spelle L, Williams MT, Moret J. Endovascular treatment of anterior choroidal artery aneurysms. AJNR Am J Neuroradiol. 2004;25(2):314–8.
Kang HS, Kwon BJ, Kwon OK, Jung C, Kim JE, Oh CW, Han MH. Endovascular coil embolization of anterior choroidal artery aneurysms. J Neurosurg. 2009;111(5):963–9.
Senturk C, Bandeira A, Bruneau M, Dewindt A, Balériaux D, De Witte O, Lubicz B. Endovascular treatment of anterior choroidal artery aneurysms. J Neuroradiol. 2009;36(4):228–32.
Bohnstedt BN, Kemp WJ 3rd, Li Y, Payner TD, Horner TG, Leipzig TJ, Cohen-Gadol AA. Surgical treatment of 127 anterior choroidal artery aneurysms: a cohort study of resultant ischemic complications. Neurosurgery. 2013;73(6):933–40.
Kim BM, Kim DI, Shin YS, Chung EC, Kim DJ, Suh SH, Kim SY, Park SI, Choi CS, Won YS. Clinical outcome and ischemic complication after treatment of anterior choroidal artery aneurysm: comparison between surgical clipping and endovascular coiling. AJNR Am J Neuroradiol. 2008;29(2):286–90.
Djamshidian A, Friedman JH. Anxiety and depression in Parkinson’s disease. Curr Treat Options Neurol. 2014;16(4):285.
Cho MS, Kim MS, Chang CH, Kim SW, Kim SH, Choi BY. Analysis of clip-induced ischemic complication of anterior choroidal artery aneurysms. J Korean Neurosurg Soc. 2008;43(3):131–4.
Suzuki K, Kodama N, Sasaki T, Matsumoto M, Konno Y, Sakuma J, Oinuma M, Murakawa M. Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg. 2003;98(3):507–14.
Naggara ON, White PM, Guilbert F, Roy D, Weill A, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010;256(3):887–97.
Kim BM, Kim DI, Chung EC, Kim SY, Shin YS, Park SI, Kim DJ, Suh SH, Choi CS, Won YS. Endovascular coil embolization for anterior choroidal artery aneurysms. Neuroradiology. 2008;50(3):251–7.
Suh W, Cho HK, Kee C. Evaluation of peripapillary choroidal thickness in unilateral normal-tension glaucoma. Jpn J Ophthalmol. 2014;58(1):62–7.
Morgenstern LB, Hankins LL, Grotta JC. Anterior choroidal artery aneurysm and stroke. Neurology. 1996;47(4):1090–2.
Shapiro M, Babb J, Becske T, Nelson PK. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol. 2008;29(9):1777–81.
Hernesniemi J, Romani R, Niemela M. Skull base and aneurysm surgery. Surg Neurol. 2009;71(1):30–1.
Lieber BB, Sadasivan C. Endoluminal scaffolds for vascular reconstruction and exclusion of aneurysms from the cerebral circulation. Stroke. 2010;41(10 Suppl):S21–5.
Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002;24(Suppl 1):33–S42.
Tremmel M, Xiang J, Natarajan SK, Hopkins LN, Siddiqui AH, Levy EI, Meng H. Alteration of intraaneurysmal hemodynamics for flow diversion using enterprise and vision stents. World Neurosurg. 2010;74(2–3):306–15.
Raz E, Shapiro M, Becske T, Zumofen DW, Tanweer O, Potts MB, Riina HA, Nelson PK. Anterior choroidal artery patency and clinical follow-up after coverage with the pipeline embolization device. AJNR Am J Neuroradiol. 2015;36(5):937–42.
Brinjikji W, Kallmes DF, Cloft HJ, Lanzino G. Patency of the anterior choroidal artery after flow-diversion treatment of internal carotid artery aneurysms. AJNR Am J Neuroradiol. 2015;36(3):537–41.
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005;103(6):982–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. André, A.-L. Boch, F. Di Maria, A. Nouet, N. Sourour, S. Clémenceau, J. Gabrieli, V. Degos, C. Zeghal, J. Chiras, P. Cornu and F. Clarençon declare that they have no competing interests.
Ethical standards
Neither approval of the institutional review board nor patient informed consent are required by the ethics committee of our institution for retrospective analyses of patient records and imaging data.
Additional information
Contributions
A. André: Project development, Data Collection, Manuscript writing; A.L. Boch: Data collection, procedures; F. Di Maria: Data collection, procedures; A. Nouet: Data collection, procedures; N. Sourour: Data collection, procedures; S. Clemenceau: Data collection, procedures; J. Gabrieli: Data collection, procedures; V. Degos: Data collection, procedures; C. Zeghal: Data collection, procedures; J. Chiras: Data collection, procedures; P. Cornu: Data collection, procedures; F. Clarençon: Project development, Data collection, Manuscript writing
Rights and permissions
About this article
Cite this article
André, A., Boch, AL., Di Maria, F. et al. Complication Risk Factors in Anterior Choroidal Artery Aneurysm Treatment. Clin Neuroradiol 28, 345–356 (2018). https://doi.org/10.1007/s00062-017-0575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0575-y