Abstract
Purpose
The development of the premaxillary–maxillary suture (PMS) in human fetuses and a possible association between the fusion time of the PMS and maxillary deficiency were investigated. Expression of transforming growth factor beta (TGF-β1 and TGF-β3) and of fibulins (fibulin‑1 and fibulin-5) were also investigated.
Methods
We analyzed 36 human fetus cadavers (19 males, 17 females; average age 23.97 ± 2.57 gestational weeks [gws], range 11–35 gws). Two cases, diagnosed with Down syndrome (DS), were characterized with maxillary deficiency; 34 fetus cadavers did not show any craniofacial abnormalities. The PMS was analyzed anatomically, followed by semi-quantitative immunohistochemical (IHC)-based expression analyses (i.e., TGF-β1/-β3, fibulin-1/-5). Spearman correlation test was conducted to investigate correlations.
Results
In the fetuses without DS, the labial region of the PMS was open at 11 gws, after which it began to ossify from the middle to the upper and lower ends of the suture, typically fusing completely at 27 gws. Fetuses with DS demonstrated complete fusion of the labial region of PMS with a spongy bone structure at 23 gws and those without DS at 27 gws. IHC revealed similar patterns of TGF-βs and fibulins expression in the PMS during the human fetal period. There were significant positive correlations between the expression of TGF-β1 and TGF-β3 (r = 0.64, p = 0.009), TGF-β1 and fibulin‑1 (r = 0.66, p = 0.008), and TGF-β3 and fibulin‑1 (r = 0.67, p = 0.006).
Conclusion
Premature fusion of the PMS in the labial region during the human fetal period may be associated with maxillary deficiency, which is related to a class III malocclusion. Overall, the similar expression patterns of TGF-β1, TGF-β3 and fibulin‑1 suggested a close relationship between these factors in regulating the development of the PMS.
Zusammenfassung
Ziel
Die Entwicklung der prämaxillär-maxillären Naht (PMS) bei menschlichen Feten und ein möglicher Zusammenhang zwischen dem Fusionszeitpunkt der PMS und einer maxillären Hypoplasie wurden untersucht. Ebenfalls untersucht wurde die Expression der transformierenden Wachstumsfaktoren β (TGF‑β1 und TGF‑β3) und von Fibulinen (Fibulin‑1 und Fibulin-5).
Methoden
Wir analysierten 36 verstorbene menschliche Feten (19 männliche, 17 weibliche; Durchschnittsalter 23,97 ± 2,57 Schwangerschaftswochen [SSW], Range 11-36 SSW). Zwei Fälle, bei denen das Down-Syndrom (DS) diagnostiziert wurde, waren durch eine maxilläre Hypoplasie gekennzeichnet; 34 Feten wiesen keine kraniofazialen Anomalien auf. Das PMS wurde anatomisch analysiert, gefolgt von semiquantitativen immunhistochemischen (IHC) Expressionsanalysen (d. h. TGF-β1/-β3, Fibulin-1/-5). Zur Untersuchung von Korrelationen wurde der Spearman-Korrelationstest durchgeführt.
Ergebnisse
Bei den Feten ohne DS war die labiale Region des PMS bei 11 SSW offen, danach begann sie von der Mitte bis zum oberen und unteren Ende der Naht zu verknöchern und verschmolz typischerweise bei 27 SSW vollständig. Feten mit DS zeigten eine vollständige Verschmelzung der labialen Region des PMS mit einer schwammartigen Knochenstruktur bei 23 SSW und Feten ohne DS bei 27 SSW. Die IHC zeigte ähnliche Muster der TGF-βs und Fibulinexpression im PMS während der menschlichen Fetalperiode. Es gab signifikante positive Korrelationen zwischen der Expression von TGF-β1 und TGF-β3 (r = 0,64, p = 0,009), TGF-β1 und Fibulin‑1 (r = 0,66, p = 0,008), TGF-β3 und Fibulin‑1 (r = 0,67, p = 0,006).
Schlussfolgerung
Die vorzeitige Fusion des PMS in der Labialregion während der fetalen Periode des Menschen kann mit einer maxillären Hypoplasie assoziiert sein, das mit einer Klasse-III-Malokklusion verbunden ist. Insgesamt deuten die ähnlichen Expressionsmuster von TGF-β1, TGF-β3 und Fibulin‑1 auf eine enge Beziehung zwischen diesen Faktoren bei der Regulierung der Entwicklung des PMS hin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angle class III malocclusion is one of the most common malocclusions in eastern Asian populations with a high prevalence rate of 15.8% [1], which seriously affects maxillofacial appearance and psychological health [2]. Class III malocclusion is a complex abnormality of soft and hard tissue, including maxillary deficiency, mandibular protrusion, or both. Among all known etiological types, maxillary deficiency with or without an abnormal mandible accounts for the largest proportion of class III malocclusions with a rate of approximately 61.5% [3]. The treatment of a class III malformation is challenging owing to a high probability of recurrence over time [2, 4, 5]. This has been attributed to a lack of knowledge regarding the pathogenesis of the maxillary deficiency. Therefore, a comprehensive understanding of the pathogenesis of maxillary deficiency is necessary based on further knowledge of anatomy and embryology.
The maxilla is the main part of the midfacial structure, whose development is closely related to the development of the human midface. However, another very often neglected but indispensable bone in front of the maxilla, termed the premaxilla, has been rarely mentioned in most anatomical assessments [6,7,8]. Nevertheless, not only the number of ossification centers of premaxilla has been questioned, but even its existence as a separate entity has been disputed [6,7,8]. The resistance to recognizing the premaxilla probably results from the rapid obliteration of the labial region of premaxillary–maxillary suture (PMS) during the early human fetal period [7,8,9]. While some researchers reported that the fusion of the labial PMS occurs as early as in the first trimester of the human fetal period [7, 8], other researchers reported that no PMS opening was seen in the labial region at 16 weeks of intrauterine life [9]. The development of PMS in humans has not been fully described, and the timing of PMS fusion still remains controversial.
Sagittal and vertical growth of the midface is largely dependent on the surrounding sutures of the maxilla, including the midpalatal suture, the zygomaticomaxillary suture, and the PMS. Several studies showed that the labial region of the PMS remain patent in all mammals, except in humans [10,11,12,13,14,15]. In fact, close primate relatives, such as orangutans and apes, present a more pronounced facial projection in the midfacial morphology compared to human beings. It was hypothesized that fusion of the labial PMS may cause abrupt termination of maxillofacial development, which as a consequence is associated with the unique facial structures of humans, such as the anterior nasal spine (ANS) [11, 12], and the maxillofacial characteristics of modern humans namely the straight face [13,14,15]. These altered configurations represented a characteristic maxillofacial feature that distinguished modern humans from the archaic Homo [13]. Mooney et al. reported that the size of the ANS was correlated with facial prognathism and the timing of PMS fusion, suggesting that the timing of PMS fusion may have implications on midfacial growth [11, 12]. Furthermore, Holton et al. reported that rigid plate fixation of the PMS in a pig model resulted in a reduction in facial protrusion and overall size [13]. In our previous study, we showed that artificially induced PMS fusion resulted in extensive craniomaxillofacial abnormalities in rats, which were similar to the morphological characteristics of maxillary deficiency [14, 15]. Therefore, we hypothesize that the premature fusion of the PMS may be associated with maxillary deficiency, which may relate to class III malocclusion. Unfortunately, up to now, no anatomical basis for this theory has been established in humans.
Down syndrome (DS), also known as trisomy 21, is a genetic abnormality that generates intellectual impairment, mental retardation, and also can affect craniofacial development [16]. A series of studies have determined that maxillary deficiency was a typical craniomaxillofacial feature of DS patients [17,18,19]. Among them, Silva et al. concluded that the craniofacial linear dimensions of DS were significantly shorter than those observed in a control group, especially the maxillary length (Co-A) [20]. In addition, in an ultrasound study, Vos et al. revealed that human fetuses with DS had significantly smaller maxillary–nasal–mandibular (MNM) angles relative to healthy fetuses (12.90° vs 13.53°) in the second trimester, indicating significant maxillary deficiency in the fetuses with DS [21]. Therefore, it was concluded that patients with DS exhibited maxillary deficiency as early as in the embryonic stage [21]. However, no anatomicopathological study has explored the development of the premaxillary region in human fetuses with DS, which may be associated with maxillary deficiency.
The osteoblasts, derived from mesenchymal stem cells within the suture, play an important role in the ossification of sutures. The proliferation and differentiation of mesenchymal cells to osteoblasts are regulated by several extracellular matrix proteins, such as transforming growth factors beta (TGF-βs), fibulins, fibroblast growth factor, platelet-derived growth factor, and insulin-like growth factors [22,23,24,25]. Especially, TGF-βs and fibulins were reported to play an important regulatory role in craniofacial skeletal morphogenesis [22, 25,26,27,28]. Researchers have reported that up-regulation of TGF-β1 was correlated with posterior frontal cranial suture fusion [26]. The expression of chondroitin sulfate proteoglycan on the surface of epithelial seam cells, which is key in palatal shelf fusion, was regulated by TGF-β3 [27, 28]. Furthermore, fibulin‑1 was required for bone formation and BMP-2-mediated induction of osterix [25]. Fibulin‑5 was reported to be expressed in the PMS during the early postnatal period in rats, and a deficiency of fibulin‑5 would inhibit the development of the premaxilla [22]. Notably, emerging studies have reported regulatory interactions between fibulins and TGF-βs signaling [25, 29,30,31,32]. On the one hand, expression of fibulin‑5 was enhanced by TGF-βs [25, 29, 30], and TGF-β1 treatment suppressed fibulin‑1 mRNA expression and protein abundance [31]. On the other hand, fibulin‑5 promoted TGF-β-induced EMT through activating MMP‑2 and -9 in mammary epithelial cells [33]. Fibulin‑1 and fibulin‑2 were found to regulate TGF-β/BMP signals, thus, playing a crucial role in the differentiation and maturation of osteoblasts [32]. However, no study has yet explored the expression of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 simultaneously, and investigated the relationship between them in the development of the PMS during the human fetal period.
In the present study, we investigated the development of the PMS in a series of aborted human fetuses with different gestational ages, using anatomical and immunohistochemical analyses. The fetuses with DS characterized by maxillary deficiency were compared with the fetuses without DS to ascertain the relationship between the fusion time of the PMS and maxillary deficiency. In addition, we aimed to investigate the correlations between the expression of TGF-βs (TGF-β1 and TGF-β3) and fibulins (fibulin‑1 and fibulin-5) in the development of the PMS.
Methods
Study subjects
A total of 36 aborted human fetus cadavers from Hangzhou Fuyang Women and Children Hospital between 2016 and 2018 were examined. The reasons of abortion, including congenital malformation (CM) and unintended pregnancy (UP), were recorded. Among the fetuses aborted because of CM, two of them were diagnosed with DS by prenatal karyotype analysis and were characterized by maxillary deficiency. The rest of the fetus cadavers did not show any craniofacial abnormalities. Exclusion criterion was the inability to examine the regions of the PMS visually. The gestational age of the fetuses was determined by the end of the last menstrual period of maternity. Gestational stages were divided into 4 periods, namely the first, second, third, and full-term trimesters, with time periods before 16, 17–25, 26–37, and 38–40 gws, respectively. Written consent was obtained from the families, and all procedures were approved by the Children’s Hospital, Zhejiang University School of Medicine Research Ethics Committee (2012-GJ-049).
Anatomical analysis of the PMS region
All the fresh frozen human fetus cadavers, across different gestational stages, were individually sealed and stored at a temperature of −80 ℃. Dissections were performed by one experienced pathologist using standard tools. Before dissection, the fetal cadavers were removed from the freezer and thawed at room temperature for 12 h. After cleaning, they were placed in a supine position, then an incision was made from the lateral edge of the nasal root, along the bridge, the lateral edge, and the base of the nose, straight to the bone wall. A full and midline incision was performed at the upper lip and along the vestibule from anterior to posterior. The skin–mucosal flap was successively turned back to expose the premaxilla’s alveolar process, its body, nasal floor region, and the maxillary process, which formed the lateral wall of the nasal cavity. The labial and palatal regions of the PMS were visually evaluated and photographs of the local anatomical areas were taken (Canon EOS 600D, Canon Inc., Tokyo, Japan).
Immunohistochemical analysis
Tissue samples from each fetus’ PMS region were immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS; pH 7.4) for 24 h, dehydrated with dH2O for 30 min, rinsed twice in 75% ethanol for 1 h, and twice in 100% ethanol for 1 h. The tissues thereafter were immersed twice in absolute xylene for 20 min and then embedded in paraffin for 3 h at 58–60 ℃. After embedding [34], 4‑μm-thick coronal serial sections were cut (Microm HM-340E microtome, Microm, Walldorf, Germany).
The sections were stained with EnVisionTM two-step strategy and high-temperature antigen retrieval (pressure cooker, Supor Co, Hangzhou, Zhejiang, China). The sections were then deparaffinized twice with 100% xylene for 10 min, then hydrated twice with 100% ethanol for 5 min, once with 95% ethanol for 3 min, and once with 80% ethanol for 5 min. Slides were soaked for 2–5 min in distilled water, then were incubated in a pressure cooker filled with 1000 ml of boiling sodium citrate buffer (pH 9.0) and heated under pressure for 2 min after steaming. The pressure cooker was removed from the heating source and cooled to room temperature using tap water. The container was opened, and the slides rinsed 3 times with phosphate-buffered saline (PBS) for 2 min. Thereafter, the slides were incubated with 3% hydrogen peroxide for 10 min, then rinsed for 2 min in PBS. For immunohistochemical localization of TGF-βs and fibulins, the slides were treated with primary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), namely rabbit anti-human TGF-β1, rabbit anti-human TGF-β3, mouse anti-human fibulin1, and mouse anti-human fibulin5 antibodies, in a moist chamber at 37 ℃ for 1 h. After rinsing 3 times in PBS for 2 min, the slides were incubated with HRP polymer (EnVisionTM detection kit, DAKO company, Glostrup, Denmark) at 37 ℃ for 30 min. Thereafter, DAB chromagen was added and the tissue sections were counterstained with hematoxylin and eosin (HE). Based on randomized selection, two slides in which the primary antibody was omitted were selected and substituted with PBS as the negative control.
To estimate the levels of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 expression in the PMS, one researcher semi-quantitatively scored immunoreactivity against TGF-βs and fibulins in a blinded manner [35, 36]. All the slides were examined under a light microscope (Carl Zeiss Inc, Thornwood, NY, USA) at 200 × magnification. Intensity of immunostaining was scored as follows: 0 = nonstaining, 1 = light yellow, 2 = brownish yellow, and 3 = brown. Sections with light yellow to brown staining in the cytoplasm were considered positive cells. The percentage of positive cells denoted the number of positive cells in the field of view and were classified as follows: 0 = 0–5%, 1 = 6–25%, 2 = 26–50%, 3 = 51–75%, and 4 = more than 75%. Five fields of view were randomly selected, for each section, and each field scored by multiplying the score of staining intensity and the percentage of positive cells. The final scores were calculated as a geometric mean of the 5 series.
Data analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, version 23.0, IBM, Armonk, NY, USA). The descriptive statistics of central trend (mean) and dispersion (standard deviation) of the data were calculated. The data were not normally distributed; therefore, the statistical analysis was performed using nonparametric tests. The correlations between the expression of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 were assessed applying the Spearman correlation test (R, version 3.6.1). The level of significance was set at 0.05.
Results
General information
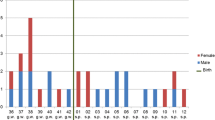
A summary of the clinical information of all fetus cadavers is outlined in Table 1. In summary, the 36 human fetus cadavers (19 males and 17 females) had an average age of 23.97 ± 6.18 gestational weeks (gws; range 11–35 gws). The average of maternal age was 26.06 ± 6.52 years (range 15–40 years). The reasons of abortion included CM (15/36, 41.67%) and UP (21/36, 58.33%). Among the fetuses aborted because of CM, two of them were diagnosed with DS by prenatal karyotype analysis and were characterized by maxillary deficiency. The other fetus cadavers did not show any craniofacial abnormalities.
Anatomy of the PMS region
We dissected the 36 human fetus cadavers, and the regions of the PMS were visually evaluated. Several important changes in PMS development during fetal period are summarized in Figs. 1 and 2.
Premaxillary–maxillary suture (PMS) in human fetuses without Down syndrome (DS). All the inset boxes are magnified in the right panel. The ages of fetuses are shown in the bottom left of the image as gestational weeks (gws)+days. a The labial region of PMS was completely open at 11 gws. The white arrow points to the labial region of PMS. The premaxilla is in front of the white arrow. b The palatal region of PMS (##) was open at 11 gws. ** premaxilla, $$ the process palatinus of maxilla. c At 17 gws, the premaxilla exhibited a butterfly shape, with an irregular S‑shaped PMS in the labial region (*). Some areas of the labial PMS were fused, whereas the ossification points were concentrated in the middle of the suture (#), leaving large gaps between the two ends of the suture (@ and white arrow). d At 17 gws, the palatal region of PMS (*) remained open. e At 20 gws, the labial region of PMS (white arrow) continued to fuse. * infraorbital foramen. f At 20 gws, the palatal region of PMS (#) remained completely open. g At 24 gws, the labial region of PMS was almost fused, but a gap still existed between the two ends of the suture (#) in an irregular S‑shape. h At 27 gws, the labial region of PMS had fused completely, while the bone suture between the premaxilla and contralateral side remained open (#)
PMS (prämaxillär-maxilläre Naht) bei menschlichen Feten ohne Down-Syndrom (DS). Alle eingefügten Kästchen sind im rechten Panel vergrößert dargestellt. Das Alter der Feten wurde unten links im Bild in Schwangerschaftswochen (SSW)+Tagen angegeben. a Der labiale Bereich der PMS war bei 11 SSW vollständig geöffnet. Der weiße Pfeil zeigt auf die Labialregion des PMS. Der Prämaxilla befindet sich vor dem weißen Pfeil. b Der palatinale Bereich des PMS (##) war bei 11 SSW offen. ** Prämaxilla, $$ Processus palatinus des Oberkiefers. c Mit 17 SSW wies die Prämaxilla die Form eines Schmetterlings auf, mit einem unregelmäßigen S‑förmigen PMS im Labialbereich (*). Einige Bereiche des labialen PMS waren verschmolzen, während sich die Verknöcherungspunkte in der Mitte der Naht konzentrierten (#) und große Lücken zwischen den beiden Enden der Naht hinterließen (@ und weißer Pfeil). d Mit 17 SSW blieb der palatinale Bereich des PMS (*) offen. e Mit 20 SSW fusionierte der labiale Bereich des PMS (weißer Pfeil) weiterhin. Foramen infraorbitale (*). f Mit 20 SSW blieb der palatinale Bereich des PMS (#) vollständig offen. g Mit 24 SSW war der labiale Bereich des PMS fast verschmolzen, aber es bestand noch eine Lücke zwischen den beiden Enden der Naht (#) in einer unregelmäßigen S‑Form. h Mit 27 SSW war der labiale Bereich des PMS vollständig verschmolzen, während die Knochennaht zwischen Prämaxilla und kontralateraler Seite offen blieb (#)
Premaxillary–maxillary suture (PMS) in human fetuses with Down syndrome (DS). a A fetus with DS at 23 gestational weeks (gws) had no nasal bone (@) and the labial region of the PMS had completely fused (#). The premaxilla was sparse with a spongy architecture (*). b A fetus with DS at 25 gws showed complete fusion in the labial region of the PMS (#), but the bone was sparsely formed
PMS (prämaxillär-maxilläre Naht) bei menschlichen Feten mit Down-Syndrom (DS). a Bei einem Feten mit DS in der 23. Schwangerschaftswoche (SSW) fehlte der Nasenknochen (@) und die labiale Region der PMS war vollständig verwachsen (#). Die Prämaxilla war spärlich mit einer schwammigen Architektur (*). b Ein Fetus mit DS in der 25. SSW zeigte eine vollständige Fusion im Labialbereich des PMS (#), aber der Knochen war nur spärlich ausgebildet
In fetuses without DS, anatomical analysis revealed that the premaxilla was located in front of the maxilla and that the labial and palatal regions of the PMS were completely open at 11 gws (Fig. 1a, b). At 17 gws, the premaxilla exhibited a butterfly shape, with an irregular S‑shaped PMS in the labial region. Some areas of the labial PMS were fused, whereas the ossification points were concentrated in the middle of the suture, leaving large gaps between the two ends of the suture (Fig. 1c). At 17 gws, the palatal region of the PMS remained open (Fig. 1d). At 20 gws, the premaxilla was growing and the alveolar process had formed to accommodate the incisor tooth (Fig. 1e, f). The labial region of the PMS continued to fuse but the palatal region of the PMS was completely open, which was not synchronized (Fig. 1e, f). The labial region of the PMS fused earlier than the palatal region. At 24 gws, the labial region of the PMS was almost fused but a gap still existed between the two ends of the suture in an irregular S‑shape (Fig. 1g). At 27 gws, the labial region of the PMS had fused completely, while the suture in the palatal region as well as the bone suture between the premaxilla and the contralateral side remained open (Fig. 1h).
Fetuses with DS exhibited complete fusion in the labial region of the PMS already at 23 gws and 25 gws, and the bone was sparse with a spongy architecture (Fig. 2). The fusion time of the PMS in the labial region in fetuses with DS occurred earlier than in those without DS, while their bone architecture was also much sparser (Fig. 1 and 2).
Immunohistochemical results
Results from immunostaining of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 in the PMS at different gestational weeks in fetuses without DS are summarized in Fig. 2. TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 were expressed in the PMS for most of the fetal period, but the expression levels varied over time (Fig. 3). The expression of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 in PMS appeared to have a similar pattern during the fetal period (Fig. 3b). All of the expression levels of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 had peaks in the first trimester (14 gws), second trimester (22 gws), and third trimester (27 gws for TGF-βs and fibulin‑1, 34 gws for fibulins; Fig. 3b).
a Expression profiles for TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 in premaxillary–maxillary suture (PMS) of fetuses without Down syndrome (DS). Immunohistochemical staining for TGF-β1 (A1–A5), TGF-β3 (B1–B5), fibulin‑1 (C1–C5), and fibulin‑5 (D1–D5) in PMS at 11th gestational weeks (gws), 22 gws, 27 gws, 31 gws, and 34 gws. Red arrows point to positive cells. Magnification: ×200. Scale bar, 50 μm. b The expression scores of TGF-β1, TGF-β3, fibulin‑1 and fibulin‑5 in PMS over time. GWs gestational weeks
a Expressionsprofile für TGF-β1, TGF-β3, Fibulin‑1 und Fibulin‑5 in der prämaxillär-maxillären Naht (PMS) von Feten ohne Down-Syndrom (DS). Immunhistochemische Färbung für TGF-β1 (A1–A5), TGF-β3 (B1–B5), Fibulin‑1 (C1–C5) und Fibulin‑5 (D1–D5) in PMS bei 11. SSW (Schwangerschaftswoche), 22 SSW, 27 SSW, 31 SSW und 34 SSW. Rote Pfeile weisen auf positive Zellen hin. Vergrößerung 200:1. Maßstab: 50 μm. b Die Expressionswerte von TGF-β1, TGF-β3, Fibulin‑1 und Fibulin‑5 im PMS im Zeitverlauf
Spearman correlation test revealed significant positive correlations between the expression of TGF-β1 and TGF-β3 (r = 0.64, p = 0.009), TGF-β1 and fibulin‑1 (r = 0.66, p = 0.008), and TGF-β3 and fibulin‑1 (r = 0.67, p = 0.006; Fig. 4). However, the expression of fibulin‑5 showed no significant correlation with the expression of TGF-β1 (r = 0.39, p = 0.147), TGF-β3 (r = 0.48, p = 0.069), or fibulin‑1 (r = 0.50, p = 0.056; Fig. 4). The Spearman correlation coefficients between TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 are summarized in Fig. 4.
Correlations between the expression of TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 in premaxillary–maxillary suture (PMS) during the gestational period. Spearman correlation test revealed significant positive correlations between the expression of TGF-β1 and TGF-β3 (r = 0.64, p = 0.009), TGF-β1 and fibulin‑1 (r = 0.66, p = 0.008), and TGF-β3 and fibulin‑1 (r = 0.67, p = 0.006). However, the expression of fibulin‑5 showed no significant correlation with the expression of TGF-β1 (r = 0.39, p = 0.147), TGF-β3 (r = 0.48, p = 0.069), and fibulin‑1 (r = 0.50, p = 0.056). Red positive correlation. Blue negative correlation. The size of the circle represents the r‑value. “X” in the circle indicates no significant correlation (p > 0.05)
Korrelationen zwischen der Expression von TGF-β1, TGF-β3, Fibulin‑1 und Fibulin‑5 in der prämaxillär-maxillären Naht (PMS) während der Gestationsperiode. Der Spearman-Korrelationstest ergab signifikante positive Korrelationen zwischen der Expression von TGF-β1 und TGF-β3 (r = 0,64, p = 0,009), TGF-β1 und Fibulin‑1 (r = 0,66, p = 0,008), TGF-β3 und Fibulin‑1 (r = 0,67, p = 0,006). Die Expression von Fibulin‑5 zeigte jedoch keine signifikante Korrelation mit der Expression von TGF-β1 (r = 0,39, p = 0,147), TGF-β3 (r = 0,48, p = 0,069) und Fibulin‑1 (r = 0,50, p = 0,056). Rot positive Korrelation. Blau negative Korrelation. Die Größe des Kreises gibt den r‑Wert an. Ein „X“ im Kreis bedeutet keine signifikante Korrelation (p > 0,05)
Discussion
Angle class III malocclusion is common and severely affects the maxillofacial appearance and psychological health in eastern Asian populations [2]. A deeper understanding of the pathogenesis of maxillary deficiency, which is related to a class III malocclusion, is key to obtain a better clinical outcome in the long-term. The present study suggested that premature fusion of the PMS in the labial region during the human fetal period may be associated with maxillary deficiency. Our study holds promise for providing an updated understanding of the pathogenesis of maxillary deficiency during the human fetal period.
Previous studies have shown that inter- and intraspecific variations in midfacial appearance occurred as early as in the embryonic stage [37]. However, the development of the midfacial area during the embryonic period has not been fully described [6, 38]. To date, the premaxilla, a very often neglected but indispensable bone in front of the maxilla, remains controversial not only with respect to the number of ossification centers, but even the existence of a separate entity is discussed [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In a previous study, Wood et al. reported the absence of a separate formation center for the premaxilla in the human embryos [39]. In contrast, other researchers reported that the premaxilla exists in an independent way within the maxillary complex during the embryonic stage [6]. The anatomical results in the present study were in line with the latter view. We confirmed the existence of the premaxilla as an independent but transient bony element that gradually unifies with the maxilla during the human fetal period. The resistance regarding recognition of the premaxilla probably results from the early obliteration of the labial PMS, which supposedly occurs early in the fetal period [7,8,9]. In the present study, we analyzed a series of aborted human fetuses throughout the embryonic period, with a minimum age of 11 gws. Thus, it was possible to assess the development of the PMS at an extremely early embryonic stage and to gain a more accurate and convincing conclusion than previous studies.
In the present study the labial region of the PMS was completely open at 11 gws, after which the suture began to ossify, eventually fusing completely at 27 gws. However, some studies reported different timing of PMS fusion from ours [7,8,9]. For example, Trevizan et al. reported that the closure of the PMS in the labial region occurred at 16 gws [9]. The variability of results between studies may be related to ethnic differences, as interspecific morphological differences in the middle face appear as early as in the embryonic stage [9, 37]. All the human fetuses studied in this study were Han offspring.
The difference in the fusion time between the labial and palatal region of the PMS may be another reason why the premaxilla was rarely studied as an independent bone [9, 40, 41]. In the present study, we found that the PMS was three-dimensional, including both labial and palatal regions, and the ossification of the PMS in the labial and palatal region was not synchronized. Even though the labial region of the PMS fused completely at 27 gws, the suture in the palatal regions remained open. Our results are in agreement with several previous studies [9, 40, 41]. They reported that the PMS in the labial region disappeared, while the palatal region remained patent throughout early childhood [40, 41]. Even in adults, the palatal region of the PMS had gaps of varying depth [9, 42, 43]. Therefore, it was believed that such a suture may provide an explanation for the action of some appliances to achieve orthodontic development in this area, especially for premaxillary protraction in the treatment of skeletal class III malocclusion [42].
Cranial sutures are membranous gaps that remain between the boney plates of the skull. Sutures provide the firm bond of union to allow a little movement for bones in response to mechanical force and serve as sites of active bone growth. The growth of the midface in humans is largely dependent on sutures in the maxillofacial area, such as the midpalatal suture, the zygomaticomaxillary suture, and the PMS [11,12,13]. From published documents, it is suggested that the PMS plays an important role in the formation of craniofacial morphology [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Maureille et al. found the fusion of PMS was later in Neandertal children than in modern homo sapiens [44]. Persistence of an open PMS represented the potential for an extended period of growth in the midface of Neandertals, distinguishing them from modern humans [44]. Mooney et al. recognized a significant relationship between the degree of the PMS fusion and the ANS prominence, and that such a difference in facial morphology was established early in fetal development (by the second trimester) and maintained postnatally [11, 12]. Furthermore, our previous animal studies showed that artificially induced premature fusion of the PMS resulted in extensive abnormal craniofacial formation in rats, which was markedly similar to facial features of maxillary deficiency in humans [14, 15]. Based on all these findings, it was hypothesized that premature ossification of PMS may be associated with maxillary deficiency in human. Unfortunately, no anatomic basis for this possibility has been established. In the present study, fetuses with DS characterized by maxillary deficiency and fetuses without DS were examined, and the development of the maxillary–premaxillary regions were compared with each other. The anatomical findings revealed that the fusion of the labial PMS was much earlier in fetuses with DS (23 gws, second trimester) than in fetuses without DS (27 gws, third trimester). Moreover, fetuses with DS exhibited much sparser premaxilla and maxilla. The present findings were consistent with the previous findings mentioned above. Our results confirmed the suspicion that premature fusion of PMS in the labial region in the human fetal period may be associated with maxillary deficiency, which is related to a class III malocclusion. However, the strength of our conclusion was limited by the small sample size. Further studies including a larger number of participants are needed for a more convincing conclusion.
Several previous studies reported that TGF-βs and fibulins were expressed in craniofacial sutures and play an important regulatory role in craniofacial skeletal morphogenesis [22, 25,26,27,28]. For example, previous studies have reported the expression of TGF-β1, TGF-β2, TGF-β3, and Msx2 during the development of the frontonasal suture [23]. Fibulin‑5 was reported to be expressed in the PMS during the early postnatal period in rats and is thought to play an important role in the development of premaxilla [22]. Consistent with these findings, in the present study, TGF-β1, TGF-β3, fibulin‑1, and fibulin‑5 were expressed in the PMS for most of the human fetal period but the expression levels varied over time.
In the present study, the expression of TGF-βs and fibulins showed a certain degree of regularity, each developing peaks in the first trimester (14 gws), second trimester (22 gws), and third trimester (27 gws for TGF-βs and fibulin‑1, 34 gws for fibulins). Particularly, the expression of TGF-βs and fibulin‑1 reached a small peak at 27 gws and then rapidly declined, which was the timing of complete fusion of labial PMS in fetuses without DS (27 gws). This consistency led us to speculate on the possible role of TGF-β1, TGF-β2, and fibulin‑1 in regulating PMS ossification during the human fetal period. However, the association between their expression and PMS ossification remains unknown, necessitating further investigations.
Emerging studies have reported regulatory interactions between fibulins and TGF-βs signaling during the embryonic development, tissue repair, and pathological processes [25, 29,30,31,32, 45, 46]. TGF-βs were found to mediate down-regulation of fibulin‑1 mRNA in airway smooth muscle cells and human bone marrow stromal HS-5 cells [31, 45]. Furthermore, smoking failed to induce secretion of TGF-βs in mice with genetic or therapeutic inhibition of fibulin‑1, suggesting that fbulin‑1 exerted a promotional effect on TGF-β secretion [46]. In the osteogenesis process, fibulin‑1 and fibulin‑2 were found to regulate TGF-β/BMP signals and hence played a crucial role in differentiation and osteoblast maturation [32]. In line with their findings, our study revealed significant positive correlations between the expression of TGF-β1 and TGF-β3, TGF-β1 and fibulin‑1, and TGF-β3 and fibulin‑1. We speculated that there was an intrinsic correlation between TGF-β1, TGF-β3, and fibulin‑1 during the development of PMS, although this may be a partial interaction in the diverse network of cross-talks involving TGF-β1, TGF-β3, and fibulin‑1.
Kuang et al. found that TGF-β stimulated the transcription and mRNA expression of fibulin‑5 in human lung fibroblasts through the PI3K/AKT pathway [47]. Expression of fibulin‑5 was reported to be promoted by TGF-β in human endometrial epithelial cancer cells [29]. However, in the present study the expression of fibulin‑5 showed no significant correlation with the expression of TGF-β1 TGF-β3 or fibulin‑1. This difference may be due to the different physiological process been studied. Further studies were required to determine the detailed molecular interaction of TGF-βs and fibulins during the development of PMS.
It is important to recognize the limitations of the present study. The main limitation was that no radiographic assessment was involved because we failed to obtain valid information owing to equipment problems. However, depending on mineralization, radiographic analysis may provide more important information to explore along with the presented results. In addition, the strength of our conclusion was limited by the small sample size and the heterogeneity of the participants groups. The inclusion of only 2 fetuses with DS mitigated the ability to draw convincing conclusions regarding perceived differences with fetuses without DS. Thus, the conclusion of this study should be interpreted with caution due to the minimal presentation of quantitative results. Finally, in the immunohistochemistry study section, only correlations between the expression levels of TGF-βs and fibulins in the development of the PMS during the fetal period were analyzed, which lacked the investigation of the molecular interactions between TGF-βs and fibulins in regulating PMS ossification. Further studies including a larger number of participants, radiographic assessment, and the molecular interaction analysis of TGF-βs and fibulins will be needed for a more convincing conclusion. However, we believe that the relatively rare human fetal samples assessed in this study hold promise for providing new information and results for widespread use.
Conclusion
Our results revealed that the premaxilla is an independent bone and that it is subsequently integrated into the maxilla during the human fetal period. The premature fusion of the PMS in the labial region may be associated with maxillary deficiency, which is related to a class III malocclusion. Overall, the similar expression patterns of TGF-β1, TGF-β3, and fibulin‑1 suggest a close interrelationship in the development of the PMS. It is hoped that these findings will guide future determination of pathogenesis, abnormalities, and variations associated with the PMS, and to further guide the development of treatment options, such as orthodontics, plastic surgery, and maxillofacial surgery.
References
Ngan P, Moon W (2015) Evolution of Class III treatment in orthodontics. Am J Orthod Dentofacial Orthop 148(1):22–36. https://doi.org/10.1016/j.ajodo.2015.04.012
Liu Y, Hou R, Jin H et al (2021) Relative effectiveness of facemask therapy with alternate maxillary expansion and constriction in the early treatment of Class III malocclusion. Am J Orthod Dentofacial Orthop 159(3):321–332. https://doi.org/10.1016/j.ajodo.2019.12.028
Guyer EC, Ellis EE 3rd, McNamara JA Jr., Behrents RG (1986) Components of class III malocclusion in juveniles and adolescents. Angle Orthod 56(1):7–30. https://doi.org/10.1043/0003-3219
Stojanović Z, Nikolić P, Nikodijević A, Milić J, Stojanović B (2013) Cephalometric assessment of maxillary length in Serbian children with skeletal class III. VOJNOSANIT PREGL 70(7):645–652. https://doi.org/10.2298/vsp110224042s
Szuhanek C, Gâdea Paraschivescu E, Sişu AM, Motoc A (2011) Cephalometric investigation of Class III dentoalveolar malocclusion. Rom J Morphol Embryol 52(4):1343–1346
Barteczko K, Jacob M (2004) A re-evaluation of the premaxillary bone in humans. Anat Embryol 207(6):417–437. https://doi.org/10.1007/s00429-003-0366-x
Vogel C (1965) Varianten des Zwischenkiefers und ihre Abhangigkeit von der Gebissdifferenzierung bei den Primaten einschliesslich der Hominiden. Fortschr Kieferorthop 26(4):357–376. https://doi.org/10.1007/BF02168478
Noback CR, Moss ML (1953) The topology of the human premaxillary bone. Am J Phys Anthropol 11(2):181–188. https://doi.org/10.1002/ajpa.1330110221
Trevizan M, Filho NP, Franzolin SOB, Consolaro A (2018) Premaxilla: up to which age it remains separated from the maxilla by a suture, how often it occurs in children and adults, and possible clinical and therapeutic implications: Study of 1,138 human skulls. Dental Press J Orthod 23(6):16–29. https://doi.org/10.1590/2177-6709.23.6.016-029.oin
Carmody KA, Mooney MP, Cooper GM et al (2008) Relationship of premaxillary bone and its sutures to deciduous dentition in nonhuman primates. Cleft Palate Craniofac J 45(1):93–100. https://doi.org/10.1597/06-197.1
Mooney MP, Siegel MI (1986) Developmental relationship between premaxillary-maxillary suture patency and anterior nasal spine morphology. Cleft Palate J 23(2):101–107
Mooney MP, Siegel MI (1991) Premaxillary-maxillary suture fusion and anterior nasal tubercle morphology in the chimpanzee. Am J Phys Anthropol 85(4):451–456. https://doi.org/10.1002/ajpa.1330850408
Holton NE, Franciscus RG, Nieves MA et al (2010) Sutural growth restriction and modern human facial evolution: an experimental study in a pig model. J Anat 216(1):48–61. https://doi.org/10.1111/j.1469-7580.2009.01162.x
Ruan WH, Winger JN, Yu JC, Borke JL (2008) Induced premaxillary suture fusion: class III malocclusion model. J Dent Res 87(9):856–860. https://doi.org/10.1177/154405910808700901
Ruan WH, Winger JN, Yu JC, Borke JL (2008) Effects of induced premaxillary suture fusion on the craniofacial morphology in growing rats. Arch Oral Biol 53(1):79–86. https://doi.org/10.1016/j.archoralbio.2007.07.002
de Campos Gomes F, de Melo-Neto JS, Goloni-Bertollo EM, Pavarino ÉC (2020) Trends and predictions for survival and mortality in individuals with Down syndrome in Brazil: A 21-year analysis. J Intellect Disabil Res 64(7):551–560. https://doi.org/10.1111/jir.12735
Diéguez-Pérez M, de Nova-García MJ, Mourelle-Martínez MR, Bartolomé-Villar B (2016) Oral health in children with physical (Cerebral Palsy) and intellectual (Down Syndrome) disabilities: Systematic review I. J Clin Exp Dent 8(3):e337–43. https://doi.org/10.4317/jced.52922
Allareddy V, Ching N, Macklin EA et al (2016) Craniofacial features as assessed by lateral cephalometric measurements in children with Down syndrome. Prog Orthod 17(1):35. https://doi.org/10.1186/s40510-016-0148-7
Korayem MA, AlKofide EA (2014) Characteristics of Down syndrome subjects in a Saudi sample. Angle Orthod 84(1):30–37. https://doi.org/10.2319/030813-195.1
Jesuino SFA, Valladares-Neto J (2013) Craniofacial morphological differences between Down syndrome and maxillary deficiency children. Eur J Orthod 35(1):124–130. https://doi.org/10.1093/ejo/cjr105
Vos FI, de Jong-Pleij EA, Bakker M, Tromp E, Kagan KO, Bilardo CM (2015) Fetal facial profile markers of Down syndrome in the second and third trimesters of pregnancy. Ultrasound Obstet Gynecol 46(2):168–173. https://doi.org/10.1002/uog.14720
Noda K, Nakamura T, Komatsu Y (2015) Fibulin‑5 deficiency causes developmental defect of premaxillary bone in mice. Biochem Biophys Res Commun 466(3):585–591. https://doi.org/10.1016/j.bbrc.2015.09.089
Adab K, Sayne JR, Carlson DS, Opperman LA (2002) Tgf-beta1, Tgf-beta2, Tgf-beta3 and Msx2 expression is elevated during frontonasal suture morphogenesis and during active postnatal facial growth. Orthod Craniofac Res 5(4):227–237. https://doi.org/10.1034/j.1600-0544.2002.02227.x
Gosain AK, Recinos RF, Agresti M, Khanna AK (2004) TGF-beta1, FGF‑2, and receptor mRNA expression in suture mesenchyme and dura versus underlying brain in fusing and nonfusing mouse cranial sutures. Plast Reconstr Surg 113(6):1675–1684. https://doi.org/10.1097/01.prs.0000117362.33347.43
Cooley MA, Harikrishnan K, Oppel JA et al (2014) Fibulin‑1 is required for bone formation and Bmp-2-mediated induction of Osterix. Bone 69:30–38. https://doi.org/10.1016/j.bone.2014.07.038
Most D, Levine JP, Chang J et al (1998) Studies in cranial suture biology: up-regulation of transforming growth factor-beta1 and basic fibroblast growth factor mRNA correlates with posterior frontal cranial suture fusion in the rat. Plast Reconstr Surg 101(6):1431–1440. https://doi.org/10.1097/00006534-199805000-00001
Knudsen TB, Bulleit RF, Zimmerman EF (1985) Histochemical localization of glycosaminoglycans during morphogenesis of the secondary palate in mice. Anat Embryol (Berl) 173(1):137–142. https://doi.org/10.1007/BF00707312
Gato A, Martinez ML, Tudela C et al (2002) TGF-beta(3)-induced chondroitin sulphate proteoglycan mediates palatal shelf adhesion. Dev Biol 250(2):393–405
Winship AL, Rainczuk K, Ton A, Dimitriadis E (2016) Fibulin‑5 localisation in human endometrial cancer shifts from epithelial to stromal with increasing tumour grade, and silencing promotes endometrial epithelial cancer cell proliferation. Oncol Lett 12(1):651–657. https://doi.org/10.3892/ol.2016.4650
Topalovski M, Hagopian M, Wang M, Brekken RA (2016) Hypoxia and transforming growth factor β cooperate to induce Fibulin‑5 expression in pancreatic cancer. J Biol Chem 291(42):22244–22252. https://doi.org/10.1074/jbc.M116.730945
Lertsuwan K, Choe LH, Marwa IR, Lee K, Sikes RA (2017) Identification of Fibulin‑1 as a Human Bone Marrow Stromal (HS-5) Cell-Derived Factor That Induces Human Prostate Cancer Cell Death. Prostate 77(7):729–742. https://doi.org/10.1002/pros.23303
Nistala H, Lee-Arteaga S, Smaldone S et al (2010) Fibrillin‑1 and -2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J Cell Biol 190(6):1107–1121. https://doi.org/10.1083/jcb.201003089
Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP (2008) Fibulin‑5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis 29(12):2243–2251. https://doi.org/10.1093/carcin/bgn199
Mullink H, Henzen-Logmans SC, Tadema TM, Mol JJ, Meijer CJ (1985) Influence of fixation and decalcification on the immunohistochemical staining of cell-specific markers in paraffin-embedded human bone biopsies. J Histochem Cytochem 33(11):1103–1109. https://doi.org/10.1177/33.11.2414361
Tsuda T (2018) Extracellular Interactions between Fibulins and Transforming Growth Factor (TGF)-beta in Physiological and Pathological Conditions. Int J Mol Sci. https://doi.org/10.3390/ijms19092787
Roth DA, Longaker MT, McCarthy JG et al (1997) Studies in cranial suture biology: Part I. Increased immunoreactivity for TGF-beta isoforms (beta 1, beta 2, and beta 3) during rat cranial suture fusion. J Bone Miner Res 12(3):311–321. https://doi.org/10.1359/jbmr.1997.12.3.311
Nicholas CL (2016) Fetal and neonatal maxillary ontogeny in extant humans and the utility of prenatal maxillary morphology in predicting ancestral affiliation. Am J Phys Anthropol 161(3):448–455. https://doi.org/10.1002/ajpa.23043
Som PM, Naidich TP (2014) Illustrated review of the embryology and development of the facial region, part 2: Late development of the fetal face and changes in the face from the newborn to adulthood. AJNR Am J Neuroradiol 35(1):10–18. https://doi.org/10.3174/ajnr.A3414
Wood NK, Wragg LE, Stuteville OH (1967) The premaxilla: embryological evidence that it does not exist in man. Anat Rec 158(4):485–489. https://doi.org/10.1002/ar.1091580411
Woo JK (1949) Ossification and growth of the human maxilla, premaxilla and palate bone. Anat Rec 105(4):737–761. https://doi.org/10.1002/ar.1091050408
Sejrsen B, Kjaer I, Jakobsen J (1993) The human incisal suture and premaxillary area studied on archaeologic material. Acta Odontol Scand 51(3):143–151. https://doi.org/10.3109/00016359309041160
Behrents RG, Harris EF (1991) The premaxillary-maxillary suture and orthodontic mechanotherapy. Am J Orthod Dentofacial Orthop 99(1):1–6. https://doi.org/10.1016/S0889-5406(05)81673-7
Trevizan M, Consolaro A (2017) Premaxilla: an independent bone that can base therapeutics for middle third growth! Dental Press J Orthod 22(2):21–26. https://doi.org/10.1590/2177-6709.22.2.021-026.oin
Maureille B, Bar D (1999) The premaxilla in Neandertal and early modern children: ontogeny and morphology. J Hum Evol 37(2):137–152. https://doi.org/10.1006/jhev.1999.0312
Chen L, Ge Q, Black JL, Deng L, Burgess JK, Oliver BG (2013) Differential regulation of extracellular matrix and soluble fibulin‑1 levels by TGF-β1 in airway smooth muscle cells. PLoS ONE 8(6):e65544. https://doi.org/10.1371/journal.pone.0065544
Liu G, Cooley MA, Jarnicki AG et al (2016) Fibulin‑1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. https://doi.org/10.1172/jci.insight.86380
Kuang PP, Joyce-Brady M, Zhang XH, Jean JC, Goldstein RH (2006) Fibulin‑5 gene expression in human lung fibroblasts is regulated by TGF-beta and phosphatidylinositol 3‑kinase activity. Am J Physiol, Cell Physiol 291(6):C1412–21. https://doi.org/10.1152/ajpcell.00087.2006
Acknowledgements
This work was supported by the key project of the Health Commission of Zhejiang province (No. 2012ZDA028). The authors thank all the fetal cadaver donor families.
We give our great thanks to Robert Dorazio, the Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang province, P.R. China for reviewing this manuscript before submission.
Author information
Authors and Affiliations
Contributions
Conceptualization: Wen-hua Ruan, Ling Zhu; methodology: Wei-zhong Gu, Wu-qun Han, Wen-hua Ruan; formal analysis and investigation: Ling Zhu, Wei-zhong Gu, Wen-hua Ruan; writing—original draft preparation: Ling Zhu; writing—review and editing: Ling Zhu, Wen-hua Ruan; funding acquisition, resources, and supervision: Wen-hua Ruan; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
L. Zhu, W.-h. Ruan, W.-q. Han and W.-z. Gu declare that they have no competing interests.
Ethical standards
All procedures in studies were approved by the Children’s Hospital, Zhejiang University school of medicine Research Ethics Committee (2012-GJ-049). Consent to participate: Written informed consent was obtained from the families.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, L., Ruan, Wh., Han, Wq. et al. Anatomical and immunohistochemical analyses of the fusion of the premaxillary–maxillary suture in human fetuses. J Orofac Orthop 85, 123–133 (2024). https://doi.org/10.1007/s00056-022-00410-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-022-00410-w