Abstract
Purpose
The goal of this work was to examine the relationship between sagittal facial pattern and thickness of alveolar bone in conjunction with root morphology of teeth by using cone beam computed tomography (CBCT).
Methods
The study was carried out on the CBCT scans from 3 group of patients (n = 20 in each group). The first group involved skeletal class 1, the second group involved skeletal class 2, and the third group involved skeletal class 3 patients. In all, 14 permanent teeth and interdental regions in the maxilla and mandible were evaluated. Root length and root width were measured on each tooth. Buccal cortical bone thickness, cancellous bone thickness, and lingual cortical bone thicknesses were measured in each interdental region. Analysis of variance, Kruskall–Wallis H and Mann–Whitney U tests were used for statistical comparisons.
Results
No significant difference was found between the groups for root length, root width, buccal cortical bone and lingual cortical bone thickness. A significant difference was observed between the groups for cancellous bone thickness as it was thicker in skeletal class 2 group. Cortical bone was thicker in the mandible compared to maxilla on both buccal and lingual sides and it was thicker in the posterior region compared to the anterior region on the buccal side.
Conclusions
Differences in cancellous bone thickness between different sagittal facial patterns and differences in cortical bone thickness between different alveolar regions should be taken into consideration when planning orthodontic tooth movements and anchorage mechanics.
Zusammenfassung
Ziel
Ziel der vorliegenden Studie war es, die Beziehungen zwischen der sagittalen skelettalen Klasse und der Dicke des Alveolarknochens sowie der Zahnwurzelmorphologie mittels digitaler Volumentomographie (DVT) zu untersuchen.
Methoden
Die Studie wurde an DVTs von drei Patientengruppen durchgeführt. In der ersten Gruppe waren Patienten mit skelettaler Klasse 1 (n = 20), in der zweiten Patienten mit skelettaler Klasse 2 (n = 20) und in der dritten Gruppe Patienten mit skelettaler Klasse 3. Insgesamt wurden 14 bleibende Zähne und Interdentalbereiche in Ober- und Unterkiefer bewertet. Wurzellänge und Wurzelbreite wurden an jedem Zahn gemessen. In jeder Interdentalregion wurde die Dicke der bukkalen und lingualen Kortikalis sowie der Spongiosa bestimmt. Zur statistischen Auswertung dienten Varianzanalysen, Kruskal-Wallis-H- und Mann-Whitney-U-Tests.
Ergebnisse
Es wurde kein signifikanter Unterschied zwischen den Gruppen in Bezug auf Wurzellänge, Wurzelbreite, Dicke der bukkalen und lingualen Kortikalis festgestellt. Es wurde ein signifikanter Unterschied zwischen den Gruppen für die Spongiosadicke beobachtet, die bei Patienten mit skelettaler Klasse 2 dicker war. Kortikaler Knochen war im Unterkiefer im Vergleich zum Oberkiefer sowohl auf der bukkalen als auch auf der lingualen Seite dicker und im posterioren im Vergleich zum anterioren Bereich auf der bukkalen Seite dicker.
Schlussfolgerungen
Bei der Planung von orthodontischen Zahnbewegungen und der Verankerungsmechanik sollten die Unterschiede in der Dicke der Spongiosa zwischen verschiedenen sagittalen skelettalen Klassen und die Unterschiede in der Kortikalis zwischen den verschiedenen Alveolarregionen berücksichtigt werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthodontic tooth movement is initiated by mechanical force and proceeds by remodeling of periodontal ligament and alveolar bone [8]. The thickness of alveolar bone determines the limits of orthodontic tooth movement and exceeding these limits may result in damage to periodontal tissues [12]. Extreme buccal–lingual movements of teeth can decentralize teeth within the dentoalveolar bone envelope, resulting in dehiscence, fenestration, and gingival recession, depending on the initial alveolar bone morphology. Therefore, buccal and lingual bone thickness should be taken into consideration during orthodontic treatment planning.
Alveolar bone dimensions, more particularly buccal and lingual cortical bone thicknesses, are important factors that affect the stability and success of miniscrews which are commonly used by many orthodontists [4]. Miniscrews require mechanical retention to bone rather than osseointegration with bone to provide anchorage. Being more strong and resistant to deformation, cortical bone provides higher anchorage for miniscrews and it is responsible for their primary stability [13]. Hence, thick cortical areas are recommended for to increase the primary stability of miniscrews and prevent their failure at early stages [10, 11, 20, 22].
Thickness and height of buccal and lingual cortical bone layers may be altered in conjunction with alignment of teeth, inclination of root and occlusal forces [5]. The vertical growth pattern has been shown to influence alveolar bone thickness and many researchers have reported that hypodivergent individuals have thicker alveolar bone morphology compared to hyperdivergent individuals [14, 20, 25].
Numerous papers investigating the relationship between vertical skeletal dimensions and alveolar bone thickness can be found in the literature [9, 14, 20, 25]. Nevertheless, the relationship between the sagittal skeletal pattern and alveolar bone thickness related with root morphology has not been studied until now. The diversity in cortical bone thickness among different alveolar regions has also not been evaluated comprehensively using cone beam computed tomography (CBCT) images. Hence, the primary aim of this study is to examine the relationship between sagittal facial pattern and thickness of alveolar cortical and cancellous bone in conjunction with root morphology of teeth by using CBCT. The H0 hypothesis is that alveolar bone thickness and root morphology of teeth is similar in individuals having different sagittal facial pattern, whereas the H1 hypothesis is that alveolar bone thickness and root morphology of teeth changes due to the sagittal facial pattern. The secondary aim of this study is to examine the diversity in cortical bone thickness between the maxillary and mandibular or between the anterior and posterior alveolar regions.
Materials and methods
This retrospective study carried out using the CBCT scans of 3 groups of patients obtained from the archive of a private maxillofacial scanning and screening center (Tomoloji Maksillofasiyal Görüntüleme Merkezi, Ankara, Turkey) was approved by Baskent University Institutional Review Board and Ethics Committee (project number D‑DA13/06). A power analysis was performed based on 1:1 ratio between the groups by using data obtained from the literature [10, 14, 25]. The analysis revealed that a sample size of n = 18 patients per group was needed to achieve a power of 80% and n = 20 patients per group was needed to achieve a power of 85% at α = 0.05 significance level to detect statistically significant differences between the groups with a 0.30 (medium) effect size. Sample size estimation was performed by using NCSS and PASS software (Number Cruncher Statistical Systems. Version 2000. Kaysville, UT, USA).
Patients were included in the study based on the following criteria:
-
No craniofacial deformity
-
Permanent dentition stage
-
No congenitally missing or extracted teeth
-
No impacted or supernumerary teeth
-
Root apices of all permanent teeth closed, except for third molars
-
Postpubertal stage (CS5, CS6) according to cervical vertebrae maturation
-
GoGn/SN angle between 28 and 36°
-
CBCT scans present involving the area from the nasal bone to chin
-
Maximum intercuspidation in CBCT scans
-
No periodontal disease causing horizontal or vertical bone loss in CBCT scans
-
No restorations involving the cementoenamel junction
-
No history of orthodontic treatment
From a total of 201 CBCT scans, 60 scans which met the inclusion criteria for the 3 study groups were incorporated into the study and a power of 85% was achieved. The first group consisted of the CBCT scans of 20 skeletal class 1 patients with ANB angle between 0 and 4° (18 girls, 2 boys, mean age 18.20 ± 3.33 years). The second group consisted of the CBCT scans of 20 skeletal class 2 patients with ANB angle greater than 4° (11 girls, 9 boys, mean age 18.25 ± 4.92 years). The third group consisted of the CBCT scans of 20 skeletal class 3 patients with ANB angle less than 0° (10 girls, 10 boys, mean age 18.90 ± 4.97 years). The demographic distributions of the groups are presented in Tables 1 and 2.
The scans were obtained by using a CBCT device (ILUMA, IMTEC Europa, Oberursel, Germany) making 360° rotation, working with 120 kVp, 3.8 mA, scanning a 14 × 19.5 cm area within 40 s, and having 0.3 mm voxel size. The patients were sitting, their heads were oriented by locating the Frankfurt plane parallel to the horizontal plane and their teeth were in maximum intercuspidation during the CBCT scan. The raw data obtained from the CBCT scan were reconstructed using software provided by the producer (ILUMA Vision, IMTEC Europa, Oberursel, Germany) and saved as viewer files. All measurements were performed at a window level of 1000 and a window width of 4000 to provide the finest images for accurate measurements. Lateral cephalometric images were obtained from the CBCT scanning data for measuring GoGnSN and ANB angles. Sagittal, axial and coronal cross-sections were used to measure alveolar buccal cortical, lingual cortical and cancellous bone thicknesses or root length and width.
A total of 14 permanent teeth (central incisor, lateral incisor, canine, first premolar, second premolar, first molar, second molar) and 12 interdental regions between them were evaluated in the maxilla and mandible on the right side. Root length and root width were measured on each tooth while buccal cortical bone thickness, cancellous bone thickness, and lingual cortical bone thickness were measured in each interdental region evaluated. Axial, coronal, and sagittal orientation planes were constructed for accurate positioning of the tooth or the interdental region to be evaluated before measuring.
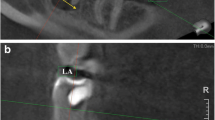
Central incisor, lateral incisor, and canine were defined as anterior teeth, while the first premolar, second premolar, first molar, and second molar were defined as posterior teeth both in upper and lower jaws in this study. Root length and width measurements were performed on the buccal root in the upper premolars with two roots, on the mesial root in lower molars with two roots and on the mesiobuccal root in the upper molars with three roots. Root length measurements were performed on the long axis of the root from the cementoenamel junction to the root apex, on the sagittal cross-section for anterior teeth and on coronal cross-section for posterior teeth. Root width measurements were performed from the buccal cementoenamel junction to the lingual cementoenamel junction, on the sagittal cross-section for anterior teeth and on the coronal cross-section for posterior teeth (Fig. 1).
Orientation of a right maxillary central tooth on axial (a) and coronal cross-sections (b). Measurement of right maxillary central root length (c) and root width (d) on the sagittal cross-section. Orientation of a right maxillary first premolar on axial (e) and sagittal cross-sections (f). Measurement of right maxillary first premolar root length (g) and root width (h) on the coronal cross-section
Rechter mittlerer Schneidezahnes im axialen Schnitt (a) und im koronalen Querschnitt (b). Messung der Wurzellänge des oberen rechten Schneidezahns (c) und Wurzelbreite (d) am sagittalen Querschnitt. Oberer rechter ersten Prämolar im axialen Schnitt (e) und sagittalem Querschnitt (f). Messungen der Wurzellänge (g) und der Wurzelbreite (h) am oberen rechten ersten Prämolar am koronalen Querschnitt
Buccal cortical bone thickness, cancellous bone thickness, and lingual cortical bone thickness measurements were performed at the mesiodistal center point of each interdental region 5 mm apical from the alveolar crest, on the sagittal cross-section for the anterior region, and on the coronal cross-section for the posterior region. Buccal and lingual cortical bone thicknesses were measured as the shortest buccolingual distances from the outer surface of cortical bone to the border between the cortical and cancellous bone. Cancellous bone thickness was measured as the shortest buccolingual distances from the buccal border between the cortical and cancellous bone to the lingual border between the cortical and cancellous bone (Fig. 2). All measurements were performed by the same observer (IC). A total of 64 parameters (14 root length, 14 root width, 12 buccal cortical bone thickness, 12 lingual cortical bone thickness, 12 cancellous bone thickness) were evaluated. Two weeks after the first measurements, 30 CBCT images (10 images randomly selected from each group) were remeasured by the same observer (IC) to check intraobserver reliability.
Orientation of the interdental region between right maxillary lateral and canine teeth on axial (a) and coronal cross-sections (b). Measurement of buccal cortical bone, cancellous bone and lingual cortical bone thicknesses on sagittal cross-sections (c). Orientation of the interdental region between right maxillary premolar teeth on axial (d) and sagittal cross-sections (e). Measurement of buccal cortical bone, cancellous bone and lingual cortical bone thicknesses on coronal cross-sections (f)
Interdentalregion zwischen oberem rechten lateralen Schneidezahn und Eckzahn am axialen (a) und koronalen Querschnitt (b). Messung der Dicken von bukkaler Kortikalis, Spongiosa und lingualer Kortikalis an sagittalen Querschnitten (c). Interdentalregion zwischen den oberen rechten Prämolaren an axialen (d) und sagittalen Querschnitten (e). Messung der Dicken von bukkaler Kortikalis, Spongiosa und lingualer Kortikalis an koronalen Querschnitten (f)
Statistical analysis
Data analysis was performed by using SPSS for Windows, version 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics for gender are presented as percentage, whereas all other parameters are presented as mean and standard deviation (SD). The differences between the three study groups were analyzed by the Analysis of Variance (ANOVA) test for variables showing normal distribution and by the Kruskall–Wallis H test for variables not showing normal distribution. The Scheffe test was used to distinguish the groups between which a statistically significant difference was observed if ANOVA revealed a significant difference. The Mann–Whitney U test was used for subgroup comparisons where the differences between 2 groups were analyzed and the variables were not showing normal distribution. A p value less than 0.05 was considered statistically significant with a 95% confidence interval (CI). The Wilcoxon’s signed test was used to determine intraobserver reliability by evaluation of data obtained from the remeasurement of 30 CBCT images. The observer was found to be consistent in the repeated measurements for all variables (p ≥ 0.05).
Results
No significant difference was found between skeletal class 1, class 2, and class 3 groups for mean age, root length, root width, buccal cortical bone, and lingual cortical bone thickness measurements (Tables 2, 3, 4, 5 and 6; Figs. 3, 4, 5 and 6). However, significant differences were observed between skeletal class 1, class 2, and class 3 groups for cancellous bone thickness. Thus, skeletal class 2 group generally had greater cancellous bone thickness between maxillary molars and in all interdental regions in the mandible (Table 7 and Fig. 7).
The subgroup comparisons revealed significant differences between upper and lower buccal cortical bone thickness (p = 0.0001) and between upper and lower lingual cortical bone thickness (p = 0.0001) in class 1, 2, and 3 groups. Cortical bone was thicker in the mandible compared to the maxilla on both the buccal and lingual sides (Table 8).
Another subgroup comparison disclosed significant differences between anterior and posterior buccal cortical bone thickness (p = 0.0001) in class 1, 2, and 3 groups. Cortical bone was observed to be thicker in the posterior region compared to the anterior region on the buccal side (Table 9). Nevertheless, no significant differences were observed between the anterior and posterior lingual cortical bone thickness.
Discussion
It is known that facial types of individuals have an effect on the morphology of their maxillary and mandibular cortical plates. Despite the number of recently published papers emphasizing the influence of vertical growth pattern on alveolar bone morphology, no research examining the influence of sagittal growth pattern on the alveolar bone thickness between all tooth roots have been presented in detail [1, 9, 14, 20, 25, 27, 36]. In addition, the relationship between root length or width and alveolar morphology on either type of growth pattern has not been examined until now. Therefore, the primary objective of this study was to understand the relationship between sagittal facial pattern and thickness of alveolar cortical and cancellous bone layers in combination with root morphology of teeth. The secondary objective of this study was to examine the diversity in cortical bone thickness between the maxillary and mandibular or between the anterior and posterior alveolar regions.
Examination of average root length and root width values in conjunction with alveolar cortical and cancellous bone thickness is thought to be important and necessary for establishing normative data related to root and bone morphology for all permanent teeth and all interdental areas between them. This data can be used as a reference in all stages of orthodontic treatment planning by providing assistance for anchorage considerations, determination of tooth movement limitations and protection or improvement of peridontal conditions [1, 9, 12, 14, 16, 20, 25, 27]. In addition to establishing detailed normative data for individuals with normal vertical facial pattern, revealing the differences in root and bone morphology among skeletal class 1, class 2 and class 3 individuals may help orthodontists by indicating the potential advantageous or hazardous factors related with sagittal facial pattern.
Previous studies have shown that hormonal and functional changes associated with age have an influence on cortical bone thickness and patients under 14 years were reported to have low success rates with miniscrews due to the thin buccal cortical bone layer [10, 11, 26, 31, 33]. Thus, patients in the postpubertal stage (CS5, CS6) according to cervical vertebrae maturation and older than 15 years were included in this study, whereas no statistically significant difference was found between the groups for age. Additionally, patients with only normal vertical growth patterns (GoGn/SN angle between 28 and 36°) were included in this study to eliminate the potential influence of vertical growth pattern on dentoalveolar bone morphology.
The influence of gender on dentoalveolar morphology was not evaluated in this study since there were fewer males and the male/female ratios were unequal in the groups. Many researchers presented that although males have larger masticatory muscles and higher maximum biting forces, these high forces are rarely achieved in daily life and no significant difference was observed between males and females for cortical bone thickness [7, 10, 29, 33]. On the other hand, Rossi et al. [28] observed differences between class 3 males and females in cortical bone thickness at some mandibular regions apart from the alveolar bone.
A limitation of this study was the relatively small sample size which was 20 patients in each group due to a great number of inclusion criteria applied for elimination of any imperfect examination and measurement. Despite the fact that a power of 85% with a medium effect size was achieved, obtaining a higher power with a larger effect size would be favored.
Root length and root width measurements revealed no significant difference between the skeletal class 1, class 2 and class 3 groups in this study. Root length measurements were performed on the long axis of the root from the cementoenamel junction to the root apex, whereas root width measurements were performed from the buccal cementoenamel junction to the lingual cementoenamel junction. Being a junction point of two tissues with different hydroxyapatite densities, the cementoenamel junction can be easily identified on CBCT images and has been used as a reliable reference for both root length and root width measurements with high levels of repeatability [5, 16, 18, 30]. No study evaluating all permanent teeth in individuals having similar facial characteristics with this study was found in the literature. Therefore, the results of this study of the relationships of root length and root width measurements can not be compared with other studies. Hence, they can be used as reference data for skeletal class 1, class 2 and class 3 individuals with a normal vertical facial pattern.
Buccal and lingual cortical bone thickness measurements did not present statistically significant difference between skeletal class 1, class 2 and class 3 groups in this study. However, cancellous bone thickness revealed significant difference between the groups especially in the mandible and was thicker in the skeletal class 2 group. This difference can be attributed to the relatively greater buccal inclination of tooth crowns in the mandibular arch in the class 2 group. This inclination may also cause the roots of these teeth to be inclined and occupy larger spaces in the alveolus which gives rise to bone modeling. Being the dominant process of facial growth and adaptation to functional loads, bone modeling may be the reason of the thicker cancellous bone observed in the skeletal class 2 group. Furthermore, cancellous bone thickness increased gradually from the canine region to the posterior region in all 3 groups. A study inspecting cortical and cancellous bone thicknesses in all interdental regions in both the maxilla and mandible in individuals with different sagittal skeletal patterns does not exist in the literature. On the other hand, studies with a somewhat similar group design examined cortical bone thicknesses either in buccal/lingual regions of teeth or in only certain interdental regions instead of all [1, 3, 9, 14, 17, 18, 20, 23, 25, 27]. Therefore, these results can not be compared with this study.
In this study, cortical bone was observed to be significantly thicker in mandible compared to maxilla both on the buccal and lingual sides in all three groups. This finding is compatible with other studies [7, 10, 14, 24, 29]. This situation can be explained with the anatomical inability of the mandible to distribute heavy biting forces to the whole cranium like the maxilla does, which causes a much stronger and stiffer structure to absorb entire load and resist distortion [35]. This structural difference between the maxilla and mandible is also clinically evident when orthodontically moving a tooth, placing a miniscrew, or predrilling [14].
Cortical bone was observed to be significantly thicker in the posterior region compared to the anterior region on the buccal side both in the maxilla and the mandible in all three groups of this study. Nevertheless, no significant difference was observed between anterior and posterior lingual cortical bone thickness. The gradual increase in buccal cortical bone thickness from anterior to posterior regions is one of the unique findings of this study as it was not examined up to now and should be taken into consideration during treatment planning if expansion of dental arch or protrusion, retrusion and torqueing of teeth is part of the treatment plan [1, 9, 14, 20, 25, 27].
In this study, lingual cortical bone thickness was greater than buccal cortical bone thickness both in the maxilla and mandible in all three groups. The only exception is between the mandibular first and second molars, where the buccal cortical bone is thicker. This finding is compatible with the study of Horner et al. [14] who also reported that cortical bone was thicker on the lingual side compared to the buccal side in both jaws. This information can be helpful in clinical practice for arranging buccal or lingual tooth movements and skeletal anchorage mechanics with miniscrews.
The thickest cortical bone on the lingual side was observed in the mandibular canine region in this study, which was formerly shown to face the greatest stress on the lingual side [14]. On the other hand, the thickest cortical bone on the buccal side was observed in the mandibular molar region, which is very close to the attachment region of masticatory muscles and therefore faces the greatest stress on the buccal side. This finding is also compatible with other studies that reported thicker buccal cortical bone compared to lingual cortical bone only between mandibular molars for the same reason [10, 14, 19, 25]. It is thought that similar with the lingual canine region, the buccal molar region of the mandible is exposed to greater masticatory forces and higher stress levels and the cortical bone in these regions is thickened to withstand these [6, 15, 34].
Studies examining cortical bone thickness aimed to find the most suitable areas for miniscrew placement which are also easy to locate on CBCT images for being able to make standard repeated measurements [11, 14]. These areas should be within attached gingiva, have low risk of inflammation, and thus be 5 mm apical to the alveolar crest at the mesiodistal center point of each interdental region which is why they are preferred in this study. It is reported that a minimum 1 mm cortical bone thickness is required to obtain successful results with miniscrews [22]. Motoyoshi et al. [21] remarked that failure rates at regions with less than 1 mm cortical bone thickness are 6.9 times higher than the regions with more than 1 mm cortical bone thickness. The mean buccal and lingual cortical bone thicknesses measured in this study were greater than 1 mm for all interdental regions evaluated. However, some studies reported less than 1 mm cortical bone thicknesses in some regions of the maxilla and mandible. This difference may be related with evaluation of both young and adult patients in those studies, as age is known to have an influence on cortical bone thickness [10, 11, 26, 31].
Extremely thick cortical bone is another factor that may cause miniscrew failure. Placing a miniscrew in an extremely thick cortical bone layer may lead to excessive torque which causes too much compression of surrounding tissues that results in osteonecrosis and resorption rather than healing [32]. Therefore, torque values over 10 Ncm are reported to increase failure rates and predrilling is advised to avoid excessive torque for cortical bone layers thicker than 1.5 mm [2, 21].
Conclusions
-
Skeletal class 1, class 2 and class 3 individuals are similar with regard to root length, root width, and buccal/lingual cortical bone thickness.
-
Skeletal class 2 individuals have greater cancellous bone thickness in the maxillary molar region and in all mandibular interdental regions.
-
The H0 hypothesis is rejected and the H1 hypothesis is accepted.
-
Cortical bone is thicker in the mandible compared to the maxilla both on the buccal and lingual sides.
-
Cortical bone is thicker in the posterior region compared to the anterior region on the buccal side.
-
Lingual cortical bone is thicker than the buccal cortical bone both in the maxilla and mandible, except for the mandibular molar region.
References
Al-Masri MM, Ajaj MA, Hajeer MY, Al-Eed MS (2015) Evaluation of bone thickness and density in the lower incisors’ region in adults with different types of skeletal malocclusion using cone-beam computed tomography. J Contemp Dent Pract 16:630–637
Baumgaertel S (2010) Predrilling of the implant site: is it necessary for orthodontic mini-implants? Am J Orthod Dentofacial Orthop 137:825–829
Baysal A, Ucar FI, Buyuk SK, Ozer T, Uysal T (2013) Alveolar bone thickness and lower incisor position in skeletal Class I and Class II malocclusions assessed with cone-beam computed tomography. Korean J Orthod 43:134–140
Buschang PH, Carillo R, Ozenbaugh B, Rossouw PE (2008) 2008 survey of AAO members on miniscrew usage. J Clin Orthod 42:513–518
Carranza FA, Bernard GW (2002) The tooth-supporting structures. In: Carranza F, Newman M, Takei H (eds) Clinical periodontology, 9th edn. W. B. Saunders, Philadephia, pp 45–52
Daegling DJ, Hotzman JL (2003) Functional significance of cortical bone distribution in anthropoid mandibles: an in vitro assessment of bone strain under combined loads. Am J Phys Anthropol 122:38–50
Deguchi T, Nasu M, Murakami K, Yabuuchi T, Kamioka H, Takano-Yamamoto T (2006) Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am J Orthod Dentofacial Orthop 129(721):e7–12
Enhos S, Uysal T, Yagci A, Veli I, Ucar FI, Ozer T (2012) Dehiscence and fenestration in patients with different vertical growth patterns assessed with cone-beam computed tomography. Angle Orthod 82:868–874
Eraydın F, Germec-Cakan D, Tozlu M, Ozdemir FI (2018) Three-dimensional evaluation of alveolar bone thickness of mandibular anterior teeth in different dentofacial types. Niger J Clin Pract 21:519–524
Farnsworth D, Rossouw PE, Ceen RF, Buschang PH (2011) Cortical bone thickness at common miniscrew implant placement sites. Am J Orthod Dentofacial Orthop 139:495–503
Fayed MM, Pazera P, Katsaros C (2010) Optimal sites for orthodontic miniimplant placement assessed by cone beam computed tomography. angle Orthod 80:939–951
Garib DG, Yatebe MS, Ozawa TO, Silva OGF (2010) Alveolar bone morphology under the perspective of the computed tomography: Defining the biological limits of tooth movement. Dental Press J Orthod 15:192–205
Holmes DC, Loftus JT (1997) Influence of bone quality on stress distribution for endosseous implants. J Oral Implantol 23:104–111
Horner KA, Behrents RG, Kim KB, Buschang PH (2012) Cortical bone and ridge thickness of hyperdivergent and hypodivergent adults. Am J Orthod Dentofacial Orthop 142:170–178
Ichim I, Kieser JA, Swain MV (2007) Functional significance of strain distribution in the human mandible under masticatory load: numerical predictions. Arch Oral Biol 52:465–473
Kim SY, Lim SH, Gang SN, Kim HJ (2013) Crown and root lengths of incisors, canines, and premolars measured by cone-beam computed tomography in patients with malocclusions. korean J Orthod 43:271–278
Kim Y, Park JU, Kook YA (2009) Alveolar bone loss around incisors in surgical skeletal Class III patients. Angle Orthod 79:676–682
Kook YA, Kim G, Kim Y (2012) Comparison of alveolar bone loss around incisors in normal occlusion samples and surgical skeletal class III patients. Angle Orthod 82:645–652
Li H, Zhang H, Smales RJ, Zhang Y, Ni Y, Ma J, Wang L (2014) Effect of 3 vertical facial patterns on alveolar bone quality at selected miniscrew implant sites. Implant Dent 23:92–97
Masumoto T, Hayashi I, Kawamura A, Tanaka K, Kasai K (2001) Relationship among facial type, buccolingual molar inclination and cortical bone thickness of the mandible. Eur J Orthod 23:15–23
Motoyoshi M, Matsuoka M, Shimizu N (2007) Application of orthodontic mini-implants in adolescents. Int J Oral Maxillofac Surg 36:695–699
Motoyoshi M, Yoshida T, Ono A, Shimizu N (2007) Effect of cortical bone thickness and implant placement torque on stability of orthodontic mini-implants. Int J Oral Maxillofac Implants 22:779–284
Nahm KY, Kang JH, Moon SC, Choi YS, Kook YA, Kim SH, Huang J (2012) Alveolar bone loss around incisors in Class I bidentoalveolar protrusion patients: a retrospective three-dimensional cone beam CT study. Dentomaxillofac Radiol 41:481–488
Ono A, Motoyoshi M, Shimizu N (2008) Cortical bone thickness in the buccal posterior region for orthodontic mini-implants. Int J Oral Maxillofac Surg 37:334–340
Ozdemir F, Tozlu M, Germec-Cakan D (2013) Cortical bone thickness of the alveolar process measured with cone-beam computed tomography in patients with different facial types. Am J Orthod Dentofacial Orthop 143:190–196
Park J, Cho HJ (2009) Three-dimensional evaluation of interradicular spaces and cortical bone thickness for the placement and initial stability of microimplants in adults. Am J Orthod Dentofacial Orthop 136(314):e1–12
Park JH, Hong JY, Ahn HW, Kim SJ (2018) Correlation between periodontal soft tissue and hard tissue surrounding incisors in skeletal Class III patients. Angle Orthod 88:91–99
Rossi M, Bruno G, De Stefani A, Perri A, Graccco A (2017) Quantitative CBCT evaluation of maxillary and mandibular cortical bone thickness and density variability for orthodontic miniplate placement. Int Orthod 15:610–624
Schwartz-Dabney CL, Dechow PC (2003) Variations in cortical material properties throughout the human dentate mandible. Am J Phys Anthropol 120:252–277
Sherrard JF, Rossouw PE, Benson BW, Carrillo R, Buschang PH (2010) Accuracy and reliability of tooth and root lengths measured on cone-beam computed tomographs. Am J Orthod Dentofacial Orthop 137:100–108
Swasty D, Lee JS, Huang JC, Maki K, Gansky SA, Hatcher D, Miller AJ (2009) Anthropometric analysis of the human mandibular cortical bone as assessed by cone-beam computed tomography. J Oral Maxillofac Surg 67:491–500
Ueda M, Matsuki M, Jacobsson M, Tjellström A (1991) Relationship between insertion torque and removal torque analyzed in fresh temporal bone. Int J Oral Maxillofac Implants 6:442–447
Usui T, Uematsu S, Kanegae H, Morimoto T, Kurihara S (2007) Change in maximum occlusal force in association with maxillofacial growth. Orthod Craniofac Res 10:226–234
Van Essen NL, Anderson IA, Hunter PJ, Carman J, Clarke RD, Pullan AJ (2005) Anatomically based modelling of the human skull and jaw. Cells Tissues Organs 180:44–53
Weinmann J, Sicher H (1955) Bone and bones, 2nd edn. C. V. Mosby, St Louis
Yagci A, Veli I, Uysal T, Ucar FI, Ozer T, Enhos S (2012) Dehiscence and fenestration in skeletal Class I, II and III malocclusions assessed with cone-beam computed tomography. angle Orthod 82:67–74
Acknowledgements
We would like to thank our radiology technician Sadettin Gürer and our biostatistician Dr. Ahmet Gül for their contributions to this work which was supported by Baskent University Research Fund (project number D‑DA13/06).
Funding
This study was supported by Baskent University Research Fund (project number D‑DA13/06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
İ. Coşkun and B. Kaya declare that they have no competing interests.
Ethical standards
This retrospective study was performed after consultation with the institutional ethics committee and in accordance with national legal requirements.
Rights and permissions
About this article
Cite this article
Coşkun, İ., Kaya, B. Relationship between alveolar bone thickness, tooth root morphology, and sagittal skeletal pattern. J Orofac Orthop 80, 144–158 (2019). https://doi.org/10.1007/s00056-019-00175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-019-00175-9