Abstract
In the subfamily Polistinae, caste dimorphism is not pronounced and differences among females are primarily physiological and behavioral. We investigated factors that indicate the reproductive status in females of Polistes ferreri Saussure. We analyzed females from nine colonies and evaluated morphometric parameters, ovarian development, occurrence of insemination, relative age, and cuticular chemical profile. The colony females showed three kinds of ovarian development: type A, filamentous ovarioles; type B, ovarioles containing partially developed oocytes; and type C, long and well-developed ovarioles containing two or more mature oocytes. The stepwise discriminant analysis of the cuticular chemical profile showed that it was possible to distinguish the three groups of females: workers 1, workers 2, and queens. However, the stepwise discriminant analysis of the morphological differences did not show significant differences among these groups. The queens were among the older females in the colony and were always inseminated, while the age of the workers varied according to the stage of colony development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Hymenoptera is one of the most diverse groups of insects, with approximately 130,000 species, including bees, wasps, and ants. Wasps of the family Vespidae are extremely important for understanding the origin and evolution of social behavior in insects (Markiewicz & O’Donnell 2001) because the group includes a range of solitary and eusocial species (Evans & West-Eberhard 1970, Wilson 1971, Carpenter 1991). In this family, caste distinction is an essential evolutionary feature, since the presence of large differences between castes indicates a higher degree of sociability (Jeanne 2003).
The patterns of caste development among the families of social wasps vary widely. In the subfamily Stenogastrinae, for example, queens are similar in size to the workers and show only physiological and behavioral differences (Pardi & Piccioli 1981, Turillazzi 1991). In the Vespinae, the queen is larger than the workers, i.e., the caste differences are typically pronounced (Spradbery 1991). Members of the subfamily Polistinae show an intermediate condition between the other two subfamilies (Carpenter 1982), and its species range from those with morphologically similar castes to others with very distinct castes (Richards 1978, O’Donnel 1998).
Although caste differences in Polistinae are less evident than those in Vespinae (Spradbery 1973), the morphological and behavioral characteristics can be quite different among species (Jeanne 1980, Jeanne et al 1995). This distinction between castes can be determined in two ways. In the pre-imaginal stage, the caste is determined before the individual emerges and usually morphological differences are present. Pre-imaginal caste determination occurs in more-derived species (Hunt 1991, O’Donnel 1998). The other, post-imaginal, occurs in less-derived species, in which the caste is determined at least in part when the wasp is an adult. Generally in these species, morphological differences are small or absent (Gadagkar 1991).
Several studies have examined caste distinctions in Polistinae. For the tribe Polistini, studies have involved behavioral aspects, ovarian development, morphology and cuticular hydrocarbon profile of Polistes females (Strassmann et al 1984, Solis & Strassmann 1990, Dani 1994, Giannotti & Machado 1999, Gobbi et al 2006, Tannure-Nascimento et al 2008, Torres et al 2009). Studies analyzing the determination, differentiation, and characterization of castes in Mischocyttarini were also performed (Noda et al 2001, Torres et al 2012, Murakami et al 2009, 2013). All these studies involved behavioral analyses. In the tribe Ropalidiini, the best known species is Ropalidia marginata (Fabricius), for which studies range from behavioral aspects of caste and chemical communication to determination and caste differentiation (Gadagkar & Joshi 1983, Gadagkar et al 1991, Premnath et al 1996, Gadagkar 2001, Mitra et al 2011, Mitra & Gadagkar 2012). Other studies that investigated the division of labor in this tribe were performed with Ropalidia rufoplagiata (Cameron) (Sinha et al 1993) and Ropalidia romandi (Le Guillou) (Fukuda et al 2003).
The ability of colony individuals to recognize each other is an important factor in establishing and maintaining reproductive dominance. Communication by means of chemical compounds is the most widely used recognition mechanism in colonies of social wasps, as well as in other social insects (Matthews & Matthews 2010, Bagnères & Blomquist 2010). The compounds involved in this type of communication and identified as acting in intra- and interspecific recognition are the cuticular hydrocarbons (CHCs), which have received increased attention in recent decades (Monnin 2006, Bagnères & Blomquist 2010).
Cuticular hydrocarbons are constituents of the lipid layer that composes the insect cuticle and acts to prevent desiccation (Lockey 1988) and to form a barrier against microorganisms (Provost et al 2008). CHCs also act as contact pheromones, allowing identification of conspecifics; this attribute helps to maintain the colony hierarchy and identify the physiological status (Monnin 2006, Provost et al 2008), functioning as a specific chemical signature of the individual.
Several studies have demonstrated the importance of CHCs for the recognition of the role individuals have in the colony (Dapporto et al 2004), recognition of nestmates (Layton et al 1994, Tannure-Nascimento et al 2007, Antonialli-Junior et al 2007), reproductive status (Sledge et al 2001, Monnin 2006), and fertility (Izzo et al 2010). Such studies have highlighted the role of the cuticle chemical composition in establishing and maintaining the colony hierarchy (Dapporto et al 2005, Cotoneschi et al 2009).
According to Monnin (2006) there is a strong correlation between the reproductive status and the CHC profile in social insects, which is important for the establishment and recognition of reproductive dominance in colonies of species with independent foundation, as in many Polistinae. In these species, it was formerly believed that the queen maintains her reproductive status only by behaving aggressively towards other females. However, in recent decades, many studies have demonstrated the importance and role of CHCs in communication among members of the colony and in maintaining the status of the queen (Bonavita-Cougourdan et al 1991, Peeters et al 1999, Liebig et al 2000, Sledge et al 2001, Dapporto et al 2005).
Polistes ferreri (Saussure) is a Neotropical eusocial wasp found in Brazil, Argentina, Uruguay, and Bolivia (Richards 1978). Studies with this species have examined foraging activity (Andrade & Prezoto 2001, De Souza et al 2008), the role of males in the colony (Sinzato et al 2003), dominance relationships in the colony (Tannure & Nascimento 1999, De Souza et al 2010), the colony cycle aspects, strategy for colony founding, and the use of comb cells (Sinzato et al 2011). Few studies have analyzed the reproductive status and CHC profile, especially in Neotropical species of the social wasps.
Among the techniques used to measure the CHC profile in social insects, gas chromatography-mass spectrometry (GC-MS) is most often used (Dietermann et al 1992). GC-MS is reliable and provides a sensitive quantitative analysis; however, it requires several steps of sample preparation and a lengthy process to quantify each hydrocarbon present in the sample. Recently, Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS) has been used; this technique is able to identify different types of compounds to study the distinctions among castes, sexes, and species of ants (Antonialli-Junior et al 2007, 2008) and acquisition of the chemical profile in polistine parasitic wasps (Neves et al 2012, 2013).
This study applied a new analytical method to the chemical profiling of social wasps by adding morphological and physiological analyses to test the hypothesis that the ovary development leads to a difference in the chemical profile of P. ferreri females.
Material and Methods
Nine colonies of P. ferreri were collected in Mundo Novo, MS (23°56′23″S, 54°17′25″W) from April 2011 through March 2012, and 90 females were evaluated. Three colonies were in the pre-emergence stage, five in post-emergence (pre-male), and one in post-emergence (post-male), according to the classification proposed by Jeanne (1972).
After the colonies were collected, the gaster of each female was placed in an individual Eppendorf vial containing absolute ethyl alcohol (PA 99.8%) for checking the ovary development stage, insemination, and relative age. The rest of the body was preserved by freezing and later used for morphometric analysis and CHC profiling. Thus, the status of each female was determined by means of several combined parameters.
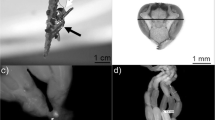
Ovaries were classified according to the stage of ovariole development stage based on Baio et al (2003a) in workers, females with filamentous or partially developed ovarioles; and queens, females with fully developed ovarioles. The insemination status was checked by staining the spermatheca in a 1:1 solution of acid fuchsin (1%) for observation of sperm cells under a light microscope.
Nine morphometric measurements were made on each female, modified from Shima et al (1994) and Noll et al (1997): head: width (HW), minimum interorbital distances (IDx); mesosome: width, length, and height of mesoscutum (MSW, MSL, and MSH, respectively); metasome: basal and apical heights of tergite 2 (T2BH and T2AH), length of tergite 2 (T2L); and wing: partial length of the forewing (WL).
The relative age was determined according to the pigmentation of the transverse apodeme, as follows: LY (light yellow), LB (light brown), DB (dark brown), and BA (black). According to Richards (1971) and West-Eberhard (1973), this color sequence indicates a progression in the age of individuals.
For cuticular chemical profile analysis, the thorax of each female was analyzed by FTIR-PAS after drying for 48 h in a vacuum oven to minimize the water content following Antonialli-Junior et al (2007, 2008) and Neves et al (2012). The FTIR-PAS technique measures the radiation absorbed by the sample. The radiation absorbed by the sample (thorax) in the mid-infrared spectral region, including wavelengths from 400–4000 cm−1, was used. Thus, it was possible to identify and distinguish molecular radicals and chemical bonds in the samples.
For statistical analyses, values corresponding to 17 peaks of absorbance in each absorption spectrum were used, which represent the compounds of the cuticle, mainly CHCs. These peaks were defined by Antonialli-Junior et al (2007) and are listed in Table 1 with their respective wave number, functional group, and vibrancy mode.
The discrimination among defined groups of females was based on the degree of ovarian development, morphometric data, and the cuticular chemical profile. These differences were evaluated by stepwise discriminant analyses and revealed the group of variables that best explains the evaluated groups in case of a difference, which is indicated by Wilks’ lambda, a measure of the difference, if any, between the groups (Quinn & Keough 2002). For all analyses, the variable was considered significant when the level reached was <0.05, and calculations were performed in Systat 11 software.
Results
After the evaluation of the ovary development (Fig 1) and the insemination status (Fig 2), the females were categorized into three groups: workers 1: females with filamentous ovarioles without visible development of oocytes (type A), which could be inseminated or not; workers 2: females containing partially developed ovarioles (type B), inseminated or not; and queens: females with fully developed ovarioles containing two or more mature oocytes (type C) and that were always inseminated.
Type A ovaries were found in 21.1% of females, type B in 68.9%, and type C in 10% (Table 2). In 77.8% of the colonies, only one inseminated female was present (Fig 2 and Table 2), except colonies 3 and 4, which had four and two inseminated females, respectively, with ovarian development type A or B. However, in each of these colonies, only one female was the queen, with type C ovary and inseminated (Fig 1c and Table 2). In all colonies analyzed, regardless of stage, most females had partially developed ovarioles (type B).
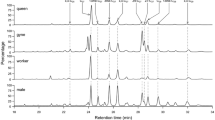
From the spectra analyzed by FTIR-PAS, a mean curve (Fig 3 and Table 1) was constructed for each group of females (workers 1, workers 2, and queens). The curves showed 11 peaks that were meaningful for separation of the groups, corresponding to the functional groups of compounds formed by CHCs and chitin, which were indicated by stepwise discriminant analysis (Wilks’ lambda = 0.045, F = 11.722, p < 0.001). The chemical profiles showed a clear separation among the three groups of females (Fig 4). The first canonical root explained 78% of the results and the second 22%.
In relation to morphometric parameters, stepwise discriminant analysis (Wilks’ lambda = 0.757, F = 4.086, p < 0.001) indicated no significant differences among these three groups of females. Although the p value was low, the Wilks’ lambda value was high; there was a high degree of overlap among the groups of females.
In the pre-emergence and post-emergence colony stages, older females, i.e., those with a darker apodeme, predominated (Fig 5). Younger females predominated in the colonies in the post-emergence (post-male) stage. The queens were always older females with a black or brown apodeme and, therefore, were always among the older females of the colony (Fig 5).
Discussion
We showed that P. ferreri colonies may contain morphologically similar females with three conditions of ovarian development, but that differ on their cuticular chemical profile.
The absence of significant morphological differences among females is a common feature of independent-foundation species (Jeanne 1986, Keeping 2000). However, differences in body size and/or physiological state can be correlated with the reproductive condition of a female (Fukuda et al 2003, Tannure-Nascimento et al 2005, Gobbi et al 2006, Murakami & Shima 2006). Yet, environmental changes can lead to the production of larger females that are able to survive harsher temperatures and start new colonies later, as observed in Polistes wasps in temperate climates (West-Eberhard 1969, Solis & Strassmann 1990, Dani 1994). The existence of little morphological differentiation among castes is also a common trait in some genera of Epiponini such as Protopolybia, Parachartergus, Pseudopolybia, Polybia, Angiopolybia, Chartergellus, and Brachygastra (Mateus et al 2004, Noll et al 2004).
In species with morphologically similar females, the castes are distinguished mainly at the behavioral level, where the dominant individuals specialize in certain tasks, while the subordinate individuals specialize in others (Jeanne 1986). Therefore, status is determined mostly during the adult stage, as indicated by Gelin et al (2008) in Apoica pallens (Lepeletier) and by O’Donnell (1998) in Polistes species. However, in some cases, the development of caste distinction, at least in part, may be pre-imaginal (Gadagkar et al 1991, Keeping 2002, Dapporto et al 2011, Hunt et al 2011).
In our study, most colonies (77.8%) had only one inseminated female. However, even in colonies with more than one inseminated female, only one had type C ovarian development, suggesting that colonies of this species contain only a single female laying eggs.
On the other hand, the other inseminated females probably would be able to replace the queen, a common feature in species of independent foundation (Murakami & Shima 2006). Thus, the difference between reproductive and nonreproductive females is flexible and complex, depending on physiological, behavioral, and ecological aspects (Murakami & Shima 2006). According to Murakami et al (2009), this strategy may minimize the effect of predation and parasitism, as observed in Mischocyttarus cassununga.
Analysis of the chemical cuticular profile indicated a variation among the three groups of females: workers 1, workers 2, and queens. This indicates that not only the physiological parameters but also the cuticle compounds are important for the recognition of the reproductive status of females. According to Monnin (2006), this relationship between reproductive status and the CHC profile is important for establishing a hierarchy in independent-foundation species. Sledge et al (2001) found differences among the CHC profile of alpha females, subordinates, and workers in colonies of Polistes dominula after the first workers in the colony emerged. These authors also reported that the removal of the alpha female leads to her replacement, and that the new female assuming this position acquires a CHC profile similar to that of the previous alpha female. Bonckaert et al (2012) analyzed colonies of Vespula vulgaris and observed that reproductive queens, spring-collected queens, virgin queens, and workers had different degrees of ovarian development, and that these corresponded to different profiles of cuticular hydrocarbons. Furthermore, females are able to recognize even subtle differences in chemical profiles (Bonavita-Cougourdan et al 1987, Espelie et al 1990, Dani et al 2001, Lorenzi et al 2004, Van Zweden & d’Ettorre 2010).
The occurrence of females with partially developed ovarioles, termed intermediate, was first reported by Richards & Richards (1951), revealing a degree of complexity in the hierarchy establishment, and thus, the role of the female in the colony. Some authors, such as Richards (1971), suggested that the role of the intermediates is to produce trophic eggs and males; however, West-Eberhard (1978) and Gastreich et al (1993) considered them as possible young, uninseminated queens. In any event, the degree of ovarian development coupled with the occurrence of insemination makes the position of the female in the hierarchy more evident.
According to our results (Fig 5), older females, including the queen, predominated in most colonies, as queens are among the older females of the colony (Baio et al 2003b, Murakami et al 2009, Felippotti et al 2010).
Therefore, in P. ferreri, although females do not differ morphologically, it is possible to distinguish three groups of females with significantly different cuticular chemical profiles. Most colonies had only one inseminated female; however, even in those with more than one inseminated female, only one had the degree of ovarian development typical of the queen, and these were among the older females of the colony.
References
Andrade FR, Prezoto F (2001) Horários de atividade forrageadora e material coletado por Polistes ferreri Saussure, 1853 (Hymenoptera, Vespidae), nas diferentes fases de seu ciclo biológico. Rev Bras Zool 3:117–128
Antonialli-junior WF, Lima SM, Andrade LHC, Súarez YR (2007) Comparative study of the cuticular hydrocarbon in queens, workers and males of Ectatomma vizzotoi (Hymenoptera: Formicidae) by Fourier transform-infrared photoacoustic spectroscopy. Genet Mol Res 6:492–499
Antonialli-Junior WF, Andrade LHC, Súarez YR, Lima SM (2008) Intra- and interspecific variation of cuticular hydrocarbon composition in two Ectatomma species (Hymenoptera: Formicidae) based on Fourier transform infrared photoacoustic spectroscopy. Genet Mol Res 7(2):559–566
Bagnères AG, Blomquist GJ (2010) Site of synthesis, mechanism of transport and selective deposition of hydrocarbons. In: Blomquist GJ, Bagnères AG (eds) Insect hydrocarbons: biology, biochemistry and chemical ecology. Cambridge University Press, Cambridge, pp 75–99, 506p
Baio MV, Noll FB, Zucchi R (2003a) Morphological caste differences, variation according to colony cycle, and non-sterility of workers in Brachygastra augusti (Hymenoptera, Vespidae, Epiponini), a Neotropical swarm-founding wasp. J N Y Entomol Soc 111(4):242–253
Baio MV, Noll FB, Zucchi R (2003b) Shape differences rather than size differences between castes in the Neotropical swarm-founding wasp Metapolybia docilis (Hymenoptera: Vespidae, Epiponini). BMC Evol Biol 3:1–9. doi:10.1186/1471-2148-3-10
Bonavita-Cougourdan A, Clément JL, Lange C (1987) Nestmate recognition: the role of cuticular hydrocarbons in the ant Camponotus vagus Scop. J Entomol Sci 22:1–10
Bonavita-Cougourdan A, Theraulaz G, Bagnères AG, Roux M, Pratte M, Provost E, Clément JL (1991) Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp Biochem Physiol 100:667–680. doi:10.1016/0305-0491(91)90272-F
Bonckaert W, Drijfhout FP, D’Ettorre P, Billen J, Wenseleers T (2012) Hydrocarbon signatures of egg maternity, caste membership and reproductive status in the common wasp. J Chem Ecol 38(1):42–51. doi:10.1007/s10886-011-0055-9
Carpenter JM (1982) The phylogenetic relationships and natural classification of the Vespoidea (Hymenoptera). Syst Entomol 7:11–38
Carpenter JM (1991) Phylogenetic relationships and the origin of social behavior in the Vespidae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 7–32
Cotoneschi C, Dani FR, Cervo R, Scala C, Strassmann JE, Queller DC, Turillazzi S (2009) Polistes dominulus (Hymenoptera, Vespidae) larvae show different cuticular patterns according to their sex: workers seem not use this chemical information. Chem Senses 34:195–202
Dani FR (1994) Caste size differences in Polistes gallicus (L) (Hymenoptera, Vespidae). Ethol Ecol Evol 3:67–73
Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S (2001) Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim Behav 62:165–171
Dapporto L, Theodora P, Spacchini C, Pieraccini G, Turillazzi S (2004) Rank and epicuticular hydrocarbons in different populations of the paper wasp Polistes dominulus (Christ) (Hymenoptera, Vespidae). Insect Soc 51:279–286. doi:10.1007/s00040-004-0738-0
Dapporto L, Sledge FM, Turillazzi S (2005) Dynamics of cuticular chemical profiles of Polistes dominulus workers in orphaned nests (Hymenoptera, Vespidae). J Insect Physiol 51(3):969–973. doi:10.1016/j.jinsphys.2005.04.011
Dapporto L, Petrocelli I, Turillazzi S (2011) Incipient morphological caste in Polistes gallicus (Vespidae, Hymenoptera). Zoomorphology 130:197–201. doi:10.1007/s00435-011-0130-3
De Souza AR, Rodrigues IL, Rocha JVA, Reis WAA, Lopes JFS, Prezoto F (2008) Foraging behavior and dominance hierarchy in colonies of the Neotropical social wasp Polistes ferreri Saussure, 1853 (Hymenoptera, Vespidae) in different stages of development. Sociobiology 52:293–303
De Souza AR, Rocha JVA, Rodrigues IL, Prezoto F (2010) Dominance interactions among females of the Neotropical eusocial wasp Polistes ferreri Saussure, 1853 (Hymenoptera: Vespidae). Sociobiology 55:547–555
Dietermann V, Peeters C, Liebig J, Thivet V, Hölldobler B (1992) Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc Natl Acad Sci U S A 100:10341–10346
Espelie KE, Wenzel JW, Chang G (1990) Surface lipids of social wasp Polistes metricus say and its nest and nest pedicel and their relation to nestmate recognition. J Chem Ecol 16(7):2229–2241
Evans HE, West-Eberhard MJ (1970) The wasps. University of Michigan Press, Ann Arbor, 265p
Felippotti GT, Mateus L, Mateus S, Noll, FB, Zucchi R (2010) Morphological caste differences in three species of the Neotropical genus Clypearia (Hymenoptera: Polistinae: Epiponini). Psyche: A J Entomol 1–8. doi:10.1155/2010/410280
Fukuda H, Kojima J, Jeanne RL (2003) Colony specific morphological caste differences in an Old World, swarm-founding polistine, Ropalidia romandi (Hymenoptera: Vespidae). Entomol Sci 6:37–47. doi:10.1046/j.1343-8786.2003.00002.x
Gadagkar R (1991) Belonogaster, Mischocyttarus, Parapolybia, and independent-founding Ropalidia. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 149–190
Gadagkar R (2001) Division of labour and organization of work in the primitively eusocial wasp Ropalidia marginata. Proc Indian Natl Sci Acad B 67:397–422
Gadagkar R, Joshi NV (1983) Quantitative ethology of social wasps: time-activity budgets and caste differentiation in Ropalidia marginata (Lep.) (Hymenoptera: Vespidae). Anim Behav 31:26–31
Gadagkar R, Bhagavan S, Chandrashekara K, Vinutha C (1991) The role of larval nutrition in pre-imaginal biasing of caste in the primitively eusocial wasp Ropalidia maginata (Hymenoptera: Vespidae). Ecol Entomol 16:435–440. doi:10.1111/j.1365-2311.1991.tb00236.x
Gastreich KR, Strassmann JE, Queller DC (1993) Determinants of high genetic relatedness in the swarm-founding wasp, Protopolybia exigua. Ethol Ecol Evol 5:529–539
Gelin LFF, Cruz JD, Noll FB, Giannotti E, Santos GMM, Bichara-Filho CC (2008) Morphological caste studies in the Neotropical warm-founding Polistinae wasp Angiopolybia pallens (Lepeletier) (Hymenoptera: Vespidae). Neotrop Entomol 37(6):691–701
Giannotti E, Machado VLL (1999) Behavioral castes in the primitively eussocial wasp Polistes lanio Fabricius (Hymenoptera: Vespidae). Rev Bras Entomol 43:185–190
Gobbi N, Noll FB, Penna MAH (2006) “Winter” aggregations, colony cycle, and seasonal phenotypic change in the paper wasp Polistes versicolor in subtropical Brazil. Naturwissenschaften 93(10):487–494
Hunt JH (1991) Nourishment and the evolution of the Social Vespidae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 426–450
Hunt JH, Mutti NS, Havukainen H, Henshaw MT, Amdam GV (2011) Development of an RNA interference tool, characterization of its target, and an ecological test of caste differentiation in the eusocial wasp Polistes. PLoS One 6(11):e26641. doi:10.1371/journal.pone.0026641
Izzo A, Wells M, Huang Z, Tibbetts E (2010) Cuticular hydrocarbons correlate with fertility, not dominance, in a paper wasp, Polistes dominulus. Behav Ecol Sociobiol 64:857–874. doi:10.1007/s00265-010-0902-7
Jeanne RL (1972) Social biology of the neotropical wasp Mischocyttarus drewseni. Bull Mus Comp Zool 3:63–150
Jeanne RL (1980) Evolution of social behavior in Vespidae. Annu Rev Entomol 25:371–395
Jeanne RL (1986) The evolution of the organization of work in social insects. Monit Zool Ital 20:119–133
Jeanne RL (2003) Social complexity in the Hymenoptera, with special attention to the wasps. In: Kikuchi T, Azuma N, Higashi S (eds) Genes, behaviors and evolution of social insects. Hokkaido University Press, Sapporo
Jeanne RL, Graf CA, Yandell BS (1995) Non-size-based morphological castes in a social insect. Naturwissenschaften 82:296–298
Keeping MG (2000) Morpho-physiological variability and differentiation of reproductive roles among foundresses of the primitively eusocial wasp, Belonogaster petiolata (Deeger) (Hymenoptera, Vespidae). Ins Soc 47:147–154
Keeping MG (2002) Reproductive and worker castes in the primitively eusocial wasp, Belonogaster petiolata (Degeer) (Hymenoptera: Vespidae) evidence for pre-imaginal differentiation. J Insect Physiol 48:867–879
Layton JM, Camann MA, Espelie KE (1994) Cuticular lipids profiles of queens, workers and males of social wasp Polistes metricus say are colony-specific. J Chem Ecol 20(9):2307–2321. doi:10.1007/BF02033205
Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B (2000) Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathossaltator? Proc Natl Acad Sci U S A 97(8):4124–4131. doi:10.1073/pnas.97.8.4124
Lockey KH (1988) Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol 89:595–645. doi:10.1016/0305-0491(88)90305-7
Lorenzi MC, Sledge MF, Laiolo P, Sturlini E, Turillazzi S (2004) Cuticular hydrocarbon dynamics in young adult Polistes dominulus (Hymenoptera: Vespidae) ant the role of linear hydrocarbons in nestmate recognition systems. J Insect Physiol 50:935–941
Markiewicz DA, O’Donnell S (2001) Social dominance, task performance and nutrition: implications for reproduction in eusocial wasps. J Comp Physiol A Sens Neural Behav Physiol 187:327–333
Mateus S, Noll FB, Zucchi R (2004) Caste flexibility and variation according to the colony cycle in the swarm-founding wasp, Parachartergus fraternus (Gribodo) (Hymenoptera: Vespidae, Polistinae). J Kansas Entomol Soc 77(4):470–483
Matthews RW, Matthews JR (2010) Insect behavior. Springer, New York, 519p
Mitra A, Gadagkar R (2012) Road to royalty—transition of potential queen to queen in the primitively eusocial wasp Ropalidia marginata. Ethology 118:694–702. doi:10.1111/j.1439-0310.2012.02059.x
Mitra A, Sahaa P, Elihu C, Bhadraa A, Gadagkar R (2011) Communication in Ropalidiamarginata: Dufour’s gland contains queen signal that is perceived across colonies and does not contain colony signal. J Insect Physiol 57:280–284. doi:10.1016/j.jinsphys.2010.11.014
Monnin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fenn 43:515–530
Murakami ASN, Shima SN (2006) Nutritional and social hierarchy establishment of the primitively eusocial wasp Mischocyttarus cassununga (Hymenoptera, Vespidae, Mischocyttarini) and related aspects. Sociobiology 48(1):183–207
Murakami ASN, Shima SN, Desuó IC (2009) More than one inseminated female in colonies of the independent-founding wasp Mischocyttarus cassununga von Ihering (Hymenoptera, Vespidae). Rev Bras Entomol 53(4):653–662. doi:10.1590/S0085-56262009000400017
Murakami ASN, Desuó IC, Shima SN (2013) Division of labor in stable social hierarchy of the independent-founding wasp Mischocyttarus (Monocyttarus) cassununga, Von Ihering (Hymenoptera, Vespidae). Sociobiology 60(1): 114–122. doi: 10.13102/sociobiology.v60i1.114-122
Neves EF, Andrade LHC, Súarez YR, Lima SM, Antonialli-Junior WF (2012) Age-related changes in the surface pheromones of the wasp Mischocyttarus consimilis (Hymenoptera: Vespidae). Genet Mol Res 11(3):1891–1898. doi:10.4238/2012.July.19.8
Neves EF, Montagna TS, Andrade LHC, Súarez YR, Lima SM, Antonialli-Junior WF (2013) Social parasitism and dynamics of cuticular hydrocarbons in paper wasps of the genus Mischocyttarus. J Kansas Entomol Soc 86(1):69–77. doi:10.2317/JKES1207610.1
Noda SCM, Rodrigues ER, Giannotti E (2001) Dominance hierarchy in different stages of development in colonies of the primitively eusocial wasp Mischocyytarus cerberus styx (Hymenoptera, Vespidae). Sociobiology 38(3):603–614
Noll FB, Simões D, Zucchi R (1997) Morphological caste differences in the neotropical swarm-founding wasps: Agelaia m. multipicta and A. p. pallipes (Hymenoptera, Vespidae). Ethol Ecol Evol 4(9):361–372
Noll FB, Wenzel JW, Zucchi R (2004) Evolution of caste in neotropical swarm-founding wasps (Hymenoptera, Vespidae, Epiponini). Am Mus Novit 3467:1–24
O’Donnel S (1998) Reproductive caste determination in eusocial wasps (Hymenoptera, Vespidae). Annu Rev Entomol 43:323–346
Pardi L, Piccioli MTM (1981) Studies on the biology of Belonogaster (Hymenoptera:Vespidae). 4. In caste differences on Belonogaster griseus (Fab.) and the position of this genus among social wasps. Monit Zool Ital 16(9):131–146
Peeters C, Monnin T, Malosse C (1999) Cuticular hydrocarbons correlated with reproductive status in a queen less ant. Proc R Soc B 266:1323–1327. doi:10.1098/rspb.1999.0782
Premnath S, Sinha A, Gadagkar R (1996) Dominance relationship in the establishment of reproductive division of labour in a primitively eusocial wasp (Ropalidiamarginata). Behav Ecol Sociobiol 39:125–132
Provost E, Blight O, Tirard A, Renucci M (2008) Hydrocarbons and insects’ social physiology. In: Maes RP (ed) Insect physiology, new research. Nova, New York, pp 19–72
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, 530p
Richards OW (1971) The biology of the social wasps (Hymenoptera, Vespidae). Biol Rev 46:483–528
Richards OW (1978) The social wasps of the Americas, excluding the Vespinae. London, British Museon (Natural History). vii+580p
Richards OW, Richards MJ (1951) Observations on the social wasps of South America (Hymenoptera, Vespidae). Trans R Entomol Soc Lond 102:1–170
Shima SN, Yamane S, Zucchi R (1994) Morphological caste differences in some Neotropical swarm-founding Polistinae wasps I. Apoica flavissima (Hymenoptera, Vespidae). Jpn J Entomol 64(10):811–822
Sinha A, Premnath R, Chandrashekara K, Gadagkar R (1993) Ropalidia rufoplagiata: a polistine wasp society probably lacking permanent reproductive division of labour. Ins Soc 40:69–86
Sinzato DMS, Prezoto F, Del-Claro K (2003) The role of males in a neotropical paper wasp, Polistes ferreri Saussure, 1853 (Hymenoptera, Vespidae, Polistinae). Rev Bras Zool 5(1):89–100
Sinzato DMS, Andrade FR, De Souza AR, Del-Claro K, Prezoto F (2011) Colony cycle, foundation strategy and nesting biology of a Neotropical paper wasp. Rev Chil Hist Nat 84:357–363
Sledge MF, Boscaro F, Turillazzi S (2001) Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav Ecol Sociobiol 49:401–409. doi:10.1007/s002650000311
Solis CR, Strassmann JE (1990) Presence of brood affects caste differentiation in the social wasp, Polistes exclamansVierek (Hymenoptera: Vespidae). Funct Ecol 4:531–541
Spradbery JP (1973) Wasps: an account of the biology and natural history of solitary and social wasps. University of Washington Press, Seattle, 408p
Spradbery JP (1991) Evolution of queen number and queen control. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 191–231
Strassmann JE, Lee RE Jr, Rojas RR, Baust JG (1984) Caste and sex diferences in cold-hardiness in the social wasps, Polistes annularis and P. exclamans (Hymenoptera: Vespidae). Insects Soc 31(3):291–201
Tannure IC, Nascimento FS (1999) Influência do conflito de dominância entre fundadoras em colônias de vespas sociais pertencentes ao gênero Polistes (HYMENOPTERA: VESPIDAE). Rev Bras Zooc 1(1):31–40
Tannure-Nascimento IC, Nascimento FS, Zucchi R (2005) Size and colony cycle in Polistes satan, a Neotropical paper wasp (Hymenoptera, Vespidae). Ethol Ecol Evol 17:105–119
Tannure-Nascimento IC, Nascimento FS, Turatti IC, Lopes NP, Trigo JR, Zucchi R (2007) Colony membership is reflected by variations in cuticular hydrocarbon profile in a Neotropical paper wasp, Polistes satan (Hymenoptera, Vespidae). Genet Mol Res 6(2):390–396
Tannure-Nascimento IC, Nascimento FS, Zucchi R (2008) The look of royalty: visual and odour signals of reproductive status in a paper wasp. Proc R Soc B 275:2555–2561
Torres VO, Antonialli-Junior WF, Giannotti E (2009) Divisão de trabalho em colônias da vespa social neotropical Polistes canadensis canadensis Linnaeus (Hymenoptera, Vespidae). Rev Bras Entomol 53(4):593–599
Torres VO, Montagna TS, Raizer J, Antonialli-Junior WF (2012) Division of labor in colonies of the eusocial wasp, Mischocyttarus consimilis. J Insect Sci 12:21. doi:10.1673/031.012.2101
Turillazzi S (1991) The Stenogastrinae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 74–98
Van Zweden JS, d’Ettorre P (2010) Nestmate recognition in social insects and the role of hydrocarbons. In: Blomquist GJ, Bagnères AG (eds). Cambridge University Press, Cambridge p. 222–243, 506p
West-Eberhard MJ (1969) The social biology of polistine wasps. Misc Publ Mus Zool Univ Mich 140:1–110
West-Eberhard MJ (1973) Monogyny in “polygynous” social wasps. Proc. 7th Cong. I.U.S.S.I. London, p. 396–403
West-Eberhard MJ (1978) Temporary queens in Metapolybia wasps: non-reproductive helpers without altruism? Science 200:441–443
Wilson EO (1971) The insect societies. Belknap Press, Cambridge, 548p
Acknowledgments
The authors thank Orlando T. Silveira (Museu Paraense Emílio Goeldi) for the identification of the species and Janet W. Reid (JWR Associates) for the revision of the English text. We are grateful to CAPES for a doctoral fellowship awarded to the second author. WFAJ acknowledges his research grants from CNPq and Fundect for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando B Noll – UNESP
Rights and permissions
About this article
Cite this article
Soares, E.R.P., Torres, V.O. & Antonialli-Junior, W.F. Reproductive Status of Females in the Eusocial Wasp Polistes ferreri Saussure (Hymenoptera: Vespidae). Neotrop Entomol 43, 500–508 (2014). https://doi.org/10.1007/s13744-014-0242-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-014-0242-9