Abstract
Chemical characterization of damage-released alarm cues in ostariophysan fishes has lagged far behind the study of the ecological role that these cues play in behavioral decision-making of prey fishes. Chondroitin sulfate has been identified as a putative component of alarm cue based on two laboratory studies of zebrafish, Danio rerio, and the northern studfish, Fundulus catenatus. The fathead minnow, Pimephales promelas, is a model organism in the study of chemically mediated predator–prey interactions, in part because they can be studied in the laboratory and under field conditions. Here, we conducted a field experiment on wild populations of fathead minnows, to test for area avoidance of chondroitin sulfate relative to conspecific skin extract (containing alarm cues = positive control) or water (negative control). We repeated the experiment in two small lakes in central Minnesota using minnow traps containing blocks of sponge with one of the three test cues. We found that fathead minnows avoided traps chemically labeled with conspecific alarm cue more than control traps labeled with water, and that the number of minnows caught in traps labeled with chondroitin sulfate was intermediate between alarm cue traps and water traps. These data are consistent with laboratory findings that chondroitin sulfate is a component of alarm cue, but that other species-specific compounds are needed for a full behavioral response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a major agent of natural selection, which has selected for a wide range of antipredator strategies (Lima and Dill 1990). Those best able to detect and respond to risk of predation have relatively high fitness. Trade-offs between predator avoidance and other behavioral activities such as habitat selection, food acquisition, and reproduction indirectly drive the evolution of many behaviors.
Maximum benefits to prey occur when risk of predation is detected at an early stage in the predation sequence before predators have detected prey, or initiated an attack (Weldon 1983; Lima and Dill 1990; Smith 1992). In aquatic habitats, chemicals released as passive by-products of predation reliably indicate risk of predation to nearby prey. These effects are well studied and have generated a large and active literature (see Chivers and Smith 1998; Ferrari et al. 2010; Smith 1992; Wisenden 2015a for reviews). Much of this work has used fathead minnows as test subjects because they adapt well to laboratory aquaria and they are relatively accessible to study in the field. Kairomones are chemicals released by predators that prey use to detect the presence of predation risk. A pulse release of urinary ammonia by startled or disturbed prey that alerts others in the area of a threat is referred to as a disturbance cue. When epidermal tissue is harmed during an attack, damage-released alarm cues permeate the surrounding water and alert conspecifics and ecologically similar species of an actively foraging predator. Post-ingestion “dietary cues” are released from the digestive tract of predators. The release of these chemical creates opportunities to associate novel stimuli with predation risk (Brown 2003; Kelley and Magurran 2003), which greatly increases the ability of prey to detect the presence of predation risk.
Despite intense research activity, little progress has been done on the chemical characterization of these compounds, specifically damage-released alarm cues, although it has not been for lack of trying. The full history of attempts to elucidate the chemistry of alarm cues in fish from 1941 to 2004 is succinctly summarized in a review by Døving et al. (2005). A leading candidate molecule emerged in the 1970s as the likely active ingredient in minnow alarm cue: hypoxanthine-(3N)-oxide (Argentini 1976). Hypoxanthine-(3N)-oxide is effective in evoking an alarm response from black tetras Gymnocorymbus ternetzi (Pfeiffer et al. 1985), zebrafish (Parra et al. 2009) and fathead minnows (Brown et al. 2000, 2001). However, other studies have found weak (Mathuru et al. 2012) or no response (Tuvikene and Freiberg, unpublished data, cited in Døving et al. 2005) to hypoxanthine-(3N)-oxide. Other work on the chemistry of alarm cues in fish point to a role for protein or polypeptides as either the active agent or as a carrier molecule (Kasumyan and Lebedeva 1979; Kasumyan and Ponomarev 1987; Wisenden et al. 2009).
Recent research revealed that purified chondroitin sulfate, a glycosaminoglycan (GAG) found in mucus, triggers antipredator behavior in zebrafish Danio rerio (Mathuru et al. 2012). This finding has been corroborated independently on northern studfish Fundulus catenatus (Farnsley et al. 2016). Mathuru et al. (2012) reported antipredator behaviors of reduced activity, movement to the bottom and dashing (rapid erratic swimming), which are all well-known components of alarm behavior (Ferrari et al. 2010). However, the behavioral response to chondroitin sulfate reported by Mathuru et al. (2012) was not as intense as the behavioral response to crude skin extract, suggesting that chondroitin sulfate was a component of alarm cue, but that other compounds present in skin, perhaps ones that denote species specificity, are necessary to evoke a full alarm response. Northern studfish reduced activity and spent more time near the bottom of the tank in response to chondroitin sulfate relative to a water control, but this study did not include a positive control treatment of crude skin extract to allow a comparison against full potency alarm cue (Farnsley et al. 2016).
Experimentation with the role of chondroitin sulfate as a putative chemical component of alarm cue has, thus, far been restricted to laboratory conditions (Farnsley et al. 2016; Mathuru et al. 2012) and not been tested on the fathead minnow, which is arguably the model organism for chemically mediated predator–prey interactions in fishes. Here, we report the result of two field experiments that recorded area avoidance by wild populations of fathead minnows in response to chondroitin sulfate relative to conspecific skin extract (containing alarm cues = positive control) or water (=negative control). The experiment was conducted in two small lakes in central Minnesota using minnow traps containing blocks of sponge soaked with one of the three test cues.
Methods and materials
Field sites
We conducted two independent tests of the aversive effects of chondroitin sulfate in June 2017; one in Deming Lake (47°09′59.78″ N, 95°10′28.90″ W, elev. 485.5 m), and the other in nearby Budd Lake (47°10′13.14″ N, 95°10′07.12″ W, elev. 476.1 m), both located in Itasca State Park, Minnesota, USA, and the site of the Itasca Biological Field Station of the University of Minnesota. These lakes have been the focus of previous field study of alarm cues in littoral fishes (Wisenden and Barbour 2005; Wisenden 2008; Wisenden et al. 2009). Both lakes are meromictic, which creates low dissolved oxygen levels during periods of ice and snow cover (December to April) that is fatal to large piscivores. Consequently, these lakes support dense populations of small fishes amenable to field experiments. Deming Lake is approximately 5 ha in area and during the time of this study contained fathead minnows (Pimephales promelas), northern redbelly dace (Chrosomus eos), blacknose shiners (Notropis heterolepis), golden shiners (Notemigonus crysoleucas), pumpkinseed sunfish (Lepomis gibbosus), brook stickleback (Culaea inconstans), central mudminnows (Umbra limi), black bullhead catfish (Amieurus melas) and Iowa darters (Etheostoma exile). Budd Lake is approximately 3 ha in area and contains only two fish species: fathead minnows and northern redbelly dace.
Stimulus preparation
Minnow alarm cue for the Deming Lake experiment was prepared from locally collected fathead minnows purchased at a bait shop. Minnows were humanely killed by severing the spinal cord with a razor blade. We carefully removed sheets of skin (=dermal + epidermal tissue) from each flank of seven fathead minnows (mean ± 1SE total length = 6.26 ± 0.14 cm), and placed the skin in deionized water maintained near 0 °C to minimize biochemical degradation. The total area of skin harvested was 41.49 cm2. We homogenized the skin with a hand blender for 60 s. The resulting solution was filtered through a loose wad of cheesecloth to remove large sheets of connective tissue. The filtrate was diluted to a final volume of 240 ml with deionized water and aliquoted into 20 ml doses infused into 12 blocks of cellulose sponge of dimensions 38 × 36 × 42 mm. Thus, each sponge contained the equivalent of 3.46 cm2 of minnow skin. The test solution for chondroitin sulfate (Sigma-Aldrich CAS 39455-18-0, chondroitin sulfate derived from bovine trachea) was prepared by dissolving 4.0 g of chondroitin sulfate into 240 ml of deionized water and aliquoting this solution into 12, 20 ml doses infused into 12 sponge blocks (0.33 g per sponge). Deionized water was infused into a third set of 12 sponges to be used as the negative control. Alarm cue for the Budd Lake experiment was prepared using fathead minnows collected by seine net from Deming Lake. A total of 49.28 cm2 of skin was collected from 12 fish (mean ± 1SE total length = 5.71 ± 0.16 cm) as described above and infused into identical sponge blocks (4.1 cm2 skin per sponge). Chondroitin sulfate and water sponges used for the Budd Lake experiment were prepared with the same protocols as described for the Deming Lake experiment. Sponges were frozen at −20 °C until needed.
Field protocol
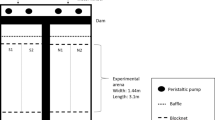
Thirty-six Gee® wire mesh minnow traps (23 cm in diameter, 44.5 cm long) were used for each experiment. Sponges were transported to the field on ice so that they remained frozen until placed in the water. We set traps in triplicate (one trap for each sponge treatment type) approximately 10 m apart along the shore of each lake, approximately 1 m from shore and at a depth of about 0.5–1.0 m. Sponges were affixed to the inside of each sponge using a small piece of stainless steel wire and positioned mid-way between the funnel entrances at each end of the trap. We waited 8 min before setting the next trap triplicate. We set 12 triplicates (representing one of each of the three sponge treatments) for a total of 36 traps per lake. We began sampling trap triplicates when the first triplicate had been in the water for 2 h, and counted and identified fish caught in each trap within 8 min so that the subsequent trap triplicate was sampled when it too had been in the lake for exactly 2 h, thus ensuring that all traps were available to catch fish for exactly 2 h. All fishes were returned to the lake as they were processed. Field data were collected from Deming Lake on June 2, 2017, and from Budd Lake on June 20, 2017.
Statistical method
Data were not normally distributed (Kilmogorov-Smirnov test, P < 0.05); therefore, Kruskal–Wallis ANOVAs were conducted to compare the number of fish caught per trap among treatment groups. One outlier datum was identified as being 3.8 standard deviations above the mean and excluded from further analysis.
Results
We caught 2775 fish on June 2 in Deming Lake and 2392 fish in Budd Lake on June 20 (Table 1). Free-living fathead minnows in their natural habitat avoided traps that were chemically labeled with conspecific alarm cues relative to control traps labeled with water (KW ANOVA: Χ 2 = 6.268, df = 2, P = 0.044; Fig. 1). The number of fathead minnows caught in traps labeled with chondroitin sulfate was intermediate between that of water and alarm cue-labeled traps, indicating partial, but not full avoidance of chondroitin sulfate. Post hoc pairwise tests (Siegel and Castellan 1988) indicated Water and Chondroitin Sulfate > Chondroitin Sulfate and Alarm Cue (P < 0.05). There was no effect of lake (Mann–Whitney U test: U = 611, P = 0.824).
The number of redbelly dace caught per trap was not affected by sponge treatment (KW ANOVA: Χ 2 = 2.566, df = 2, P = 0.277; Fig. 2) nor was there any effect of lake (Mann–Whitney U test: U = 614, P = 0.699).
Traps set in Deming Lake caught several other fish species but none of them responded to sponge treatments: (pumpkinseed sunfish KW ANOVA: Χ 2 = 0.353, df = 2, P = 0.830, Fig. 3; blacknose shiners KW ANOVA: Χ 2 = 0.761, df = 2, P = 0.684, Fig. 4). Catch rates for brook stickleback were too low to be useful for testing the effect of sponge treatment (Table 1).
Discussion
Avoidance of chondroitin sulfate by free-living fathead minnows was intermediate between avoidance of conspecific alarm cue (positive control) and water (negative control). This is the first report of behavioral responses by fathead minnows to chondroitin sulfate, and the first test to be conducted under field conditions. Although behavioral responses are more difficult to demonstrate under field conditions because so many variables are not controlled, field data are valuable because they occur in the ecological context in which these behaviors evolved (Mathis and Smith 1992; Wisenden et al. 2004). Our findings are consistent with the findings of Mathuru et al. (2012) who found partial antipredator behavioral response to chondroitin relative to raw skin extract. Chondroitin sulfate has biological activity but it is not the sole component of alarm cue in zebrafish (a member of the minnow family) or fathead minnows. Alarm cue derived from damaged epithelial tissue is likely a mixture of two or more compounds that confer predator activity and information about species specificity of the prey (Wisenden 2015a), and in some cases size or life-stage specificity (Mirza and Chivers 2002). Perhaps other components of alarm cue are compounds such as hypoxanthine-(3N)-oxide or a similar molecule with a N–O side group (Brown et al. 2000, 2001) and/or a variety of polypeptides that could be a rich source of chemical information about the individual fish releasing the cue (Decho et al. 1998; Ferrari et al. 2010; Wisenden et al. 2009).
We found one outlier datum in which a very high number of fish entered a trap chemically labeled with alarm cue. Previous research suggests that this probably resulted from a synergy between a chemical cue about predation risk and a social cue created by fish already in the trap (Wisenden et al. 2003). Once a trap begins to catch fish, the response to the chemical alarm cue switches from area avoidance to increased shoal cohesion with fish inside the trap, even though the trap is the source of the alarm cue (Wisenden et al. 2003). Limiting the duration of the experiment to 2 h usually precludes this from occurring, but the existence of this phenomenon is a good example of why it is important to test these effects in the field.
There were no detectable responses by other fish species to fathead minnow alarm cue or to chondroitin sulfate. This was unexpected because cross-species reactions by redbelly dace to alarm cues of fathead minnows are ecologically relevant (Chivers et al. 2002) and have been observed in previous studies at these study sites by redbelly dace and by brook stickleback (Wisenden et al. 2003; Wisenden 2008). However, it is apparent that catch rates of cyprinid species (redbelly dace, blacknose dace) trended in the rank order of alarm cue < chondroitin sulfate < water. We have never observed pumpkinseed sunfish to respond to chemical alarm cues of cyprinids in previous studies (Wisenden 2008). The study on northern studfish indicates that chondroitin sulfate is biologically active for fishes outside of the Cyprinidae or Ostariophysi (Farnsley et al. 2016).
The emergence of chondroitin sulfate as a likely component of alarm cues reprises interest in the role that epidermal club cells may play as a contributor to alarm cues. These cells are a defining characteristic of fishes in the speciose superorder Ostariophysi (Nelson 2006), most of which are obligate schooling species, but also in non-ostariophysan groups such as the speciose percidae (Smith 1992). However, for these cells to be maintained by natural selection for the function of producing alarm cue, there must be one or more mechanisms by which they can contribute to the inclusive fitness of those that invest in them (Weldon 1983; Wisenden 2015b). Leading hypotheses include the presence of kin among the receivers (evidence is not compelling but it is possible, Russell et al. 2004), nearby predators to attempt to pirate food from the primary predator allowing the prey that released the alarm cue to escape (it is unclear how often these circumstances occur in nature, Chivers et al. 1996), or that these cells simultaneously serve another function that provides a direct fitness benefit, such as an immune response (Chivers et al. 2007; Smith 1992). Because chondroitin sulfate is now implicated as a component of alarm cue (Farnsley et al. 2016; Mathuru et al. 2012; this study), and confirmed to be present in epidermal club cells and serve a role in the immune system (Ralphs and Benjamin 1992), there may now be renewed justification for studying the interactions between immunocompetence and the evolution of alarm cues and alarm signals (Smith 1986; Wisenden 2015b).
This is the first field test of chondroitin sulfate as a putative component of alarm cues in fishes. The partial support for a role of chondroitin sulfate reported here opens possibilities for future research testing chondroitin sulfate in combination with other test compounds such as hypoxanthine-(3N)-oxide, biochemical components of fractionated skin extract (e.g., Kasumyan and Lebedeva 1979; Kasumyan and Ponomarev 1987; Lebedeva et al. 1975; Mathuru et al. 2012), and for application to the management of nuisance species in the field (Sorensen and Johnson 2016).
References
Argentini M (1976) Isolerung des Schreckstoffes aus der Haut der Elritze Phoxinus phoxinus L. Dissertation, Universität Zürich
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234. doi:10.1046/j.1467-2979.2003.00132.x
Brown GE, Adrian JC, Smyth E, Leet H, Brennan S (2000) Ostariophysan alarm pheromones: laboratory and field tests of the functional significance of nitrogen oxides. J Chem Ecol 26:139–154. doi:10.1023/A:1005445629144
Brown GE, Adrian JC, Shih ML (2001) Behavioural responses of fathead minnows to hypoxanthine-3-N-oxide at varying concentrations. J Fish Biol 58:1465–1470. doi:10.1111/j.1095-8649.2001.tb02301.x
Chivers D, Smith R (1998) Chemical alarm signaling in aquatic predator-prey systems: a review and prospectus. Écoscience 5:338–352. doi:10.1080/11956860.1998.11682471
Chivers DP, Brown GE, Smith RJF (1996) The evolution of chemical alarm signals: attracting predators benefits alarm signal senders. Am Nat 148:649–659. doi:10.1086/285945
Chivers DP, Mirza RS, Johnston JG (2002) Learned recognition of heterospecific alarm cues enhances survival during encounters with predators. Behaviour 139:929–938. doi:10.1163/156853902320387909
Chivers DP, Wisenden BD, Hindman CJ, Michalak TA, Kusch RC, Kaminskyj SGW, Jack KL, Ferrari MCO, Pollock RJ, Halbgewachs CF, Pollock MS, Alemadi S, James CT, Savaloja RK, Goater CP, Corwin A, Mirza RS, Kiesecker JM, Brown GE, Adrian JCJr, Krone PH, Blaustein AR, Mathis A (2007) Epidermal “alarm substance” cells of fishes are maintained by non-alarm functions: possible defence against pathogens, parasites and UVB radiation. Proc Roy Soc Lond B 274:2611–2620. doi:10.1098/rspb.2007.0709
Decho AW, Browne KA, Zimmer-Faust RK (1998) Chemical cues: why basic peptides are signal molecules in marine environments. Limnol Oceanogr 43:1410–1417. doi:10.4319/lo.1998.43.7.1410
Døving KB, Hamdani EH, Hoglund E, Kasumyan AO, Tuvikene A (2005) Review of the chemical and physiological basis of alarm reactions in cyprinids. In: Reutter K, Kapoor BG (eds) Fish chemosenses. Science Publishers, Enfield NH, pp 133–164
Farnsley S, Kuhajda B, George A, Klug H (2016) Fundulus catenatus (northern studfish) response to the potential alarm cue chondroitin sulfate. Southeast Nat 15:523–533. doi:10.1656/058.015.0315
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724. doi:10.1139/Z10-029
Kasumyan AO, Lebedeva NY (1979) New data on the nature of the alarm pheromone in cyprinids. J Ichthyol 19:109–114
Kasumyan AO, Ponomarev VY (1987) Biochemical features of alarm pheromone in fish of the order cypriniformes. J Evol Biochem Physiol - Eng Tr 23:20–23
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish Fish 4:216–226. doi:10.1046/j.1467-2979.2003.00126.x
Lebedeva NY, Malyukina GA, Kasumyan AO (1975) The natural repellent in the skin of cyprinids. J Ichthyol 15:472–480
Lima S, Dill L (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. doi:10.1139/z90-092
Mathis A, Smith RJF (1992) Avoidance of areas marked with a chemical alarm substance by fathead minnows (Pimephales promelas) in a natural habitat. Can J Zool 70:1473–1476. doi:10.1139/z92-203
Mathuru AS, Kibat C, Cheong WF, Shui G, Wenk MR, Friedrich RW, Jesuthasan S (2012) Chondroitin fragments are odorants that trigger fear behavior in fish. Curr Biol 22:538–544. doi:10.1016/j.cub.2012.01.061
Mirza RS, Chivers DP (2002) Brook charr (Salvelinus fontinalis) can differentiate chemical alarm cues produced by different size classes of conspecifics. J Chem Ecol 28:555–564. doi:10.1023/A:1014544112763
Nelson JS (2006) Fishes of the world. Wiley, New Jersey
Parra KV, Adrian JCJr, Gerlai R (2009) The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio). Behav Brain Res 205:336–341. doi:10.1016/j.bbr.2009.06.037
Pfeiffer W, Riegelbauer G, Meier G, Scheibler B (1985) Effect of hypoxanthine-3(N)-oxide and hypoxanthine-1(N)-oxide on central nervous excitation of the black tetra Gymnocorymbus ternetzi (Characidae, Ostariophysi, Pisces) indicated by dorsal light response. J Chem Ecol 11:507–523. doi:10.1007/BF00989562
Ralphs JR, Benjamin M (1992) Chondroitin and keratan sulphate in the epidermal club cells of teleosts. J Fish Biol 40:473–475. doi:10.1111/j.1095-8649.1992.tb02594.x
Russell ST, Kelley JL, Graves JA, Magurran AE (2004) Kin structure and shoal composition dynamics in the guppy, Poecilia reticulata. Oikos 106:520–526. doi:10.1111/j.0030-1299.2004.12847.x
Siegel S, Castellan NJJr (1988) Nonparametric statistics for the behavioral sciences. McGraw-Hill, New York
Smith RJF (1986) Evolution of alarm signals: role of benefits of retaining group members or territorial neighbors. Am Nat 128:604–610
Smith RJF (1992) Alarm signals in fishes. Rev Fish Biol Fish 2:33–63. doi:10.1007/BF00042916
Sorensen PW, Johnson NS (2016) Theory and application of semiochemicals in nuisance fish control. J Chem Ecol 42:698–715. doi:10.1007/s10886-016-0729-4
Weldon PJ (1983) The evolution of alarm pheromones. In: Muller-Schwarze D, Silverstein RM (eds) Chemical Signals in Vertebrates 3. Plenum, New York, pp 309–312. doi:10.1007/978-1-4757-9652-0_20
Wisenden BD (2008) Active space of chemical alarm cue in natural fish populations. Behaviour 145:391–407. doi:10.1163/156853908783402920
Wisenden BD (2015a) Chemical cues that indicate risk of predation. In: Sorensen PW, Wisenden BD (eds) Fish pheromones and related cues. Wiley-Blackwell Press, Ames IA, pp 131–148
Wisenden BD (2015b) The cue-signal continuum: an evolutionary trajectory for semiochemicals in fishes. In: Sorensen PW, Wisenden BD (eds) Fish pheromones and related cues. Wiley-Blackwell Press, Ames IA, pp 149–158
Wisenden BD, Barbour K (2005) Antipredator responses to skin extract of redbelly dace, Phoxinus eos, by free-ranging populations of redbelly dace and fathead minnows, Pimephales promelas. Environ Biol Fish 72:227–233. doi:10.1007/s10641-004-8753-6
Wisenden BD, Pollock MS, Tremaine RJ, Webb JM, Wismer ME, Chivers DP (2003) Synergistic interactions between chemical alarm cues and the presence of conspecific and heterospecific fish shoals. Behav Ecol Sociobiol 54:485–490. doi:10.1007/s00265-003-0653-9
Wisenden BD, Vollbrecht KA, Brown JL (2004) Is there a fish alarm cue? Affirming evidence from a wild study. Anim Behav 67:59–67. doi:10.1016/j.anbehav.2003.02.010
Wisenden BD, Rugg M, Korpi N, Fuselier L (2009) Lab and field estimates of active time of chemical alarm cues of a cyprinid fish and an amphipod crustacean. Behaviour 146:1423–1442. doi:10.1163/156853909X440998
Acknowledgements
Protocols used in the course of this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota (protocol 1412-32136A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Rights and permissions
About this article
Cite this article
Faulkner, A.E., Holstrom, I.E., Molitor, S.A. et al. Field verification of chondroitin sulfate as a putative component of chemical alarm cue in wild populations of fathead minnows (Pimephales promelas). Chemoecology 27, 233–238 (2017). https://doi.org/10.1007/s00049-017-0247-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-017-0247-z