Abstract
A series of derivatives of 5-hydroxyalkylamino-1,3-oxazoles were synthesized. Among these derivatives, 5 compounds have been evaluated for their activities against a normal laboratory human cytomegalovirus (HCMV) strain, AD169, in human foreskin fibroblast (HFF) cells. Bioassays showed that among these, 2 compounds containing a remainder of glucamine exhibited moderate antiviral activity (compounds 9 and 15, EC50 = 5.42 µM, CC50 > 150 µM, SI50 > 28 and EC50 = 4.91 µM, CC50 > 150 µM, SI50 > 31, respectively). The difference between these compounds is that the first contains a phtalimide radical at position 4 and phosphoryl at position 5, whereas the latter contains a phenyl radical and a cyano group in the corresponding positions. However, they were considerably less active than the control drug, ganciclovir (EC50 = 0.32 µM, CC50 > 150 µM and SI50 > 476), in this assay. These and previously obtained data allow us to consider 1,3-oxazole as a promising backbone for the synthesis of new compounds with anti-HCMV activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human cytomegalovirus (HCMV) is a widespread herpesvirus of the Betaherpesvirinae subfamily characterized by intermittent lytic replication and its ability to exist in latent form within a host (Hanson et al. 2019). The manifestation of HCMV infection is of most concern in those with secondary or congenital immunodeficiencies, whereas HCMV disease is typically mild and self-limited in immunocompetent persons. When activated, HCMV can affect almost all systems and organs of an infected person (Kaushik et al. 2019). In current clinical practice, therapeutic targets against HCMV infection include inhibition of viral DNA polymerase (ganciclovir), the viral terminase complex (letermovir), and the viral kinase UL97 (maribavir) (Krishna et al. 2019). When resistance to ganciclovir appears, second-line drugs include the pyrophosphate analog foscarnet, and the nucleotide analog cidofovir, but these agents are associated with significant toxicity. In addition, resistance to second-line drugs sometimes leads to multi-resistance, which makes it impossible to use traditional drugs against HCMV (Krishna et al. 2019). Due to the increasing number of immunocompromised patients, the toxicity and emerging resistance associated with currently available drugs, and the fact that effective vaccines are still under development, there is an unmet need for new anti-HCMV drugs.
Among the chemical compounds, derivatives of 1,3-oxazole belong to the most useful heterocyclic structures with a wide spectrum of biological activity (see rev. Kakkar and Narasimhan 2019). The results obtained by several authors allow us to consider oxazoles as a promising framework for the synthesis of new derivatives with antiviral activity (see rev. Zhang et al. 2018). Indeed, we showed that substituted 1,3-oxazole derivatives are a promising structure class of chemical compounds for the development of antiviral drugs against HPV lesions (Kachaeva et al. 2017). Moreover, we synthesized 5-functionalized derivatives of 1,3-oxazole, one of which, (5-((2-hydroxyethyl)(methyl)amino)-2-(4-methylphenyl)-1,3-oxazole-4-carbonitrile), showed high efficacy and low toxicity in the primary assays against a normal laboratory HCMV strain (AD-169), and the HCMV resistant isolate (GDGr K17) in human foreskin fibroblast cells (EC50 < 0.05, CC50 > 150 µM and SI50 > 3000) (Kachaeva et al. 2019). These data provided evidence that derivatives of 1,3-oxazole could be useful for developing new anti-HCMV drugs. This study relates to the anti-HCMV activity of 1,3-oxazole derivatives designed and synthesized in Kyiv. The evaluation of their cytotoxicity and efficacy was performed at UAB.
Material and methods
General chemistry methods
1H, 13C, 31P NMR spectra were obtained on a Bruker AVANCE DRX-500 (500, 100 and 202 MHz, respectively) spectrometer in CDCl3 or DMSO-d6. IR spectra were recorded on a Vertex 70 spectrometer in KBr pellets. GC-MS spectra were recorded on an LC-MS system - HPLC Agilent 1100 Series equipped with a diode array detector Agilent LC\MSD SL. Parameters of GC-MS analysis: Zorbax SB - C18 column (1.8 μm, 4.6–15 mm, PN 821975-932), solvent water – acetonitrile mixture (95: 5), 0.1% of aqueous trifluoroacetic acid; eluent flow 3 mL min–1; injection volume 1 μL; UV detecting at 215, 254, 265 nm; chemical ionization at atmospheric pressure (APCI), scan range m/z 80–1000. UV-Vis absorption spectra were recorded on a Shimadzu UV-3100 spectrophotometer in toluene of spectral grade. Elemental analysis was carried out in the Analytical Laboratory of the V.P.Kukhar Institute of Bioorganic and Petrochemistry of the National Academy of Sciences of Ukraine by manual methods. M. p. were determined on a Fisher–Johns apparatus and are uncorrected. Reactions and purity of the products were monitored by thin-layer chromatography on Silufol UV-254 plates using 9:1(v/v) chloroform–methanol as eluent. All reagents and solvents were purchased from Aldrich and used as received.
General procedure for the synthesis of compounds 1–9
Hydroxyalkylamine (0.045 mol) was added to a solution of 0.01 mol of corresponding diethyl 1-acylamino-2,2,2-trichloroethylphosphonate A in 50 mL of methanol. The mixture was stirred for 36–72 h at 18–25 °C. The solvent was removed in a vacuum. The residue was treated with distilled water and extracted with tert-butyl methyl ether. The extract was dried over sodium sulfate. The solvent was removed in a vacuum, compounds 1–9 were purified by column chromatography (gradient elution with dichloromethane/methanol, 98:2, 95:5, 90:10).
General procedure for the synthesis of compounds 10–15
To a solution of 0.01 mol of compound B or C in 50 mL of methanol was added 0.035 mol of corresponding amine. The mixture was stirred for 12 h at a temperature of 22–25 °C. The precipitated amine hydrochloride was filtered off. After removing the solvent, 20 mL of acetonitrile was added and the mixture was refluxed for 3–5 min. The resulting precipitate was filtered off, the solvent was removed in vacuum, and compounds 10–15 recrystallized from EtOH.
Diethyl (5-((2-hydroxyethyl)(methyl)amino)-1,3-oxazol-4-yl)phosphonate (1)
Colorless oil; 86% Yield. IR (KBr) νmax/cm−1 3373.1, 2982.9, 1599.5, 1231.3, 1015.6, 795.0, 760.6, 596.6. 1H NMR (500 MHz, CDCl3) δ 7.30 (1H, s, CHoxazol), 4.66 (1Н, br. s, ОН), 4.12–4.00 (4Н, m, 2 ОСН2СН3), 3.75 (2Н, t, 3JHH = 5.1 Hz, СН2), 3.62 (2Н, t, 3JHH = 5.5 Hz, СН2), 3.06 (3Н, s, СН3), 1.29 (6Н, t, 3JHH = 7.0 Hz, 2 ОСН2СН3). 13C NMR (100 MHz, CDCl3) δ 162.7 (d, 2JРС = 39.2 Hz, С-5oxazol), 140.5 (d, 3JРС = 22.0 Hz, С-2oxazol), 96.4 (d, JРС = 259.7 Hz, С-4oxazol), 62.6 (d, 2JРС = 6.0 Hz, РОСН2СН3), 59.0 (СН2), 54.9 (СН2), 36.8 (СН3), 16.2 (d, 3JРС = 7.0 Hz, РОСН2СН3). 31P NMR (202 MHz, CDCl3) δ 14.2. LCMS, m/z [M + 1]+ 279.0. Anal.calcd for C10H19N2O5P: C, 43.17; H, 6.88; N, 10.07; P, 11.13. Found: C, 43.35; H, 7.02; N, 10.21; P, 10.97.
Diethyl (2-benzyl-5-((2-hydroxyethyl)(methyl)amino)-1,3-oxazol-4-yl)phosphonate (2)
Colorless oil; 98% Yield; IR (KBr) νmax/cm−1 3291.8, 2982.1, 1628.8, 1585.1, 1230.1, 1016.6, 963.0, 727.7, 697.9, 536.6, 482.7. 1H NMR (500 MHz, CDCl3) δ 7.42–7.21 (5H, m, CHarom), 4.76 (1H, t, JHH = 5.1 Hz, OH), 4.07–3.85 (6H, m, 3CH2), 3.62–3.51 (2H, m, CH2), 3.49–3.42 (2H, m, CH2), 3.07 (3H, s, CH3), 1.20 (6H, t, 3JHH = 7.0 Hz, 2OCH2CH3). 13C NMR (125 MHz, CDCl3) δ 162.00 (2JPC = 36.9 Hz, C-5oxazol), 151.80 (3JPC = 21.9 Hz, C-2oxazol), 136.46, 129.16, 129.14, 127.40 (Carom), 97.44 (JPC = 255.3 Hz, C-4oxazol), 62.05 (2JPC = 5.5 Hz, OCH2CH3), 54.87 (CH2O), 38.94 (CH), 33.80 (CH), 16.69 (3JPC = 5.5 Hz, OCH2CH3). 31P NMR (202 MHz, CDCl3) δ 13.79. LCMS, m/z [M + 1]+ 369.2. Anal.calcd for C17H25N2O5P: C, 55.43; H, 6.84; N, 7.60; P, 8.41. Found: C, 55.55; H, 6.90; N, 7.60; P, 8.40.
Diethyl (5-((2-hydroxyethyl)(methyl)amino)-2-(4-nitrophenyl)-1,3-oxazol-4-yl)phosphonate (3) have been decribed in (Abdurakhmanova et al. 2016)
Diethyl (5-((2-hydroxypropyl)(methyl)amino)-2-(4-methylphenyl)-1,3-oxazol-4-yl)phosphonate (4)
Colorless solid; 88% Yield; mp 83–85 °С; IR (KBr) νmax/cm−1 3455.9, 2971.4, 2907.8, 1608.0, 1585.7, 1299.5, 1232.3, 1214.4, 1049.5, 1026.7, 963.5, 795.1, 765.3, 710.4, 583.4. 1Н ЯМР (500 МHz, CDCl3) δ 7.73 (2Н, d, 3JHH = 8.4 Hz, Aryl), 7.79 (2Н, d, 3JHH = 8.4 Hz, Aryl), 4.24- 4.06 (5Н, m, 2ОСН2СН3, ОН), 4.05–3.94 (1Н, m, СН), 3.74 (1Н, dd, 2JHH = 14.4 Hz, 3JHH = 9.3 Hz, СН), 3.22 (1Н, dd, 2JHH = 14.4 Hz, 3JHH = 3.2 Hz, СН), 3.17 (3Н, s, СН3), 2.33 (3Н, s, СН3), 1.33 (6Н, t 3JHH = 7.0 Hz, 2ОСН2СН3), 1.22 (3Н, d, 3JHH = 6.0 Hz, СН3). 13С ЯМР (100 МHz, CDCl3) δ 162.3 (d, 2JРС = 39.2 Hz, С-5oxazol), 151.3 (d, 3JРС = 22.1 Hz, С-2oxazol), 139.6, 129.3, 125.4, 124.3 (Aryl), 98.7 д (JРС = 267.5 Hz, С-4oxazol), 64.7 (СН), 62.8 (d, 2JРС = 6.0 Hz, РОСН2СН3), 62.5 (d, 2JРС = 6.0 Hz, РОСН2СН3), 60.0 (СН2), 37.9 (СН3), 21.4 (СН3), 21.1 (СН3), 16.3 (d, 3JРС = 7.0 Hz, РОСН2СН3), 16.2 (d, 3JРС = 6.5 Hz, РОСН2СН3). 31Р ЯМР (202 МHz, CDCl3) δ 14.9. LCMS, m/z [M + 1]+ 383.2. Anal.calcd for C18H27N2O5P: C, 56.54; H, 7.12; N, 7.33; P, 8.10. Found: C, 56.83; H, 7.36; N, 7.58; P, 8.01.
Diethyl (5-((2,3-dihydroxypropyl)amino)-2-phenyl-1,3-oxazol-4-yl)phosphonate (5) have been described in (Abdurakhmanova et al. 2016)
Diethyl (5-((2,3-dihydroxypropyl)amino)-2-(4-methylphenyl)-1,3-oxazol-4-yl)phosphonate (6)
Colorless solid; 79% Yield; mp 118–120 °С; IR (KBr) νmax/cm−1 3335.0, 2983.9, 2924.9, 2865.9, 1627.7, 1503.8, 1450.5, 1393.2, 1296.3, 1253.2, 1215.1, 1190.45, 1105.4, 1026.0, 977.7, 923.9, 844.6, 770.1, 593.3. 1Н ЯМР (500 МHz, CDCl3) δ 7.74 (2Н, d, 3JHH = 8.0 Hz, Aryl), 7.17 (2Н, d, 3JHH = 8.0 Hz, Aryl), 6.26 (1Н, t, 3JHH = 5.6 Hz, NH), 4.38 (1Н, br. s, ОН), 4.18–4.02 (4Н, m, 2ОСН2СН3), 3.95 (1Н, ws, ОН), 3.82–3.71 (2Н, m, СН2), 3.69–3.61 (1Н, m, СН), 3.60–3.50 (1Н, m, СН2), 3.48–3.37 (1Н, m, СН2), 2.34 (3Н, s, СН3), 1.31 (6Н, t, 3JHH = 7.0 Hz, 2ОСН2СН3). 13С ЯМР (100 МHz, CDCl3) δ 163.5 (d, 2JРС = 39.4 Hz, С-5oxazol), 152.5 (d, 3JРС = 22.4 Hz, С-2oxazol), 139.8, 129.4, 125.6, 124.3 (Aryl), 96.3 (d, JРС = 258.8 Hz, С-4oxazol), 70.9 (СН), 64.6 (СН), 62.7 (d, 2JРС = 5.0 Hz, РОСН2СН3), 46.3 (СН2), 21.5 (СН3), 16.2 (d, 3JРС = 6.5 Hz, РОСН2СН3). 31Р ЯМР (202 МHz, CDCl3) δ 14.32. LCMS, m/z [M + 1]+ 385.2. Anal.calcd for C17H25N2O6P: C, 53.12; H, 6.56; N, 7.29; P, 8.06. Found: C, 53.42; H, 6.73; N, 7.12; P, 8.01.
Diethyl (5-(2-(hydroxymethyl)pyrrolidin-1-yl)-2-(4-methylphenyl)-1,3-oxazol-4-yl)phosphonate (7)
Colorless oil; 81% Yield; IR (KBr) νmax/cm−1 3380.9, 2976.0, 1603.4, 1576.3, 1226.9, 1019.0, 957.8, 820.2, 795.8, 760.4, 638.2, 553.5. 1Н ЯМР (500 МHz, CDCl3) δ 7.75 (2Н, d, 3JHH = 8.2 Hz, Aryl), 7.19 (2Н, d, 3JHH = 8.2 Hz, Aryl), 4.46 (1Н, t, 3JHH = 5.3 Hz, OH), 4.24–4.11 (4Н, m, 2ОСН2СН3), 3.87–3.79 (1Н, m, СН), 3.64–3.54 (3Н, m, СН), 2.38 (3Н, s, СН3), 2.06–1.89 (4Н, m, 2СН2), 1.38 (6Н, t, 3JHH = 6.9 Hz, 2ОСН2СН3). 13С ЯМР (100 МHz, CDCl3) δ 160.2 (d, 2JРС = 39.4 Hz, С-5oxazol), 151.5 (d, 3JРС = 21.9 Hz, С-2oxazol), 139.6, 129.3, 125.5, 124.4 (Aryl), 97.8 (d, JРС = 258.8 Hz, С-4oxazol), 64.7 (d, 2JРС = 5.5 Hz, РОСН2СН3), 62.5 (d, 2JРС = 5.5 Hz, РОСН2СН3), 61.6 (СН), 50.0 (СН), 28.7 (СН), 23.7 (СН), 21.5 (СН3), 16.4-16.26 (m, РОСН2СН3). 31Р ЯМР (202 МHz, CDCl3) δ 14.82. LCMS, m/z [M + 1]+ 395.4. Anal.calcd for C19H27N2O5P: C, 57.86; H, 6.90; N, 7.10; P, 7.85. Found: C, 57.71; H, 6.73; N, 7.28; P, 7.89.
Diethyl (5-(3-(hydroxymethyl)pyrrolidin-1-yl)-2-phenyl-1,3-oxazol-4-yl)phosphonate (8)
Colorless solid; 85% Yield; mp 87–88 °С; IR (KBr) νmax/cm−1 3374.7, 2985.7, 2874.1, 1615.1, 1584.3, 1480.4, 1449.4, 1392.6, 1221.9, 1023.1, 968.5, 799.3, 768.5, 693.1, 620.2, 590.1. 1Н ЯМР (500 МHz, CDCl3) δ 7.85 (2Н, d, C6H5,3JHH = 7.9 Hz), 7.42–7.28 (3Н, m, Aryl), 4.19–4.10 (4Н, m, 2ОСН2СН3), 3.81–3.54 (6Н, m, СН), 3.24 (1Н, br. s, ОН), 2.61–2.47 (1Н, m, СН), 2.16–2.03 (1Н, m, СН), 1.85–1.73 (1Н, m, СН), 1.34 (6Н, t, 3JHH = 7.0 Hz, 2ОСН2СН3). 13С ЯМР (100 МHz, CDCl3) δ 159.7 (d, 2JРС = 38.6 Hz, С-5oxazol), 150.9 (d, 3JРС = 22.6 Hz, С-2oxazol), 129.2, 128.5, 127.1, 125.4 (Aryl), 97.9 (d, JРС = 261.5 Hz, С-4oxazol), 64.0 (СН), 62.5 (d, 2JРС = 5.5 Hz, РОСН2СН3), 62.4 (d, 2JРС = 5.5 Hz, РОСН2СН3), 62.1 (СН), 48.8 (СН), 41.3 (СН), 27.9 (СН), 16.3 (t, 3JPC = 7.0 Hz, РОСН2СН3). 31Р ЯМР (202 МHz, CDCl3) δ 14.3. LCMS, m/z [M + 1]+ 381.2. Anal.calcd for C18H25N2O5P: C, 56.84; H, 6.62; N, 7.36; P, 8.14. Found: C, 57.03; H, 6.73; N, 7.48; P, 8.10.
Diethyl(2-((1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl)-5(methyl(2,3,4,5,6-pentahydroxyhexyl)amino)-1,3-oxazol-4-yl)phosphonate (9) and ethyl 5-(methyl(2,3,4,5,6-pentahydroxyhexyl)amino)-2-phenyl-1,3-oxazole-4-carboxylate (10) have been described in (Abdurakhmanova et al. 2015).
5-((2-Hydroxyethyl)(methyl)amino)-2-methyl-1,3-oxazol-4-carbonitrile (11)
Colorless solid; 60% Yield; mp 58–59 °С; IR (KBr) νmax/cm−1 3343.4, 2941.3, 2881.5, 2198.7, 1650.0, 1603.7, 1427.3, 1383.9, 1354.9, 1189.1, 1148.6, 1113.8, 1071.4, 1051.2, 937.4, 652.9, 612.4, 511.1. 1H NMR (500 MHz, DMSO-d6) δ 4.19–3.83 (1H, m, OH), 3.61–3.56 (2H, m, CH2), 3.46–3.41 (2H, m, CH2), 3.13–3.08 (3H, m, CH3), 2.25 (3H, s, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.2 (C-5oxazol), 150.3 (C-2oxazol), 117.4 (C≡N), 82.3 (C-4oxazol), 58.6 (CH2O), 53.7 (CH), 37.5 (CH), 13.5 (CH3). LCMS, m/z [M + 1]+ 182.2. Anal.calcd. for C8H11N3O2: C, 53.03; H, 6.12; N, 23.19. Found: C, 53.07; H, 6.06; N, 23.01.
5-((2-Hydroxyethyl)(methyl)amino)-2-phenyl-1,3-oxazol-4-carbonitrile (12)
Colorless solid; 97% Yield; mp 119–120 °С; IR (KBr) νmax/cm−1 3329.9, 2937.5, 2857.4, 2199.7, 1638.5, 1589.3, 1455.2, 1420.5, 1075.3, 769.6, 691.5, 506.3. 1H NMR (500 MHz, DMSO-d6) δ 7.86–7.78 (2H, m, Aryl), 7.52–7.43 (3H, m, Aryl), 4.94 (1H, t, JHH = 5.3 Hz, OH), 3.68–3.62 (2H, m, CH2), 3.59–3.54 (2H, m, CH2), 3.21 (3H, s, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.1 (C-5oxazol), 149.8 (C-2oxazol), 130.6, 129.6, 126.5, 125.6 (Aryl), 117.3 (C≡N), 84.4 (C-4oxazol), 58.6 (CH2O), 54.0 (CH), 37.5 (CH). LCMS, m/z [M + 1]+ 244.2. Anal.calcd for C13H13N3O2: C, 64.19; H, 5.39; N, 17.27. Found: C, 64.15; H, 5.43; N, 17.44.
2-(4-Chlorophenyl)-5-((2-hydroxyethyl)(methyl)amino)-1,3-oxazol-4-carbonitrile (13)
Colorless solid; 75% Yield; mp 142–143 °С; IR (KBr) νmax/cm−1 3450.5, 3175.6, 2950.0, 2896.9, 2199.7, 1634.6, 1604.7, 1486.1, 1406.0, 1087.8, 1047.3, 847.7, 527.5. 1H NMR (500 MHz, DMSO-d6) δ 7.93–7.77 (2H, m, Aryl), 7.64–7.49 (2H, m, Aryl), 5.18–4.72 (1H, m, OH), 3.69–3.62 (2H, m, CH2), 3.60–3.53 (2H, m, CH2), 3.26–3.17 (3H, m, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 161.3 (C-5oxazol), 149.0 (C-2oxazol), 135.3, 129.8, 127.4, 125.2 (Aryl), 117.2 (C≡N), 84.7 (C-4oxazol), 58.7 (CH2O), 54.1 (CH), 37.6 (CH). LCMS, m/z [M + 1]+ 278.2. Anal.calcd for C13H12ClN3O2: C, 56.23; H, 4.36; Cl, 12.77; N, 15.13. Found: C, 56.35; H, 4.45; Cl, 12.75; N, 15.25.
5-((3-Hydroxypropyl)amino)-2-phenyl-1,3-oxazol-4-carbonitrile (14)
Colorless solid; 92% Yield; mp 63–64 °С; IR (KBr) νmax/cm−1 3482.3, 3358.9, 3278.8, 3214.2, 3014.6, 2915.1, 2878.4, 2208.4, 1646.2, 1589.3, 1445.6, 1070.4, 925.8, 774.4, 697.2, 521.7. 1H NMR (500 MHz, DMSO-d6) δ 8.53–8.37 (1H, m, NH), 7.79–7.73 (2H, m, Aryl), 7.56–7.43 (3H, m, Aryl), 4.58 (1H, br. s, OH), 3.54–3.48 (2H, m, CH2), 2.53–2.47 (2H, m, CH2), 1.79–1.72 (2H, m, CH3). 13C-NMR (100 MHz, DMSO-d6) δ 163.3 (C-5oxazol), 149.5 (C-2oxazol), 130.2, 129.2, 125.9, 125.1 (Aryl), 116.1 (C≡N), 83.3 (C-4oxazol), 58.0 (CH2O), 32.3 (CH), 30.5 (C, CH). LCMS, m/z [M + 1]+ 244.2. Anal.calcd for C13H13N3O2: C, 64.19; H, 5.39; N, 17.27. Found: C, 64.21; H, 5.28; N, 17.34.
5-(Methyl(2,3,4,5,6-pentahydroxyhexyl)amino)-2-phenyl-1,3-oxazole-4-carbonitrile (15) have been described in (Abdurakhmanova et al. 2015).
Antiviral and cytotoxicity assays
Five synthesized derivatives of 1,3-oxazoles were selected for testing their antiviral activity. Compounds 3, 5, 10, and 15 were chosen because they belong to the phenyloxazole class known to be potent inhibitors of histone demethylases (Dulla et al. 2013), pharmacological inhibition of which leads to severely impaired viral immediate-early gene expression by blocking HCMV life cycle (Gan et al. 2017). Compound 9 was taken in view of the broad biological activities of compounds functionalized with phthalimide (Imran et al. 2019).
Primary antiviral and CellTiter Glo Cytotoxicity Assays were performed in a primary cell line of human foreskin fibroblasts (HFF) as described previously (Kachaeva et al. 2019). In breaf, plaque reduction assays were used to assess antiviral activity against HCMV by methods reported previously (Prichard et al. 2008). The assays were performed essentially as follows: human foreskin fibroblast (HFF) cells cultivated in minimum essential media (MEM) with Earle’s salts supplemented with 10% fetal bovine serum (FBS) at 37 °C and standard concentrations of penicillin and gentamicin. Monolayers were infected with both normal strain (AD169) as well as resistant isolate (GDGr K17) of HCMV (American Type Culture Collection, Manassas, VA) to yield approximately 20–30 plaques per well. Drug dilutions were prepared in growth media and were added to duplicate wells in six-well plates. The infected plates were incubated for 8 days, the overlays were removed and monolayers were stained with a 1.5% solution of neutral red in phosphate-buffered saline. The stain was aspirated, and plaques were counted by using a stereomicroscope at ×10 magnification. Then drug concentrations sufficient to reduce plaque number 50% (EC50) values were interpolated from the experimental data by comparing drug-treated with untreated wells.
Neutral Red Cytotoxicity Assays: HFF cells were seeded into 96-well tissue culture plates at 2.5 × 104 cells/well. After a 24-h incubation, medium was replaced with MEM containing 2% FBS, and drug was added to the first row and then diluted serially fivefold from 100 to 0.03 μM. The plates were incubated for 7 days, and cells were stained with neutral red and incubated for 1 h. Plates were shaken on a plate shaker for 15 min, and neutral red was solubilized with 1% glacial acetic acid-50% ethanol. The optical density was read at 540 nm to determine the number of viable cells. Cytotoxicity is expressed as the concentration of drug that reduced cell viability by 50% (CC50), and was interpolated from the experimental data.
In both assays, the final concentration of compound-derived DMSO was maintained at 1% (v/v) in all samples. In control samples (with no compound), an appropriate volume of pure DMSO was added for a final concentration of 1%.
Results and discussion
Chemistry
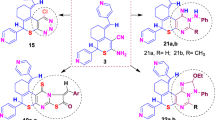
Syntheses of 5-hydroxyalkylamino-1,3-oxazoles 1–15 are depicted on Scheme 1. As the starting material were selected the available diethyl 1-acylamino-2,2,2-trichloroethylphosphonates A (Drach and Sviridov 1974; Lukashuk et al 2013), ethyl 1-benzoylamino-2,2-dichloroacrylate B (Drach and Miskevich 1974) and 1-acylamino-2,2-dichloroacrylonitriles C (Drach et al. 1973). By the action of an access of alkanolamines they underwent cyclization into the substituted 5-hydroxyalkylamino-1,3-oxazoles 1–15 in yields of 60–98 %. Structures of synthesized compounds were confirmed by the IR, 1H, 13C and 31P (compounds 1–9) NMR, and GC-MS spectra.
Synthesis of derivatives of 5-hydroxyalkylamino-1,3-oxazoles 1–15: (i) (2-hydroxyethyl)(methyl)amine, (2-hydroxypropyl)(methyl)amine, (2,3-dihydroxypropyl)(methyl)amine, 2-(hydroxymethyl)pyrrolidine, 3-(hydroxymethyl)pyrrolidine or N-methyl-D-glucamine, MeOH, 18–25 °C, 36–72 h, (ii) (2-hydroxyethyl)(methyl)amine, (2-hydroxypropyl)amine or N-methyl-D-glucamine, MeOH, rt, 22–25 °C, 12 h
Biology
The five 1,3-oxazole derivatives were evaluated in HFF for antiviral efficacy against HCMV strain AD169 as well as for cytotoxicity using a CellTiter-Glo (Cytopathic effect/Toxicity) assay (Table 1). The selectivity index (SI) was calculated for each compound as an indicator of antiviral activity.
Among tested derivatives of 5-hydroxyalkylamino-1,3-oxazoles, compounds 9 and 15 exhibited moderate antiviral activity (defined as having an SI50 within the range of 10–49.9 μM) against HCMV strain AD169 (EC50 = 5.42 and 4.91 µM, respectively). Although these results are worse than the control compound ganciclovir (EC50 = 0.32 µM), cytotoxicity does not exceed the cytotoxicity of ganciclovir. Among pentahydroxyhexylamine-containing compounds, only compound 10 did not display biological activity. Replacing pentahydroxyhexylamine with methylaminoethanol (compound 3) or aminopropane-1,2-diol (compound 5) at the position 5 of the 1,3-oxazole ring in these compounds did not improve efficacy. Furthermore, attaching a nitro group to the phenyl ring (compound 3) resulted in increased cytotoxicity without any significant gain in antiviral efficacy (EC50 > 30.00 µM, CC50 = 139.09 µM). The introduction of cyano group into the fourth position (compound 15) instead of carboxylate significantly enhanced antiviral activity and selectivity of compound 10 (Table 1).
It has been shown that some compounds comprising a phthaleimide radical possess antiviral activity blocking either integrase (Kushwaha and Kaushik 2016) or reverse transcriptase (Al-Masoudi et al. 2016). Taking into account structural analogy, it is possible that compound 9 may also act in a similar manner. It has also been shown that integrase inhibitors block replication of alpha-, beta-, and gammaherpesviruses including HCMV (Yan et al. 2014). It is possible that the target of compound 15, as indicated above, could be histone demethylase.
These and previously obtained data allow us to consider 1,3-oxazole as a promising backbone for the synthesis of new compounds with anti-HCMV activity.
Conclusion
Fifteen substituted 5-hydroxyalkylamino-1,3-oxazoles have been synthesized and evaluated for in vitro antiviral efficacy and cytotoxicity. Five of these compounds were tested for anti-HCMV activity. Bioassays showed that 2 of the tested compounds exhibited the moderate antiviral activity against a normal laboratory HCMV strain (AD169) in HFF cells, which was an order of magnitude smaller than that of the control drug ganciclovir. Further functional modifications of these compounds may lead to development of new derivatives of 5-hydroxyalkylamino-1,3-oxazoles possessing higher anti-HCMV activity.
Disclaimer
This material should not be interpreted as representing the viewpoint of the National Institute of Allergy and Infectious Diseases (USA) and its Collaborative Antiviral Testing Group.
References
Abdurakhmanova ER, Lukashuk EI, Golovchenko AV, Pil’o SG, Brovarets VS (2015) N-methyl-d-glucamine-derived 4-substituted 1,3-oxazoles. Russ J Gen Chem 85(4):851–857
Abdurakhmanova ER, Lukashuk EI, Golovchenko AV, Brovarets VS (2016) Synthesis and properties of 4-phosphorylated derivatives of 5-hydroxyalkylamino-1,3-oxazoles. Russ J Gen Chem 86(7):1584–1596
Al-Masoudi NA, Abood E, Al-Maliki ZT, Al-Masoudi WA, Pannecouque C (2016) Amino acid derivatives. Part 6. Synthesis, in vitro antiviral activity and molecular docking study of new N-α-amino acid derivatives conjugated spacer phthalimide backbone. Med Chem Res 25(11):2578–2588
Drach BS, Sviridov EP, Kisilenko AA, Kirsanov AV (1973) Interaction of secondary amines with N-acyl-2,2-dichlorovinylamines and N-acyl-1-cyano-2,2-dichlorovinylamines. Zh Org Khim 9:1818–1824
Drach BS, Sviridov EP (1974) Acyl derivatives of 1,2,2-trichloro-2-bromoethylamine. Zh Obshch Khim 44:348–352
Drach BS, Miskevich GN (1974) Interaction of azlactone α-benzamido-β,β-dichloroacrylic acid with amines and alcohols. Zh Org Khim 10:2315–2319
Dulla B, Kirla KT, Rathore V, Deora GS, Kavela S, Maddika S, Chatti K, Reiser O, Iqbal J, Pal M (2013) Synthesis and evaluation of 3amino/guanidine substituted phenyl oxazoles as a novel class of LSD1 inhibitors with antiproliferative properties. Org Biomol Chem 11:3103–3107
Gan X, Wang H, Yu Y, Yi W, Zhu S, Li E, Liang Y (2017) Epigenetically repressing human cytomegalovirus lytic infection and reactivation from latency in THP-1 model by targeting H3K9 and H3K27 histone demethylases. PLoS ONE 12(4):e0175390
Hanson DJ, Tsvetkova O, Rerolle GF, Greninger AL, Sette A, Jing L, Campbell VL, Koelle DM (2019) Genome-wide approach to the CD4 T-cell response to human herpesvirus 6B. J Virol 93(14):e00321–19
Imran M, Bisht AS, Asif M (2019) A review on biological and chemical potential of phthalimide and maleimide derivatives. Acta Sci Pharm Sci 3(9):51–67
Kachaeva MV, Pilyo SG, Kornienko AM, Prokopenko VM, Zhirnov VV, Prichard MN, Keith KA, Yang G, Wang HK, Banerjee NS, Chow LT, Broker TR, Brovarets VS (2017) In vitro activity of novel 1,3-oxazole derivatives against human papillomavirus. Ibnosina J Med Biomed Sci 9:111–118
Kachaeva MV, Pilyo SG, Hartline CB, Harden EA, Prichard MN, Zhirnov VV, Brovarets VS (2019) In vitro activity of novel derivatives of 1,3-oxazole-4-carboxylate and1,3-oxazole-4-carbonitrile against human cytomegalovirus. Med Chem Res 28:1205–1211
Kakkar S, Narasimhan B (2019) A comprehensive review on biological activities of oxazole derivatives. BMC Chem 13(16):1–24
Kaushik AC, Mehmood A, Upadhyay AK, Paul S, Srivastava S, Mali P, Xiong Y, Dai X, Wei DQ Sahi S (2019) Cytomegalovirus infection database: a public omics database for systematic and comparable information of CMV. Interdiscip Sci. https://doi.org/10.1007/s12539-019-00350-x
Krishna BA, Wills MR, Sinclair JH (2019) Advances in the treatment of cytomegalovirus. Br Med Bull 131(1):5–17
Kushwaha N, Kaushik D (2016) Recent advances and future prospects of phthalimide derivatives. J Appl Pharm Sci 6(3):159–171
Lukashuk OI, Kondratyuk KM, Golovchenko AV, Brovarets VS, Kukhar VP (2013) A novel synthetic approach to phosphorylated peptidomimetics. Heteroat Chem 4:289–297
Prichard MN, Hartline CB, Harden EA, Daily SL, Beadle JR, Valiaeva N, Kern ER, Hostetler KY (2008) Inhibition of herpesvirus replication by hexadecyloxypropyl esters of purine- and pyrimidine-based phosphonomethoxyethyl nucleoside phosphonates. Antimicrob Agents Chemother 52(12):4326–4330
Zhang HZ, Zhao ZL, Zhou CH (2018) Recent advance in oxazole-based medicinal chemistry. Eur J Med Chem 144:444–492
Yan Z, Bryant KF, Gregory SM, Angelova M, Dreyfus DH, Zhao XZ, Coen DM, Burke Jr TR, Knipe DM (2014) HIV integrase inhibitors block replication of alpha-, beta-, and gammaherpesviruses. MBio 5(4):e01318–14
Acknowledgements
We would like to thank Enamine Ltd for the material and technical support. These studies were funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN272201100016I (MNP) and HHSN75N93019D00016 (SHJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Abdurakhmanova, E.R., Brusnakov, M.Y., Golovchenko, O.V. et al. Synthesis and in vitro anticytomegalovirus activity of 5-hydroxyalkylamino-1,3-oxazoles derivatives. Med Chem Res 29, 1669–1675 (2020). https://doi.org/10.1007/s00044-020-02593-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02593-6