Abstract
A series of new C28-amino-lupanes bearing A-azepano- and A-seco-3-amino-fragments was synthesized from 3,28-dioximino-betulin and evaluated for cytotoxicity toward the NCI-60 cancer cell line panel and antimicrobial activity against key ESKAPE pathogens. A-azepano-28-amino-betulin exhibited remarkable activities with GI50 ranging from 1.16 to 2.27 μM against all panel with the highest activity toward leukemia, colon cancer, non-small cell lung cancer and breast cancer. The replacement of the hydroxyl group at C28 in the structure of azepanobetulin to the amino group did not show a strong effect on the cytotoxic activity. Both compounds were ∼5 and ∼4 times more active than doxorubicin against colon cancer HCT-15 and ovarian cancer NCI/ADR-RES cell lines, thus these A-azepano-lupane triterpenoids are the promising agents for future anticancer drug development. The ability of A-azepanobetulin to inhibit cell growth may be associated with its cytostatic effect, which, depending on the cell line, is associated with the arrest either S or G1 phase of cell cycle. 3-Amino-3,4-seco-28-amino-lup-4(23),20(29)-dien exhibited significant bacteriostatic effect against methicillin-resistant Staphylococcus aureus (MIC ≤ 0.25 μg/mL) that exceeds the effect of the clinically used antibiotic vancomycin.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lupane type triterpenoids (lupeol, betulin, betulinic, and betulonic acids) are widespread in the plant kingdom including the bark of birch trees and display important biological properties such as anticancer, antiviral, antibacterial, and antimalarial activities, among others (Tolstikov et al. 2005; Tolstikova et al. 2006; Krasutsky 2006; Csuk 2014; Sousa et al. 2019; Bildziukevich et al. 2019). In particular, the antitumor properties of lupanes have attracted considerable attention worldwide since many synthetic derivatives of these triterpenoids have shown promising results as chemotherapeutic agents for different types of cancer (Csuk 2014; Zhang et al. 2015; Ali-Seyed et al. 2016; Sousa et al. 2019; Bildziukevich et al. 2019). Recently, pentacyclic triterpenoids were identified as antimicrobial and antibiofilm agents, and as adjuvants in restoring the activity of common antibiotics against Staphylococcus aureus (Chung 2019; Catteau et al. 2018). Since bacteria are the most common pathogens causing infections in oncologic patients (Hoz et al. 2019), the search of agents combining both anticancer and antimicrobial activities is actual.
Over the past years, a large number of lupane triterpenoids have been chemically modified in order to improve their bioactivity and bioavailability and to enhance their protective and/or therapeutic effects. Among these derivatives, triterpenoids with A-azepane ring are a group of new and promising modificants with anticancer (Kazakova et al. 2014; Lopatina et al. 2019; Giniyatullina et al. 2019; Smirnova et al. 2019), antimicrobial (Medvedeva et al. 2018; Kazakova et al. 2019a, b) and alpha-glucosidase inhibitory (Khusnutdinova et al. 2016) activities. Based on the results we obtained with A-azepanes of lupane, oleanane, ursane, and dammarane types, the recent investigation has been focused on the synthesis of A-azepano- and A-seco-3-amino-lupanes with C28-amino-substituent as well as A-azepano-lupeol, and evaluation of their cytotoxicity with cell cycle analysis and antimicrobial activity.
Experimental
General
The spectra were recorded at the Center for the Collective Use “Chemistry” of the Ufa Institute of Chemistry of the UFRC RAS and RCCU “Agidel” of the UFRC RAS. X-ray diffraction experiments were carried out using the equipment of Center for molecular composition studies of INEOS RAS. 1H and 13C-NMR spectra were recorded on a “Bruker AM-500” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz, respectively, δ, ppm, Hz) in CDCl3, internal standard tetramethylsilane. Mass spectra were obtained on a liquid chromatograph–mass spectrometer LCMS-2010 EV (Shimadzu, Kyoto, Japan). Single crystal X-ray diffraction study was carried out with SMART APEX II CCD diffractometer (λ(Mo-Kα) = 0.71073 A, graphite monochromator, ω-scans) at 120 K. Collected data were processed by the SAINT and SADABS programs incorporated into the APEX2 program package (Bruker 2014). The structures were solved by the direct methods and refined by the full-matrix least-squares procedure in anisotropic approximation for non-hydrogen atoms (Sheldrick 2015). Melting points were detected on a micro table “Rapido PHMK05” (Nagema, Dresden, Germany). Optical rotations were measured on a polarimeter “Perkin-Elmer 241 MC” (Perkin-Elmer, Waltham, MA, USA) in a tube length of 1 dm. Elemental analysis was performed on a Euro EA-3000 CHNS analyzer (Eurovector, Milan, Italy); the main standard is acetanilide. Thin-layer chromatography analyses were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russian Federation), using the solvent system chloroform–ethyl acetate, 40:1. Substances were detected by 10% H2SO4 with subsequent heating to 100–120 °C for 2–3 min. Betulin dioxime 1 (Flekhter et al. 2002), compounds 2 (Khusnutdinova et al. 2019), 3 (Tolmacheva et al. 2018), and 10 (Lopatina et al. 2019) were obtained according to the methods described previously.
Chemistry
Crystallographic data for compound (2)
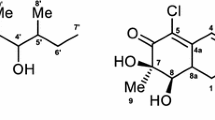
C30H46N2O are monoclinic, space group P21: a = 6.6272(7) Å, b = 27.629(3) Å, c = 14.252(2) Å, β = 90.409(3)°, V = 2609.5(5) Å3, Z = 4, M = 450.69, dcryst = 1.147 g cm−3. wR2 = 0.1259 calculated on F2hkl for all 11,545 independent reflections with 2θ < 27.1°, (GOF = 0.955, R = 0.0656 calculated on Fhkl for 6124 reflections with I > 2σ(I)). Crystallographic data (excluding structure factors) for the structure have been deposited at the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication No. CCDC 1986798.
Synthesis of compounds (4) and (6)
To a solution of 1 mmol of compound 2 or 3 in anhydrous THF (40 mL) 2.2 mmol (0.08 g) of LiAlH4 was added and a mixture was refluxed for 1 h, then poured into 5% HCl (100 mL), the product was extracted with CHCl3, chromatographed on a column with Al2O3, eluting with CHCl3 and a mixture of CHCl3—EtOH (100:1, 50:1).
3-deoxy-3a-homo-3a-aza-28-amino-lup-20(29)-en (4)
Yield 0.36 g (82%); the spectral and physicochemical data are in agreement with those from the literature (Kazakova et al. 2019b).
3-amino-3,4-seco-28-amino-lup-4(23),20(29)-dien (6)
Yield 0.34 g (77%); MP 121 °С; [α]D20 + 163° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.85, 0.98, 1.21, 1.33, 1.68 (5 s, 15H, 5CH3), 1.72–2.42 (m, 25H, CH and CH2), 2.60 and 3.00 (both d, 2J = 12.9 Hz, 2H, H-28), 3.01–3.13 (m, 4H, H-3, H-2), 3.36 (br. s, 2H, NH2), 4.64 and 4.66 (both d, 2J = 1.8 Hz, 2H, H-24), 4.69 and 4.78 (both d, 2J = 2.0 Hz, 2H, H-29); δC (125.76 MHz, CDCl3) 14.75, 16.05, 19.12, 19.40, 20.51, 21.41, 26.85, 27.28, 29.22, 29.40, 32.01, 33.40, 34.26, 37.58, 38.79, 39.26, 40.18, 40.83, 42.74 (C-3), 43.16, 47.83, 48.77 (C28), 49.48, 50.43, 51.26, 56.60, 109.71 (C-29), 112.86 (C-24), 148.28 (C-4), 150.52 (C-20); Anal. Calcd for C30H52N2: C, 81.75; H, 11.89; N, 6.36. Found: C, 81.71; H, 11.87; N, 6.32.
3-oxo-3а-homo-3а-aza-28-amino-lup-20(29)-en (5)
To a solution of 1 mmol (0.45 g) of compound 2 in anhydrous THF (20 mL), 2.2 mmol (0.08 g) of LiAlH4 was added and a mixture was stirred at room temperature for 1 h, then poured into 5% HCl (50 mL), the products were extracted with CHCl3 and chromatographed on a column with Al2O3, eluting with CHCl3 and a mixture of CHCl3—EtOH (150:1, 100:1, 50:1). Yield 0.035 g (8%) of compound 4, 0.12 g (27%) of compound 5, and recovery of compound 2 0.26 g (58%). For compound 5 MP 145 °С; [α]D20 + 127 (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.83, 1.03, 1.05, 1.20, 1.28, 1.62 (6 s, 18H, 6CH3), 1.70–2.58 (m, 25H, CH and CH2), 2.62 and 3.03 (both d, 2J = 12.9 Hz, 2H, H-28), 3.33 (br. s, 2H, NH2), 4.56 and 4.71 (both d, 2J = 2.0 Hz, 2H, H-29), 5.59 (br. s, 1H, NH); δC (125.76 MHz, CDCl3) 14.63, 15.94, 18.26, 19.21, 21.92, 22.51, 25.41, 26.85, 27.28, 29.22, 29.40, 32.01, 33.40, 34.26, 37.54, 38.81, 39.26, 40.20, 40.88, 42.74, 46.09, 47.02 (С-28), 47.06, 48.98, 50.78, 53.00, 56.26, 110.22 (C-29), 149.52 (C-20), 176.31 (C-3); Anal. Calcd for C30H50N2O: C, 79.24; H, 11.08; N, 6.16. Found: C, 80.19; H, 11.84; N, 6.20.
Synthesis of compounds (7–9)
A rapid stream of ozone was passed through a solution of compound 2 or 3 or 4 (1 mmol) in CH2Cl2 (30 mL) at −40 °C until the starting compound disappeared (TLC control). The solvent was removed under reduced pressure, and the residue was purified by column chromatography on Al2O3 eluting with CHCl3 and a mixture of CHCl3—EtOH (100:1, 50:1) giving compound 7 or 8 or 9.
3-oxo-3a-homo-3-aza-20-oxo-17-nitrilo-29-nor-lupane (7)
Yield 0.40 g (88%); MP 195 °С; [α]D20 + 21° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.88, 1.01, 1.04, 1.24, 1.29 (5 s, 15H, 5CH3), 1.40–2.10 (m, 19H, CH and CH2), 2.12 (s, 3H, H-30), 2.23–2.90 (m, 6H, CH and CH2), 5.81 (br. s, 1H, NH); δC (125.76 MHz, CDCl3) 14.56, 15.72, 18.20, 21.64, 22.43, 27.21, 27.36, 27.46, 28.89, 30.19, 30.42, 31.98, 33.27, 33.55, 35.74, 39.33, 40.20, 40.28, 40.58, 42.38, 48.84, 49.01, 50.61, 51.63, 52.86, 56.17, 122.05 (C28), 176.26 (C-3), 209.78 (C-20); Anal. Calcd for C29H44N2O2: C, 76.95; H, 9.80; N, 6.16. Found: C, 77.00; H, 9.84; N, 6.12.
3,4-seco-4,20-dioxo-2,17-dinitrilo-23,29-dinorlupane (8)
Yield 0.32 g (74%); MP 81 °С; [α]D20 −66° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.91, 0.93, 1.11, 2.11, 2.18 (5 s, 15H, 5CH3), 1.22–2.42 (m, 23H, CH and CH2), 2.88–2.90 (m, 2H, CH); δC (125.76 MHz, CDCl3) 11.50, 14.59, 15.92, 19.48, 20.77, 21.62, 26.53, 27.45, 28.95, 30.13, 30.41, 30.64, 31.83, 34.67, 35.71, 38.95, 39.97, 40.17, 40.33, 42.61, 48.75, 48.91, 51.77, 56.25, 119.83 (C28), 122.75 (C-3), 209.66 (C-4), 211.49 (C-20); Anal. Calcd for C28H40N2O2: C, 77.02; H, 9.23; N, 6.42. Found: C, 77.69; H, 9.26; N, 6.50.
3-deoxy-3a-homo-3a-aza-20-oxo-28-amino-29-norlupane (9)
Yield 0.34 g (76%); MP 221 °С; [α]D20 + 73° (с 0.05, CHCl3); δH (500.13 MHz, CDCl3) 0.85, 0.99, 1.05, 1.17, 1.41 (5 s, 15H, 5CH3), 2.15 (s, 3H, H-30), 1.65–2.40 (m, 28H, CH and CH2), 2.78–2.90 (2H, m, H-28), 3.35–3.29 (1H, m, H-3a), 3.80–3.78 (1H, m, H-3b); δC (125.76 MHz, CDCl3) 14.51, 16.55, 16.61, 19.15, 21.30, 21.66, 22.08, 22.78, 22.93, 23.08, 25.79, 26.86, 27.77, 28.99, 29.66, 33.96, 37.71, 40.72, 40.91, 41.22, 43.10, 47.45 (C28), 47.61, 47.84, 48.59, 54.74, 60.51, 67.98 (C-3), 211.49 (C-20); Anal. Calcd for C29H50N2O: C, 78.68; H, 11.38; N, 6.33. Found: C, 78.55; H, 11.99; N, 6.40.
3-deoxy-3a-homo-3a-aza-lup-20(29)-en (11) was synthesized according to Kumar et al. (2008). Yield 0.34 g (79%); MP 178 °С; [α]D20 + 155° (с 0.05, CHCl3); Liter. (Kumar et al. 2008) MP 185–188 °С; δH (500.13 MHz, CDCl3) 0.78, 0.90, 1.09, 1.15, 1.48, 1.57, 1.68 (7 s, 21H, 7CH3), 1.70–2.50 (m, 25H, CH and CH2), 3.00 (br. s, 1H, NH), 3.30 (br. s, 2H, H-2), 4.50 and 4.70 (both d, 2J = 2.0 Hz, 2H, H-29); δC (125.76 MHz, CDCl3) 14.29, 16.47, 16.58, 18.07, 19.35, 21.33, 21.51, 22.85, 23.05, 25.74, 27.27, 27.83, 29.78, 33.64, 35.40, 38.39, 39.47, 39.95, 40.77, 41.09, 41.17, 43.04, 43.10, 47.37, 47.76, 48.32, 54.39, 63.15, 109.49 (C-29), 150.73 (C-20); Anal. Calcd for C30H51N: C, 84.64; H, 12.07; N, 3.29. Found: C, 84.70; H, 12.00; N, 3.30.
Pharmacological studies
Anticancer assay
In vitro cancer screen in National Cancer Institute (NCI), USA
The screening is a two-stage process, beginning with the evaluation of all compounds against the 60 cell lines at a single dose of 10−5 M. Compounds that exhibit significant growth inhibition are evaluated against the 60 cell panel at five concentration levels. The human tumor cell lines of the cancer-screening panel are grown in RPMI 1640 medium containing 5% fetal bovine serum and 2 mM L-glutamine. For a typical screening experiment, cells are inoculated into 96-well microtiter plates in 100 mL at plating densities ranging from 5000 to 40,000 cells/well depending on the doubling time of individual cell lines. After cell inoculation, the microtiter plates are incubated at 37 °C, 5% CO, 95% air, and 100% relative humidity for 24 h prior to addition of experimental drugs. After 24 h, two plates of each cell line are fixed in situ with TCA, to represent a measurement of the cell population for each cell line at the time of drug addition (time zero (Tz)). Experimental drugs are solubilized in dimethylsulfoxide at 400-fold the desired final maximum test concentration and stored frozen prior to use. At the time of drug addition, an aliquot of frozen concentrate is dissolved and diluted to twice the desired final maximum test concentration with complete medium containing 50 mg/mL gentamicin. Additional four, 10-fold or ½ log serial dilutions are made to provide a total of five drug concentrations plus control. Aliquots of 100 mL of these different drug dilutions are added to the appropriate microtiter wells already containing 100 mL of medium, resulting in the required final drug concentrations. Following drug addition, the plates are incubated for an additional 48 h at 37 °C, 5% CO, 95% air, and 100% relative humidity. For adherent cells, the assay is terminated by the addition of cold TCA. Cells are fixed in situ by the gentle addition of 50 mL of cold 50% TCA (final concentration, 10% TCA) and incubated for 60 min at 4 °C. The supernatant is discarded, and the plates are washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (100 mL) at 0.4% in 1% acetic acid is added to each well, and plates are incubated for 10 min at room temperature. After staining, the unbound dye is removed by washing five times with 1% acetic acid and the plates are air dried. The bound stain is subsequently solubilized with 10 nm Trizma base, and the absorbance is read on an automated plate reader at a wavelength of 515 nm. For suspension cells, the methodology is the same except that the assay is terminated by fixing settled cells at the bottom of the wells by gently adding 50 mL of 80% TCA (final concentration, 16% TCA). Using the seven absorbance measurements [Tz, control growth (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth is calculated at each of the drug concentrations levels. Percentage growth inhibition is calculated as:
Three dose response parameters are calculated for each experimental agent. Growth inhibition of 50% (GI50) is calculated from [(Ti_Tz)/(C_Tz)]_100 ¼ 50, which is the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. The drug concentration resulting in total growth inhibition (TGI) is calculated from (Ti ¼ Tz). The LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared with that at the beginning) indicating a net loss of cells following treatment is calculated from:
Values are calculated for each of these three parameters if the level of activity is reached; however, if the effect is not reached or is exceeded, the value for that parameter is expressed as greater or less than the maximum or minimum concentration tested (Boyd and Paul 1995; Grever et al. 1992; Monks et al. 1991, 1997; Weinstein et al. 1997).
Cell cycle analysis
HEK293 (2 × 103 cells/well), SH-SY5Y (2.5 × 105 cells/well), A549 (5 × 104 cells/well) cells were seeded in 24-wells plates and cultured for 24 h followed by treatment with compound 10 for 72 h (at IC50 values previously determined for distinct cell line). Cells of control group were treated with 0.1% DMSO. Cells were harvested and fixed in 70% ice-cold ethanol overnight. Subsequently, the cells were centrifuged, the supernatant was discarded and the pellet was treated with RNase A (50 μg/mL) for 5 min at room temperature. The treated cells were stained with propidium iodide (50 µg/mL) for 15 min at room temperature in the dark. The cells were then analyzed for cell cycle distribution by flow cytometry (“Novocyte® 2060,” “ACEA Bioscience Inc,” USA) and the changes in the cell cycle profiles were analyzed using “NovoExpress 1.2.5” (“ACEA Bioscience Inc,” USA). Experiments were repeated independently two times in triplicate.
Antimicrobial and antifungal assays
Sample preparation
Samples were prepared in DMSO and water to a final testing concentration of 32 µg/mL, in 384-well, nonbinding surface plate for each bacterial/fungal strain and keeping the final DMSO concentration to a maximum of 1% DMSO. All the sample preparations were done using liquid handling robots. Compounds that showed solubility issues during stock solution preparation are detailed in the datasheet.
Antimicrobial assay
Procedure
All bacteria were cultured in cation-adjusted Mueller–Hinton broth at 37 °C overnight. A sample of each culture was then diluted 40-fold in fresh broth and incubated at 37 °C for 1.5–3 h. The resultant mid-log phase cultures were diluted (CFU/ml measured by OD600), then added to each well of the compound-containing plates, giving a cell density of 5 × 105 CFU/mL and a total volume of 50 µl. All the plates were covered and incubated at 37 °C for 18 h without shaking.
Analysis
Inhibition of bacterial growth was determined measuring absorbance at 600 nm (OD600), using a Tecan M1000 Pro monochromator plate reader. The percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (bacteria without inhibitors) on the same plate as references. The significance of the inhibition values was determined by modified Z-scores, calculated using the median and MAD of the samples (no controls) on the same plate. Samples with inhibition value above 80% and Z-score above 2.5 for either replicate were classed as actives. Samples with inhibition values between 50 and 80% and Z-score above 2.5 for either replicate were classed as partial actives.
Hit confirmation
The percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (bacteria without inhibitors) on the same plate. The MIC was determined as the lowest concentration at which the growth was fully inhibited, defined by an inhibition ≥ 80%. In addition, the maximal percentage of growth inhibition is reported as DMax, indicating any compounds with partial activity. Hits were classified by MIC ≤ 16 μg/mL in either replicate.
Antifungal assay
Procedure
Fungi strains were cultured for 3 days on yeast extract-peptone dextrose agar at 30 °C. A yeast suspension of 1 × 106 to 5 × 106 CFU/mL (as determined by OD530) was prepared from five colonies. The suspension was subsequently diluted and added to each well of the compound-containing plates giving a final cell density of fungi suspension of 2.5 × 103CFU/mL and a total volume of 50 µL. All plates were covered and incubated at 35 °C for 24 h without shaking.
Analysis
Growth inhibition of Candida albicans was determined measuring absorbance at 530 nm (OD530), while the growth inhibition of Cryptococcus neoformans was determined measuring the difference in absorbance between 600 and 570 nm (OD600–570), after the addition of resazurin (0.001% final concentration) and incubation at 35 °C for additional 2 h. The absorbance was measured using a Biotek Synergy HTX plate reader. The percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (fungi without inhibitors) on the same plate. The significance of the inhibition values was determined by modified Z-scores, calculated using the median and MAD of the samples (no controls) on the same plate. Samples with inhibition value above 80% and Z-score above 2.5 for either replicate (n = 2 on different plates) were classed as actives. Samples with inhibition values between 50 and 80% and Z-score above 2.5 for either replicate were classed as partial actives.
Hit confirmation
In both cases, the percentage of growth inhibition was calculated for each well, using the negative control (media only) and positive control (fungi without inhibitors) on the same plate. The MIC was determined as the lowest concentration at which the growth was fully inhibited, defined by an inhibition ≥ 80% for C. albicans and an inhibition ≥ 70% for C. neoformans. Due to a higher variance in growth and inhibition, a lower threshold was applied to the data for C. neoformans. In addition, the maximal percentage of growth inhibition is reported as DMax, indicating any compounds with marginal activity. Hits were classified by MIC ≤ 16 μg/mL in either replicate.
Antibiotic, cytotoxic, and hemolytic standards preparation and quality control (QC)
Colistin and vancomycin were used as positive bacterial inhibitor standards for gram-negative and gram-positive bacteria, respectively. Fluconazole was used as a positive fungal inhibitor standard for C. albicans and C. neoformans.
The QC of the assays was determined by Z′-factor, calculated from the negative (media only) and positive controls (bacterial, fungal, or cell culture without inhibitor), and the standards. Plates with a Z′-factor of ≥0.4 and standards active at the highest and inactive at the lowest concentration were accepted for further data analysis.
Results and discussion
Chemistry
3,28-Dioximino-betulin 1 was taken as the starting compound for the designed A-azepano- and A-seco-3-amino-lupanes with C17-CH2NH2 substituent. Dioxime 1 is available from the betulonic aldehyde (Flekhter et al. 2002) and possesses promising activities (Yli-Kauhaluoma et al. 2010). The Beckmann rearrangement of dioxime 1 by the treatment with SOCl2 in dioxane according to the previously described method (Medvedeva et al. 2018) led to a mixture A-azepanono-17-nitrile 2 and 3,4-seco-4(23)-en-2,17-dinitrile 3 with yields of 55% and 38%, respectively, after isolation by column chromatography. The spectral and physicochemical data for the obtained compounds 2 and 3 were in agreement with those from the literature (Khusnutdinova et al. 2019; Tolmacheva et al. 2018). Moreover, the X-ray analysis of compound 2 (Fig. 1) confirms the location of lactame system at C3a position and that the nitrogen atom is situated between C-3 and C-4 atoms. Therefore, the C-3-oxime group in compound 1 reveals E configuration which is in agreement with those for oleanolic acid lactame (Bednarczyk-Cwynar et al. 2013). The reduction of compound 2 or 3 with LiAlH4 under reflux in THF for 1 h affected both lactame and nitrile-groups with the formation of amines 4 and 6 in good yields. The same reaction provided at room temperature led to a mixture of products with full (compound 4) and fractional regioselective reduction (compound 5) and yields of 8% and 27%, respectively, and recovery of initial compound 2 (58%) after purification by column chromatography (Scheme 1). In the 13C-NMR spectra of compounds 4–6 the signal of the carbon atom C28 is observed at δ 47.02–47.77 ppm and in the 1H NMR spectra characteristic signals of H-28 protons were appeared as two doublets at δ 2.60–2.62 and 3.00–3.03 ppm, as well as broadened signals of the NН2 group at δ 3.30–3.36 ppm. The C-3 carbon atom signal for compound 4 is detected at δ 63.2 ppm which is in agreement with those for A-azepanotriterpenoids (Medvedeva et al. 2018), for compound 6 — at δ 42.74 ppm (Giniyatullina et al. 2020). The presence of the lactam cycle in compound 5 was confirmed by the characteristic C-3 carbon signal at δ 176.31 ppm and NH-proton of lactame ring at δ 5.59 ppm.
Ozonolysis of compound 2, 3, or 4 led to 20-oxo-29-nor-derivative 7, 8, or 9, respectively. In the 13C-NMR spectra of these compounds C-20(29) double bond signals were not observed but the C-20 signal of the oxo-group appeared at δ 209.78–213.50 ppm. To compare the influence of substituent at C17 on the biological activities of A-azepano-lupanes azepanobetulin 10 and azepano-lupeol 11 were also synthesized as previously described (Lopatina et al. 2019; Kumar et al. 2008).
Biological activity
Evaluation of cytotoxicity activity
The synthesized lupane derivatives 2–11 were evaluated for their in vitro antitumor activity (cytotoxicity) toward 60 cell lines of nine different types of human cancers (lung, colon, central nervous system, ovary, renal, prostate, breast tumors, leukemia, and melanoma) according to the protocols available at the NCI (Bethesda, USA) in a single concentration 10−5 M. Each cell line was inoculated and preincubated on a microtiter plate after that test compound was added and the culture was incubated for 48 h. Results were reported as the percent of growth of the treated cells compared with the untreated control cells (negative numbers indicate cell kill). According to the NCI criteria (reduction of the growth of any one of the cancer cell lines to ca. 32% or less), compounds 2, 3, and 7 did not show cytotoxic activity against the studied cell lines. Compound 6 was active against leukemia (K-562 and SR) and colon cancer (HT29). Compound 8 inhibited the cell growth of the leukemia cell lines (CCRF-CEM, HL-60(TB), and MOLT-4), CNS cancer (SF-295 and SNB-75) and cell line renal cancer (RXF 393) and breast cancer (HS 578T). Compound 9 showed activity against one colon cancer cell line (HT29), whereas compound 11 was active toward leukemia (K-562, RPMI-8226, SR), colon cancer (COLO 205, HCT-116, HCT-15, HT29, SW-620), CNS cancer (U251), and breast cancer (MDA-MB-468). Compound 5 inhibits leukemia cell lines (CCRF-CEM, HL-60(TB), K-562, MOLT-4, SR), colon cancer (HCT-116, HT29), and melanoma (MALME-3M, UACC-257). Compound 4 showed the greatest antiproliferative activity toward 56 cell lines, resulting in 48 cases of cancer cell lethality from −9.72 to −97.20% (Table 1).
Compound 4, which exhibited the most promising results in single-dose test and fulfilling the NCI criteria for activity in that preliminary assay, was further investigated in a five-dose testing mode at ten-fold dilution (100–0.01 µM) over the full panel. For this compound, three response parameters, the GI50 (the concentration producing 50% GI, a measure of compound potency), TGI (the concentration producing 100% GI, a measure of compound efficacy), and LC50 (the concentration causing 50% lethality, a measure of compound efficacy and cytotoxicity), were determined and are summarized in Table 2.
Compound 4 exhibited remarkable activities with GI50 ranging from 1.16 to 2.27 μM against all panels of NCI-60. The highest anticancer activity was observed against leukemia cell lines with the values of GI50 1.16 μM (K-562) and 1.21 μM (SR), colon cancer cell lines with GI50 1.19 μM (HCT-15), non-small cell lung cancer cell line with GI50 1.24 μM (NCI-H460) and breast cancer with GI50 1.27 μM (MCF7). Azepanobetulin 10 was taken for the comparison and exhibited remarkable activities with GI50 ranging from 1.08 to 2.17 μM against all panels of NCI-60 (Lopatina et al. 2019) (Table 2).
In general, the replacement of the hydroxyl group by the amino group in compound 4 does not have a strong effect on the cytotoxic activity of these compounds. Pronounced activity against cell lines of leukemia (K-562), colon cancer (HCT-15), and breast cancer (MCF7) remains the same for both compounds 4 and 10, but cytotoxicity against colon cancer cell lines HCT-116 and HT29 was lower for compound 4 (GI50 1.29 and 1.27 μM for compound 10 and GI50 1.42 and 1.65 μM for compound 4, respectively), while cytotoxicity against leukemia cell line SR (GI50 1.21 μM for compound 4 instead of GI50 1.59 μM for compound 10) and non-small cell lung cancer cell line NCI-H460 (GI50 1.24 μM for compound 4 instead of GI50 1.63 μM for compound 10) was increased (Table 2).
A raw comparison of the activities of compounds 4 and 10 with respect to the activity reported for the standard drug doxorubicin, used by NCI as control (Montoya et al. 2014), reflects that the activity displayed for 4 or 10 was lower than for the standard drug except for cell lines of colon cancer HCT-15 and ovarian cancer NCI/ADR-RES (compounds 4 and 10 were ∼5 and ∼4 times more active than doxorubicin, respectively). Furthermore, at the LC50 level of cytotoxicity, compounds 4 and 10 were more efficient against six cell lines of non-small cell lung cancer, three cell lines of colon cancer, three cell lines of CNS cancer, two cell lines of melanoma, five cell lines of renal cancer, all prostate cancer cell lines, and four breast cancer cell lines. These results suggest that A-azepano-lupanes 4 and 10 are the promising triterpene structures for our future drug development antitumor studies.
The mechanism of cell-growth inhibitory activity
In non-cancerous HEK293 cells, A-azepano-betulin 10 causes cell accumulation in the G1 phase, a decrease in the S and G2/M phases and an increase of the apoptotic cells (as evidenced by the appearance of cells in subG1) (Fig. 2). Accordingly, a decrease of HEK293 cell viability upon the action of compound 10 may be due to suppression of proliferation as a result of arrest of the G1 phase. The effect in this case is considered as cytostatic rather than cytotoxic. In SH-SY5Y neuroblastoma cells, compound 10 contributes to a slight increase in the number of cells in the S phase and a decrease in the percentage of cells in phases G1 and G2/M. A decrease in the content of apoptotic cells is statistically significant. In A549 lung carcinoma cells, compound 10 causes an increase in cells in the G1 phase, followed by a decrease in the percentage of cells in the S phase and, more pronounced, in the G2/M phase. The accumulation of apoptotic cells was also noted. The cell cycle arrest in phase G1 testifies in favor of the possible cytostatic action of A-azepanobetulin 10 and suggests the presence of a differentiating effect. Thus, the data on the effect of compound 10 on the cell cycle progression suggest that the suppression of cell growth is mainly governed by the cytostatic effect, which, depending on the cell line, is associated with the arrest either S or G1 phase.
Evaluation of antimicrobial activity
The lupane triterpenoids 2, 3, 5, 6, and 10 were evaluated at the University of Queensland (Australia) against key ESKAPE pathogens: five bacterial strains (gram-negative Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, and Acinetobacter baumannii and gram-positive methicillin-resistant S. aureus (MRSA)). The antifungal activity was determined against C. albicans and C. neoformans. Colistin and vancomycin were used as positive bacteria inhibitor standards for gram-negative and gram-positive bacteria, respectively. Fluconazole was used as a positive fungal inhibitor standard for C. albicans and C. neoformans.
In the primary screening the compounds 5, 6, and 10 showed antimicrobial activity against S. aureus bacteria cultures and culture of fungi Cryptococcus neoformans var. grubii. Compound 6 was also active against bacteria Acinetobacter baumannii and fungi C. albicans (Table 3).
For these compounds, a minimum inhibitory concentration was determined for the above cultures of bacteria and fungi. Compound 5 showed weak activity against MRSA (MIC = 16 μg/mL), while the compound 6 exhibited significant bacteriostatic effect against MRSA (MIC ≤ 0.25 μg/mL) that exceeds the effect of the clinically used antibiotic vancomycin (MIC = 1 μg/mL). Compound 10 did not show antibacterial activity after repeated screening (Table 4).
Conclusions
Starting from 3,28-dioximino-betulin we have synthesized a series of C28-amino-lupanes holding A-azepano- and A-seco-3-amino-fragments, their cytotoxicity toward the NCI-60 cancer cell line panel and antimicrobial activity against key ESKAPE pathogens were evaluated. A-azepano-28-amino-betulin exhibited remarkable activities with GI50 ranging from 1.16 to 2.27 μM against all panel with the highest activity toward leukemia, colon cancer, non-small cell lung cancer, and breast cancer. The replacement of the hydroxyl group at C28 in A-azepano-betulin to the amino group did not show a strong effect on the cytotoxic activity. Both compounds were ∼5 and ∼4 times more active than doxorubicin against colon cancer HCT-15 and ovarian cancer NCI/ADR-RES cell lines that make them promising structures for the future anticancer drug development. The ability of A-azepanobetulin to inhibit cell growth may be associated with its cytostatic effect, which, depending on the cell line, is associated with the arrest of either S or G1 phase of the cell cycle. 3-Amino-3,4-seco-28-amino-lup-4(23),20(29)-dien exhibited significant bacteriostatic effect against MRSA (MIC ≤ 0.25 μg/mL) that exceeds the effect of the clinically used antibiotic vancomycin. The combination of cytotoxicity and antimicrobial activity in a series of A-azepano- and A-seco-3-amino-C28-aminolupanes makes them perspective scaffolds for further research.
References
Ali-Seyed M, Jantan I, Vijayaraghavan K, Bukhari SNA (2016) Betulinic acid: recent advances in chemical modifications, effective delivery, and molecular mechanisms of a promising anticancer therapy. Chem Biol Drug Des 87(4):517–536. https://doi.org/10.1111/cbdd.12682
Bednarczyk-Cwynar B, Zaprutko L, Froelich A (2013) Beckmann rearrangement of oxime obtained from oleanolic acid. Structure elucidation of the initial oxime. J Mol Str 1053:115–121. https://doi.org/10.1016/j.molstruc.2013.09.006
Bildziukevich U, Özdemir Z, Wimmer Z (2019) Recent achievements in medicinal and supramolecular chemistry of betulinic acid and its derivatives. Molecules 24(19):3546. https://doi.org/10.3390/molecules24193546
Boyd MR, Paul KD (1995) Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Res Rep. 34:91–109
Bruker (2014) APEX2 and SAINT. Bruker AXS Inc, Madison, WI
Catteau GL, Zhu L, Bambeke FV, Quetin-Leclercq J (2018) Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: a review. Phytochem Rev 17:1129–1163. https://doi.org/10.1007/s11101-018-9564-2
Chung PY (2019) Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: a systematic review. Phytomedicine: 152933. https://doi.org/10.1016/j.phymed.2019.152933
Csuk R (2014) Betulinic acid and its derivatives: a patent review (2008–2013). Expert Opin Ther Pat 24:913–923. https://doi.org/10.1517/13543776.2014.927441
Flekhter OB, Ashavina OY, Boreko EI, Karachurina LT, Pavlova NI, Kabal’nova NN, Savinova OV, Galin FZ, Nikolaeva SN, Zarudii FS, Baltina LA, Tolstikov GA (2002) Synthesis of 3-O-acetylbetulinic and betulonic aldehydes according to Svern and the pharmacological activity of related oximes. Pharm Chem J 36:303–306. https://doi.org/10.1023/A:1020824506140
Grever MR, Schepartz SA, Chabner BA (1992) The National Cancer Institute: cancer drug discovery and development program. Semin Oncol 19:622–638
Giniyatullina GV, Kazakova OB, Baikova IP, Yamansarov EY, Osterman IA, Komarova ES, Skvortsov DA, Saltikova IV, Majouga AG, Ivanenkov YA (2019) Synthesis and cytotoxicity of N-methylpiperazinylamide azepanobetulinic acid. Nat Prod Comm 14:1–5. https://doi.org/10.1177/1934578X19860670
Giniyatullina GV, Mustafin AG, Kazakova OB (2020) Synthesis and antitumor activity of 3-amino-3,4-Seco-Lupa-4(23),20(29)-Diene Derivatives. Chem Nat Comp 1:84–88. https://doi.org/10.1007/s10600-020-02951-1
Hoz ADe la, Cortés JA (2019) Bacterial and atypical infections in critically Ill cancer patients. Oncol Crit Care: 379–1400. https://doi.org/10.1007/978-3-319-74588-6_123
Kazakova OB, Brunel JM, Khusnutdinova EF, Negrel S, Giniyatullina GV, Lopatina TV, Petrova AV (2019b) A-ring modified triterpenoids and their spermidine-aldimines with strong antibacterial activity. Molbank: M1078. https://doi.org/10.3390/M1078
Kazakova OB, Giniyatullina GV, Medvedeva NI, Lopatina TV, Baikova IP, Tolstikov GA, Apryshko GN (2014) Synthesis and cytotoxicity of triterpenoids seven members cyclic amines. Russ J Bioorg Chem 40:217–225. https://doi.org/10.1134/s106816201402006x
Kazakova OB, Rubanik LV, Smirnova IE, Savinova OV, Petrova AV, Poleschuk NN, Khusnutdinova EF, Boreko EI, Kapustsina YM (2019a) Synthesis and in vitro activity of oleanane type derivatives against Chlamydia trachomatis. Org Commun 12:169–175. https://doi.org/10.25135/acg.oc.66.19.07.1352
Khusnutdinova EF, Petrova AV, Thi Thu HN, Thi Tu AL, Thanh TN, Ba Thi C, Babkov DA, Kazakova OB (2019) Structural modifications of 2,3-indolobetulinic acid: design and synthesis of highly potent α-glucosidase inhibitors. Bioorg Chem 88:102957. https://doi.org/10.1016/j.bioorg.2019.102957
Khusnutdinova EF, Smirnova IE, Giniyatullina GV, Medvedeva NI, Yamansarov EY, Kazakov DV, Kazakova OB, Linh PT, Viet Q, Huong DT (2016) Inhibition of alpha-glucosidase by synthetic derivatives of lupane, oleanane, ursane and dammarane triterpenoids. Nat Prod Comm 11:33–35. https://doi.org/10.1177/1934578X1601100112
Krasutsky PA (2006) Birch bark research and development. Nat Prod Rep. 23:919–942
Kumar S, Misra N, Raj K, Srivastava K, Puri SK (2008) Novel class of hybrid natural products derived from lupeol as antimalarial agents. Nat Prod Res 22(4):305–319. https://doi.org/10.1080/14786410701766349
Lopatina TV, Medvedeva NI, Baikova IP, Iskhakov AS, Kazakova OB (2019) Synthesis and cytotoxicity of O- and N-acyl derivatives of azepanobetulin. Russ J Bioorg Chem 45:292–301. https://doi.org/10.1134/S106816201904006X
Medvedeva NI, Kazakova OB, Lopatina TV, Smirnova IE, Giniyatullina GV, Baikova IP, Kataev VE (2018) Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur J Med Chem 143:464–472. https://doi.org/10.1016/j.ejmech.2017.11.035
Monks A, Scudiero DA, Johnson GS, Paull KD, Sausville EA (1997) The NCI anti-cancer drug screen: a smart screen to identify effectors of novel targets. Anticancer Drug Des 12:533–541
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull KD, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd MJ (1991) Feasibility of a highflux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Nat Cancer Inst 183:757–766
Montoya A, Quiroga J, Abonia R, Nogueras M, Cobo J, Insuasty B (2014) Synthesis and in vitro antitumor activity of a novel series of 2-pyrazoline derivatives bearing the 4-aryloxy-7-chloroquinoline fragment. Molecules 19:18656–18675. https://doi.org/10.3390/molecules191118656
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C71:3–8
Smirnova IE, Petrova AV, Kazakova OB (2019) Synthesis and cytotoxicity of A-azepanodammaradiene. Chem Nat Compd 55:883–889. https://doi.org/10.1007/s10600-019-02838-w
Sousa JLC, Freire CSR, Silvestre AJD, Silva AMS (2019) Recent developments in the functionalization of betulinic acid and its natural analogues: a route to new bioactive compounds. Molecules 24(2):355. https://doi.org/10.3390/molecules24020355
Tolmacheva IA, Nazarov AV, Eroshenko DV, Grishko VV (2018) Synthesis, cytotoxic evaluation, and molecular docking studies of the semi-synthetic “triterpenoid-steroid” hybrids. Steroids 140:131–143. https://doi.org/10.1016/j.steroids.2018.10.005
Tolstikov GA, Flekhter OB, Shul’ts EE, Baltina LA, Tolstikov AG (2005) Betulin and its derivatives. Chemistry and biological activity. Khim Interes Ustoich Razvit 13:1–30
Tolstikova TG, Sorokina IV, Tolstikov GA, Tolstikov AG, Flekhter OB (2006) Biological activity and pharmacological prospects of lupane terpenoids: I. natural lupane derivatives. Russ J Bioorg Chem 32:37–49. https://doi.org/10.1134/S1068162006010031
Weinstein JN, Myers TG, O’Connor PM, Friend Jr. SH, Fornace AJ, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD (1997) An information-intensive approach to the molecular pharmacology of cancer. Science 275:343–349. https://doi.org/10.1126/science.275.5298.343
Yli-Kauhaluoma J, Alakurtti S, Minkkinen J, Sarcerdoti-Sierra N, Jaffe CL, Heiska T (2010) Betulin derived compounds useful as antiprotozoal agents. US Patent 2010190795, filled 6 June 2007, issued 28 Oct 2010
Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu XM, Wang GJ, Chen WM, Ye WC (2015) Betulinic acid and its derivatives as potential antitumor agents. Med Res Rev 35:1127–1155. https://doi.org/10.1002/med.21353
Acknowledgements
This work was supported by Federal programs (N AAAA-A20-120012090023-8, AAAA-A20-120012090029-0, and AAAA-A17-117011910028-7). The synthesis of compounds 2, 4, 5, and 9-11 and evaluation of their biological activity were supported by the Russian Foundation for Basic Research (project no. 18-33-00364 for TVL). We thank National Cancer Institute for the screening of cytotoxicity of compounds 2–11. The antimicrobial screening was performed by CO-ADD (The Community for Antimicrobial Drug Discovery) and funded by the Wellcome Trust (UK) and The University of Queensland (Australia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Kazakova, O.B., Lopatina, T.V., Baikova, I.P. et al. Synthesis, evaluation of cytotoxicity, and antimicrobial activity of A-azepano- and A-seco-3-amino-C28-aminolupanes. Med Chem Res 29, 1507–1519 (2020). https://doi.org/10.1007/s00044-020-02577-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02577-6