Abstract
Antioxidant potencies of an ethanolic extract and its components from Lepidium latifolium were investigated. In this study, we found that the ethyl acetate soluble fraction of L. latifolium was a rich source of antioxidant, resulting from its high total phenolic content. To determine the antioxidant components of the ethyl acetate fraction, a bioassay-guided fractionation approach using 1,1-diphenyl-2-picrylhydrazyl, 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate), and ferric reducing antioxidant power assays were conducted. Nine compounds were isolated and their structures were identified by MS and NMR spectral data and comparison to reported data. They are Quercetin-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (1), Apetalumoside B6 (2), Kaempferol-3-O-β-d-glucopyranosyl-(1-2)-β-d-glucopyranoside-7-O-β-d-glucopyranoside (3), Kaempferol-7-O-α-l-rhamnopyranoside (4), Kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (5), Kaempferol-3-O-(2-O-feruloyl-β-d-glucopyranosyl-(1-2)-β-d-glucopyranoside)-7-O-glucopyranoside (6), Kaempferol-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (7), Kaempferol-3-O-robinoside-7-O-(2″″-(E)-feruloyl)-sophoroside (8), Quercetin-3-O-(2,6-di-O-β-d-glucopyranosyl)-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (9), compounds 1, 2, 4, and 8 had potent free radical scavenging activity. The IC50 values of these compounds were 9.8–12.3 and 7.4–31.4 μg/mL in DPPH and ABTS assays, respectively. The results indicate that L. latifolium is a potential natural source of antioxidants to treat several diseases related to oxidant by-products of human metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organisms that utilize oxygen are able to produce reactive oxygen species (ROS) through metabolic processes. Excessive ROS production in human metabolism can surpass the antioxidant capacity of the cell and lead to oxidative stress (Erlânio et al. 2015). Free radical mediated oxidative stress is a major causative factor in many chronic diseases, including cardiovascular diseases, cataracts, cancer, and diabetes (Cheng et al. 2015; Reetika et al. 2015). Antioxidants are substances that are capable of inhibiting oxidative chain reactions, either by delaying or inhibiting the oxidation, leading to lower concentrations of free radicals in the body. Foods with these properties can prevent peroxidation damage (Maria et al. 2016).
In recent years, natural antioxidants have attracted interest in antioxidant research as an alternative to synthetic antioxidant substances. This is mainly bacause synthetic antioxidants have numerous disadvantages, such as high manufacturing cost, environmental pollution, side-effects, and sometimes lower efficiency than natural antioxidants (Reetika et al. 2015). Therefore, studies on antioxidants, especially plant-derived natural compounds with high activity and low cytotoxicity, have become an important research field.
Lepidium latifolium, a perennial in the Brassicaceae plant family, has attracted the attention of ecologists after been recognized as a noxious weed on the western coast of North America (Seyed et al. 2015). It is an endemic species in Asia and is distributed at altitudes of 1300–2700 m in river bed wetlands or wasteland (Editorial Committee of Flora of China 1979). However, the Western Himalayan ecotype of this plant is used as a phytofood and gastrointestinal treatment (Xu et al. 2015; Zhang et al. 1994).
Previous studies had investigated essential oil from L. latifolium and described changes in protein and amino acid content over different development periods (Zhang et al. 1994). Extracts of L. latifolium have been shown to possess various biological activities, including anthelmintic, anti-protozoal, and insecticidal properties (Liu et al. 1999; Seyed et al. 2015). Although few studies have been carried out with L. latifolium, it is possible that this plant, which represents an immense biomass, is potentially rich in compounds with functional activities, such as flavonoids. In view of this, the present study was conducted to evaluate the antioxidant activities of L. latifolium extract and derived fractions, followed by isolation of its major active components. The L. latifolium extract was separated by means of an activity-guided fractionation approach using 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and ferric-reducing antioxidant power assay (FRAP) assays, followed by identification of the chemical structures of these purified antioxidants by mass spectrometry (MS) and nuclear magnetic resonance (NMR).
Material and methods
Chemicals
DPPH, ABTS, tripyridyltriazine (TPTZ), Folin–Ciocalteu reagent, Rutin and Gallic acid were purchased from Sigma Chemical Co. (USA). Quercetin, natrium aceticum, ferrous sulfate, ferric chloride, and ferrous chloride were purchased from Tianjin chemical reagent factory. Solvents used in high-performance liquid chromatography (HPLC) analysis were HPLC grade, and other chemicals were at least of analytical grade.
Plant material
The aerial parts of L. latifolium plants were collected from Haidong, Qinghai Province, China, in August 2015, and identified by Prof. Mei Lijuan of the Northwest Institute of Plateau Biology, Chinese Academy of Sciences. A voucher specimen (0324183) has been deposited in the Herbarium of Northwest Institute of Plateau Biology.
Extraction and identification of compounds
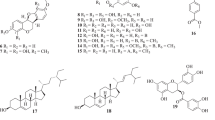
The aerial parts of L. latifolium were air-dried and powdered, and then a 2 kg portion of the powdered material was extracted three times by refluxing with 85% ethanol (20 L, 70 °C, 3 h each time). The combined extracts were filtered and evaporated under reduced pressure at 50 °C to produce a crude extract. The residue was suspended in water and successively partitioned with petroleum ether (60–90 °C), ethyl acetate (EtOAc), and then n-butanol. Part of EtOAc extract (5 g) was subjected to silica gel column chromatography and eluted with a CH2Cl2–MeOH gradient (10:0–3:7, v/v), yielding ten fractions (Fr. 1–10). Fraction 7 (503 mg) was subjected to semi-preparative HPLC (column: HC C18, 10 μm, 250 × 20 mm; Water-CH3CN: 88–12; flow rate 15 mL/min; detection 210 nm) to obtain compounds 1 (31 mg), 2 (42 mg), 3 (21 mg), 4 (26 mg), 5 (19 mg), and subfraction 7–1 (219 mg). Subfraction 7–1 (219 mg) was separated by semi-preparative HPLC (column: HCE C18, 10 μm, 250 × 20 mm; Water-CH3CN: 85–15; flow rate 15 mL/min; detection 210 nm) to obtain compounds 6 (33 mg), 7 (45 mg), 8 (65 mg), and 9 (17 mg) (Fig. 1).
1,1-Diphenyl-2-picrylhydrazyl assay (DPPH assay)
The DPPH free radical scavenging activity of the extracts and compounds from L. latifolium was evaluated using the method of Nirupama et al. (2016) with some modifications. In brief, 100 µL of 0.2 mM DPPH prepared in ethanol were added to 96-well plates containing 100 µL of samples at different concentrations. The samples were incubated for 30 min in the dark at room temperature. The absorbance of the mixture was recorded at 517 nm (A i ) and compared with that of quercetin as the positive control. The absorbance of the mixture, in which the DPPH solution was replaced by ethanol was recorded as A j . When the sample solution was replaced by solvent, the absorbance of the mixture was used as a control (A c ). All determinations were carried out more than three times. The radical scavenging activity of the tested samples was calculated as “inhibition percentage” according to the equation: Inhibition percentage (%) = [1−(A i− A j )/A c ] × 100%.
Superoxide radical scavenging assay (ABTS assay)
The ABTS assay described by Vítor et al. (2016) was used with minor modifications. ABTS radical cation was generated by mixing ABTS (7 mM) and potassium persulfate (2.45 mM) and allowing the mixture to incubate for 12 h at room temperature in the dark. The working solution was then diluted with phosphate buffered saline (pH 7.0) to an absorbance of 0.70 ± 0.02 at 734 nm. A series of 20 μL samples were mixed with 180 μL of ABTS·+ in individual wells of a 96-well microplate and incubated for 6 min at 30 °C. The absorbance of the mixture was immediately recorded at 734 nm (A i ). The absorbances of the mixtures without extract (A c ) and without ABTS (A j ) were also recorded. All determinations were carried out more than three times.The ABTS free radical scavenging activity was calculated using the formula: Inhibition percentage (%) = [1−(A i −A j )/(A i −A c )] × 100%.
FRAP assay
The ability to reduce ferric ions was measured using the modified method of Halil and Ilkay (2016). FRAP solution (180 μL, prepared daily by mixing ten volumes of 300 mM sodium acetate buffer (pH 3.6) with 1 volume of 10 mM TPTZ solution and 1 volume of 20 mM ferric chloride) was added to 20 μL of sample solution and the reaction mixture was incubated at 37 °C for 30 min. The absorbance was measured at 593 nm. Aqueous solutions of ferrous sulfate (0.05–0.5 mM) were used for calibration, and the results were expressed as FeSO4 concentration (mmol/L). All determinations were carried out more than three times.
Total phenolic content (TPC)
TPC was estimated in the extract and fractions of L. latifolium using the Folin–Ciocalteau method (Luísa et al. 2015). In brief, 0.1 mL of the extracts were diluted appropriately and mixed with 0.5 mL Folin–Ciocalteu reagent. After 6 min, 0.4 mL of 7.5% (w/v) sodium carbonate solution was added and incubated in the dark at room temperature for 1 h. The absorbance was measured by UV–Visible spectrophotometry at 765 nm. Blanks were prepared similarly but containing 50% ethanol instead of Folin–Ciocalteu reagent. TPC was expressed as gallic acid equivalents (GAE) in milligrams per gram of sample (mg GAE/g). All determinations were carried out in triplicate.
Total flavonoid content (TFC)
TFC of the extract and fractions were examined by the AlCl3 method (Kosalec et al. 2004). Plant extract (2 mL) was added to 0.6 mL sodium nitrite (5%, w/v), 0.5 mL aluminum chloride (10%, w/v), 3 mL sodium hydroxide (4.3%, w/v) and the volume was adjusted to 10 mL with ultra-pure water. After addition of each solution, the reaction mixture was shaken vigorously for 10 min at ambient temperature to complete the reaction. The absorbance of the mixture was measured at 490 nm. The calibration curve was prepared using rutin solutions. TFC was estimated using the calibration curve and expressed as rutin equivalents (mg RE/g extract). All determinations were carried out in triplicate.
Statistical analyses
The statistical significance of difference was analyzed with SPSS software, and values p < 0.05 were considered significant. The calculations of TPC and TFC in the extracts were performed using MS Excel software. All data are expressed as the mean ± SD for triplicate analyzes.
Results and discussion
DPPH radical scavenging activity of L. latifolium extracts and its soluble fractions
DPPH free radical is frequently employed to measure antioxidant activity. It is a stable free radical with an intense purple color and a strong absorption band at wave-length of 515–520 nm. The DPPH radical is scavenged by antioxidant compounds present in the extracts via proton donation to form reduced DPPH, which can be quantified by the decrease of absorbance (Shimada et al. 1992). In this study, the antioxidant activities of crude extract and the derived soluble fractions from L. latifolium were investigated. The DPPH radical scavenging activities are shown in Fig. 2. The scavenging effects of crude extract (15.2–84.5%), water (16.6–47.6%), BuOH (16.0–79.4%), EtOAc (19.3–83.1%), and Hex (13.5–27.2%) soluble fractions increased linearly with increasing concentrations (6.25, 12.5, 25, 50, 100, and 200 μg/mL). At a concentration of 50 μg/mL, the EtOAc extract (70.1%) showed higher scavenging activity than ethanolic (64.4%), water (47.6%), BuOH (55.2%) and Hex (26.4%) extract. Quercetin scavenges 82.0% of the DPPH radical at a concentration of 50 μg/mL. The IC50 values of the crude extract, water soluble fraction, BuOH soluble fraction, EtOAc soluble fraction and Hex soluble fraction were 34.3, >100.0, 46.6, 23.9, and >100.0 μg/mL, respectively (Table 1), compared to 6.6 μg/mL for quercetin. The results showed that antioxidant phytochemicals were abundantly present in ethanolic extracts of L. latifolium, particularly in the EtOAc soluble fraction. According to the results reported by Yuan et al. (2014), an ethanolic extract of Forsythia suspensa leaves effectively scavenges DPPH radical with an IC50 value of 86.77 μg/mL. Zhigang et al. (2014) also reported that the IC50 value of an ethanolic extract of Musa basjoo flowers was 21.6 μg/mL in a DPPH assay. The results indicate that the ethanolic extract and EtOAc soluble fraction from L. latifolium have superior DPPH free radical scavenging activity.
ABTS radical scavenging activity of L. latifolium extracts and its soluble fractions
ABTS·+ is frequently employed to evaluate the antioxidant activities of plant materials. Cationic ABTS·+ is reactive toward most antioxidants, including phenolic compounds (Walker and Everette 2009). The color of the stable ABTS·+ radical cation is diminished during incubation with antioxidant. The antioxidant capacity of plant extracts is related to the phenolic compound content, since these compounds are able to donate electrons or hydrogen atoms and to capture free radicals (Awe et al. 2013). Figure 3 shows the ABTS·+ scavenging activities of the ethanolic extract of L. latifolium, its soluble fractions and quercetin. The scavenging effects of crude extract (11.9–65.1%), water (8.1–43.0%), BuOH (9.0–63.2%), EtOAc (12.0–74.1%), and Hex (7.0–24.0%) soluble fractions increased linearly with increasing concentration over the range of 6.25–200 μg/mL. The IC50 values of the crude extract, water soluble fraction, BuOH soluble fraction, EtOAc soluble fraction, and Hex soluble fraction were 70.4, >100.0, 86.8, 46.5, and >100.0 μg/mL, respectively (Table 1). The IC50 of quercetin was 11.8 μg/mL. These results demonstrated that the ABTS·+ scavenging ability of the EtOAc soluble fraction from the ethanolic extract of L. latifolium was greater than those of fractions and the crude extract. Similar results were observed in the DPPH radical scavenging assay. Haifa et al. (2016) reported that the IC50 values of ethanolic extracts of Capparis aegyptia, Capparis orientalis, Capparis ovata subsp. ovata, Capparis sicula subsp. sicula, Capparis spinosa subsp. spinosa var. spinosa, and Capparis zoharyi ranged between 40.98 and 62.63 μg/mL in the ABTS assay. The methanol extract from leaves of Quercus suber showed excellent inhibitory activity against ABTS radical with 67% inhibition at a concentration of 1 mg/mL (Luísa et al. 2015). Comparing these results indicated that the EtOAc soluble fraction of L. latifolium extract would be an excellent source of natural antioxidants.
Reducing power of L. latifolium extracts and its soluble fractions
The reducing power method reflects the electron donating ability of antioxidants present in the extracts to convert Fe3+ into Fe2+ (Gordon et al. 1990). Therefore, the absorbance (OD value) can be monitored at 593 nm to measure production of Fe2+ (Nemanja et al. 2016). Increased absorbance indicates an increase in the reducing power activity. The reducing capacities of ethanolic extracts of L. latifolium and its soluble fractions are shown in Fig. 4. The data show that reduction of iron by the ethanolic extract and its soluble fractions increased with increasing concentrations over the range of 6.25–200 μg/mL. The reducing power of the crude extract and its derived soluble fractions increased at a concentration of 50 μg/mL in the following order: quercetin (OD value = 2.8) >EtOAc soluble fraction (OD value = 1.3) > BuOH soluble fraction (OD value = 1.0) > crude extract (OD value = 0.8) > water soluble fraction (OD value = 0.3) > Hex soluble fraction (OD value = 0.1). These results demonstrated that the EtOAc soluble fraction had greater capacity to reduce Fe3+ to Fe2+ than the other fractions and crude extract, which may be due to a higher concentration of phenolics in this extract. It has previous been reported that the ethanolic extract of Cunninghamia konishii Hayata had reducing properties, giving absorption value of 0.8 at a concentration of 50 μg/mL in the reducing power assay (Cheng et al. 2015). Singh et al. (2007) reported that the reduction potential of the ethyl acetate extract of Acacia auriculiformis bark gave an absorption value of 0.73 at a concentration of 50 μg/mL in the reducing power assay. Comparing these data indicated that the extract of L. latifolium, especially the EtOAc soluble fraction, had better reducing power.
TPC and TFC of L. latifolium extracts and its soluble fractions
It is very important to investigate the phenolic and flavonoid compound content, since the antioxidant activity of various plant extracts is attributed to the presence of these compounds, particularly the flavonoids. The total phenolic content, evaluated in the Folin–Ciocalteu assay, was expressed as mg GAE/g of crude extract and the derived fractions of L. latifolium. The TPC ranged from 0.0 ± 0.0 to 172.4 ± 1.5 mg GAE/g (Table 2). The order of TPC was: EtOAc soluble fraction (172.4 ± 1.5 mg GAE/g) > crude extract (124.1 ± 2.1 mg GAE/g) > BuOH soluble fraction (67.2 ± 1.1 mg GAE/g) > water soluble fraction (31.5 ± 0.5 mg GAE/g) > Hex soluble fraction (0.0 ± 0.0 mg GAE/g). According to these results, there was a high correlation between the TPC and antioxidant activities for all samples, including DPPH radical scavenging activity, ABTS assay, and FRAP assay. A similar finding was observed in studies by Zhigang et al. (2014), Ji-Sun et al. (2015), and Jing et al. (2016) in which extracts containing abundant phenolic components were well correlated with their antioxidant activity. The EtOAc soluble fraction of L. latifolium extract was further investigated to isolate and identify the antioxidant compounds.
The aluminum chloride complexation method was used to determine the flavonoid content. TFC in the crude extract and its derived soluble fractions were calculated as RE in mg/g sample. TFC values showed a similar distribution to TPC (Table 2). The highest concentration was found, as for phenolics, in the EtOAc soluble fraction (110.8 ± 1.9 mg RE/g), followed by crude extract (52 ± 0.9 mg RE/g), water soluble fraction (14.3 ± 0.2 mg RE/g), BuOH soluble fraction (10 ± 0.3 mg RE/g), and Hex soluble fraction (0 ± 0.0 mg RE/g). The results showed that TFC of an ethanolic extract of L. latifolium was enriched in the EtOAc soluble fraction. Because of their strong ability to donate electrons or hydrogen atoms, the high level of total flavonoids suggests that the extract has an antioxidant activity. Therefore, the EtOAc soluble fraction was further investigated in this study to determine its phytochemical characteristics and antioxidant activity.
Antioxidant activities and TPC of subfractions from the EtOAc soluble fraction
In view of its higher antioxidant activity, the EtOAc soluble fraction was separated on silica gel column chromatography with a gradient of MeOH–CH2Cl2 to give nine fractions (Fr. 1–Fr. 9). Antioxidant activities of the fractions at a concentration of 50 μg/mL were determined using DPPH, ABTS, and FRAP assays, and the results are shown in Table 3. Fractions 3, 5, 6, and 7 showed high antioxidant activity in the DPPH assay (>60%), particularly Fr. 7 (84.6%). In the ABTS assay, Fractions 3 and 7 showed the highest antioxidant activity (60.1 and 59.0%, respectively). The data in Table 3 show the reducing power (as indicated by absorbance at 593 nm) of the nine fractions, and Fr. 7 showed the greatest reducing power (OD value = 1.8) compared to the other samples (OD values = 0.4–1.5). Figure 5 shows the TPC of the nine fractions measured as GAE in mg/g of sample, which ranged from 91.2 to 268.3 mg GAE/g. Fraction 7 had the highest total phenolic content compared to the other samples. The results indicated a strong relationship between TPC and antioxidant activity. Fraction 7 was therefore further purified by semi-preparative HPLC.
Identification of active compounds
Bioassay-guided fractionation of Fr. 7 isolated nine active compunds. The structures were characterized as Quercetin-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (1) (Li et al. 2014), Apetalumoside B6 (2) (Shi et al. 2015), Kaempferol-3-O-β-d-glucopyranosyl-(1–2)-β-d-glucopyranoside-7-O-β-d-glucopyranoside (3) (Kim et al. 2002), Kaempferol-7-O-α-l-rhamnopyranoside (4) (Li et al. 2006), Kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (5) (Ouyang et al. 2003), Kaempferol-3-O-(2-O-feruloyl-β-d-glucopyranosyl-(1–2)-β-d-glucopyranoside)-7-O-glucopyranoside (6) (Kim et al. 2002), Kaempferol-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (7) (Tang et al. 2001), Kaempferol-3-O-robinoside-7-O-(2″″-(E)-feruloyl)-sophoroside(8) (Rasha et al. 2014), Quercetin-3-O-(2,6-di-O-β-d-glucopyranosyl)-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (9) (Lea et al. 2011). They were identified by spectroscopic methods, including MS and NMR, and by comparison of their data with those reported in the literature. Compounds 1, 2, and 3 were isolated previously from Lepidium apetalum (Shi et al. 2015). Compounds 4 and 5 were isolated previously from Orostachys fimbriatus (Zheng et al. 2009). Compounds 6, 7, 8, and 9 was previously isolated from Brassica juncea, Carduus crispus, Suaeda maritima, and Barbarea vulgaris, respectively (Kim et al. 2002; Tang et al. 2001; Rasha et al. 2014; Lea et al. 2011).
Antioxidant activities of isolated compounds
The isolated compounds were evaluated for their antioxidant activity in DPPH, ABTS, and the FRAP assays. The results for compounds 1–9 and quercetin are shown in Table 4. The DPPH radical scavenging activities of compounds 1, 2, and 8 were the same as that of quercetin. In the ABTS assay, compound 2 had stronger scavenging activity than quercetin. In the FRAP assay, compounds 2, 4, 8, and quercetin at a concentration of 50 μg/mL had similar reducing power with OD values of 2.6, 2.3, 2.4, and 2.8, respectively. To the best of our knowledge, this is the first time that bioactive flavonoids have been isolated from L. latifolium using the activity-guided fractionation technique, together with determination of their structure identified and antioxidant activities.
Conclusion
This is the first report demonstrating that the EtOAc soluble fraction of ethanolic extracts from L. latifolium possesses beneficial antioxidant activity based on multiple in vitro assays. The bioactive phytochemicals, Quercetin-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (1), Apetalumoside B6 (2), Kaempferol-3-O-β-d-glucopyranosyl-(1–2)-β-d-glucopyranoside-7-O-β-d-glucopyranoside (3), Kaempferol-7-O-α-l-rhamnopyranoside (4), Kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (5), Kaempferol-3-O-(2-O-feruloyl-β-d-glucopyranosyl-(1–2)-β-d-glucopyranoside)-7-O-glucopyranoside (6), Kaempferol-3-O-β-d-sophoroside-7-O-α-l-rhamnoside (7), Kaempferol 3-O-robinoside-7-O-(2″″-(E)-feruloyl)-sophoroside(8), Quercetin-3-O-(2,6-di-O-β-d-glucopyranosyl)-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside (9), were derived from the active Fr. 7 of EtOAc soluble fraction, and exhibited significant antioxidant activitiy. In particular, we isolated the presence of the flavonoids in L. latifolium for the first time. Compounds 1, 2, 4, and 8 showed potent free radical scavenging activity. The IC50 values of these compounds were 9.8–12.3 and 7.4–31.4 μg/mL in DPPH and ABTS assays, respectively. The compounds isolated in this study and the extracts are very promising, since they have great potential for use as antioxidants in the food and pharmaceutical industries. For full utilization of the species, our results provide evidence that L. latifolium is a potential natural source of antioxidants for further food industry and pharmaceutical applications.
References
Awe FB, Fagbemi TN, Ifesan BOT, Badejo AA (2013) Antioxidant properties of cold and hot water extracts of cocoa, Hibiscus flower extract, and ginger beverage blends. Food Res Int 52:490–495
Cheng SS, Yen PL, Chang ST (2015) Phytochemicals from wood extract of Cunninghamia konishii Hayata as antioxidant agents. Ind Crops Prod 64:39–44
Editorial Committee of Flora of China (1979) Flora of China, Vol. 67. Beijing Scientific Press, Beijing, p 325
Erlânio OS, Camila MBAM, Camila BN, Aline AB, Margareth LA, José GMC (2015) Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind Crops Prod 70:7–15
Gordon MH (1990) The mechanism of antioxidant action in vitro. In: Hudson BJF (ed) Food antioxidants. Elsevier Applied Science, London, p 1–18
Haifa A, Emna M, Wafa R, Lamia H, Slim R, Mohamaed NR, Zeineb G (2016) Phenolic composition and antioxidant activity of aqueous and ethanolic leaf extracts of six Tunisian species of genus Capparis Capparaceae. Ind Crops Prod 92:218–226
Halil IO, Ilkay K (2016) Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using differentextraction methods. Ind Crops Prod 91:114–124
Jing Z, Thomas SV, Yue G, Yadong Q, Kit C, Min-Hsiung P, Chi-Tang H, James ES, QinglIi W (2016) Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem 190:673–680
Ji-Sun H, Hee-Young K, Seung-Taik L (2015) Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind Crops Prod 73:99–105
Kim JE, Jung MJ, Jung HA, Woo JJ, Cheigh HS, Chung HY, Choi JS (2002) A new Kaempferol 7-O-triglucoside from the leaves of Brassica juncea L. Arch Pharm Res 25:621–630
Kosalec I, Bakmaz M, Pepeliniak S, Vladimir-Knezevic S (2004) Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm 54:65–72
Lea DB, Carl EO, Jens KN, Niels A (2011) Polymorphism for novel tetraglycosylated flavonols in an eco-model Crucifer, Barbarea vulgaris. J Agric Food Chem 59:6947–6953
Li D, Ikeda T, Matsuoka N, Nohara T, Zhang H, Sakamoto T, Nonaka GI (2006) Cucurbitane glycosides from unripe fruits of Lo Han Kuo (Siraitia grosvenori). Chem Pharm Bull 54:1425–1435
Li Y, Yili A, Liu Y, Kawuli A, Aisa HA (2014) Chemical constituents of Hippophae rhamnoides subsp. turkestanica fruits. Chem Nat Compd 50:352–360
Liu S (1999) Economic flora of Qinghai, Vol. 2. Qinghai people’s press, Qinghai, p 227
Luísa C, João P, Fernando A, Nuno RN, José MFN, Anabela R (2015) Phenolic composition, antioxidant potential and in vitro inhibitory activity of leaves and acorns of Quercus suber on key enzymes relevant for hyperglycemia and Alzheimei’s disease. Ind Crops Prod 64:45–51
Maria L, Bezerra A, Wallace ESF, Patrícia LDM, José DAS, Ricardo EA (2016) Bioactive compounds and antioxidant potential fruit of Ximenia americana L. Food Chem 192:1078–1082
Nemanja S, Tatjana M, Bojan Z, Vesna S, Violeta M, Jovana J, Ljiljana C, Branislava K, Nirit B (2016) Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. Wageningen J Life Sci 78:21–28
Nirupama G, Dilip KR, Nigel PB, Eimear G, Mohammad BH (2016) Antioxidant-guided isolation and mass spectrometric identification of the major polyphenols in barley (Hordeum vulgare) grain. Food Chem 210:212–220
Ouyang MA, He ZD, Wu CL (2003) Anti-oxidative activity of glycosides from Ligustrum sinense. Nat Prod Res 17:381–388
Rasha RAE, Ragaa MAMMS, Ahmad F (2014) Three new flavonol glycosides from Suaeda maritima. J Asian Nat Prod Res 16(5):434–439
Reetika S, Nishi K (2015) Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—a valuable medicinal tree. Ind Crops Prod 73:1–8
Seyed MR, Mohammad A, Arash J, Seyed HM (2015) The field efficacy of Lepidium latifolium and Zataria multiflora methanolic extracts against Varroa destructor. Parasitol Res 114:4233–4238
Shi P, Chao L, Wang T, Liu E, Han L, Zong Q, Li X, Zhang Y, Wang T (2015) New bioactive flavonoid glycosides isolated from the seeds of Lepidium apetalum Wild. Fitoterapia 103:197–205
Singh R, Singh S, Kumar S, Arora S (2007) Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem Toxicol 45:1216–1223
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthum on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Tang YP, Lou FC, Wang JH (2001) Two Kaempferol triglycosides from pericarps of Sophora japonica L. China J Chin Mat Med 26:839–845
Vítor S, Eulogio JL, Sandra G, Paula C (2016) Ulex europaeus: from noxious weed to source of valuable isoflavones and flavanones. Ind Crops Prod 90:9–27
Walker RB, Everette JD (2009) Comparative reaction rates of various antioxidants with ABTS radical cation. J Agric Food Chem 57:1156–1161
Xu C, Wang L, Gao W (2015) Optimization of ultrasonic-assisted extraction for polysaccharide from Lepidium latifolium by response surface methodology. Cereals Oils 28:59–62
Yuan J, Liu X, Yang J, Cui X (2014) Forsythia suspense leaves, a plant: extraction, purification and antioxidant activity of main active compounds. Eur Food Res Technol 238:527–533
Zhang X, Hu B (1994) Study on the constituents of Lpidium latifolium. Acta Bot Boreal Occident Sin 14(4):329–333
Zheng W, Zhong Y, Sun J, Zhang P (2009) Study on the constituents of Orotachys fimbriatus. Chin Trad Herb Drug 40:859–862
Zhigang T, Anyi C, Bendui Q, Le C, Yanqun X (2014) Chemical constituents and antioxidant activity of the Musa basjoo flower. Eur Food Res Technol 239:501–508
Acknowledgements
This research was sponsored by the Project of Discovery, Evaluation and Transformation of Active Natural Compounds, Strategic Biological Resources Service Network Program of Chinese Academy of Sciences (ZSTH-027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xiang, Y., Haixia, W., Lijuan, M. et al. Isolation, purification and identification of antioxidants from Lepidium latifolium extracts. Med Chem Res 27, 37–45 (2018). https://doi.org/10.1007/s00044-017-2042-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2042-3