Abstract
A new class of chalcones, aurones and flavones derived from carbazole is designed as potential antimicrobial and antioxidant agents. Synthesis of (Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-ones and 2-(9-ethyl-9H-carbazol-3-yl)-4H-chromen-4-ones was carried out by the oxidation of (E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(2-hydroxyphenyl)prop-2-en-1-ones under microwave irradiation and conventional heating. All the newly synthesized compounds were characterized on the basis of IR, 1H NMR, 13C NMR, mass and analytical data. All the synthesized compounds were evaluated for their antibacterial, antifungal and antioxidant activities. Synthesized compounds were screened in vitro for antibacterial activity against two gram-positive bacterial strains like Staphylococcus aureus and Bacillus subtilis and two gram-negative bacterial strains like Escherichia coli and Klebsiella pneumonia and antifungal activity by inhibitory action against three fungal strains like Fusarium oxysporum, Aspergillus niger and Aspergillus flavus. The synthesized compounds were also evaluated for their DPPH radical scavenging activity. All the newly synthesized compounds have shown good antibacterial, antifungal and antioxidant activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbazole scaffolds are embedded with many natural products and drug molecules (Itoigawa et al., 2000), mainly extracted from the plants of Rutaceae family, widely used in folk medicine. Carbazole skeleton containing alkaloids, ellipticine, olivacine, datelliptium, retelliptine and pazelliptine was proved as antitumor drugs. Furthermore, carbazole alkaloids extracted from Clausena heptaphylla found to exhibit antimicrobial activity. Flavonoids, medicinally important class of compounds extracted from various natural plants, possess a variety of pharmacological properties, including antioxidant, anticancer (Li et al., 2008), antihypertensive (Xuea et al., 2008) and anti-inflammatory (Lim et al., 2011) activities.
Chalcones, aurones and flavones are three major subclasses of flavonoids. Among these aurones, (Z)-2-benzylidenebenzofuran-3-(2H)-ones constitute a less studied subclass of flavonoids, which occur rarely in nature. These are responsible for the pigmentation of flowers and fruits, especially for the bright yellow color of flowers. Aurones have been reported to possess insect antifeedant (Morimoto et al., 2007), anticancer (Cheng et al., 2010), anti-inflammatory (Shin et al., 2011), antileishmanial (Kayser et al., 1998), antibacterial (Hadj-esfandiari et al., 2007) and also show inhibitory activity against a variety of enzymes and proteins (Thomas et al., 2003; Okombi et al., 2006). Surprisingly, only few studies on the antioxidant activity (Detsi et al., 2009) of aurones exist. Flavones exhibit a wide spectrum of biological activities such as anti-inflammatory (Bano et al., 2013), antiallergic (Tomohiro et al., 2008), anticancer (Lin et al., 2008) and neuroprotective properties (Groot and Rauen 1998). Chalcones are flavonoid and isoflavonoid precursors, abundant in edible plants, and display a wide spectrum of biological activities, including antioxidant (Shenvi et al., 2013), antibacterial (Tran et al., 2012), antileishmanial (Barbosa et al., 2011), anticancer (Boumendjel et al., 2008), antiangiogenic (Radha et al., 2012), anti-infective and anti-inflammatory (Wu et al., 2011). The growing interest in these compounds and their potential use in medicinal applications are proved by the growing number of publications concerning the synthesis and biological evaluation of chalcone analogues.
Microwave-assisted organic synthesis (MAOS) (Ashok et al., 2013, 2014) has emerged as a new tool in organic synthesis. This technique offers simple, clean, fast, efficient and economic for the synthesis of a large number of organic molecules. Important advantage of this technology includes highly accelerated rate of the reaction, reduction in reaction time with an improvement in the yield and quality of the product (Fig. 1).
In this study, we have synthesized carbazol substituted chalcones 3a–i, aurones 4a–i and flavones 5a–i with the aim to study the structure–activity relationship and therefore to provide variation in activity from straight-chained α, β-unsaturated carbonyl compounds, i.e., chalcones to aurones and flavones (Schemes 1, 2).
Results and discussion
Chemistry
The precursor chalcones 3a–i were synthesized by the Claisen–Schmidt condensation of substituted 2 hydroxy acetophenone with 9-ethyl-9H-carbazol-3-carbaldehyde in the presence of KOH in MeOH. Further, these 2′ hydroxy chalcones 3a–i were oxidized with cupric bromide and iodine in presence of DMSO to afford corresponding aurones 4a–i and flavones 5a–i. In the reaction of 2′ hydroxy chalcones 3a–i with CuBr2, a five-membered transition state is favorable leading to corresponding aurones 4a–i, whereas in case of iodine six-membered intermediate is favorable resulting in corresponding flavones 5a–i (Scheme 3). All the compounds were synthesized by microwave irradiation method as well as conventional heating method. In these, the microwave irradiation method proved to be environmentally benign, high yielding and high rate of acceleration (Table 1).
Structures of synthesized compounds (4a–i and 5a–i) were characterized by spectral data such as IR, 1H NMR, 13C NMR, mass and elemental analysis. In IR spectrum of the representative compound 3a showed signal for carbonyl group (–C=O) at 1693 cm−1. The 1H NMR spectrum of the representative compound 4a showed a characteristic singlet for =C–H proton at δ 7.17, a doublet of doublet for Ar–H2′ at δ 8.18 and two doublets for aromatic protons Ar–H4′, Ar–H1′ at δ 8.69 and δ 8.19, respectively. In the 13C NMR spectrum of 4a, the carbonyl carbon appeared at δ 184.4, C–O carbon at δ 165.7 and N–CH2 carbon at δ 37.8. The GC–MS spectrum exhibited M+ peak at m/z 339. In 1H NMR, the representative compound 5a showed a characteristic singlet for Ar–H3 at δ 6.91, two doublets for Ar–H1′, Ar–H2′ appeared at δ 8.36 and δ 8.18, respectively. In 13C NMR spectrum, C=O carbon appeared at δ 177.2 and C–O carbon at δ 165.5. The ESI–MS spectrum exhibited (M + H) peak at m/z 340.
Antibacterial activity
All the synthesized compounds 3a–i, 4a–i and 5a–i were screened in vitro for their antibacterial activity against gram-positive bacterial strains [Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633)] and gram-negative bacterial strains [Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 13883)] at 20 and 40 µg/ml concentrations (Figs. 2, 3). The zone of inhibition (in mm) was compared with standard drug ciprofloxacin. The results are given in Table 2.
All the compounds showed relatively better activity against gram-positive bacterial strains than gram-negative bacterial strains. There was no remarkable change in antibacterial activity from chalcones to aurones and flavones. Among all, compounds 3a, 3h, 3i, 3a, 4h, 4i, 5a, 5h and 5i were shown promising activity against gram-positive bacterial strains and compounds 3c, 3f, 4c, 5c and 5f were shown moderate zone of inhibition, indicating that compounds with electron-donating substitutions on phenyl ring, i.e., –OCH3 and –OC2H5, shown maximum zone of inhibition. Compounds with substitutions at R1 = Cl and R1 = Cl, R2 = Me on phenyl ring shown moderate activity. Compounds 3a, 4a and 5a shown maximum zone of inhibition; remaining compounds shown poor activity against gram-negative bacterial strains, indicating that compounds without substitutions on phenyl ring were active against gram-negative bacterial strains.
Compounds 3a (13 mm), 3h (13 mm), 3i (14 mm), 4a (12 mm), 4h (13 mm), 4i (14 mm), 5a (13 mm), 5h (13 mm) and 5i (13 mm) were shown maximum zone of inhibition compared with standard drug ciprofloxacin (15 mm) against S. aureus at 20 µg/ml concentration. Compounds 3h (13 mm), 4a (13 mm), 4i (13 mm) and 5i (14 mm) were showed high resistance compared with standard drug at 20 µg/ml concentration against B. faecalis.
Antifungal activity
The antifungal activity of synthesized compounds 3a–i, 4a–i and 5a–i was tested against three pathogenic fungi, Fusarium oxysporum, Aspergillus niger and Aspergillus flavus (Fig. 4). All the compounds were shown moderate activity against the tested fungal strains. Among all, chalcones shown relatively less antifungal activity compared with aurones and flavones. Compounds 4b, 5b with flouro substitution on ring showed maximum activity against A. niger and compounds 4h, 5h with methoxy substitution on ring were active against A. flavus, and compounds 4b, 5h shown promising activity against F. oxysporum.
Compounds 4b (15.8 mm) against A. niger, 4a (13.6 mm), 5g (12.8 mm) against A. flavus and 4b (17.4 mm) against F. oxysporum shown maximum zone of inhibition than standard drug amphotericin-B.
DPPH radical scavenging activity
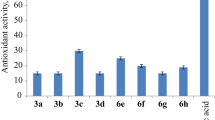
DPPH radical scavenging activity of compounds 3a–i, 4a–i and 5a–i was measured at 100 µg/ml concentration in triplicate using sodium ascorbate as standard (Fig. 4). Compounds 3g, 3h, 3i, 4f, 4g, 4h, 4i, 5c, 5d, 5h and 5i were shown promising DPPH radical scavenging activity compared with standard, indicating that compounds with electron-donating groups (OCH3 and OC2H5) and weak electron-withdrawing groups (Cl and Br) increase the antioxidant activity of the compounds. Compounds 3a, 3e, 4a, 4e, 5a and 5e and shown moderate activity compared with standard, indicating that compounds with CH3 and without substitution on ring showed moderate antioxidant activity in Table 3.
Experimental
Chemistry
Melting points were determined in open glass capillary tube on a Gallen-Kamp MFB-595 apparatus and are uncorrected. The progress of reactions was monitored by TLC (Silica gel, aluminum sheets 60 F254, Merck). The IR spectra were taken on a Perkin–Elmer FT-IR-8400s, using samples in KBr disks. Microwave reactions were carried out in the milestone multi SYNTH microwave system. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on Bruker Avance II 400 spectrometer using CDCl3 as solvent and TMS as the internal standard; the chemical shifts are expressed in δ ppm. Mass spectra were recorded on GCMS-QP 1000 EX and SHIMADZU LCMS 2020 mass spectrometers. Elemental microanalysis was performed on a Perkin–Elmer CHN-2400 analyzer.
General procedure for the synthesis of compounds 3a–i
Conventional method
To a stirred solution of substituted 2-hydroxy acetophenone 1 (1.19 ml, 10 mmol) and KOH (20 %, w/v aqueous solution) in ethanol (30 ml) was added 9-ethyl-9H-carbazol-3-carbaldehyde 2 (2.23 g, 10 mmol), and the mixture was stirred at room temperature for 6–8 h. After completion of reaction (as indicated by TLC), the reaction mixture was poured into ice-cold water and neutralized with dil. HCl. The yellow product formed was filtered and recrystallized from ethanol.
Microwave irradiation method
A mixture of substituted 2-hydroxy acetophenone 1 (1.19 ml, 10 mmol), 9-ethyl-9H-carbazol-3-carbaldehyde 2 (2.23 g, 10 mmol) and powdered KOH was taken in a quartz tube and inserted into a Teflon vial with screw capped, and then it was subjected to microwave irradiation at 180 watts for 5–8 min. As indicated by TLC, after completion of reaction, the reaction mixture was poured into ice-cold water and neutralized with dil. HCl. The yellow product formed was filtered and recrystallized from ethanol.
(E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(2-hydroxyphenyl) prop-2-en-1-one ( 3a )
Yellow colored solid; m.p.: 141–143 °C; IR (KBr, cm−1): 3442 (OH), 1630 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.13 (s, 1H, Ar–OH), 8.40 (d, 1H, J = 1.6 Hz, Ar–H4″), 8.19–8.14 (m, 2H, Ar–H1″ and H2), 8.03–8.02 (dd, 1H, J = 7.6, 1.2 Hz, Ar–H2″), 7.72 (d, 1H, J = 15.6 Hz, H2), 7.82–6.95 (m, 8H, Ar–H), 4.40 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 193.6 (C, C-1), 163.6 (C, C-2′), 147.0 (C, C-3), 141.7 (C, C-3″), 140.6 (C, C-1a″), 135.7 (C, C-4′), 129.5 (C, C-3′), 126.5 (C, C-6′), 126.5 (C, C-1′), 126.4 (C, C-4a″), 125.8 (C, C-8a″), 123.7 (C, C-1″), 122.9 (C, C-5a″), 121.8 (C, C-7″), 120.6 (C, C-5″), 120.4 (C, C-6″), 119.8 (C, C-2″), 118.5 (C, C-2), 117.0 (C, C-8″), 108.9 (C, C-4″), 37.7 (C, CH2), 13.7 (C, CH3); GC–MS: 341 (M+). Anal. Calc. for C23H19NO2: C, 80.92; H, 5.61; N, 4.10. Found: C, 81.05; H, 5.68; N, 4.15.
(E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(5-fluoro-2-hydroxyphenyl)prop-2-en-1-one ( 3b )
Yellow colored solid; m.p.: 198–200 °C; IR (KBr, cm−1): 3445 (OH), 1634 (C=O); 1H NMR (CDCl3, 400 MHz): δ 12.81 (s, 1H, Ar–OH), 8.40 (d, 1H, J = 1.2 Hz, Ar–H4″), 8.20–8.15 (m, 2H, Ar–H1″ and H2), 7.82–7.79 (dd, 1H, J = 1.6, 6.8 Hz, Ar–H2″), 7.58 (d, 1H, J = 15.2 Hz, H2), 7.68–6.98 (m, 7H, Ar–H), 4.39 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 192.6 (C, C-1), 159.7 (C, C-2′), 156.0 (C, C-5′), 153.6, 148.1 (C, C-3), 141.8 (C, C-3″), 140.5 (C, C-1a″), 126.7 (C, C-6′), 126.5 (C, C-1′), 125.3 (C, C-3′), 123.6 (C, C-8a″), 123.4 (C, C-4a″), 123.2 (C, C-1″), 122.8 (C, C-5a″), 122.2, 120.7, 120.6 (C, C-5″), 119.9 (C, C-6″), 119.7 (C, C-2), 119.6 (C, C-2″), 116.0 (C, C-8″), 114.4 (C, C-4′), 114.3 (C, C-4″), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 359 (M+). Anal. Calc. for C23H18FNO2: C, 76.86; H, 5.05; N, 3.90. Found: C, 76.95; H, 5.13; N, 3.95.
(E)-1-(5-chloro-2-hydroxyphenyl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one ( 3c )
Yellow colored solid; m.p.: 148–150 °C; IR (KBr, cm−1): 3424 (OH), 1632 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.04 (s, 1H, Ar–OH), 8.43 (d, 1H, J = 1.2 Hz, Ar–H4″), 8.21–8.17 (m, 2H, Ar–H1″ and H2), 7.96 (d, 1H, J = 2.4 Hz, Ar–H6′), 7.83–7.81 (dd, 1H, J = 1.6, 7.2 Hz, Ar–H2″), 7.76–7.30 (m, 6H, Ar–H), 6.99 (d, 1H, J = 9.2 Hz, Ar–H3′), 4.40 (q, 2H, N–CH2), 1.47 (t, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ 192.6 (C, C-1), 162.0 (C, C-2′), 148.3 (C, C-3), 141.9 (C, C-3″), 140.5 (C, C-1a″), 135.7 (C, C-4′), 128.7 (C, C-6′), 126.8 (C, C-8a″), 126.5 (C, C-1′), 125.3 (C, C-1″), 123.6 (C, C-5′), 123.3 (C, C-5a″), 122.8 (C, C-3′), 121.9 (C, C-7″), 120.9 (C, C-5″), 120.7 (C, C-6″), 119.9 (C, C-2″), 115.9 (C, C-8″), 109.0 (C, C-4″), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 375 (M+). Anal. Calc. for C23H18ClNO2: C, 73.50; H, 4.83; N, 3.73. Found: C, 73.61; H, 4.88; N, 3.77.
(E)-1-(5-bromo-2-hydroxyphenyl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one ( 3d )
Yellow colored solid; m.p.: 134–136 °C; IR (KBr, cm−1): 3442 (OH), 1627 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.06 (s, 1H, Ar–OH), 8.43 (d, 1H, J = 2.0 Hz, Ar–H4″), 8.22–8.18 (m, 2H, Ar–H1″ and H2), 8.10 (d, 1H, J = 2.8 Hz, Ar–H6′), 7.83 (dd, 1H, J = 2.0, 9.0 Hz, Ar–H2″), 7.61 (d, 1H, J = 14.8 Hz, H2), 7.58–7.30 (m, 5H, Ar–H), 6.94 (d, 1H, J = 8.8 Hz, Ar–H3′), 4.40 (q, 2H, N–CH2), 1.46 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 192.5 (C, C-1), 162.3 (C, C-2′), 148.3 (C, C-3), 141.9 (C, C-3″), 140.5 (C, C-1a″), 138.4 (C, C-4′), 131.7 (C, C-6′), 126.9 (C, C-4a″), 126.9 (C, C-1′), 126.5 (C, C-5′), 125.3 (C, C-8a″), 123.6 (C, C-1″), 122.8 (C, C-5a″), 122.3 (C, C-7″), 121.5 (C, C-5″), 120.8 (C, C-6″), 120.5 (C, C-2″), 115.9 (C, C-3′), 110.3 (C, C-8″), 109.0 (C, C-4″), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 419 (M+). Anal. Calc. for C23H18BrNO2: C, 65.73; H, 4.32; N, 3.33. Found: C, 65.78; H, 4.38; N, 3.36.
(E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(2-hydroxy-5-methylphenyl)prop-2-en-1-one ( 3e )
Yellow colored solid; m.p.: 174–176 °C; IR (KBr, cm−1): 3430 (OH), 1636 (C=O); 1H NMR (CDCl3, 400 MHz): δ 12.90 (s, 1H, Ar–OH), 8.42 (d, 1H, J = 1.6 Hz, Ar–H4″), 8.18–8.14 (m, 2H, Ar–H1″ and H2), 7.84–7.81 (dd, 1H, J = 1.6, 7.2 Hz, Ar–H2″), 7.78 (s, 1H, Ar–H6′), 7.71 (d, 1H, J = 15.6 Hz, H2), 7.54–7.28 (m, 5H, Ar–H), 6.94 (d, 1H, J = 8.4 Hz, Ar–H3′), 4.40 (q, 2H, N–CH2), 2.39 (s, 3H, Ar–CH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 191.9 (C, C-1), 161.5 (C, C-2′), 147.0 (C, C-3), 141.5 (C, C-3″), 140.5 (C, C-1a″), 137.0 (C, C-4′), 131.1 (C, C-5′), 129.2 (C, C-4a″), 127.7 (C, C-5a″), 126.7 (C, C-1′), 126.5 (C, C-6′), 126.4 (C, C-8a″), 125.7 (C, C-1″), 122.8 (C, C-7″), 121.9 (C, C-6″), 119.8 (C, C-5″), 118.3 (C, C-2″), 116.8 (C, C-3′), 107.5 (C, C-4″), 101.0, 37.8 (C, CH2), 20.7 (C, Ar–CH3), 13.8 (C, CH3); GC–MS: 355 (M+). Anal. Calc. for C24H21NO2: C, 81.10; H, 5.96; N, 3.94. Found: C, 81.17; H, 6.02; N, 3.96.
(E)-1-(5-chloro-2-hydroxy-4-methylphenyl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one ( 3f )
Yellow colored solid; m.p.: 188–190 °C; IR (KBr, cm−1): 3436 (OH), 1624 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.00 (s, 1H, Ar–OH), 8.42 (d, 1H, J = 2.0 Hz, Ar–H4″), 8.19–8.15 (m, 2H, Ar–H1″ and H2), 7.93 (s, 1H, Ar–H6′), 7.82 (dd, 1H, J = 1.6, 6.8 Hz, Ar–H2″), 7.59 (d, 1H, J = 15.6 Hz, H2), 7.54–7.29 (m, 4H, Ar–H), 6.91 (s, 1H, Ar–H3′), 4.41 (q, 2H, N–CH2), 2.40 (s, 3H, Ar–CH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 192.2 (C, C-1), 162.0 (C, C-2′), 147.7 (C, C-3), 145.0 (C, C-4′), 141.8 (C, C-3″), 140.5 (C, C-1a″), 129.1 (C, C-6′), 126.8 (C, C-5′), 126.5 (C, C-1′), 124.0 (C, C-1″), 123.6 (C, C-3′), 122.8 (C, C-5a″), 122.1 (C, C-7″), 120.7 (C, C-5″), 120.5 (C, C-2″), 120.3 (C, C-6″), 119.9 (C, C-2), 119.2 (C, C-4a″), 116.1 (C, C-8″), 109.0 (C, C-4″), 37.8 (C, CH2), 20.8 (C, Ar–CH3), 13.8 (C, CH3); GC–MS: 389 (M+). Anal. Calc. for C24H20ClNO2: C, 73.94; H, 5.17; N, 3.59. Found: C, 74.04; H, 5.19; N, 3.65.
(E)-1-(3,5-dichloro-2-hydroxyphenyl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one ( 3g )
Yellow colored solid; m.p.: 168–170 °C; IR (KBr, cm−1): 3443 (OH), 1629 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.84 (br. s, 1H, Ar–OH), 8.44 (d, 1H, J = 1.6 Hz, Ar–H4″), 8.25 (d, 1H, J = 15.2 Hz, H2), 8.18 (d, 1H, J = 8.0 Hz, Ar–H1″), 7.90 (d, 1H, J = 2.8 Hz, Ar–H6′), 7.83 (dd, 1H, J = 2.0, 7.6 Hz, Ar–H2″), 7.59 (d, 1H, J = 15.2 Hz, H2), 7.64–7.32 (m, 5H, Ar–H), 4.41 (q, 2H, N–CH2), 1.48 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 192.2 (C, C-1), 162.5 (C, C-2′), 149.4 (C, C-3), 142.1 (C, C-3″), 140.5 (C, C-1a″), 135.2 (C, C-4′), 129.6 (C, C-6′), 128.4 (C, C-5′), 127.3 (C, C-4a″), 127.0 (C, C-3′), 126.6 (C, C-1′), 125.1, 123.7 (C, C-8a″), 123.0 (C, C-1″), 122.8 (C, C-5a″), 122.5 (C, C-7″), 121.3 (C, C-5″), 120.7 (C, C-6″), 120.1 (C, C-2″), 115.2 (C, C-8″), 109.1 (C, C-4″), 109.0 (C, C-2), 37.9 (C, CH2), 13.8 (C, CH3); GC–MS: 409 (M+). Anal. Calc. for C23H17Cl2NO2: C, 67.33; H, 4.18; N, 3.41. Found: C, 67.41; H, 4.21; N, 3.46.
(E)-3-(9-ethyl-9H-carbazol-3-yl)-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one ( 3h )
Pale yellow colored solid; m.p.: 156–160 °C; IR (KBr, cm−1): 3436 (OH), 1648 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.72 (s, 1H, Ar–OH), 8.39 (d, 1H, J = 1.6 Hz, Ar–H4″), 8.17–8.14 (m, 2H, Ar–H1″ and H2), 7.93 (d, 1H, J = 8.8 Hz, Ar–H6′), 7.81–7.78 (dd, 1H, J = 2.0, 6.8 Hz, Ar–H2″), 7.63 (d, 1H, J = 15.6 Hz, H2), 7.54–7.28 (m, 4H, Ar–H), 6.53–6.49 (m, 2H, Ar–H), 4.39 (q, 2H, N–CH2), 3.87 (s, 3H, –OCH3), 1.46 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 191.9 (C, C-1), 166.6 (C, C-4′), 165.9 (C, C-2′), 146.1 (C, C-3), 141.5 (C, C-3″), 140.5 (C, C-1a″), 131.1 (C, C-6′), 126.5 (C, C-1′), 126.4 (C, C-4a″), 125.8 (C, C-1″), 123.5 (C, C-5a″), 122.8 (C, C-5′), 121.7 (C, C-7″), 120.6 (C, C-5″), 119.7 (C, C-6″), 116.9 (C, C-2″), 114.3 (C, C-2), 108.9 (C, C-8″), 108.8 (C, C-4″), 107.5 (C, C-3′), 101.0, 55.5 (C, O–CH3), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 371 (M+). Anal. Calc. for C24H21NO3: C, 77.61; H, 5.70; N, 3.77. Found: C, 77.65; H, 5.75; N, 3.76.
(E)-1-(4-ethoxy-2-hydroxyphenyl)-3-(9-ethyl-9H-carbazol-3-yl)prop-2-en-1-one ( 3i )
Pale yellow colored solid; m.p.: 138–140 °C; IR (KBr, cm−1): 3441 (OH), 1629 (C=O); 1H NMR (CDCl3, 400 MHz): δ 13.7 (s, 1H, Ar–OH), 8.39 (d, 1H, J = 2.0 Hz, Ar–H4″), 8.16–8.10 (m, 2H, Ar–H1″ and H2), 7.91 (d, 1H, Ar–H6′), 7.63 (d, 1H, J = 15.6 Hz, H2), 7.53–7.27 (m, 5H, Ar–H), 6.51–6.47 (m, 2H, Ar–H), 4.39 (q, 2H, N–CH2), 4.10 (q, 2H, O–CH2), 1.43–1.48 (m, 6H, 2×–CH3); 13C NMR (CDCl3, 100 MHz) δ 191.8 (C, C-1), 166.6 (C, C-4′), 165.3 (C, C-2′), 146.0 (C, C-3), 141.5 (C, C-3″), 140.5 (C, C-1a″), 131.1 (C, C-6′), 126.5 (C, C-1′), 126.4 (C, C-4a″), 125.8 (C, C-8a″), 123.5 (C, C-1″), 122.8 (C, C-5a″), 121.7 (C, C-7″), 120.6 (C, C-5″), 119.7 (C, C-6″), 116.9 (C, C-2″), 114.1 (C, C-2), 108.9 (C, C-5′), 108.8 (C, C-8″), 107.9 (C, C-4″), 101.4, 63.9 (C, O–CH2), 37.8 (C, CH2), 14.6 (C, CH3), 13.8 (C, CH3); GC–MS: 385 (M +). Anal. Calc. for C25H23NO3: C, 77.90; H, 6.01; N, 3.65. Found: C, 77.93; H, 6.04; N, 3.66.
General procedure for the synthesis of aurones 4a–i
Conventional method
To a stirred solution of cupric bromide (7.2 mmol) in DMSO (30 ml) was added substituted 2′ hydroxy chalcone 3a–i (10 mmol) at room temperature and refluxed for 6–8 h. After completion of reaction, the reaction mixture was poured into ice-cold water and extracted with dichloromethane (2 × 30 ml) and dried over Na2SO4, purified by column chromatography using n-hexane: ethylacetate (9:1).
Microwave irradiation method
A mixture of substituted 2′ hydroxy chalcone 3a–i (10 mmol) and cupric bromide (7.2 mmol) in DMSO (2 ml) was taken in a quartz tube and inserted into a Teflon vial with screw capped, and then it was subjected to microwave irradiation at 320 watts for 4–7 min. After completion of reaction (as indicated by TLC), the reaction mixture was poured into ice-cold water and extracted with dichloromethane (2 × 30 ml) and dried over Na2SO4, purified by column chromatography using n-hexane: ethylacetate (9:1).
(Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-one ( 4a )
Yellow colored solid; m.p.: 176–178 °C; IR (KBr, cm−1): 2976 (C–H), 1693 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.69 (d, 1H, J = 1.6 Hz, Ar–H4′), 8.19 (d, 1H, J = 8 Hz, Ar–H1′), 8.08 (dd, 1H, J = 1.6, 7.2 Hz, Ar–H2′), 7.84–7.21 (m, 8H, Ar–H), 7.17 (s, 1H, =C–H), 4.40 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 184.4 (C, C-3), 165.7 (C, C-7a), 145.7 (C, C-2), 140.8 (C, C-3′), 140.4 (C, C-1a′), 136.3 (C, C-6), 129.8 (C, C-4), 126.3 (C, C-3a), 124.6 (C, C-5), 124.5 (C, C-7), 123.6 (C, C-8a′), 123.2 (C, C-1′), 123.1 (C, C-5a′), 122.9 (C, C-7′), 122.2 (C, C-5′), 120.7 (C, C-6′), 117.8 (C, C-2′), 115.4, 112.9 (C, C-4′), 109.0 (C, C-8′), 108.9 (C, =C–H), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 339 (M+). Anal. Calc. for C23H17NO2: C, 81.40; H, 5.05; N, 4.13. Found: C, 81.53; H, 5.09; N, 4.22.
(Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)-5-fluorobenzofuran-3(2H)-one ( 4b )
Yellow colored solid; m.p.: 168–170 °C; IR (KBr, cm−1): 2972 (C–H), 1690 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.68 (s, 1H, Ar–H4′), 8.20 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.07 (d, 1H, J = 8.4 Hz, Ar–H2′), 7.56–7.22 (m, 7H, Ar–H), 7.20 (s, 1H, =C–H), 4.41 (q, 2H, N–CH2), 1.49 (s, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): δ 183.8 (C, C-3), 164.0 (C, C-7a), 145.9 (C, C-2), 145.3 (C, C-5), 140.9 (C, C-3′), 140.4 (C, C-1a′), 129.8 (C, C-3a), 129.4 (C, C-7), 128.3 (C, C-6), 124.6 (C, C-4), 124.4 (C, C-8a′), 123.6 (C, C-1′), 122.9 (C, C-5a′), 121.2 (C, C-7′), 120.7 (C, C-5′), 119.8 (C, C-6′), 115.8 (C, C-2′), 114.7 (C, C-8′), 108.9 (C, =C–H), 37.7 (C, CH2), 13.8 (C, CH3); GC–MS: 357 (M+). Anal. Calc. for C23H16FNO2: C, 77.30; H, 4.51; N, 3.93. Found: C, 77.35; H, 4.59; N, 3.95.
(Z)-5-chloro-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-one ( 4c )
Yellow colored solid; m.p.: 178–181 °C IR (KBr, cm−1): 2986 (C–H), 1686 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.67 (d, 1H, J = 1.2 Hz Ar–H4′), 8.19 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.06 (dd, 1H, J = 2 Hz, 7.6 Hz, Ar–H2′), 7.80–7.31 (m, 7H, Ar–H), 7.19 (s, 1H, =C–H), 4.39 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): δ 192.6 (C, C-3), 162.0 (C, C-7a), 148.3 (C, C-2), 141.9 (C, C-3′), 140.5 (C, C-1a′), 135.7 (C, C-6), 128.7 (C, C-4), 126.8 (C, C-4a′), 126.5 (C, C-3a), 125.3 (C, C-4′), 123.6 (C, C-8a′), 123.3 (C, C-1′), 122.8 (C, C-5a′), 122.3 (C, C-7′), 120.9 (C, C-5′), 120.7 (C, C-6′), 120.1 (C, C-7), 119.9 (C, C-2′), 115.8 (C, C-8′), 109.0 (C, =C–H), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 373 (M+). Anal. Calc. for C23H16ClNO2: C, 77.90; H, 4.31; N, 3.75. Found: C, 77.95; H, 4.36; N, 3.79.
(Z)-5-bromo-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-one ( 4d )
Yellow colored solid; m.p.: 134–136 °C IR (KBr, cm−1): 2923 (C–H), 1692 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.65 (s, 1H, Ar–H4′), 8.17 (d, 1H, J = 8 Hz, Ar–H1′), 8.04 (d, 1H, J = 8.0 Hz, Ar–H2′), 7.94 (s, 1H, Ar–H4), 7.74–7.11 (m, 6H, Ar–H), 7.18 (s, 1H, =C–H), 4.39 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 192.6 (C, C-3), 162.0 (C, C-7a), 148.3 (C, C-2), 141.9 (C, C-3′), 140.5 (C, C-1a′), 135.7 (C, C-6), 128.7 (C, C-4), 126.8 (C, C-3a), 126.5 (C, C-7), 125.3 (C, C-8a′), 123.6 (C, C-1′), 123.3 (C, C-4a′), 122.8 (C, C-5a′), 122.3 (C, C-7′), 120.9 (C, C-4′), 120.7 (C, C-5′), 120.1 (C, C-6′), 119.9 (C, C-2′), 115.8 (C, C-8′), 109.0 (C, =C–H), 37.8 (C, CH2), 13.8 (C, CH3); GC–MS: 417 (M+). Anal. Calc. for C23H16BrNO2: C, 66.04; H, 3.86; N, 3.35. Found: C, 66.09; H, 3.92; N, 3.39.
(Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)-5-methylbenzofuran-3(2H)-one ( 4e )
Yellow colored solid; m.p.: 138–140 °C; IR (KBr, cm−1): 2976 (C–H), 1693 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.68 (d, 1H, J = 1.2 Hz, Ar–H4′), 8.19 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.07 (dd, 1H, J = 1.2, 7.2 Hz, Ar–H2′), 7.62 (s, 1H, Ar–H6′), 7.51–7.29 (m, 6H, Ar–H), 7.14 (s, 1H, =C–H), 4.40 (q, 2H, N–CH2), 2.42 (s, 3H, Ar–CH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): δ 182.7 (C, C-3), 168.0 (C, C-7a), 146.5 (C, C-2), 140.6 (C, C-1a′), 137.4 (C, C-3′), 132.8 (C, C-6), 129.7 (C, C-5), 128.8 (C, C-4), 126.2 (C, C-3a), 125.5 (C, C-8a′), 124.4 (C, C-1′), 124.0 (C, C-4a’), 123.5 (C, C-7′), 122.8 (C, C-5a′), 121.9 (C, C-5′), 120.6 (C, C-6′), 119.7 (C, C-2′), 115.2 (C, C-8′), 114.9 (C, C-7), 112.4 (C, C-4′), 108.8 (C, =C–H), 37.7 (C, CH2), 26.6, 13.8 (C, CH3); GC–MS: 353 (M+). Anal. Calc. for C24H19NO2: C, 81.56; H, 5.42; N, 3.96. Found: C, 81.61; H, 5.52; N, 3.99.
(Z)-5-chloro-2-((9-ethyl-9H-carbazol-3-yl)methylene)-6-methylbenzofuran-3(2H)-one ( 4f )
Yellow colored solid; m.p.: 168–170 °C; IR (KBr, cm−1): 2919 (C–H), 1692 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.62 (s, 1H, Ar–H4′), 8.16 (d, 1H, J = 8.0 Hz, Ar–H1′), 8.01 (d, 1H, J = 8.0 Hz, Ar–H2′), 7.76 (s, 1H, Ar–H4), 7.73–7.53 (m, 4H, Ar–H), 7.28 (s, 1H, Ar–H6), 7.11 (s, 1H, =C–H), 4.37 (q, 2H, N–CH2), 2.50 (s, 3H, Ar–CH3), 1.46 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): δ 188.8 (C, C-3), 164.0 (C, C-7a), 145.9 (C, C-2), 145.3 (C, C-6), 140.9 (C, C-3′), 140.4 (C, C-1a′), 129.8 (C, C-4), 129.4 (C, C-5), 128.3 (C, C-4a′), 124.6 (C, C-8a′), 124.1 (C, C-1′), 123.6 (C, C-5a′), 122.9 (C, C-7′), 121.2 (C, C-5′), 120.7 (C, C-6′), 119.8 (C, C-2′), 118.5 (C, C-7), 115.8 (C, C-3a), 114.7 (C, C-8′), 109.0 (C, =C–H), 108.9 (C, C-4′), 37.7 (C, CH2), 21.7 (C, Ar–CH3), 13.8 (C, CH3); GC–MS: 387 (M+). Anal. Calc. for C24H18ClNO2: C, 74.32; H, 4.68; N, 3.61. Found: C, 74.35; H, 4.71; N, 3.65.
(Z)-5,7-dichloro-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-one ( 4g )
Yellow colored solid; m.p.: 238–241 °C; IR (KBr, cm−1): 2923 (C–H), 1692 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.76 (s, 1H, Ar–H4′), 8.15 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.05–8.03 (m, 2H, Ar–H2′ and Ar–H5), 7.56–7.30 (m, 5H, Ar–H), 7.16 (s, 1H, Ar–H3), 4.39 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3) 13C NMR (CDCl3, 100 MHz): δ 186.2 (C, C-3), 163.5 (C, C-7a), 145.0 (C, C-2), 140.6 (C, C-3′), 135.2 (C, C-1a′), 130.2 (C, C-6), 129.5 (C, C-4), 129.3 (C, C-5), 128.6 (C, C-4a′), 127.6 (C, C-5a′), 126.8 (C, C-4′), 126.5 (C, C-3a), 125.9 (C, C-8a′), 125.4 (C, C-1′), 124.6 (C, C-7′), 123.8 (C, C-7), 122.4 (C, C-5′), 120.4 (C, C-6′), 118.1 (C, C-2′), 109.2 (C, C-8′), 109.0 (C, =C–H), 31.4 (C, CH2), 13.5 (C, CH3); GC–MS: 407 (M+). Anal. Calc. for C23H15Cl2NO2: C, 67.66; H, 3.70; N, 3.43. Found: C, 67.71; H, 3.75; N, 3.47.
(Z)-2-((9-ethyl-9H-carbazol-3-yl)methylene)-6-methoxybenzofuran-3(2H)-one ( 4h )
Yellow colored solid; m.p.: 196–198 °C; IR (KBr, cm−1): 2978 (C–H), 1691 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.66 (d, 1H, J = 2.0 Hz Ar–H4′), 8.19 (d, 1H, J = 8.4 Hz, Ar–H1′), 8.03 (dd, 1H, J = 2.0, 8.4 Hz, Ar–H2′), 7.73 (d, 1H, J = 8.8 Hz, Ar–H4), 7.53–7.28 (m, 4H, Ar–H), 7.08 (s, 1H, =C–H), 6.85–6.75 (m, 2H, Ar–H), 4.39 (q, 2H, N–CH2), 3.95 (s, 3H, –OCH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 182.8 (C, C-3), 168.1 (C, C-7a), 167.0 (C, C-6), 146.6 (C, C-2), 140.6 (C, C-3′), 140.4 (C, C-1a′), 129.5 (C, C-4), 126.3 (C, C-3a), 125.6 (C, C-8a′), 124.2 (C, C-1′), 123.6 (C, C-7′), 123.3 (C, C-5′), 122.9 (C, C-5′), 120.7 (C, C-6′), 119.6 (C, C-2′), 115.4 (C, C-8′), 114.1 (C, C-5), 111.9 (C, C-4′), 108.8 (C, =C–H), 96.6 (C, C-7), 56.0 (C, O–CH3), 37.7 (C, CH2), 13.8 (C, CH3); GC–MS: 369 (M+). Anal. Calc. for C24H19NO3: C, 78.03; H, 5.18; N, 3.79. Found: C, 78.08; H, 5.23; N, 3.83.
(Z)-6-ethoxy-2-((9-ethyl-9H-carbazol-3-yl)methylene)benzofuran-3(2H)-one ( 4i )
Yellow colored solid; m.p.: 145–147 °C; IR (KBr, cm−1): 2957 (C–H), 1690 (C=O); 1H NMR (CDCl3, 400 MHz): δ 8.64 (d, 1H, J = 2.0 Hz, Ar–H4′), 8.18 (d, 1H, J = 8.8 Hz, Ar–H1′), 8.04 (dd, 1H, J = 2.0, 8.4 Hz, Ar–H2′), 7.72 (d, 1H, J = 8.8 Hz, Ar–H4), 7.56–7.25 (m, 4H, Ar–H), 7.10 (s, 1H, =C–H), 6.85–6.77 (m, 2H, Ar–H), 4.39 (q, 2H, N–CH2), 4.10 (q, 2H, O–CH2), 1.49–1.51 (m, 6H, 2×–CH3); 13C NMR (CDCl3, 100 MHz) δ 182.8 (C, C-3), 168.1 (C, C-7a), 166.4 (C, C-6), 146.6 (C, C-2), 140.6 (C, C-3′), 140.4 (C, C-1a′), 129.5 (C, C-4), 126.2 (C, C-3a), 126.1 (C, C-8a′), 125.6 (C, C-5a′), 124.4 (C, C-1′), 123.6 (C, C-7′), 123.3, 122.9 (C, C-5′), 120.7 (C, C-6′), 119.6 (C, C-2′), 115.2 (C, C-8′), 114.0 (C, C-5), 112.3 (C, C-4′), 108.8 (C, =C–H), 96.9 (C, C-7), 64.4, 37.7 (C, CH2), 14.6 (C, CH3), 13.8 (C, CH3); GC–MS: 383 (M+). Anal. Calc. for C25H21NO3: C, 78.31; H, 5.52; N, 3.65. Found: C, 78.36; H, 5.57; N, 3.69.
General procedure for the synthesis of flavones 5a–i
Conventional method
To a stirred solution of iodine (20 mol%) in DMSO (20 ml) was added substituted 2′ hydroxy chalcone 3 (10 mmol) at room temperature and refluxed for 4–6 h. After completion of reaction, the reaction mixture was poured into ice-cold water and extracted with dichloromethane (2 × 30 ml) and dried over Na2SO4, purified by column chromatography using hexane: ethylacetate (9:1).
Microwave irradiation method
A mixture of substituted 2′ hydroxy chalcone 3 (10 mmol) and iodine (20 mol%) in 2 ml of DMSO was taken in a quartz tube and inserted into a Teflon vial with screw capped, and then it was subjected to microwave irradiation at 320 watts for 4–7 min. After completion of reaction (as indicated by TLC), the reaction mixture was poured into ice-cold water and extracted with dichloromethane (2 × 30 ml) and dried over Na2SO4, purified by column chromatography using hexane: ethylacetate (9:1).
2-(9-Ethyl-9H-carbazol-3-yl)-4H-chromen-4-one ( 5a )
Pale yellow colored solid; m.p.: 168–171 °C; IR (KBr, cm−1): 1652 (C=O), 1586 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.71 (d, 1H, Ar–H4′), 8.27–8.19 (m, 2H, Ar–H), 8.03 (dd, 1H, J = 1.6, 8.4 Hz, Ar–H), 7.75–7.32 (m, 7H, Ar–H), 6.94 (s, 1H, Ar–H3), 4.41 (q, 2H, N–CH2), 1.48 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): 174.4 (C, C-4), 164.7 (C, C-2), 156.3 (C, C-8a), 141.8 (C, C-1a′), 140.5 (C, C-7), 133.4 (C, C-3′), 126.6 (C, C-4a), 125.6 (C, C-8a′), 125.0 (C, C-1′), 124.0 (C, C-4a′), 123.9 (C, C-5), 123.3 (C, C-5a′), 122.8 (C, C-6), 122.1 (C, C-8), 120.7 (C, C-5′), 119.9 (C, C-7′), 119.0 (C, C-4′), 118.0 (C, C-2′), 109.0 (C, C-6′), 108.8 (C, C-8′), 106.1 (C, C-3), 37.8 (C, CH2), 13.8 (C, CH3); ESI–MS: 340 (M + H)+. Anal. Calc. for C23H17NO2: C, 81.40; H, 5.05; N, 4.13. Found: C, 81.49; H, 5.11; N, 4.19.
2-(9-Ethyl-9H-carbazol-3-yl)-6-fluoro-4H-chromen-4-one ( 5b )
Pale yellow colored solid; m.p.: 222–224 °C; IR (KBr, cm−1): 1640 (C=O), 1597 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.67 (s, 1H, Ar–H4′), 8.18 (d, 1H, J = 7.6 Hz, Ar–H1′), 7.99 (d, 1H, J = 8.4 Hz, Ar–H2′), 7.88–7.30 (m, 7H, Ar–H), 6.90 (s, 1H, Ar–H3), 4.41 (q, 2H, N–CH2), 1.48 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz): 177.5 (C, C-4), 165.0 (C, C-6), 160.7 (C, C-2), 152.5 (C, C-8a), 141.9 (C, C-7), 140.6 (C, C-1a′), 126.7 (C, C-3′), 125.3 (C, C-4a), 123.9 (C, C-8a′), 122.8 (C, C-1′), 121.8 (C, C-4a′), 121.6 (C, C-5), 121.3 (C, C-5a′), 120.0 (C, C-6), 119.1 (C, C-8), 110.7 (C, C-5′), 110.5 (C, C-7′), 109.0 (C, C-4′), 108.9 (C, C-8′), 105.5 (C, C-6′), 37.8 (C, CH2), 13.8 (C, CH3); ESI–MS: 358 (M + H)+. Anal. Calc. for C23H16FNO2: C, 77.30; H, 4.51; N, 3.92. Found: C, 77.35; H, 4.57; N, 3.96.
6-Chloro-2-(9-ethyl-9H-carbazol-3-yl)-4H-chromen-4-one ( 5c )
Pale yellow colored solid; m.p.: 210–212 °C; IR (KBr, cm−1): 1658 (C=O), 1596 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.69 (d, 1H, J = 1.2 Hz, Ar–H4′), 8.40 (s, 1H, Ar–H5), 8.20 (m, 2H, Ar–H2′ and H1′), 7.93–7.31 (m, 6H, Ar–H), 6.93 (s, 1H, Ar–H3), 4.42 (q, 2H, N–CH2), 1.48 (t, 3H, –CH2–CH3); 13C NMR (CDCl3, 100 MHz) δ 188.9 (C, C-4), 152.4 (C, C-2), 142.7 (C, C-8a), 140.6 (C, C-1a′), 139.4 (C, C-7), 134.7 (C, C-3′), 133.5 (C, C-6), 131.1 (C, C-4a), 130.1 (C, C-8a′), 129.6 (C, C-1′), 129.0 (C, C-4a′), 127.3 (C, C-5), 126.6 (C, C-5a′), 123.2 (C, C-6), 122.8 (C, C-8), 122.3 (C, C-5′), 121.9 (C, C-7′), 120.7 (C, C-4′), 119.4 (C, C-6′), 118.6 (C, C-3), 109.0 (C, C-8′), 39.9 (C, CH2), 13.8 (C, CH3); ESI–MS: 374 (M + H)+. Anal. Calc. for C23H16ClNO2: C, 77.90; H, 4.31; N, 3.75. Found: C, 77.96; H, 4.35; N, 3.79.
6-Bromo-2-(9-ethyl-9H-carbazol-3-yl)-4H-chromen-4-one ( 5d )
Pale yellow colored solid; m.p.: 178–180 °C; IR (KBr, cm−1): 1640 (C=O), 1596 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.69 (d, 1H, J = 2.0 Hz, Ar–H4′), 8.38 (d, 1H, J = 2.4 Hz, Ar–H5), 8.20 (d, 1H, J = 8.0 Hz, Ar–H1′), 8.00 (dd, 1H, J = 1.6, 6.8 Hz, Ar–H2′), 7.78–7.32 (m, 6H, Ar–H), 6.94 (s, 1H, Ar–H3), 4.43 (q, 2H, N–CH2), 1.49 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 177.0 (C, C-4), 165.0 (C, C-2), 155.1 (C, C-8a), 141.9 (C, C-7), 140.6 (C, C-1a′), 136.4 (C, C-3′), 128.3 (C, C-4a), 126.7 (C, C-8a′), 125.4 (C, C-1′), 123.4 (C, C-4a′), 122.8 (C, C-5), 121.7 (C, C-5a′), 120.7 (C, C-6), 120.0 (C, C-8), 119.2 (C, C-5′), 118.4 (C, C-7′), 109.1 (C, C-4′), 108.9 (C, C-8′), 106.1 (C, C-2′), 37.8 (C, CH2), 13.8 (C, CH3); ESI–MS: 418 (M + H)+. Anal. Calc. for C23H16BrNO2: C, 66.04; H, 3.86; N, 3.35. Found: C, 66.11; H, 3.91; N, 3.37.
2-(9-Ethyl-9H-carbazol-3-yl)-6-methyl-4H-chromen-4-one ( 5e )
Pale yellow colored solid; m.p.: 226–228 °C; IR (KBr, cm−1): 1629 (C=O), 1610, 1598 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.70 (d, 1H, J = 1.6 Hz, Ar–H4′), 8.18 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.04–8.00 (m, 2H, Ar–H2′ and Ar–H5), 7.57–7.31 (m, 6H, Ar–H), 6.92 (s, 1H, Ar–H3), 4.41 (q, 2H, N–CH2), 2.48 (s, 3H, Ar–CH3), 1.48 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 178.4 (C, C-4), 164.6 (C, C-2), 164.5 (C, C-8a), 141.7 (C, C-7), 140.5 (C, C-1a′), 134.8 (C, C-1a′), 134.6 (C, C-6), 126.5 (C, C-3′), 125.0 (C, C-4a), 123.9 (C, C-8a′), 123.7 (C, C-1′), 123.3 (C, C-4a′), 122.8 (C, C-5), 122.2 (C, C-5a′), 120.7 (C, C-8), 119.9 (C, C-5′), 119.0 (C, C-7′), 117.7 (C, C-4′), 109.0 (C, C-6′), 108.9 (C, C-8′), 106.0 (C, C-2′), 37.4 (C, CH2), 20.9 (C, Ar–CH3), 13.8 (C, CH3); ESI–MS: 354 (M + H)+. Anal. Calc. for C24H19NO2: C, 81.56; H, 5.42; N, 3.96. Found: C, 81.60; H, 5.49; N, 3.99.
6-Chloro-2-(9-ethyl-9H-carbazol-3-yl)-7-methyl-4H-chromen-4-one ( 5f )
Pale yellow colored solid; m.p.: 219–221 °C; IR (KBr, cm−1): 1638 (C=O), 1594 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.66 (d, 1H, J = 1.2 Hz, Ar–H4′), 8.36 (s, 1H, Ar–H5), 8.18 (d, 1H, J = 8.0 Hz, Ar–H1′), 7.98 (dd, 1H, J = 1.6, 8.0 Hz, Ar–H2′), 7.77–7.30 (m, 5H, Ar–H), 6.89 (s, 1H, Ar–H3), 4.39 (q, 2H, N–CH2), 2.52 (s, 3H, Ar–CH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 176.9 (C, C-4), 164.9 (C, C-2), 155.0 (C, C-8a), 141.9 (C, C-7), 140.5 (C, C-1a′), 136.2 (C, C-3′), 128.2 (C, C-6), 126.7 (C, C-4a), 125.3 (C, C-8a′), 123.9 (C, C-1′), 123.3 (C, C-4a′), 122.7 (C, C-5), 121.6 (C, C-5a′), 120.6 (C, C-6), 120.0 (C, C-8), 119.9 (C, C-5′), 118.3 (C, C-7′), 109.0 (C, C-4′), 108.9 (C, C-8′), 105.9 (C, C-6′), 37.8 (C, CH2), 22.6 (C, Ar–CH3), 13.8 (C, CH3); ESI–MS: 388 (M + H)+. Anal. Calc. for C24H18ClNO2: C, 74.32; H, 4.68; N, 3.61. Found: C, 74.37; H, 4.75; N, 3.67.
6,8-Dichloro-2-(9-ethyl-9H-carbazol-3-yl)-4H-chromen-4-one ( 5g )
Pale yellow colored solid; m.p.: 216–218 °C; IR (KBr, cm−1): 1637 (C=O), 1593 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.67 (d, 1H, J = 1.6 Hz, Ar–H4′), 8.15 (d, 1H, J = 7.6 Hz, Ar–H1′), 8.03–8.00 (m, 2H, Ar–H2′ and Ar–H5), 7.62–7.30 (m, 5H, Ar–H), 6.89 (s, 1H, Ar–H3), 4.39 (q, 2H, N–CH2), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 176.3 (C, C-4), 164.3 (C, C-2), 155.2 (C, C-8a), 140.6 (C, C-1a′), 139.8 (C, C-7), 135.4 (C, C-3′), 133.3 (C, C-6), 130.4 (C, C-8), 129.5 (C, C-4a), 126.7 (C, C-8a′), 124.0 (C, C-1′), 123.7 (C, C-4a′), 122.6 (C, C-5), 121.1 (C, C-5a′), 119.2 (C, C-6′), 117.3 (C, C-5′), 115.8 (C, C-7′), 112.7 (C, C-4′), 110.1 (C, C-8′), 109.1 (C, C-2′), 105.5 (C, C-3), 37.9 (C, CH2), 13.8 (C, CH3); ESI–MS: 408 (M + H)+. Anal. Calc. for C23H15Cl2NO2: C, 67.66; H, 3.70; N, 3.43. Found: C, 67.73; H, 3.76; N, 3.47.
2-(9-Ethyl-9H-carbazol-3-yl)-7-methoxy-4H-chromen-4-one ( 5h )
Yellow colored solid; m.p.: 214–216 °C; IR (KBr, cm−1): 1620 (C=O), 1593 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.67 (d, 1H, J = 1.6 Hz, Ar–H4′), 8.20–8.14 (m, 2H, Ar–H1′ and Ar–H6), 7.98 (dd, 1H, J = 2.0, 6.8 Hz, Ar–H2′), 7.55–6.98 (m, 6H, Ar–H), 6.97 (s, 1H, Ar–H3), 4.40 (q, 2H, N–CH2), 3.96 (s, 3H, –OCH3), 1.47 (t, 3H, –CH3); 13C NMR (CDCl3, 100 MHz) δ 177.9 (C, C-4), 164.3 (C, C-2), 163.9 (C, C-7), 157.9 (C, C-8a), 141.6, 140.5 (C, C-1a′), 126.9 (C, C-3′), 126.5 (C, C-4a), 123.8 (C, C-8a′), 123.2 (C, C-1′), 122.8 (C, C-4a′), 122.1 (C, C-6), 120.6 (C, C-5), 119.8 (C, C-5a′), 118.8 (C, C-7′), 117.8 (C, C-4′), 114.1 (C, C-6′), 109.0 (C, C-2′), 108.8 (C, C-8′), 105.9 (C, C-3), 100.3 (C, C-8), 55.8 (C, OCH3), 37.8 (C, CH2), 13.8 (C, CH3); ESI–MS: 370 (M + H)+. Anal. Calc. for C24H19NO3: C, 78.03; H, 5.18; N, 3.79. Found: C, 78.07; H, 5.21; N, 3.85.
2-(9-Ethyl-9H-carbazol-3-yl)-7-methoxy-4H-chromen-4-one ( 5i )
Pale yellow colored solid; m.p.: 208–210 °C; IR (KBr, cm−1): 1620 (C=O), 1591 (C=C); 1H NMR (CDCl3, 400 MHz): δ 8.67 (d, 1H, J = 1.6 Hz, Ar–H4′), 8.19 (d, 1H, J = 8.8 Hz, Ar–H1′), 8.18 (d, 1H, J = 8.8 Hz, Ar–H5), 8.14 (d, 1H, J = 8.8 Hz, Ar–H2′), 7.56–7.26 (m, 4H, Ar–H), 7.03–6.94 (m, 2H, Ar–H), 6.46 (s, 1H, Ar–H3), 4.38 (q, 2H, N–CH2), 4.19 (q, 2H, O–CH2), 1.53–1.46 (m, 6H, 2×–CH3); 13C NMR (CDCl3, 100 MHz): δ 176.9 (C, C-4), 163.2 (C, C-2), 162.3 (C, C-7), 157.0 (C, C-8a), 140.6 (C, C-1a′), 139.5 (C, C-3′), 125.9 (C, C-4a), 125.5 (C, C-8a′), 122.8 (C, C-1′), 122.2 (C, C-4a′), 121.8 (C, C-5), 121.2 (C, C-5a′), 119.6 (C, C-7′), 118.8 (C, C-4′), 117.8 (C, C-6′), 116.7 (C, C-6), 113.4 (C, C-5), 107.9 (C, C-8′), 107.8 (C, C-8), 105.0 (C, C-2′), 99.8 (C, C-3), 63.2 (C, OCH2), 36.8 (C, CH2), 13.6 (C, N–CH2–CH3), 12.8 (C, O–CH2–CH3); ESI–MS: 384 (M + H)+. Anal. Calc. for C25H21NO3: C, 78.31; H, 5.52; N, 3.65. Found: C, 78.37; H, 5.55; N, 3.71.
Biological assay
Antimicrobials are one class of drugs prescribed for the treatment of simple infection to life-threatening infections. Nowadays, the antimicrobials resistance toward infection increases, and the toxic effects produced by these antimicrobials decrease its significance. So the scope for the synthesis of newer antimicrobials always exists.
The synthesized compounds were assayed against gram-positive and gram-negative bacterial and fungal cultures.
Antibacterial assay
All the synthesized compounds were evaluated for their in vitro antibacterial activity against gram-positive bacteria such as S. aureus, B. subtilis and gram-negative bacteria E. coli and K. pneumoniae. The bacterial cultures were grown in nutrient agar media and subcultured for the better growth (log-phase cultures) in a liquid nutrient broth medium and further subcultured onto the Petri plates for the experiments. The broth cultures were diluted with sterilized saline to bring the final size of inoculum approximately to 105–106 CFU/ml. The compounds were diluted in acetone, DMSO and diethyl ether for biological assays. Among the three solvents, diethyl ether is taken as the best solvent than the remaining two solvents. The bacterial culture inoculum was placed on the media and incubated at 37 °C for 24–48 h along with the chemical disks dipped and placed over the media. The zones of bacterial growth inhibition were measured using the diameter of the zone as an unit to measure the antibacterial activity. All the experiments were carried out in triplicates, and the results were expressed as zone of inhibition in millimeter. The results were compared with the activity of the standard antibiotic ciprofloxacin (20 and 40 µg/ml). For disk diffusion method, the test compound was introduced onto the disk and then allowed to dry. Once the disk was completely saturated with the test compound, then it was introduced onto the upper layer of the medium containing the bacterial inoculum. The Petri dishes were incubated overnight at 37 °C for 24 h.
Antifungal assay
The antifungal activity of synthesized compounds was tested against three pathogenic fungi, F. oxysporum, A. niger and A. flavus, by the poison plate technique. Test compounds were dissolved in diethyl ether (10 ml) before mixing with potato dextrose agar medium (PDA, 90 ml). The final concentration of compounds in the medium was maintained to be 100 µg/ml. Above-mentioned types of fungi were incubated in PDA at 25 ± 1 °C for 3–4 days to get good mycelium growth for antifungal assay; then, a mycelia disk of approximately 0.45 cm diameter cut from the culture medium was picked up with a sterilized inoculation needle and inoculated in the center of PDA plate. The inoculated plates were incubated at 25 ± 1 °C for 5 days. Diethyl ether in sterilized distilled water was used as control, while amphotericin-B and clotrimazole were used as standards for all the treatment; three replicates were performed. The radial growth of the fungal colonies was measured on the fourth day, and the data were statistically analyzed. The in vitro inhibition effects of the test compounds on the fungi were calculated by the given formula CV = A − B/A, where A represents the diameter of fungi growth on untreated PDA, B represents the diameter of fungi on treated PDA and CV represents the rate of inhibition.
DPPH radical scavenging activity
DPPH radical scavenging activity was measured by following the method of Cotelle et al. (1996), after standardization with some modifications. 3 ml of reaction mixture containing 0.2 ml of DPPH (100 μM in methanol) and 2.0 ml of test solution, at various concentrations (50, 100, 200 μg/ml) of the synthesized extracts, was incubated at 37 °C for 30 min; absorbance of the resulting solution was measured at 517 nm using Beckman model DU-40 spectrophotometer. Among these three concentrations, 100 μg/ml gave significant results, and we performed the reactions in triplicate. Compounds 3c, 3g, 3h, 3i, 4a, 4g, 4h, 4i, 5c, 5d, 5e, 5h and 5i showed promising DPPH radical scavenging activity as compared with the standard.
Conclusion
Three series of nine chalcones, nine aurones and nine flavones possessing a carbazole group in lieu of phenyl ring were synthesized and screened for their antimicrobial and antioxidant activities. For antibacterial activity, S. aureus, B. subtilis, E. coli and K. pneumonia were used. For antifungal activity, F. oxysporum, A. niger and A. flavus were used. The results revealed that most of the compounds shown promising activity against tested microorganisms which may be due to the increased lipophilic character of the molecules, which facilitates the crossing through biological membrane of the microorganism and therefore inhibits their growth, and the antioxidant activity of these compounds was very close to standard. In conclusion, we report a convenient, simple and high yielding route for the synthesis of chalcones, aurones and flavones derived from carbazole.
References
Ashok D, Ravi S, Sreenivas P (2013) Solvent-free microwave-assisted synthesis of 3-((E)-3-(2,4-dihydroxy-5-((E)-3-(4-oxo-4H-3-chromenyl)-2-propenoyl)phenyl)-3-oxo-1-propenyl)-4H-chromen-4-ones. Indian J Heterocycl Chem 23:11–14
Ashok D, Gandhi DM, Srinivas G, Vikas KA (2014) Microwave-assisted synthesis of novel 1,2,3-triazole derivatives and their antimicrobial activity. Med Chem Res 23:3005–3018
Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Chaitanya M, Arunasree KM, Alam MS (2013) Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur J Med Chem 65:51–59
Barbosa TP, Suervy SCO, Amorim FM, Rodrigues YKS, De Assis PAC, Caldas JPA, Oliveira MR, Vasconcellos MLAA (2011) Design, synthesis and antileishmanial in vitro activity of new series of chalcones-like compounds: a molecular hybridization approach. Bioorg Med Chem 19(14):4250–4256
Boumendjel A, Boccard J, Carrupt PA, Nicolle E, Blanc M, Geze A, Choisnard L, Wouessidjewe D, Matera EL, Dumontet C (2008) Antimitotic and antiproliferative activities of chalcones: forward structure–activity relationship. J Med Chem 51:2307–2310
Cheng H, Zhang L, Liu Y, Chen S, Cheng H, Lu X, Zheng Z, Zhou GC (2010) Design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur J Med Chem 45:5950–5957
Cotelle N, Bemier JL, Catteau JP, Pommery J, Wallet JC, Gaydou EM (1996) Antioxidant properties of hydroxy flavones. Free Radic Biol Med 20:35–43
Detsi A, Majdalani M, Kontogiorgis CA, Hadjipavlou-Litina D, Kefalas P (2009) Natural and synthetic 2'-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem 17(23):8073–8085
Groot HD, Rauen U (1998) Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol 12:249–255
Hadj-esfandiari N, Navidpour L, Shadnia H, Amini M, Samadi N, Faramarzid MA, Shafiee A (2007) Synthesis, antibacterial activity and quantitative structure–activity relationships of new (Z)-2-(nitroimidazolylmethylene)-3(2H)-benzofuranone derivatives. Bioorg Med Chem 17:6354–6363
Itoigawa M, Kashiwada Y, Ito C, Furukawa H, Tachibana Y, Bastow KF, Lee KH (2000) Antitumor agents. 203. Carbazole alkaloid murrayaquinone A and related synthetic carbazolequinones as cytotoxic agents. J Nat Prod 63:893–897
Kayser O, Kiderlen AF (1998) Leishmanicidal activity of aurones. Tokai J Exp Clin Med 23:423–426
Li N, Liu JH, Zhang J, Yu BY (2008) Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem 56:1429–1433
Lim H, Jin JH, Park H, Kim HP (2011) New synthetic anti-inflammatory chrysin analog, 5,7-dihydroxy-8-(pyridine-4yl)flavones. Eur J Pharmacol 670:617–622
Lin HJ, De YH, Fang L, Wei X, Xia Z (2008) Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorg Med Chem 16:1337–1344
Morimoto M, Fukumoto H, Nozoe T, Hagiwara A, Komai K (2007) Synthesis and insect antifeedant activity of aurones against Spodoptera litura larvae. J Agric Food Chem 55:700–704
Okombi S, Rival D, Bonnet S, Mariotte AM, Perrier E, Boumendjel A (2006) Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 49:329–333
Radha K, Youra K, Chul HK, Kyungrook K, Jung AK, Eung SL (2012) Hydroxychalcones as potential anti-angiogenic agent. Bull Korean Chem Soc 33:2925–2929
Shenvi S, Kumar K, Hatti KS, Rijesh K, Diwakar L, Reddy GC (2013) Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: structure activity relationship. Eur J Med Chem 62:435–442
Shin SY, Shin JS, Lee YS (2011) Synthesis of aurones and their inhibitory effects on nitric oxide and PGE productions in LPS-induced RAW 264.7 cells. Bioorg Med Chem Lett 21:4520–4523
Thomas MG, Lawson C, Allanson NM, Leslie BW, Bottomley JR, McBride A, Olusanya OA (2003) A series of 2(Z)-2-benzylidene-6,7-dihydroxybenzofuran-3[2H]-ones as inhibitors of chorismate synthase. Bioorg Med Chem Lett 3:423–426
Tomohiro I, Kenji O, Munekazu I, Yoshinori N, Yukihiro A (2008) Inhibitory effects of polymethoxy flavones isolated from Citrus reticulate on degranulation in rat basophilic leukemia RBL-2H3: enhanced inhibition by their combination. Bioorg Med Chem 16:7592–7598
Tran TD, Do TH, Tran NC, Ngo TD, Huynh TNP, Tran CD, Thai KM (2012) Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg Med Chem Lett 22:4555–4560
Wu J, Li J, Cai Y, Pan Y, Ye F, Zhang Y, Zhao Y, Yang S, Li X, Lian G (2011) Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem 54(23):8110–8123
Xuea B, Lib J, Chaic Q, Liuc Z, Chenb L (2008) Effect of total flavonoid fraction of Astragalus complanatus R. Brown on angiotensin II-induced portal-vein contraction in hypertensive rats. Phytomedicine 15:759–762
Acknowledgments
We are thankful to the Head, Department of Chemistry, Osmania University, for providing laboratory facilities and CFRD OU, for providing spectral analysis. S.R. is thankful to the Council of Scientific and Industrial Research, New Delhi, for the award of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashok, D., Ravi, S., Ganesh, A. et al. Microwave-assisted synthesis and biological evaluation of carbazole-based chalcones, aurones and flavones. Med Chem Res 25, 909–922 (2016). https://doi.org/10.1007/s00044-016-1537-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1537-7