Abstract
We report here, the synthesis of 1-(1-ethyl-1H-indol-3-yl)-3-pyridin-4-yl-prop-2-en-1-one (2) which was used as a base to the synthesis of new 3-(pyrimidin-4-yl)-1H-indole derivatives; their thioglycoside and N-glycoside derivatives 3–10a, b; pyrane derivatives and pyranopyrimidine derivative 11–13; and tricyclic pyranotriazolo[1, 5-a]pyrimidine 14. Moreover, reaction of N-ethyl-3-acetylindole 2 with phenylhydrazine and hydroxylamine hydrochloride gave pyrazolyl-indole and oxazolyl-indole derivatives 15 and 16, respectively. The structures of the products obtained were confirmed by elemental analysis, IR, 1HNMR and 13C NMR. The newly synthesized compounds were investigated for their antimicrobial activity, and some of them showed high growth inhibition activities. Anti-bacterial activity of the synthesized compounds was further analyzed by the molecular docking approach, a method of simulation of fitting ligands into binding site(s) of macromolecular targets. AutoDock Vina results showed that compounds 5a and 6a, b were located in a pocket in the active site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
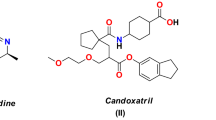
The chemistry of indole compounds has received a great interest because of their interesting biological activities (El-Sayed et al., 2011; Chang et al., 2005). Indole derivatives have been found to possess anti-inflammatory (Ozdemir et al., 2015), anti-bacterial (Baharfar et al., 2015), anti-viral (Bal et al., 2005), analgesic (Dilli et al., 2012) and anti-tumor activities (El-Sayed et al., 2011). Many indole compounds were effective in causing a marked increase in growth inhibition activity against various types of bacteria, fungi and viruses (Velezheva et al., 2004). On the other hand, substituted pyrimidines are of great interest due to their diverse biological activities such as HIV-1 inhibition (Gadhachanda et al., 2007), anti-bacterial (Hegab et al., 2007), anticancer (Donger et al., 2014) and anti-inflammatory (Ahmed et al., 2014) activities. The thiopyrimidine bases and their S-, N-disubstituted analogues have shown therapeutic properties as anti-viral, anti-thyroid and anti-tumor activities (Tanaka et al., 1995; Nugent et al., 1998) due to their incorporation into poly-nucleic acids and inhibition of protein and poly-nucleic acid syntheses. As the literature survey revealed that indole and pyrimidine rings possess potential antimicrobial activity, it was thought that is worthwhile to link indole-pyrimidine-conjugated system to sugar molecule so as to get enhanced bioactivity. Substituted indolyl-pyrimidine analogues I (Fig. 1) have been reported of high antimicrobial potency (Jaiprakash and Sasidhar, 2012). Recently, new indolyl-pyrimidine analogues II (Fig. 1) were found to be equally potent as to the anti-fungal drugs Griseofulvin and Nystatin (Gaffar et al., 2012). Also, triazolopyrimidine constitutes a well-established scaffolding in crop protection chemistry (Lamberth, 2007). Such compounds revealed anti-fungal (Serey et al., 2007) and anti-mycobacterial (Abdel-Rahman et al., 2009) activities. On the other hand, it is well known that DNA gyrase A protein from Escherichia coli is a macromolecular target for several antimicrobial drugs (Morris et al., 1998, Ostrov et al., 2007). Interestingly, such target possesses several potential sites at which ligands can be bound, probably those located in the proximity of residues taking key roles in catalytic functions.
Led by the previous facts and coupled with our ongoing project aimed at investigating new bioactive molecules derived from heterocycles attached to carbohydrate moieties (Abdel-Aal et al., 2006, 2008; El-Sayed et al., 2009a, b, c), the present work involves the synthesis and antimicrobial activity of new pyrimidine glycosides and thioglycoside derivatives.
Experimental
Chemistry
General procedures
All melting points are uncorrected and measured using an Electro-thermal IA 9100 apparatus (Shimadzu, Japan). Yields listed are of the isolated compounds. The IR spectra were recorded as potassium bromide pellets on a PerkinElmer 1650 Spectrophotometer, National Research Centre, Cairo, Egypt. 1H NMR and 13C NMR spectra were determined on a Jeol-Ex-300 NMR spectrometer, and chemical shifts were expressed as parts per million, ppm (δ values) against TMS as an internal reference (Faculty of Science, Cairo University, Cairo, Egypt). Microanalyses were operated using Mario Elmentar apparatus, Organic Microanalysis Unit, National Research Centre, Cairo, Egypt. The progress of the reactions was monitored by TLC using aluminum silica gel plates 60 F245. Column chromatography was performed on (Merck) Silica gel 60 (particle size 0.06–0.20 mm). Antimicrobial activities were measured at National Research Centre, Cairo, Egypt. The chemical names given for the prepared compounds are according to the IUPAC system. The starting compound 1-(1-ethyl-1H-indol-3-yl)ethanone 1 was prepared according to a previously reported procedure (Ottoni, et al., 1998).
1-(1-Ethyl-1H-indol-3-yl)-3-pyridin-4-yl-prop-2-en-1-one (2)
To a solution of 1-(1-ethyl-1H-indol-3-yl)ethanone 1 (1.87 g, 0.01 mol) in ethanol (50 mL), potassium hydroxide (0.5 g) in water (5 mL) was added over a period of 30 min at 0 °C. Then 4-pyridine carboxaldehyde (1.07 g, 0.01 mol) was added slowly to the reaction mixture with stirring at room temperature for 12 h. Then the reaction mixture was poured into water. The solid that formed was filtered, dried and recrystallized from dioxane to afford compound 2 as a pale yellow powder, 2.62 g, (90 %); m.p. 172–174 °C. IR (KBr) cm−1: ν (C=O) 1695, ν (C=N) 1608; 1H NMR (DMSO-d6, 300 MHz): δ 1.48 (t, 3H, J = 6.2 Hz, CH3), 4.12 (q, 2H, J = 6.2 Hz, CH2), 6.25 (d, 1H, J = 7.2 Hz, CH), 6.88 (d, 1H, J = 7.2 Hz, CH), 7.38 (m, 3H, Ar–H), 7.52–7.82 (m, 3H, Ar–H), 8.55 (s, 1H, indole H-2), 8.72 (d, 2H, J = 7.6 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 109.1, 110.7, 120.1, 121.9, 123.7, 124.4, 124.9, 135.1, 136.5, 144.5, 149.3, 151.2, 152.5 (Ar-13C, 2 CH), 180.8 (C=O). Anal. Calcd. for C18H16N2O (276.33): C, 78.24; H, 5.84; N, 10.14. Found: C, 78.08; H, 5.98; N, 10.01.
4-(1-Ethyl-1H-indol-3-yl)-6-(pyridin-4-yl)-5, 6-dihydropyrimidine-2(1H)-thione (3)
A mixture of compound 2 (2.76 g, 0.01 mol), thiourea (0.1 g, 0.014 mol) and NaOH (1.0 g, 0.025 mol) in ethanol (30 mL) was refluxed for 6 h. The reaction mixture was concentrated, cooled and filtered. The formed precipitate was recrystallized from ethanol to give 3 as a pale yellow powder, 2.74 g, (82 %); m.p. 182–184 °C. IR (KBr) cm−1: ν (NH) 3312, ν (C=N) 1610, ν (C=S) 1180; 1H NMR (DMSO-d6, 300 MHz): δ 1.47 (t, 3H, J = 6.2 Hz, CH3), 3.07 (dd, 1H, J = 12.8 Hz, J = 4.8 Hz, pyrimidyl H-5), 3.67 (dd, 1H, J = 8.8 Hz, J = 12.8 Hz, pyrimidyl H-5′), 3.84–4.02 (m, 3H, CH2 and CH2CH), 7.38–7.50 (m, 3H, Ar–H), 7.62 (d, 2H, J = 7.4 Hz, Ar–H), 8.27–8.32 (m, 3H, Ar–H), 8.62 (s, 1H, indole H-2), 9.18 (s, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 49.8 (pyrimidyl C-5), 58.2 (pyrimidyl C-6), 109.2, 111.3, 120.4, 121.8, 123.1, 124.4, 125.1, 135.1, 136.1, 144.3, 149.2 (Ar-13C), 161.1 (pyrimidyl C-4), 182.8 (C=S). Anal. Calcd. for C19H18N4S (334.44): C, 68.23; H, 5.42; N, 16.75. Found: C, 68.19; H, 5.38; N, 16.64.
General procedure for synthesis of compounds 5a, b
To a solution of the compound 3 (3.34 g, 0.01 mol) in aqueous potassium hydroxide [(0.01 mol in distilled water (16 mL)], a solution of 2, 3, 4, 6-tetra-O-acetyl-α-d-galactopyranosyl bromide or 2, 3, 4-tri-O-acetyl-α-d-xylopyranosyl bromide (0.011 mol) in acetone (20 mL) was added. The reaction mixture was stirred at room temperature for 12 h. The solvent was evaporated under reduced pressure at 40 °C, and the residue was washed with distilled water to remove potassium bromide formed. The product was dried and crystallized from ethanol to give compounds 5a, b, respectively.
1-Ethyl-3-{2-[(2, 3, 4, 6-tetra-O-acetyl-ß- d -galactopyranosyl)thio]-6-(pyridin-4-yl)-5, 6-dihydropyrimidin-4-yl}-1H-indole (5a)
4.59 g, (69 %), pale yellow powder, m.p. 139–141 °C. IR (KBr) cm−1: ν (C=O) 1742, ν (C=N) 1612; 1H NMR (DMSO-d6, 300 MHz): δ 1.47 (t, 3H, J = 6.2 Hz, CH3), 1.95, 2.05, 2.11, 2.14 (4s, 12H, 4CH 3 CO), 3.11 (dd, 1H, J = 5.2 Hz, J = 12.8 Hz, pyrimidyl H-5), 3.72 (dd, 1H, J = 8.6 Hz, J = 12.8 Hz, pyrimidyl H-5′), 3.95–4.07 (m, 2H, CH2CH and H-6), 4.11–4.15 (m, 3H, CH2 and H-6′), 4.17 (dd, 1H, J = 2.8 Hz, J = 11.4 Hz, H-5), 4.96 (t, 1H, J 3, 4 = 9.3 Hz, H-4), 5.24 (dd, 1H, J 2, 3 = 9.6 Hz, J 3, 4 = 9.3 Hz, H-3), 5.38 (t, 1H, J 2, 3 = 9.6 Hz, H-2), 5.72 (d, 1H, J 1, 2 = 10.2 Hz, H-1), 7.40–7.47 (m, 3H, Ar–H), 7.55 (d, 1H, J = 7.4 Hz, Ar–H), 8.18 (d, 2H, J = 7.6 Hz, Ar–H), 8.59 (s, 1H, indole H-2), 8.72 (s, 2H, J = 7.6 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 19.3, 19.5, 20.1, 20.2 (4CH 3 CO), 44.6, (CH2), 50.8 (pyrimidyl C-5), 56.2 (pyrimidyl C-6), 62.7 (C-6), 64.2 (C-4), 68.7 (C-3), 71.2 (C-2), 71.9 (C-5), 88.9 (C-1), 109.3, 111.1, 121.4, 122.2, 123.5, 125.1, 126.1, 134.6, 136.0, 143.7, 149.6, 150.9, 152.2 (Ar-13C), 159.2 (pyrimidyl C-4), 165.1 (pyrimidyl C-2), 169.4, 170.5, 171.2, 171.7 (4C=O). Anal. Calcd. for C33H36N4O9S (664.73): C, 59.63; H, 5.46; N, 8.43. Found: C, 59.48; H, 5.38; N, 8.52.
1-Ethyl-3-{2-[(2, 3, 4-tri-O-acetyl-ß- d -xylopyranosyl)thio]-6-(pyridin-4-yl)-5, 6-dihydro-pyrimidin-4-yl}-1H-indole (5b)
2.91 g, (49 %), pale yellow powder, m.p. 141–143 °C. IR (KBr) cm−1: ν (C=O) 1739, ν (C=N) 1619; 1H NMR (DMSO-d6, 300 MHz): δ 1.48 (t, 3H, J = 6.2 Hz, CH3), 1.95, 2.05, 2.12 (3 s, 9H, 3CH 3 CO), 3.12 (dd, 1H, J = 5.2 Hz, J = 12.8 Hz, pyrimidyl H-5), 3.72 (dd, 1H, J = 8.8 Hz, J = 12.8 Hz, pyrimidyl H-5′), 3.82–4.09 (m, 2H, CH2CH and H-5), 4.12–4.17 (m, 3H, CH2 and H-5′), 4.20 (dd, 1H, J = 2.8 Hz, J = 11.4 Hz, H-4), 5.26 (dd, 1H, J 2, 3 = 9.6 Hz, J 3, 4 = 9.3 Hz, H-3), 5.38 (t, 1H, J 2, 3 = 9.6 Hz, H-2), 5.75 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.44–7.51 (m, 3H, Ar–H), 7.59–7.65 (m, 3H, Ar–H), 8.42 (s, 1H, indole H-2), 8.73 (d, 2H, J = 7.8 Hz, Ar–H) ppm; 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 19.3, 19.5, 20.2 (3CH 3 CO), 44.6 (CH2), 49.8 (pyrimidyl C-5), 55.2 (pyrimidyl C-6), 62.7 (C-5), 64.2 (C-4), 68.8 (C-3), 71.2 (C-2), 89.1 (C-1), 110.8, 111.9, 120.2, 122.9, 123.7, 124.0, 126.0, 134.1, 136.4, 141.6, 143.1, 144.3, 150.9 (Ar-13C), 159.2 (pyrimidyl C-4), 165.1 (pyrimidyl C-2), 169.4, 170.5, 171.2 (3C=O) ppm. Anal. Calcd. for C30H32N4O7S (592.66): C, 60.80; H, 5.44; N, 9.45. Found: C, 60.67; H, 5.36; N, 9.31.
General procedure for the synthesis of compounds 6a, b
The protected glycoside 5a or 5b (0.01 mol) was dissolved in methanolic ammonia (20 mL) at 0 °C and stirred for 0.5 h at the same temperature. The reaction mixture was further stirred at room temperature for about 5 h. The solvent was evaporated under reduced pressure at 40 °C to give a solid residue, which was crystallized from ethanol to give compounds 6a, 6b, respectively.
1-Ethyl-3-{2-[(ß- d -galactopyranosyl)thio]-6-(pyridin-4-yl)-5, 6-dihydropyrimidin-4-yl}-1H-indole (6a)
2.13 g, (43 %), pale yellow powder, m.p. 189–191 °C. IR (KBr) cm−1: ν (OH) 3471–3435, ν (C=N) 1622; 1H NMR (DMSO-d6, 300 MHz): δ 1.48 (t, 3H, J = 6.2 Hz, CH3), 3.18–3.34 (m, 3H, H-6, H-6′ and pyrimidyl H-5), 3.51–3.55 (m, 1H, H-5), 3.70–3.79 (m, 3H, H-3, H-4 and pyrimidyl H-5′), 3.95–4.12 (m, 3H, CH2 and CH2CH), 4.21 (t, 1H, J 2, 3 = 9.2 Hz, H-2), 4.28 (t, 1H, J = 6.4 Hz, OH exchangeable with D2O), 4.36 (m, 1H, OH exchangeable with D2O), 4.42 (t, 1H, J = 6.4 Hz, OH exchangeable with D2O), 4.84 (m, 1H, OH exchangeable with D2O), 5.76 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.39–7.45 (m, 2H, Ar–H), 7.54 (d, 2H, J = 7.4 Hz, Ar–H), 8.18 (s, 1H, indole H-2), 8.59 (d, 2H, J = 7.6 Hz, Ar–H), 8.72 (d, 2H, J = 7.8 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.3 (CH3), 43.9 (CH2), 48.2 (pyrimidyl C-5), 55.3 (pyrimidyl C-6), 63.9 (C-6), 64.1 (C-5), 65.3 (C-4), 69.5 (C-3), 70.7 (C-2), 89.6 (C-1), 109.0, 111.4, 121.8, 122.6, 123.5, 125.3, 126.0, 134.6, 136.3, 143.7, 148.6, 151.9, 153.8 (Ar-13C), 159.6 (pyrimidyl C-4), 165.8 (pyrimidyl C-2). Anal. Calcd. for C25H28N4O5S (496.58): C, 60.47; H, 5.68; N, 11.28. Found: C, 60.14; H, 5.48; N, 11.54.
1-Ethyl-3-{2-[(ß- d -xylopyranosyl)thio]-6-(pyridin-4-yl)-5, 6-dihydropyrimid-in-4-yl}-1H-indole (6b)
1.91 g, (41 %), pale yellow powder, m.p. 192–194 °C. IR (KBr) cm−1: ν (OH) 3465–3440, ν (C=N) 1614; 1H NMR (DMSO-d6, 300 MHz): δ 1.43 (t, 3H, J = 6.2 Hz, CH3), 3.18–3.39 (m, 3H, H-5, H-5′ and pyrimidyl H-5), 3.71–3.77 (m, 3H, H-3, H-4 and pyrimidyl H-5′), 3.92–4.12 (m, 3H, CH2 and CH2CH), 4.24 (t, 1H, J 2, 3 = 9.2 Hz, H-2), 4.31 (t, 1H, J = 6.4 Hz, OH exchangeable with D2O), 4.42 (m, 1H, OH exchangeable with D2O), 4.84 (m, 1H, OH exchangeable with D2O), 5.77 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.42–7.50 (m, 3H, Ar–H), 7.54–8.64 (m, 3H, Ar–H), 8.18 (s, 1H, indole H-2), 8.71 (d, 2H, J = 7.6 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 48.2 (pyrimidyl C-5), 55.2 (pyrimidyl C-6), 63.8 (C-5), 65.2 (C-4), 69.1 (C-3), 70.8 (C-2), 89.5 (C-1), 108.9, 110.7, 121.4, 122.5, 123.4, 126.1, 126.8, 134.6, 136.6, 143.7, 149.6, 150.2, 153.0 (Ar-13C), 159.4 (pyrimidyl C-4), 165.2 (pyrimidyl C-2). Anal. Calcd. for C24H26N4O4S (466.55): C, 61.78; H, 5.62; N, 12.01. Found: C, 61.54; H, 5.52; N, 11.92.
4-(1-Ethyl-1H-indol-3-yl)-6-(pyridin-4-yl)pyrimidin-2-amine (7)
To a solution of guanidine hydrochloride (0.95 g, 0.01 mol) in ethanol (50 mL), sodium ethoxide (0.011 mol) was added. The reaction mixture was refluxed for 2 h, then compound 2 (2.76 g, 0.01 mol) was added, and the reflux was continued for 8 h. The reaction mixture was cooled and poured into water. The solid that formed was filtered, dried and recrystallized from dioxane to afford compound 7 as a yellow powder, 1.86 g, (59 %), m.p. 202–204 °C. IR (KBr) cm−1: ν (NH2) 3412, 3388, ν (C=N) 1612; 1H NMR (DMSO-d6, 300 MHz): δ 1.43 (t, 3H, J = 6.2 Hz, CH3), 4.16 (q, 2H, J = 6.2 Hz, CH2), 6.25 (s, 2H, NH2 exchangeable with D2O), 7.37–7.49 (m, 3H, Ar–H), 7.55–7.69 (m, 4H, Ar–H), 8.25 (s, 1H, indole H-2), 8.70 (d, 2H, J = 7.8 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 109.3, 111.1, 121.4, 122.2, 124.5, 125.4, 126.0, 134.4, 136.8, 141.3, 143.7, 149.6, 150.9, 152.3 (Ar-13C and pyrimidyl C-5), 160.1, 164.2, 165.1 (pyrimidyl C-2, C-4, C-6). Anal. Calcd. for C19H17N5 (315.37): C, 72.36; H, 5.43; N, 22.21. Found: C, 72.18; H, 5.21; N, 22.02.
General procedure for the synthesis of compounds 9a, b
A mixture of compound 7 (3.15 g, 0.01 mol), d-galactose and/or d-xylose (0.011 mol) in ethanol (30 mL), and a catalytic amount of acetic acid (3 drops) were heated at reflux temperature for 2 h. The formed precipitate was filtered on hot, washed with water several times, dried and recrystallized from ethanol to give to give compounds 9a, b, respectively.
4-(1-Ethyl-1H-indol-3-yl)-N-(ß-d-galactopyranosyl)-6-(pyridin-4-yl)pyrimidin-2-amine (9a)
2.91 g, (61 %), pale yellow powder, m.p. 196–197 °C. IR (KBr) cm−1: ν (OH) 3515–3440, ν (NH) 3287, ν (C=N) 1615; 1H NMR (DMSO-d6, 300 MHz): δ 1.42 (t, 3H, J = 6.2 Hz, CH3), 3.40 (m, 2H, H-6, H-6′), 3.51 (m, 1H, H-5), 3.73–3.78 (m, 2H, H-3, H-4), 4.11 (q, 2H, J = 6.2 Hz, CH2), 4.24 (t, 1H, J 2, 3 = 9.2 Hz, H-2), 4.31 (t, 1H, J = 6.4 Hz, OH exchangeable with D2O), 4.37 (m, 1H, OH exchangeable with D2O), 4.45 (t, 1H, J = 6.4 Hz, OH exchangeable with D2O), 4.88 (m, 1H, OH exchangeable with D2O), 5.87 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.38–7.52 (m, 3H, Ar–H), 7.70–7.94 (m, 4H, Ar–H), 8.36 (s, 1H, indole H-2), 8.53 (d, 2H, J = 7.8 Hz, Ar–H), 10.12 (s, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 62.8 (C-6), 65.2 (C-4), 69.1 (C-3), 70.8 (C-2), 72.6 (C-5), 92.2 (C-1), 109.2, 110.4, 120.8, 121.4, 124.5, 125.5, 126.5, 134.6, 136.4, 143.7, 149.1, 150.4, 151.0 (Ar-13C and pyrimidyl C-5), 161.0, 164.2, 165.1 (pyrimidyl C-2, C-4, C-6). Anal. Calcd. for C25H27N5O5 (477.51): C, 62.88; H, 5.70; N, 14.67. Found: C, 62.69; H, 5.53; N, 14.49.
4-(1-Ethyl-1H-indol-3-yl)-N-(ß-d-xylopyranosyl)-6-(pyridin-4-yl)pyrimidin-2-amine (9b)
2.59 g, (58 %), pale white powder, m.p. 197–199 °C. IR (KBr) cm−1: ν (OH) 3510–3448, ν (NH) 3293, ν (C=N) 1612; 1H NMR (DMSO-d6, 300 MHz): δ 1.45 (t, 3H, J = 6.2 Hz, CH3), 3.42 (m, 2H, H-5, H-5′), 3.75 (m, 2H, H-3, H-4), 4.14 (q, 2H, J = 6.2 Hz, CH2), 4.25 (t, 1H, J 2, 3 = 9.2 Hz, H-2), 4.32 (m, 1H, OH exchangeable with D2O), 4.38 (m, 1H, OH exchangeable with D2O), 4.88 (m, 1H, OH exchangeable with D2O), 5.89 (d, 1H, J 1, 2 = 9.6 Hz, H-1), 7.38–7.51 (m, 3H, Ar–H), 7.54–7.69 (m, 4H, Ar–H), 8.58 (s, 1H, indole H-2), 8.62 (d, 2H, J = 7.8 Hz, Ar–H), 10.08 (s, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.7 (CH3), 44.6 (CH2), 62.8 (C-5), 65.2 (C-4), 69.1 (C-3), 70.9 (C-2), 92.8 (C-1), 110.1, 111.1, 121.4, 122.6, 123.1, 123.9, 125.9, 134.6, 136.2, 143.7, 149.6, 150.9, 151.3, 151.9 (Ar-13C and pyrimidyl C-5), 160.9, 164.2, 165.2 (pyrimidyl C-2, C-4, C-6). Anal. Calcd. for C24H25N5O4 (447.49): C, 64.42; H, 5.63; N, 15.65. Found: C, 64.11; H, 5.45; N, 15.41.
General procedure for the preparation of compounds 10a, b
To a well-stirred solution of the glycoside 9a, b (0.01 mol) in pyridine (7 mL), acetic anhydride (0.03 mol) was added. The mixture was stirred at room temperature for 15–20 h (TLC). The resulting solution was poured onto crushed ice, and the product that separated out was filtered, washed with sodium hydrogen carbonate (30 %, 50 mL) solution, followed by water (50 mL) and then dried. The products were recrystallized from ethanol–water (2:1) to give compounds 10a, b, respectively.
4-(1-Ethyl-1H-indol-3-yl)-N-(2, 3, 4, 6-tetra- O -acetyl-ß-d-galactopyranosyl)-6-(pyridin-4-yl)pyrimidin-2-amine (10a)
2.71 g, (42 %), pale yellow powder, m.p. 137–138 °C. IR (KBr) cm−1: ν (NH) 3288, ν (C=O) 1744, ν (C=N) 1615; 1H NMR (CDCl3, 300 MHz): δ 1.46 (t, 3H, J = 6.2 Hz, CH3), 1.94, 2.04, 2.11, 2.15 (4s, 12H, 4 CH 3 CO), 4.05 (m, 1H, H-6), 4.12–4.15 (m, 3H, CH2 and H-6′), 4.15 (dd, 1H, J = 2.8 Hz, J = 11.4 Hz, H-5), 4.98 (t, 1H, J 3, 4 = 9.3 Hz, H-4), 5.25 (dd, 1H, J 2, 3 = 9.6 Hz, J 3, 4 = 9.3 Hz, H-3), 5.39 (t, 1H, J 2, 3 = 9.6 Hz, H-2), 5.71 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.42–7.55 (m, 3H, Ar–H), 7.59–7.72 (m, 4H, Ar–H), 8.42 (s, 1H, indole H-2), 8.61 (d, 2H, J = 7.8 Hz, Ar–H), 10.08 (s, 1H, NH exchangeable with D2O); 13C NMR (CDCl3, 75 MHz): δ 16.7 (CH3), 19.3, 19.5, 20.2, 20.2 (4CH 3 CO), 44.6 (CH2), 62.7 (C-6), 64.2 (C-4), 68.8 (C-3), 71.2 (C-2), 72.0 (C-5), 87.1 (C-1), 110.1, 111.1, 121.3, 122.1, 123.4, 124.7, 126.1, 134.1, 136.0, 141.8, 143.7, 149.6, 150.9, 151.2 (Ar-13C and pyrimidyl C-5), 161.1, 164.2, 165.2 (pyrimidyl C-2, C-4, C-6), 169.4, 170.5, 171.3, 171.7 (4C=O). Anal. Calcd. for C33H35N5O9 (645.66): C, 61.39; H, 5.46; N, 10.85. Found: C, 61.14; H, 5.31; N, 10.73.
4-(1-Ethyl-1H-indol-3-yl)-N-(2, 3, 4-tri- O -acetyl-ß-d-xylopyranosyl)-6-(pyridin-4-yl) pyrimidin-2-amine (10b)
3.21 g, (56 %), pale white powder, m.p. 143–145 °C. IR (KBr) cm−1: ν (NH) 3294, ν (C=O) 1742, ν (C=N) 1618; 1H NMR (DMSO-d6, 300 MHz): δ 1.47 (t, 3H, J = 6.2 Hz, CH3), 1.94, 2.03, 2.14 (3 s, 9H, 3CH 3 CO), 4.04 (m, 1H, H-5), 4.12–4.15 (m, 3H, CH2 and H-5′), 4.17 (dd, 1H, J = 2.6 Hz, J = 11.4 Hz, H-4), 5.26 (dd, 1H, J 2, 3 = 9.6 Hz, J 3, 4 = 9.3 Hz, H-3), 5.38 (t, 1H, J 2, 3 = 9.6 Hz, H-2), 5.73 (d, 1H, J 1, 2 = 9.8 Hz, H-1), 7.44–7.57 (m, 3H, Ar–H), 7.59–7.73 (m, 4H, Ar–H), 8.45 (s, 1H, indole H-2), 8.71 (d, 2H, J = 7.8 Hz, Ar–H), 10.14 (s, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.5 (CH3), 19.4, 19.4, 20.2 (3CH 3 CO), 44.7 (CH2), 64.4 (C-4), 68.6 (C-3), 71.2 (C-2), 72.9 (C-5), 87.1 (C-1), 109.3, 111.1, 120.3, 121.4, 122.2, 123.5, 125.1, 126.1, 134.6, 136.0, 143.7, 149.6, 150.9, 151.6 (Ar-13C and pyrimidyl C-5), 161.4, 164.9, 165.2 (pyrimidyl C-2, C-4, C-6), 169.9, 170.1, 171.3 (3C=O). Anal. Calcd. for C30H31N5O7 (573.60): C, 62.82; H, 5.45; N, 12.21. Found: C, 62.54; H, 5.35; N, 11.93.
2-Amino-6-(1-ethyl-1H-indol-3-yl)-4-(pyridin-4-yl)-2H-pyran-3-carbonitrile (11)
A mixture of the compound 2 (2.76 g, 0.01 mol) and malononitrile (0.66 g, 0.01 mmol) in pyridine (20 mL) was refluxed for 10 h. The solution was cooled and poured onto ice/HCl, and the solid that formed was filtered, washed several times with water, dried and recrystallized from ethanol to give 11 as a yellow powder, 1.68 g, (49 %), m.p. 221–223 °C. IR (KBr) cm−1: ν (NH2) 3415, 3390, ν (CN) 2212, ν (C=N) 1610; 1H NMR (DMSO-d6, 300 MHz): δ 1.45 (t, 3H, J = 6.2 Hz, CH3), 4.17 (q, 2H, J = 6.2 Hz, CH2), 5.25 (bs, 2H, NH2 exchangeable with D2O), 5.35 (t, 1H, J = 5.2 Hz, pyran H-2), 5.64 (s, 1H, pyran H-5), 7.35–7.48 (m, 3H, Ar–H), 7.52–7.66 (m, 3H, Ar–H), 8.56 (s, 1H, Indole H-2), 8.70 (d, 2H, J = 7.8 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (CH3), 44.6 (CH2), 85.1 (pyran C-2), 104.5 (pyran C-5), 109.0, 109.9, 111.1, 119.4, 123.2, 123.8, 125.6, 126.4, 132.6, 134.3, 135.7, 140.9, 143.7, 149.6, 152.2, 154.7 (Ar-13C, CN and pyran C-3, C-4, C-6). Anal. Calcd. for C21H18N4O (342.39): C, 73.67; H, 5.30; N, 16.36. Found: C, 73.49; H, 5.20; N, 16.09.
Ethyl N-(3-cyano-6-(1-ethyl-1H-indol-3-yl)-4-(pyridin-4-yl)-2H-pyran-2-yl)formimidate (12)
A mixture of compound 11 (3.42 g, 0.01 mol) and triethylorthoformate (3 mL) was heated at 100 °C for 5 h. The excess triethylorthoformate was evaporated under reduced pressure. Ether (10 mL) was added, and the solid product obtained was filtered and crystallized from ethanol to give 12 as a yellow powder, 2.27 g, (57 %), m.p. 186–188 °C. IR (KBr) cm−1: ν (CN) 2210, ν (C=N) 1611; 1H NMR (CDCl3, 300 MHz): δ 1.35 (t, 3H, J = 6.6 Hz, CH3), 1.46 (t, 3H, J = 6.2 Hz, CH3), 3.98 (q, 2H, J = 6.6 Hz, CH2), 4.21 (q, 2H, J = 6.2 Hz, CH2), 5.37 (t, 1H, J = 5.2 Hz, pyran H-2), 5.65 (s, 1H, pyran H-5), 7.35–7.48 (m, 3H, Ar–H), 7.53–7.67 (m, 4H, Ar–H and CH=N), 8.54 (s, 1H, Indole H-2), 8.72 (d, 2H, J = 7.8 Hz, Ar–H); 13C NMR (CDCl3, 75 MHz): δ 15.2, 16.1 (2CH3), 43.1, 44.6 (2CH2), 81.2 (pyran C-2), 103.5 (pyran C-5), 109.3, 110.1, 119.1, 121.4, 122.5, 123.3, 125.7, 126.0, 134.3, 135.4, 143.3, 148.1, 150.4, 151.3, 152.4, 153.4, 155.1 (Ar-13C, CN, CH=N, and pyran C-3, C-4, C-6). Anal. Calcd. for C24H22N4O2 (398.46): C, 72.34; H, 5.57; N, 14.06. Found: C, 72.10; H, 5.31; N, 13.84.
7-(1-Ethyl-1H-indol-3-yl)-4-imino-5-(pyridin-4-yl)-4, 8a-dihydro-3H-pyrano[2, 3-d] pyrimidin-3-amine (13)
A mixture of compound 12 (3.98 g, 0.01 mol) and hydrazine hydrate (1 mL, 0.017 mol) in ethanol (30 mL) was heated under reflux for 10 h. The solvent was reduced under reduced pressure and left overnight at 5 °C. The solid product obtained was filtered, washed with cold ethanol and crystallized from ethanol as pale white powder, 1.80 g, (47 %), m.p. 234–236 °C. IR (KBr) cm−1: ν (NH2, NH) 3418, 3385, 3328, ν (C=N) 1615; 1H NMR (DMSO-d6, 300 MHz): δ 1.49 (t, 3H, J = 6.2 Hz, CH3), 4.19 (q, 2H, J = 6.2 Hz, CH2), 5.37 (t, 1H, J = 5.2 Hz, pyran H-2), 5.69 (s, 1H, pyran H-5), 6.74 (bs, 2H, NH2 exchangeable with D2O), 7.48–7.57 (m, 3H, Ar–H), 7.61–7.77 (m, 4H, CH = N and Ar–H), 8.55 (s, 1H, indole H-2), 8.72 (d, 2H, J = 7.8 Hz, Ar–H), 11.26 (bs, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (CH3), 44.6 (CH2), 84.1 (pyran C-2), 105.5 (pyran C-5), 109.2, 110.1, 121.4, 122.0, 123.2, 125.0, 126.1, 133.5, 136.6, 143.1, 149.9, 150.9, 152.2, 155.9 (Ar-13C, CH = N and pyran C-3, C-4, C-6), 158.1 (C=NH). Anal. Calcd. for C22H20N6O (384.43): C, 68.73; H, 5.24; N, 21.86. Found: C, 68.59; H, 5.18; N, 21.61.
8-(1-Ethyl-1H-indol-3-yl)-10-(pyridin-4-yl)-3, 6a-dihydro-2H-pyrano[3, 2-e][1, 2, 4] triazolo[1, 5-c]pyrimidine-2-thione (14)
To a stirred suspension of compound 13 (3.84 g, 0.01 mol) in ethanol (15 mL), ethanolic potassium hydroxide (20 mL, 0.01 mol) and CS2 (2 mL) were added dropwisely. The reaction mixture was then heated under reflux for 8 h. After cooling and evaporation of the solvent, the potassium salt obtained was dissolved in water and acidified with 2 N aqueous HCl. The solid product formed was collected and recrystallized from ethanol to give 14 as a yellow powder, 2.47 g, (58 %), m.p. 179–180 °C. IR (KBr) cm−1: ν (NH) 3319, ν (C=N) 1618, ν (C=S) 1189; 1H NMR (DMSO-d6, 300 MHz): δ 1.48 (t, 3H, J = 6.2 Hz, CH3), 4.16 (q, 2H, J = 6.2 Hz, CH2), 5.35 (t, 1H, J = 5.2 Hz, pyran H-2), 5.69 (s, 1H, pyran H-5), 7.47–7.59 (m, 3H, Ar–H), 7.63–7.80 (m, 4H, CH = N and Ar–H), 8.59 (s, 1H, indole H-2), 8.72 (d, 2H, J = 7.8 Hz, Ar–H), 12.98 (bs, 1H, NH exchangeable with D2O); 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (CH3), 44.6 (CH2), 84.2 (pyran C-2), 106.7 (pyran C-5), 109.4, 110.9, 120.1, 121.7, 124.1, 125.7, 126.6, 134.2, 136.4, 143.4, 149.8, 150.6, 152.4, 155.4 (Ar-13C, CH = N and pyran C-3, C-4, C-6), 159.1 (C=NH), 179.1 (C=S). Anal. Calcd. for C23H18N6OS (426.49): C, 64.77; H, 4.25; N, 19.70. Found: C, 64.55; H, 4.31; N, 19.58.
1-Ethyl-3-(1-phenyl-5-pyridin-4-yl-1H-pyrazol-3-yl) 1H-indol (15)
A mixture of the compound 2 (2.76 g, 0.01 mol) and phenylhydrazine (1.08 g, 0.01 mol) in ethanol (20 mL) was heated under reflux for 10 h. Then the reaction mixture was cooled, filtered, dried and recrystallized from ethanol to give the compound 15 as a pale yellow powder, 2.20 g, (60 %), m.p. 182–184 °C. IR (KBr) cm−1: ν (C=N) 1605; 1H NMR (DMSO-d6, 300 MHz): δ 1.44 (t, 3H, J = 6.2 Hz, CH3), 3.22 (dd, 1H, J = 4.8 Hz, J = 14.6 Hz, pyrazoline H-4), 3.82 (dd, 1H, J = 8.8 Hz, J = 14.6 Hz, pyrazoline H-4′), 4.16 (q, 2H, J = 6.2 Hz, CH2), 5.32 (dd, 1H, J = 4.8 Hz, J = 8.8 Hz, pyrazoline H-5), 7.14–7.26 (m, 3H, Ar–H), 7.47–7.59 (m, 4H, Ar–H), 7.69–7.82 (m, 4H, Ar–H), 8.57 (s, 1H, indole H-2), 8.65 (d, 2H, J = 7.6 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.2 (CH3), 44.1, 44.5 (2CH2), 66.0 (CH), 110.1, 110.9, 111.3, 112.5, 120.8, 122.7, 123.5, 125.6, 126.4, 134.9, 136.2, 140.3, 143.8, 149.6, 150.9, 152.0, 152.9 (Ar-19C), 152.7 (pyrazole C-3). Anal. Calcd. for C24H22N4)366.46): C, 78.66; H, 6.05; N, 15.29. Found: C, 78.48; H, 5.89; N, 15.02.
1-Ethyl-3-(5-pyridin-4-yl-isoxazol-3-yl) 1H-indol (16)
A solution of compound 2 (2.76 g, 0.01 mol) and hydroxylamine hydrochloride (0.33 g, 0.01 mol) in ethanol (15 mL) containing (0.4 g) sodium hydroxide was refluxed for 8 h. The reaction mixture was cooled and poured onto crushed ice. The precipitate was filtrated, washed with water and recrystallized from ethanol to give the compound 16 as a yellow powder, 2.03 g, (70 %), m.p. 178–179 °C. IR (KBr) cm−1: ν (C=N) 1608; 1H NMR (DMSO-d6, 300 MHz): δ 1.49 (t, 3H, J = 6.2 Hz, CH3), 3.18 (dd, 1H, J = 4.6 Hz, J = 14.8 Hz, isoxazoline H-4), 3.82 (dd, 1H, J = 9.2 Hz, J = 14.8 Hz, isoxazoline H-4′), 4.21 (q, 2H, J = 6.2 Hz, CH2), 5.28 (dd, 1H, J = 4.6 Hz, J = 9.2 Hz, isoxazoline H-5), 7.48–7.60 (m, 3H, Ar–H), 7.70–7.83 (m, 3H, Ar–H), 8.61 (s, 1H, indole H-2), 8.75 (d, 2H, J = 7.8 Hz, Ar–H); 13C NMR (DMSO-d6, 75 MHz): δ 16.1 (CH3), 44.1, 44.5 (2CH2), 66.1 (CH), 110.14, 121.4, 122.9, 123.5, 125.1, 126.4, 134.6, 136.0, 143.1, 149.6, 150.0, 150.4 (Ar-13C), 153.1 (oxazole C-3). Anal. Calcd. for C18H17N3O (291.35): C, 74.20; H, 5.88; N, 14.42. Found: C, 74.02; H, 5.65; N, 14.20.
Molecular docking
All docking simulations were performed with AutoDock Vina v1.1.2 software (Trott and Olson, 2010). Initial 3D conformations of ligands were generated with CORINA 3D structure generator v3.47. Preparation of protein and further processing of ligand data were done in AutoDock Tools suite v1.5.6. Result analysis and visualizations were performed with PyMOL v1.5.0.3.
The DNA gyrase A protein was represented by 1AB4 PDB entry. The biological assembly dimeric form was directly downloaded from RCSB Internet site [RCSB http://www.rcsb.org accessed 28/04/2013]. The protein structure was preprocessed using the AutoDock Tools suite. Partial charges were automatically added, and AutoDock-specific atom types were assigned. In order to increase accuracy of docking simulation side chains of following residuals in both chains were annotated as flexible: R32, H78, S83, D87, Y122. Initial confirmations of all 17 compounds used in docking simulations were found using the CORINA 3D structure generator. Further preprocessing was performed with AutoDock Tools suite. Partial charges were assigned, and rotatable bonds were selected.
Docking simulations were performed with AutoDock Vina. The size of the search space was set to 40 × 40 × 40 Å, and it was centered at expected binding site residues, i.e., at R32, H78, S83, D87 and Y122 residues (Cartesian coordinates of the center were 60, 60, 40 Å)—see Fig. 6. Two docking simulations were performed. In the first simulation, all 17 compounds were docked. Twenty different docking poses were obtained for each compound. The maximal allowed energy range between the best and the worst binding mode was set to 3 kcal/mol. The global search exhaustiveness parameter controlling the search algorithm accuracy was set to 12 (default value is 8; 12 means high accuracy). The second simulation was done for 3, 5a, 5b, 6a and 6b compounds only. The energy range parameter was set to 4.5 kcal/mol and the exhaustiveness to 20. Inspection and visualization of the docking results were done in PyMOL.
Antimicrobial activity
The anti-bacterial activity of the synthesized compounds was tested against Bacillus subtilis, Streptococcus lactis (Gram-positive bacteria), E. coli, Pseudomonas sp. (Gram-negative bacteria) using the nutrient agar medium. The anti-fungal activity of the compounds was tested against the yeast-like pathogenic fungi (Candida albicans, Candida gabrata) using Sabouraud dextrose agar medium.
Agar diffusion medium
All compounds were in vitro screened for their antimicrobial activity by agar diffusion method (Cruickshank et al., 1975). A suspension of the organisms was added to sterile nutrient agar media at 45 °C, and the mixture was transferred to sterile Petri dishes and allowed to solidify. Holes of 10 mm in diameter were made using a cork borer. An amount of 0.1 mL of each of the synthesized compounds was poured inside the holes. A hole filled with DMSO was also used as a control. The plates were left for 1 h at room temperature as a period of preincubation diffusion to minimize the effects of variation in time between the applications of the different solutions. The plates were then incubated at 37 °C for 24 h and observed for anti-bacterial activity. The diameters of zone of inhibition were measured and compared with that of the standard drugs, and the values are tabulated in Table 1. Nalidixic acid® and Nystatin® were used as standard drugs for anti-bacterial and anti-fungal activity, respectively.
Minimum inhibitory concentration
Minimum inhibitory concentration (MIC) of the test compounds was determined by the agar streak dilution method. A stock solution of 68 mg/mL of the synthesized compounds was made using DMSO as the solvent. From this stock solution, the following concentrations (0.17; 0.34; 0.68; 0.85; and 1.7 mg/mL) of the tested compound solutions were mixed with the known quantities of molten sterile agar media aseptically. About 20 mL of the media containing the tested compound was dispensed into each sterile Petri dish. Then the media were allowed to get solidified. Microorganisms were then streaked one by one on the agar plates aseptically. After streaking, all the plates were incubated at 37 °C for 24 h/48 h for bacterial and fungus activities, respectively. Then the plates were observed for the growth of microorganisms. The lowest concentrations of the synthesized compounds inhibiting the growth of the given bacteria/fungi were considered as the minimum inhibitory concentration (MIC) of the tested compounds against that bacteria or fungi on the plate. The MIC values of each compound against various bacteria and fungus are tabulated in Table 2. MIC values of the compounds against the tested bacteria/fungi (Table 2) were calculated as the concentration which resulted in >99 % inhibition of the microorganism on the plate.
Results and discussion
Chemistry
In this investigation, the N-alkylated indole derivative 1 was reacted (El-Sayed et al., 2009a, b, c) with 4-pyridine carboxaldehyde in the presence of potassium hydroxide to afford the substituted indolyl enone derivative 2. The later enone derivative was used as a key precursor for the preparation of 4, 6-disubstituted pyrimidine and thiopyrimidine derivatives (Scheme 1). Thus, the reaction of 2 with thiourea in the presence of sodium hydroxide led to the formation of 4, 6-disubstituted thiopyrimidine 3 in 82 % yield (El-Sayed et al., 2009a, b, c; Rathod and Kulkarni, 2011; Patel et al., 2011), while in reaction with guanidine hydrochloride the amino-4, 6-disubstituted pyrimidine derivative 7 was obtained (Scheme 1). Formation of compound 3 may be explained according to a reported mechanism starting with a 1, 2-addition product which is then dehydrated to form an intermediate possessing a sufficiently activated olefinic double bond to undergo an internal nucleophilic attack and thus forming the 5, 6-dihydro derivative (Ebraheem, 2013; Brown et al., 1985). Another mechanistic route starts with an initial 1, 4-addition to the activated olefinic double bond2, and the resulting Micheal addition product then cyclizes to tetrahydropyrimidine derivative. The later derivative readily loses a water molecule to afford the 5, 6-dihydro derivative. As the reaction was not carried out under nitrogen, no hydrogen transfer to the chalcone occurred, and thus, fully aromatic compound was not formed (Brown et al., 1985).
Glycosylation of the thiopyrimidine derivative 3 was carried out by using glycosyl halides 4a, b in the presence of potassium hydroxide to produce the corresponding thioglycosides 5a, b (Scheme 1). The regioselectivity of forming thioglycosides was confirmed on the basis of their spectral data. The 1H NMR spectra of the produced thioglycosides showed the anomeric protons of the sugar moieties in the range δ 5.72 and 5.75 ppm as doublets, with coupling constants equal to 10.2 and 9.8 Hz indicating the β-orientation of the thioglycosidic bond. The anomeric proton of β-N-glycosides having an adjacent C=S was reported to appear at higher chemical shift (δ 6.9–7.2 ppm) due to the anisotropic deshielding effect of the C=S (Ibrahim, 1996; Mansour et al., 1999). The 13C NMR spectra of 5a, b showed a signal at δ 89.1 and 88.9 ppm corresponding to the anomeric C-1, which also confirmed the β-configuration. The absence of a peak corresponding to the C=S group indicates that the attachment of the sugar has taken place at the sulfur atom and not on the nitrogen atom. Treatment of the acetylated thioglycosides 5a, b with methanolic ammonia afforded the free hydroxyl glycosides 6a, b, respectively. The spectral data of the obtained derivatives were agreements with the assigned structures.
Reaction of aminopyrimidine derivative 7 with d-galactose and d-xylose in the presence of acetic acid gave stereoselectively the corresponding β-N-glycosides 9a, b, respectively (Scheme 1). The characterization and assignment of the acyclic–cyclic nature of the produced amino sugars were investigated by their NMR spectra. Both sugars afforded products whose NMR spectral data indicated the cyclic structure of the sugar moiety. The observed lower chemical shift values assigned for the H-1 proton at δ 5.87 and 5.89 ppm with coupling constants 9.8 and 9.6 in addition to the absence of the doublet signal in the downfield region at 7.50–7.70 (for the acyclic form) indicated the cyclic form with β-configuration. Moreover, the appearance of the C-1 signal in the 13C NMR spectra at δ 92.2 and 92.8 ppm in addition to the absence of the signal in the downfield region at 153–158 (for the acyclic form) evidenced the cyclic structure. The reaction of the β-N-glycosides 9a, b with acetic anhydride at room temperature led to the acetylation of the sugar hydroxyls and formation of compounds 10a, b, respectively (Scheme 1).
On the other hand, the N-ethylindolyl enone 2 was used as a starting compound for the preparation of fused tricylic and heterocycles. Reaction of compound 2 with malononitrile afforded the aminocyano-functionalized pyran derivative 11 (Scheme 2) (Mohamed et al., 2008). Reaction of the obtained pyrane 11 with triethylorthoformate led to the formation of the formamidate derivative 12 which when allowed to react with hydrazine hydrate afforded the pyranopyrimidine derivative 13 (Scheme 2). Its 1H NMR spectrum showed triplet and quartet signals at δ 1.49, 4.19 ppm assigned for the ethyl protons and broad signals at δ 6.74, 11.26 ppm corresponding to NH2 and NH, respectively. When compound 13 was reacted with carbon disulfide, the tricyclic derivative 14 was obtained in 58 % yield (Scheme 2). The 1H NMR spectrum of 14 showed the signals corresponding to ethyl group as triplet and quartet, pyran H-5 in addition to the aromatic protons and the NH signal. Its 13C NMR spectrum showed the signal at 179.1 ppm for the C=S group.
Reaction of the N-ethylindolyl enone 2 with phenylhydrazine and hydroxylamine hydrochloride resulted in the formation of pyrazolyl-indole and oxazolyl-indole derivatives 15 and 16, respectively (Scheme 2). Their 1H NMR spectra revealed the presence of the signals corresponding to the methylene and methane protons in the newly formed rings in their structures.
Antimicrobial activity
The synthesized compounds were tested for their in vitro antimicrobial activity against a panel of standard strains of the Gram-positive bacteria (B. subtilis and S. lactis), the Gram-negative bacteria (E. coli and Pseudomonas aeuroginosa) and the yeast-like pathogenic fungi (C. albicans, C. gabrata). The primary screening was carried out using the agar disk diffusion method using Muller-Hinton agar medium. The results of the preliminary anti-bacterial and the anti-fungal activities are shown in Table 1. The results revealed that the compounds showed varying degrees of inhibition against the tested microorganisms. In general, the best anti-bacterial activity was displayed by compounds 5a, b; 6a, b; 9a, b; 11; 13; 14; and 16. Compounds 6a, b; 9a, b; and 14 showed strong activity against Gram-positive bacteria B. subtilis (inhibition zone 22–25 mm), while compounds 5a, b; 11; and 13 were moderate activity (inhibition zone 18–21 mm). Compounds 6a and 7 showed good activity against S. lactis, while compounds 3, 12 and 16 were moderately active. Compounds 2; 5a, b; 6a, b; 9a; 10a, b; 11; 13; 14; and 16 produced high activity against the Gram-negative bacteria E. coli. The compounds 2; 5a; 6a; 7; 9b; 11–13; and 15 showed good activity against Pseudomonas sp., while compounds 6b, 14 and 16 produce the highest activity. The anti-fungal activities depicted in Table 1 revealed that compounds 3, 5a; 6a, b; 9a; 10a; and 12 exhibited good anti-fungal activities.
The minimum inhibitory concentration (MIC) of the most active compounds compared to the antibiotic Nalidixic acid® and the anti-fungal drug Nystatin® is explained in Table 2. The results showed that the MICs ranged between 20 and 50 μg/disk. The MICs for the synthesized compounds were in accordance with the results obtained in the primary screening.
The antimicrobial activity results and structure–activity relationship indicated that the attachment of glycosyl moieties to the pyrimidine ring in the indolyl-pyrimidine ring system through N- or S-glycosidic linkage resulted in a marked increase in growth inhibition activity of different microbial strains. Additionally, the anti-bacterial activity observed for the N-substituted pyrimidine glycosides 6a, b and 9a, b indicated the importance of the free hydroxyl groups of S-glycopyranosyl and N-glycopyranosyl moieties, and the activity was reduced when these groups were replaced with the corresponding O-acetylated analogues. Moreover, the anti-bacterial activity observed for the xylopyranosyl derivative was relatively higher than that of the corresponding galactopyranosyl. In addition, the inhibition activity of the thioglycoside 6b against Gram-positive bacteria was found to be relatively higher than that of the similar N-glycoside 9b. The anti-bacterial activity results also indicated that the attachment of pyrano[3, 2-e][1, 2, 4]triazolo[1, 5-c]pyrimidine thione moiety to the N-ethyl indole ring as compound 14 resulted in a marked increase in activity when compared with the amino derivative 13.
For the anti-fungal properties, the results revealed that the growth inhibition activity of the thioglycoside 6b against fungi under test was found to be relatively higher than that of the similar N-glycoside 9b. Furthermore, the indolyl-pyrimidine thione 3 and the formamidate derivative 12 showed higher activity against C. gabrata. In addition to the glycosides and tricyclic pyridotriazolopyrimidine, the 2-amino-substituted pyrimidine, the N-phenylpyrazole and oxazole attached to the indolyl ring systems showed increased inhibition activities against Gram-negative bacteria E. coli and P. aeuroginosa.
Molecular docking
The anti-bacterial activity of the synthesized compounds was further analyzed by the molecular docking approach, a method of simulation of fitting ligands into binding site(s) of macromolecular targets. The docking algorithm manipulates ligands and macromolecules in the way that leads to the optimal fit. Analysis of the docking results can lead to better understanding of the molecular basis of the biological effects in question. The obvious prerequisite of successful docking is that the macromolecule and ligands selected for studies can interact in the living organisms and that this interaction plays an important role in production of the observed biological effect (Trott and Olson, 2010). One should keep in mind that the direct analysis of docking results assumes a priori that the interaction between a drug and a docking target is the main mechanism of action. This assumption can often be false. The actual mechanism is usually much more complicated and involves multiple steps or multitarget interactions, metabolic transformations and other events.
As a docking macromolecular target for compounds described in this report, DNA gyrase A protein from E. coli was used (Morris et al., 1998). DNA gyrase is a well-known target for several antimicrobial drugs which have some structural features common with our compounds (Ostrov et al., 2007). It has several potential sites where ligands can be bound. From the perspective of inhibitors, the most potent sites are probably those located in the proximity of residues taking key roles in catalytic functions. According to the literature, the most important residues constituting the active site are R32, H78 and Y122 (Morris et al., 1998; Ostrov et al., 2007). Moreover, it was reported that the residues S83 and D87 are often substituted in fluoroquinolone-resistant forms (Ostrov et al., 2007). All these residues are in the relative mutual proximity and are located around the dimer interface of the protein. We have used this part of the protein in our simulations, and side chains of the above-mentioned residues were kept flexible (10 residues in total for both chains of the dimer).
Performed simulations resulted in 20 different binding poses for each of 17 compounds. Table 3 summarizes the estimated binding affinities compared for convenience with growth inhibition of E. coli (Table 1). Figure 2 shows the plot of minimal, maximal and mean values (together with standard deviation) of scoring function plotted against the inhibition zone.
The lack of correlation between the inhibition and scoring function values is evident. Quantitative reliability of scoring functions used in docking simulations has been often questionable. However, we have found in our previous studies that docking results can be successfully discussed in a qualitative manner (Magdziarz et al., 2009). Careful analysis of docking results can reveal some interesting differences between the active and the inactive compounds.
Thiopyrimidine derivatives
Compounds 3, 5a, 5b, 6a and 6b form a group of thiopyrimidine derivatives (Scheme 1). Compounds 3 and 5b are not very active against E. coli, whereas 5a, 6a and 6b are quite active ones. Compound 3 is much smaller than the other compounds lacking the sugar moieties, and this could be the reason of its low activity.
Inspection of docking poses reveals one interesting difference between compounds 3, 5a, b and 6a, b. The formers do not bind in the pocket located between R32, P43, H78 and Y122 residues. This pocket seems to be important because it is constituted by the residues playing key roles in the catalytic function of the protein, and Y122 residue in this pocket is located at the opposite chain. Inhibitors located in this pocket, probably, interfere with these important residues and influence with the dimer interface of the protein.
Figures 3, 4 and 5 show compounds 5a and 6a, b located in this pocket as found by AutoDock Vina (Trott and Olson, 2010). Compound 5b is not placed in this pocket what seems to correspond with its slightly lower inhibition activity.
Pyrimidine derivatives
This is another group formed by 5 compounds 7, 9a, b and 10a, b. In this case, there is no clear dependency between the inhibition rate and the propensity to the R32, P43, H78 and Y122 binding sites. This seems to contradict the hypothesis of the importance of this binding pocket. On the other hand, the lack of specificity in binding can be explained by slightly less overall activity of this group as compared to thiopyrimidine derivatives. It is also very likely that the lack of sulfur atom in these compounds changes completely their interaction mode with the protein. No specific docking modes were observed for the compounds 2 and 11–16 (Fig. 6).
Thiopyrimidine derivatives: repeated simulations
In order to verify results observed for the thiopyrimidine derivatives, docking simulations were repeated using slightly different settings. The parameters controlling allowed energy range and the accuracy of search algorithm were increased—see experimental section for details. Table 4 compares the results of the first and repeated simulations.
It is interesting to notice that for compounds 5a, b and 6b, the modified settings allowed to find more poses located in the binding pocket. In particular, compound 5b was located in the pocket 2 times top two poses. Compound 6a was placed in the pocket only 2 times, but this is somehow compensated by its lowered minimal score. In spite of significantly increased search accuracy, compound 3 was not placed in the pocket what is in good agreement with its low activity.
Comparison of results between 5a and 5b shows apparent dependency between inhibition potency of these compounds and their propensity to bind to this pocket. One has to keep in mind that docking simulations performed with AutoDock Vina have stochastic nature (Trott and Olson, 2010). Therefore, further repetition of docking simulations should be performed in order to fully support observations described in this report. Moreover, additional studies should be done to compare thiopyrimidine derivatives binding modes with compounds 11, 13, 14 and 16 (all displaying high activity against E. coli).
Conclusion
In the present work, new 1H-indole derivatives were synthesized and characterized using spectral and elemental analyses. The synthesized compounds were investigated for their antimicrobial activity, and some of them showed promising anti-bacterial and anti-fungal activities. Anti-bacterial activity against E. coli of the synthesized compounds was further analyzed by the molecular docking approach. Obtained results showed qualitative dependency between activity and binding mode in the thiopyrimidine derivatives. Lack of specific binding of pyrimidine derivatives may indicate that this group exhibits different mechanism of E. coli inhibition
References
Abdel-Aal MT, El-Sayed WA, El-Ashry ESH (2006) Synthesis and antiviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch Pharm Chem Life Sci 39:656–663
Abdel-Aal MT, El-Sayed WA, El-Kosy SM, El-Ashry ESH (2008) Synthesis and antiviral evaluation of novel 5-(N-Aryl-aminomethyl-1, 3, 4-oxadiazol-2-yl) hydrazines and their sugars, 1, 2, 4-triazoles, tetrazoles and pyrazolyl derivatives. Arch Pharm Chem Life Sci 341:307–313
Abdel-Rahman HM, El-Koussi NA, Hassan HY (2009) Fluorinated 1, 2, 4-triazolo[1, 5-a]pyrimidine-6-carboxylic acid derivatives as antimycobacterial agents. Arch Pharm (Weinheim) 342:94–99
Ahmed O, Razvi SA, Rayees TKM, Shoeb MAN, Salahuddin M (2014) Synthesis, characterization and anti-inflammatory activity of some substituted pyrimidine derivatives. Indo Am J Pharm Res 4(5):2301–2306
Baharfar R, Asghari S, Kiani M (2015) Regioselective synthesis and antibacterial activity of 3-(cyanoacetyl)indole-based kojic acid derivatives. Monatsh Chem 146:336–343
Bal TR, Anand B, Yogeeswari P, Sriram D (2005) Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg Med Chem Lett 15:4451–4455
Brown DJ, Evans RF, Cowden WB, Fenn MD (ed) (1985) Chemistry of heterocyclic compounds: a series of monographs. In: The pyrimidines: supplement II, vol 16. Wiley, New York, p 408
Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF (2005) 3, 3’-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 26:771–778
Cruickshank R, Duguid JP, Marmion BP, Swain RH (1975) Medical microbiology, 12th edn. Longman group Ltd., Edinburgh, pp 180–188
Dilli VE, Mastan M, Sobha RT (2012) Synthesis and evaluation of analgesic activity of novel series of Indole derivatives linked to isoxazole moiety. Der Pharmacia Lett 4:1431–1437
Donger RS, Bhat AR, Meshram JS, Selokar RS (2014) Anticancer activity of assorted annulated pyrimidine: a comprehensive review. Am J Pharm Tech Res 4(1):138–155
Ebraheem HA (2013) Synthesis of some pyrimidine-2-one and pyrimidine-2-thione. Raf J Sci 24(120–127):2013
El-Sayed WA, Nassar IF, Abdel-Rahman AA-H (2009a) C-Furyl glycosides, II: synthesis and antimicrobial evaluation of C-furyl glycosides bearing pyrazolines, isoxazolines, and 5, 6-dihydropyrimidine-2(1H)-thiones. Monatsh Chem 140:365–370
El-Sayed WA, Ramiz MMM, Abdel-Rahman AA-H (2009b) Anti-hepatitis B virus activity of New N4-b-d-glycoside pyrazolo[3, 4-d]pyrimidine derivatives. Zeitschrift für Naturforschung 64c:323–328
El-Sayed WA, Rashad AE, Awad SM, Ali MM (2009c) Synthesis and in vitro antitumor activity of new substituted thiopyrimidine acyclic nucleosides and their thioglycoside analogs. Nucleosides, Nucleotides Nucleic Acids 28:261–274
El-Sayed WA, Abdel Magide RE, Abbas H-AS (2011) Synthesis and antimicrobial activity of new 1-[(tetrazol-5-yl)methyl]indole derivatives and their 1, 2, 4-triazole thioglycosides and acyclic analogs. Arch Pharmacal Res 34:1085–1096
Gadhachanda VR, Wu B, Wang Z, Kuhen KL, Caldwell J, Zondler H, Walter H, Havenhand M, He Y (2007) 4-Aminopyrimidines as novel HIV-1 inhibitors. Bioorg Med Chem Lett 17:260–265
Gaffar S, Nikalje A, Riyaz S, Yogesh B, Harish L (2012) Design, synthesis and evaluation of novel indolyl pyrimidine carbohydrazides as antifungal agents. Curr Pharma Res 2:612–619
Hegab MI, Hassan NA, Rashad AE, Fahmy AA, Abdel-Megeid FME (2007) Synthesis, reactions, and antimicrobial activity of some fused thieno[2, 3-d]pyrimidine derivatives. Phosphorus, Sulfur Silicon Relat Elem 182:1535–1556
Ibrahim YA (1996) Facile approach for the selective glycodisation of cyclic asymmetric amides and thioamides. Carbohydr Lett 1:425–432
Jaiprakash SB, Sasidhar BS (2012) 2, 5-Disubstituted novel indolyl pyrimidine analogues as potent antimicrobial agents. Der Pharm Lettre 4:344–348
Lamberth C (2007) Pyrazole chemistry in crop protection. Heterocycles 71:1467–1502
Magdziarz T, Mazur P, Polanski J (2009) Receptor independent and receptor dependent CoMSA modeling with IVE-PLS: application to CBG benchmark steroids and reductase activators. J Mol Model 15:41–51
Mansour AK, Ibrahim YA, Khalil NSAM (1999) Selective synthesis and structure of 6-arylvinyl-2- and 4-glucosyl-1, 2, 4-triazines of expected interesting biological activity. Nucleosides, Nucleotides Nucleic Acids 18:2265–2283
Mohamed SF, Youssef MM, Amr AE-G, Kotb ER (2008) Antimicrobial activities of some synthesized pyridines, oxazines and thiazoles from 3-Aryl-1-(2-naphthyl)prop-2-en-1-ones. Sci Pharm 76:279–303
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Nugent RA, Schlachter ST, Murphy MI, Cleek GJ, Poel TJ, Wishka DG, Graber DR, Yagi Y, Kaiser BJ, Olmsted RA, Kopta LA, Swaney SM, Poppe SM, Morris J, Tarpley WG, Thomas RC (1998) Pyrimidine thioethers: a novel class of HIV-1 reverse transcriptase inhibitors with activity against BHAP-resistant HIV. J Med Chem 41:3793–3803
Ostrov D, Hernández Prada J, Corsino PE, Finton K, Le N, Rowe TC (2007) Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob Agents Chemother 51:3688–3698
Ottoni O, Cruz R, Alves R (1998) Efficient and simple methods for the introduction of the sulfonyl, acyl and alkyl protecting groups on the nitrogen of indole and its derivatives. Tetrahedron 54:3915–13928
Ozdemir A, Altıntop MD, Turan-Zitouni G, Çiftçi GA, Ertorun I, Alatas O, Kaplancıkl ZA (2015) Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents. Eur J Med Chem 89:304–309
Patel RN, Nimavat KS, Vyas KB (2011) Study on synthesis of chalcone & pyrimidine heterocyclic compound & their antimicrobial activity. Asian J Biochem Pharm Res 4(1):49–56
Rathod AK, Kulkarni GB (2011) Synthesis of 2-mercapto-dihydropyrimidines derivatives under conventional and microwave digestion technique and their anti-cancer and anti-tuberculosis activity. Inter J PharmTech Res 3(2):728–731
Serey RA, Torres R, Latorre BA (2007) Pre- and post-infection activity of new fungicides against Botrytis cinérea and other fungi causing decay of table grapes. Cien Inv Agr 34:215–224
Tanaka H, Takashima H, Ubasawa M, Sekiya K, Inouye N, Baba M, Shigeta S, Walker RT, De Clercq E (1995) Synthesis and antiviral activity of 6-benzyl analogs of 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine (HEPT) as potent and selective anti-HIV-1 agents. J Med Chem 38:2860–2865
Trott O, Olson AJ (2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Velezheva VS, Brennan PJ, Marshakov VY, Gusev DV, Lisichkina IN, Peregudov AS, Tchernousova LN, Smirnova TG, Andreevskaya SN, Medvedev AE (2004) Novel pyridazino[4, 3-b]indoles with dual inhibitory activity against Mycobacterium tuberculosis and monoamine oxidase. J Med Chem 47:3455–3461
Acknowledgments
The authors would like to thank Dr. El-Sayed E. Mostafa, Department of Microbial Chemistry, National Research Center, Cairo, Egypt, for performing the antimicrobial activity of the synthesized compounds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Tomasz Magdziarz on leave from Institute of Chemistry, The University of Silesia, Katowice, Poland.
Rights and permissions
About this article
Cite this article
El-Sayed, W.A., Abbas, HA.S., Abdel Mageid, R.E. et al. Synthesis, antimicrobial activity and docking studies of new N-ethyl-3-indolyl heterocycles. Med Chem Res 25, 339–355 (2016). https://doi.org/10.1007/s00044-015-1488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1488-4