Abstract
Intraspecific competition can significantly alter the foraging behavior of social insects. How competition might impact closely located colonies with overlapping foraging ranges is largely unknown. Between August–November 2022 and September–November 2023, we investigated the intraspecific competition of three Aphaenogaster species (A. famelica, A. schurri, and A. japonica) in two urban forests in Kunming, China. We estimated the density of ant nest openings and measured foraging distances. Nest opening density was 0.18/m2 (0.33/m2 for A. famelica, 0.03/m2 for A. schurri, and 0.07/m2 for A. japonica), and mean foraging range was 160 cm (124 cm for A. famelica, 296 cm for A. schurri, and 228 cm for A. japonica). We then ran a series of field experiments to assess the effects of distance, food load, and intraspecific competition on ant foraging. For 36 pairs of nest openings, we placed one light and one heavy tuna bait at various distances between neighboring nest openings. We modified competition intensity by physically blocking one of the nest openings and analyzed time spent on food discovery, removal, and retrieval by ant workers. Ants spent a longer time discovering baits located farther away from, and retrieving heavier or farther baits. Blocking was correlated with a longer time for food removal (for A. schurri/japonica and A. famelica) and transportation (for A. famelica). Selectivity of the light vs heavy bait was not found, suggesting neighborhood distance was too short to generate distance-based selectivity patterns. Differences in nest spacing might be related to species-specific traveling and intraspecific competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraspecific competition is a major biotic factor that influences wildlife behavior. Both direct (interference) and indirect (exploitative) competition can be found in social insects interacting with conspecifics over a common food resource. Interference competition involves active defense of resources against rivals (Adams 2016). In exploitative competition, behaviors based on time-efficient foraging strategies might be favored (David et al. 2014), such as fast detection of food and recruitment of nestmates (Human and Gordon 1996). The strategies used may depend on species-specific foraging capacities. Under competition pressure, single-loaded foragers might increase movement speed to reduce the probability of encountering potential competitors (Detrain et al. 2000), whereas multi-loaded species might increase the load carried per foraging trip. This in turn can deplete resources at a higher rate (Pyke 1984). At the population level, foragers may adjust their strategies depending on spatial variability and quality of the food. For instance, individuals with larger body sizes or those capable of distant travel are often found at food patches farther from the nest (Wright et al. 2000). Additionally, chemical communications (e.g. pheromone trails deposited by ants) increase the number of foraging individuals and speed to reach food patches (Pyke and Starr 2020). Both interference and exploitative competition can have density-dependent fitness consequences for animals. Ants are widely distributed and often fill important niches such as seed dispersal, soil perturbation, and nutrient cycling. Therefore, understanding the foraging patterns of these ecologically important ant communities provides a theoretical basis for conservation of understudied insect groups and their habitat.
Many species of ants (Hymenoptera: Formicidae) are central-place foragers, as workers bring back food from the nest opening they initiated foraging trips from (Dornhaus and Powell 2010). Distance between ant colonies represents an interesting example for understanding foraging strategy in relation to intraspecific competition. All other variables held constant, competition pressure increases as ants travel farther from nest openings, and decreases when neighboring nests are more distant. It is expected that spatial recognition of food location is key to making foraging decisions (Reznikova 2020), which involves weighing energy gain versus cost for outbound and return trips (Mayo and Benabib 2009). Large food items take longer to retrieve, and may lead animals to preferentially select smaller food items instead. A more complex scenario may arise when food-handling time is influenced by both distance, load, and their interactions (Wetterer 1989). This is particularly important for exploitative competitors, as food-handling time is a major energy expenditure constraint (Sotillo et al. 2019).
Ants in the genus Aphaenogaster are widely distributed in forests in North America, Europe, and East Asia. Aphaenogaster workers are characterized by lengthy bodies with long legs, generalist diets, and monodomous nesting habits. Three Aphaenogaster species: A. famelica Smith, A. schurri Forel and A. japonica Forel form numerous nest openings on the forest floor of subtropical urban and suburban forests in China. They exhibit strong intraspecific competition in areas where colonies overlap in their foraging ranges. The above-ground competition may even extend to inside the ant nests, where food items may be stolen and then brought into rivals' nests with little protest. This is probably due to relatedness of closely located colonies, as Adams and Tschinkel (1995) found in A. rudis in North America). It is possible, however, for interference competitors to also have overlapping foraging ranges (Hölldobler 1976). In addition, aggression in A. japonica (previously A. smythesii japonica) has also been reported in temperate forests in Japan (Higashi et al. 1987), suggesting behavior flexibility under different ecological contexts (Gordon 1991). It is however, not known how intraspecific exploitative competition might underlie the co-existence of closely located colonies in Aphaenogaster ants. So far, no research on ant behaviors in the subtropical forest ecosystem of China has ever been conducted. The objectives of this study are (1) to understand the effects of food load and distance on ant foraging; and (2) to test if changes in competition might lead to alterations in ant foraging behavior. We hypothesized that foraging time is directly correlated with both distance and food load. In neighborhood competitions, lighter food items that are relatively easy to obtain would be preferred by the ants. However, when competitive pressure is lower, heavier foods might be preferred instead to increase energy gain.
Materials and methods
Study sites and ant species
During 26 August—20 November 2022 and 20 September—30 November 2023, data collection was carried out at two urban forests in Mt. Changchongshan (CCS) and Mt. Mianshan (MS) in Kunming, Yunnan Province, China. The study coincided with the wet (June–October) and winter dry (November–December) seasons of Kunming. The daily air temperature ranged from 9 to 21 ℃, and rainfall was from 2.3 mm to 154.88 mm (World Weather Online 2024). To reduce the negative impact of rainfall on ant activity, only days with light or no rain were selected for fieldwork. Also, observations were carried out after 10 a.m., when the soil temperature was higher with sunshine and ant foraging more active (Dai personal observation), During the experiments, mean soil temperature was 18.7 ℃, and soil moisture level ranged from 10 - 30%, measured using a soil meter soil tester (EEEkit Soil Hygrometer).
At CCS, silver wattles (Acacia dealbata, Fabaceae) dominated the canopy layer, and leaf litter depth was 0.29 cm on average (range = 0.11–0.50 cm). At MS, Chinese hackberry (Celtis sinensis, Cannabaceae) and Chinese chestnut (Castanea mollissima, Fagaceae) were the main canopy species, and leaf litter depth measured 0.36 cm on average (range = 0.13–0.50 cm). Fallen leaves and other debris were cleared upon experimentation to allow for clear observations of ant behavior.
Of the three study species, A. famelica was in CCS, and A. schurri and A. japonica were in MS. They were active from February or March to early December at the study sites. At CCS, nests of A. famelica were found in great numbers, each contained one to two openings [small, approximately circular holes on the surface of the ground or on other substrates, e.g. mushrooms; (Dai 2022)], occasionally coupled with a ventilation turret (Fig. 1). During the fruiting season of A. dealbata, A. famelica workers were observed extracting seeds from open pods and carrying them back to the nests (Fig. 1). At MS, A. schurri and A. japonica were seen harvesting insect carcasses (e.g. damselflies, moths, beetles) and plant parts. In addition, Asian wooly hackberry aphid (Shivaphis celti Das) inhabiting leaves of Chinese hackberries might be a source of sugary food. Where A. japonica was found to overlap in foraging ranges with Pheidole fervens Smith, the area was avoided for experimentation as the latter often interfered with A. japonica during its foraging activities. In addition, P. fervens also frequently attacked Aphaenogaster nests. More details on the study sites can be found in Dai (2022).
Estimating the density of ant nest openings
Distance sampling using line transects and stationed baits (Baccaro and Ferraz 2013) was used to investigate density of ant nest openings at the study sites. A total of 18 transects (10 in CCS, and eight in MS) were established, each measuring 10 m in length, with 36 m on average (14–46 m) between neighboring transects. Each transect line was composed of a white cotton string, fixed by nails at both ends and raised off of the ground > 5 cm to avoid obstructing ant movement. All transects were situated on the plain hilltops of CCS or on the terrace treads [for agricultural activities under rugged topographies (Wang et al. 2010)] of MS. Food stations containing small pieces of brightly colored egg rolls (to enable tracking of ant movement on the forest floor were positioned at every 0.5 m along the transects. Twenty-one baited stations were implemented per transect. To prevent exhaustion of food items, stations were refilled ad libitum during the observation period. On average two (range = 1–4) transect lines were established each day. Each transect was checked on a rotating basis, and food-entry nest opening locations were marked.
Perpendicular distances of each Aphaenogaster nest opening found along the transect lines weres measured using a steel tape measure. Density of Aphaenogaster ant nest openings was estimated for each species (A. famelica, A. schurri, and A. japonica) and for all species combined using R package “Distance”. The truncation value for distance sampling was set at 7 m, slightly larger than the maximum foraging distance observed during the study (see Results). Foraging range was measured as the distance between a food station and ant nest opening into which egg-roll bait entered. Diameter of individual ant nest openings and height of the ventilation turrets (when present) were also measured using a digital vernier caliper.
Kruskal–Wallis test and Dunn’s test were performed to test differences of foraging range and number of nests found along each transect line for A. famelica, A. schurri, and A. japonica.
Experimental setup
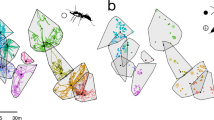
A total of 25 pairs A. famelica from CCS and 11 pairs (seven A. japonica and four A. schurri) from MS of randomly-selected Aphaenogaster nest openings were used for the experiments. Location, species, and number of trials run for each pair are shown in Online Resource 1. All study pairs were situated in relatively flat areas to reduce the impact of uneven topography on ant movement.
If two nest openings (of different or similar sizes, one major and one minor, depending on the levels of ant traffic observed) were found on the same mound (soil pile, or fresh-looking soils surrounding the nest opening hole), we assumed they led to the same nest. Paired nest openings were selected when no other nest openings were found between them. The ant nest opening towards which a bait was pulled following its discovery was referred to as “home” nest opening, and the other nest opening in the pair was “rival” nest opening. Up to five workers were collected from each nest opening for species identification after the experiment to avoid interfering with ant foraging.
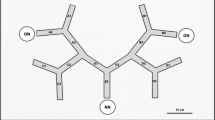
Two distance designs (“mid” and “side”) and two load types (“light” and “heavy”) were used. Distance (d) between two nest openings of each study pair was measured using the steel tape measure. The mid design divided neighborhood distance into even distance intervals, and the side design placed baits at intervals biased to one of the nest openings. A food patch containing one heavy and one light tuna bait was placed at three distance arrangements in the two designs: (1) 0.25d, 0.5d, 0.75d (mid design); (2) 0.125d, 0.25d, 0.375d, 0.5d (side design); or 3) 0.5d, 0.625d, 0.75d, 0.875d (side design) (Fig. 2). The aim of the distance arrangements was to enable diverse distance scenarios to be generated during ant-food interactions (discovery, removal and transportation; see Data Analysis), since neighboring nests might differ in the capability of food monopolization. For example, dominant nests (perhaps with more workers foraging on the ground) might be more capable of obtaining both the close and far foods, and baits positioned nearer to the less dominant nests might facilitate data collection from these nests, as they were given more access to the foods which might take lesser time to transport.
Since ants exhibit size-dependent load carrying capacity (Wills et al. 2018), for A. famelica with smaller worker size, tuna baits weighing 0.001 (light)/0.025 g (heavy) or 0.025 (light)/0.05 g (heavy) were used, and for the larger A. japonica/schurri, baits weighing 0.025 (light)/0.05 g (heavy) or 0.05 (light)/0.1 g (heavy) were used in most cases, with 0.001 /0.025 g also used; however, only in a few cases, due to difficulties tracking fast food transportation. The maximum load size for each species or species combination was determined in a preliminary test at the study site. All baits were dried at room temperature for approximately 12 h before experimentation to avoid ant consumption during transportation. Baits at different placement locations (e.g. 0.025d, 0.05d) were dyed using edible food dyes (green, red, pink, purple, and blue; Tengbao Trading Company, Luoyang, China) to avoid confusion during transportation (Fig. 1).
A blocking treatment that aimed to modify the intensity of intraspecific competition between paired ant nest openings was used, about ten minutes before experiments took place. An upside-down Petri dish (diameter = 9 cm, height = 1.5 cm) was used to cover one nest opening at a time. While blocking was expected to suppress ant foraging by limiting the number of workers moving onto the ground, it was not possible to exclude all workers under the field conditions. To reduce competition with the study ants, all (known) non-study Aphaenogaster nest openings in the nearby areas were also blocked following the same method. All blockings were removed at the end of the experimental day when observations ended.
An individual trial consisted of a unique combination of distance arrangement, food load, and presence or absence of blocking treatment, determined using a random number generator. Trials were then replicated for one to three times, with six to ten minutes between consecutive trials.
Data analysis
Individual foraging trip of the Aphaenogaster ants was divided into three phases. The time (t) spent on each phase was recorded as: (1) food discovery (t0)—from when a bait was assigned to when it was first visited by ants; (2) food removal (t1)—from when the bait was assigned to when it was pulled from the assignment spot; and (3) food transportation (t2)—from when the bait was removed to when it arrived at an ant nest opening (Fig. 2). In several cases, a single bait was being pulled by more than one Aphaenogaster worker simultaneously (often when approaching a nest opening), resulting in “circling” of the bait as it might reach the nest opening multiple times before finally entering it. We excluded the time spent as a result of disagreement among nestmates, and only the time at which the bait was first seen arriving at a nest opening was used to calculate t2. Note that the disagreement among nestmates differed from food competition by non-nestmates. In the later, a rival pulled the bait away from the study nest openings. The final location of the bait was recorded as the ant nest opening it entered after not being seen for ≥ 10 min. Missing baits were recorded as having unknown fates.
Based on the division above, four predictions were made that associated ant foraging time with distance, food load, and intraspecific competition: (1) when a bait was positioned further from the ant nest opening, a longer time was needed for ants to discover it; (2) farther and heavier baits required a longer time to be transported back to ant nest openings; and (3) if one nest opening in a pair was blocked, longer time would be needed for ants from the neighboring nest opening to remove and retrieve bait compared to when blocking was not applied; (4) lighter baits were selected first over heavier ones when neighborhood competition was high. Due to small sample sizes, data from A. schurri and A. japonica was pooled.
Survival analysis was used to assess ant foraging time (t0, t1, and t2) with censored data. A Cox Proportional Hazard Model was built for each foraging phase (food discovery, removal, and transportation) using package “coxme” (with frailty; for models with random effects) or package “survival” (for models without random effects), for A. famelica, A. schurri/japonica separately and for all species (the “all species” model) combined.
For prediction #1, that time spent to discover food, t0 (the dependent variable) increases with bait distance, the distance from the nest openings to the location workers were first seen visiting the bait was modeled as fixed effects. The total distance between neighboring ant nest openings was modeled as another fixed effect. In the “all species” model, ant species (A. famelica or A. schurri/japonica) were modeled as an additional fixed effect. To account for the influence of possible pheromone trails on food-finding (Hölldobler et al. 1995) and a possible association with distance to ant nest openings, an interaction term for “pheromone” and the distance to the ant nest openings was included. Pheromone trail presence was coded as a binary variable based on the trial number for each day. The earliest trials conducted on an experimental day were marked as “0”, indicating no or low probabilities of pheromone use, and trials that took place later on that day were marked as “1”, indicating possible pheromone usage. Nest opening ID, pair ID, and replication effect (a unique code was assigned to the same trials conducted for individual nest opening pairs) were modeled as random effects.
For prediction #2, that farther and heavier baits needed a longer time to be transported back to ant nests, time spent on food transportation, t2, was the dependent variable. An interaction term between food load and distance that a bait was transported by ants from the assignment spot to the nest opening it entered, was modeled as a fixed effect. In addition, total distance between neighboring ant nest openings was also modeled as a fixed effect. In the “all species” model, ant species was also modeled as a fixed effect. During the experiment, several stochastic events (referred to as “delay”) occurred, which potentially increased t2. The delay (marked as “1” when one or more events occurred, and “0” when none occurred) was also treated as a fixed effect, which included the following: (1) intraspecific competition: workers from a rival or non-study Aphaenogaster nests competing by gripping at the bait and pulling in another direction. (2) interspecific competition: workers from other ant species competing with Aphaenogaster workers for bait (3) immobilization: the bait fell into a soil depression or became stuck in the leaf litter, which took extra time to retrieve; (4) releasing: bait was left on the ground for no apparent reasons, and was not picked up again for ≥ 10 s; and (5) misplacing: workers carrying baits deflected from the shortest (straight-line) distance or went sideways. For prediction #3 that blocking ant nest openings increases the time required for ants from neighboring nest openings to retrieve bait, blocking treatment applied to the “rival” nest openings (see below) was modeled as a fixed effect. Nest opening ID, pair ID and replication effect were modeled as random effects. Trials were excluded from the analysis if baits did not arrive at the study ant nest openings, or when they were initially removed by ants from one nest opening, later on were grabbed, and eventually arrived at the rival nest openings.
For prediction #3, that blocking ant nest openings increases time required for ants from neighboring nest openings to remove bait; and prediction #4, that lighter baits were selected over heavier ones when neighborhood competition was high time spent on food removal, t1, was the dependent variable. Blocking treatment (marked as “1” when applied, and “0” when not applied) was modeled as a fixed effect. While blocking applied to rival nest openings was expected to influence behavior of ants from the home nest opening, blocking on home nest openings might also lead to slower food removal as less workers would be available. Hence, the effects of blocking were tested for both rival and home nest openings. To understand ant food selectivity (defined by differences of t1 for the light vs. heavy baits in a patch), an interaction term for blocking treatment (applied to the rival nest openings) and load type (light vs heavy) was included in the model as a fixed effect. To evaluate if the experimental design on food load and distance might affect ant foraging, an interaction term for distance design (two levels: mid and side) and load type (three levels: 0.001 g & 0.025 g, 0.025 g & 0.05 g, and 0.05 g & 0.1 g) was modeled as fixed effect. Additionally, the presence of other ant species (“interspecific competition", same as above) which might interfere with food acquisition by Aphaenogaster ants was also modeled as a fixed effect. In the “all species” model, ant species was also modeled as a fixed effect. Nest opening ID, pair ID, and replication effect were modeled as random effects.
To test the validity of each Cox model, the proportional hazards assumption was verified using goodness-of-fit tests. The significance of the random effects was tested using likelihood ratio tests. Kaplan–Meier curves on food removal with blocking treatments applied to the home or rival nest openings, and on food transportation between light vs heavy load type, were drawn for A. famelica, A. schurri/japonica and all species combined.
All data analysis was performed in R (R Core Team 2024) with RStudio (RStudio Team 2024).
Results
Density of ant nest openings
On average, 11.5 nest openings (range = 2–16) of A. famelica, 4 nest openings (1–5) of A. schurri, and 3.25 nest openings (2–5) of A. japonica were found along each transect line. The perpendicular distance of the ant nest openings to the transect lines was on average 98 cm (82 cm for A. famelica, 246 cm for A. schurri, and 130 cm for A. japonica). Two A. famelica nests had ventilation turrets, which measured 20 mm (15–25 mm) in height. Density estimate of ant nest openings and foraging ranges are shown in Table 1. Kruskal–Wallis test and Dunn’s test showed that the differences of foraging range were significant between A. famelica and A. schurri (z = − 3.213, p = 0.001) and between A. famelica and A. japonica (z = − 2.207, p = 0.027), however, were not significant between A. schurri and A. japonica (z = − 1.232, p = 0.218). Differences of number of nests per transect line were significant between A. famelica and A. schurri (z = 3.524, p < 0.001), A. famelica and A. japonica (z = 3.199, p = 0.001), however were not significant between A. schurri and A. japonica (z = 0.293, p = 0.769).
Experimental results
In total, 228 trials (148 for CCS, and 80 for MS) were performed involving 1,630 individual baits. Among them, 108 trials (654 baits) were conducted in the mid distance design, and 120 trials (976 baits) were in the side distance design. Baits weighing 0.001 & 0.025g (level one load design) were used in 62 trials, 0.025g & 0.05g (level two load design) were in 115 trials, and 0.05 & 0.1g (level three load design) were in 31 trials. In total, 1,297 cases of food discovery (900 for A. famelica, 204 for A. schurri, and 193 for A. japonica), 1,388 cases of food removal (946 for A. famelica, 217 for A. schurri, 225 for A. japonica), and 1,207 cases of food transportation (843 for A. famelica, 190 for A. schurri, 174 for A. japonica) were observed in Aphaenogaster ants from the study nests. The numbers of food items removed and transported for each distance scenario under the mid and side designs are in Fig. 3.
Other ant species that were observed competing with Aphaenogaster ants at the study site included Ectomomyrmex astutus, Pheidole fervens, and Kartidris nyos. In 333 cases, baits were discovered and removed by Aphaenogaster ants from the non-study nests (45 cases for A. famelica, 12 cases for A. schurri, and 32 cases for A. japonica), by other ant species (11 cases for Ectomomyrmex astutus, and 3 cases for unknown species), or by unknown ants (230 cases). In 37 cases, baits arrived at the non-study Aphaenogaster nests (35 cases for A. famelica, and 2 cases for A. schurri), or were taken away by single-foraging Ectomomyrmex astutus (n = 4 cases), or occupied by mass-recruiting ants (Pheidole fervens, n = 2 cases, and K. nyos, n = 1). In one case, the bait was first seen entering a study nest opening but was grabbed and then carried into a non-study nest opening. In another case (in MS), the bait was seized by a hornet (Vespula orbata Buysson) before ants could interact with it. In 390 cases, the fate of the baits was unknown.
In four cases (E. astutus, n = 3, K. nyos, n = 1), Aphaenogaster ants were able to re-obtain foods from interspecific competitors. When a bait was occupied by a number of K. nyos workers showing aggression to approaching Aphaenogaster workers, the latter usually moved to avoid being bit and grabbed at a part of the food item guarded by fewer competitors. When a bait was carried by individual E. astutus workers, the Aphaenogaster workers grabbed at it and pulled in a different direction, forcing the competitors to drop the bait and retreat. Thirty-seven food transportation cases were mediated by Aphaenogaster workers from the non-study nests, and in 33 cases, competition with E. astutus (n = 4), P. fervens (n = 27), or Prenolepis striata (n = 2) were observed. In these cases, the Aphaenogaster ants failed to re-obtain food from P. fervens or P. striata, of which workers aggregated at the bait in great numbers almost completely covering it; and in the cases of E. astutus, the Aphaenogaster workers seemed to have not noticed when the bait was being carried away.
The distance between two nest openings within each study pair was on average 135 cm (15–560 cm) for all three Aphaenogaster species. For individual species, the mean distance of nest-spacing was 84 cm (range = 15–236 cm) for A. famelica, 226 cm (range = 122–560 cm) for A. schurri, and 219 cm (range = 154–288 cm) for A. japonica. Two nests (one by A. famelica and the other by A. schurri) were found to have a major and a minor nest opening each (Online Resource 1).
In general, it took longer for ants to discover baits positioned farther from the nest openings (Fig. S1, Online Resource 5). In 68 cases, foraging Aphaenogaster workers discovered baits immediately after placement. When ants first visited a food patch, both the heavy and light items were often lifted but not necessarily transported. The “weighing” process was not observed when only one item was left in a patch nor when more than one worker or other ant species were close by. In these cases, a bait was carried away immediately, without delay (Figs. 4, 5).
Hazard ratios of the Cox models examining time spent on food discovery (t0) and its relationships with distance from ant nest openings, the interaction between distance from ant nest openings and pheromone presence, total neighboring distance, and ant species (A. famelica or A. schurri/japonica) all species combined (A), for A. famelica (B) and for A. schurri/japonica (C). Hazard ratios larger than 1 had a positive impact on t0, and those less than 1 had a negative impact on t0
Similarly, a longer time was needed for farther and heavier baits to be transported back to an ant nest (Fig. S2, Online Resource 5, Fig. 6). A total of 609 delays were observed (with partial overlaps, when more than one incident type occurred during an individual trial). Among all the incidents, intraspecific competition (n = 445) was the most commonly observed, followed by “releasing” (n = 210), “immobilization” (n = 197), “misplacing” (n = 138), and interspecific competition (n = 115). Kaplan–Meier curves showing the probability of food transportation versus time for light and heavy load types for A. famelica, A. schurri/japonica as well as all species combined are shown in Figs. 5 and 7, respectively.
Hazard ratios of the cox models examining time spent on food transportation (t2) and its relationships with distance from ant nest openings, food load, their interactions, total neighboring distance, blocking treatment applied to rival nest openings, incidents of “delay”, and ant species (A. famelica or A. schurri/japonica) all species combined (A), for A. famelica (B) and for A. schurri/japonica (C). Hazard ratios larger than 1 had a positive impact on t2, and those less than 1 had a negative impact on t2
Several other ant species present at the study site, including Camponotus tonkinus Santschi, Lasius niger L., Tetramorium sp., Monomorium sp. (only in MS), and two unknown species (only in CCS) were not seen competing with Aphaenogaster. In one case (in MS), an A. schurri queen was seen feeding on the bait carried by several workers.
Model results
An increased time spent on food discovery, or t0, was correlated with longer distance from ant nest openings and the interaction of distance from ant nest openings and pheromone trial presence for A. famelica, A. schurri/japonica and for all species combined, and to the total distance between neighboring ant nest openings for A. schurri/japonica. In the “all species” model, increased t0 was also marginally correlated with A. schurri/japonica (Fig. 4 and Online Resource 2).
An increased time spent on food transportation, t2, was correlated with longer distance from ant nest openings, and incidents of “delay” for A. famelica, A. schurri/japonica, and all species combined. Increased t2 was correlated with heavier food load (Fig. 6) for A. famelica and all species combined. For A. schurri/japonica, t2 was positively correlated with the interaction between distance to ant nest openings and food load. In the “all species” model, increased t2 was also correlated with ant species A. schurri/japonica (Fig. 5 and Online Resource 3).
Since load design and ant species failed to meet the proportional hazards assumption, they were removed from the models. An increased time spent on food removal, or t1, was correlated with interspecific competition for A. famelica, A. schurri/japonica and for all species combined. An increased t1 was also correlated with blocking treatment applied to the home or rival nest openings for A. schurri/japonica and for all species combined. However, t1 was not correlated with distance design, food selectivity, and the interaction between blocking treatment applied to rival nest openings and food selectivity (Fig. 8 and Online Resource 4).
Hazard ratios of the Cox models examining time spent on food removal (t1) and its relationships with blocking treatment applied to the home or rival nest openings, interspecific competition, distance design, food selectivity for all species combined (A), for A. famelica (B) and for A. schurri/japonica (C). Hazard ratios larger than 1 had a positive impact on t1, and those less than 1 had a negative impact on t1.
Discussion
In this study, we explored foraging time of three Aphaenogaster ant species in the subtropical forests of southwest China and assessed the impacts of distance, food load, and intraspecific competition on ant foraging behavior. Survival analysis showed that the ants spent more time discovering baits located farther from their nest openings and transporting farther or heavier baits back to the nests. These findings were in agreement with predictions # 1 and in part with #2. However, farther and heavier foods were not associated with an increased time for food transportation for A. famelica. For A. schurri/japonica, under the natural competition pressure (in which neighboring competitors were not experimentally suppressed using the blocking treatment), food removal took less time compared to when neighborhood competition was lower. For A. famelica, a longer time spent on food transportation was also observed when blocking treatment was applied to the rival nest openings. However, blocking treatment seemed to be less effective for A. famelica during food removal. Also, blocking treatment applied to rival nest openings did not significantly affect food transportation for A. schurri/japonica. These results did not conform to prediction #3. Selectivity over light vs heavy items in a food patch was also not found. Therefore, prediction #4 was also not supported by the experimental results.
The relationship between foraging rate and interspecific competition are also reported for other organisms, such as bees (Balfour et al. 2015) and crabs (Chakravarti and Cotton 2014). Whereas the time for food discovery might be less predictable due to the opportunistic nature of food-finding, deciding when to remove an item of known location (indicated by pheromone trails) might be easily foreseen (except perhaps for the earliest trials of a day). It is known that inter- and intra-competitions are both related to extra time cost during foraging. A higher foraging rate might lead to increased food acquisition via reducing the probabilities of encountering a competitor. However, accelerated foraging might also come with a trade-off. Chakravarti and Cotton (2014) suggested that crab handling of prey (mussels) at a higher speed might damage the claws. And David et al. (2014) proposed that faster foraging speeds are a trade-off with accuracy during food detection. Our results showed that the nearer a food was from the nest openings, the more effective pheromone might be. It was noted that while foraging alone to farther foods at fast movement speeds, some Aphaenogaster workers followed trials which apparently deviated (though the degree of the deviationwas not quantified in this study) from the shortest paths to the ant nest openings. This was possibly because the newly established trajectories had not been corrected by many nestmates (Czaczkes et al. 2013).
Differences in nest opening density of the three Aphaenogaster species suggested a possible relationship with size-dependent foraging capacity. It appeared that Aphaenogaster workers were capable of traveling farther than the distance between neighboring nest openings to forage in wider ranges to feed their colonies (Dornhaus and Powell 2010). The relative ease of covering the neighborhood space was indicated by the insignificant effect of total distance on food discovery time (except for A. schurri/japonica with a longer inter-colony spacing than A. famelica), though it might not be equally easy to locate items positioned at specific spots, as shown by the model results. The maximum traveling distance of the ants, although not tested in this study, was also likely to be greater in A. schurri/A. japonica than in A. famelica.
During the study, A. schurri and A. japonica workers were found moving larger prey items, such as earthworms, moths, and other macroinvertebrates, into their nests, whereas A. famelica workers generally harvested smaller insects and seeds. The prey-size difference might explain the differences found in their nest-opening diameters. While load alone did not significantly affect food transportation by A. schurri/japonica, workers took longer time to retrieve heavier items farther from the nests. In comparison, food transportation by A. famelica was significantly affected by load, however not by the combined effects of load and distance. This was possibly due to the difficulties for A. schurri/japonica workers carrying heavy items over a long distance, with a higher chance of encountering physical barriers (e.g. soil depressions), which made accelerated transportation less possible.
Travel capacity and neighborhood competition might both underlie the different interspacing of Aphaenogaster ant colonies (Ryti and Case 1984). Since more workers are generally found near the nest openings, larger nest spacing might help mitigate the negative impact of competition. In addition, larger colonies are better at resource monopolization, which drives smaller colonies to nest farther away (Yamaguchi 1995). However, natural obstacles such as fallen branches and leaves increase microhabitat dimensions on the forest floor, which might favor maneuvering in addition to movement speed. Ant workers which utilize the additional space might benefit from avoiding confrontation with competitors and individual capacity in resource exploitation. The potential effect of habitat complexity on ant movement and foraging efficiency needs further research.
Although both the light and heavy baits in a patch took equal time to be discovered, and heavier baits took longer to retrieve; lighter baits were not selected over heavier ones when competition pressure changed. Compared to the ants' travelling distance, the neighborhood distance might be too short to generate distance-related selectivity patterns (Mayo and Benabib 2009). Within a short space, the ants might focus more on food acquisition (i.e. using a “grab and run” strategy). However, the small sample size for longer-distance scenarios (e.g. 0.75d and 0.875d) offered insufficient evidence. This was likely because baits positioned farther were relatively easily removed by workers from the neighboring nests, resulting in sparse data and underpowered statistical analysis.
The use of blocking treatments might have caused a change in the local competition regime. When blocking was applied, baits were removed by non-study ants (of Aphaenogaster or other species) more often. In addition, blocking nest openings did not significantly impact food removal time for A. famelica. This was probably due to foraging by workers that had left their nests before nest openings were covered up, and because of the relatively short inter-spacing which failed to generate a meaningful relationship between distance and load. As more than two Aphaenogaster colonies and several other ant species appeared to be competing within the same foraging range, future studies conducted under laboratory conditions might eliminate the issue of noisy competitors and enable better control of the study populations.
Data availability
The data that support the finding of this study are available upon request.
References
Adams ES (2016) Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecol News 23:101–118
Adams ES, Tschinkel WR (1995) Divergent mating patterns and a method for estimating reproductive success in the fire ant, Solenopsis invicta. Behav Ecol Sociobiol 36:391–398
Baccaro FB, Ferraz G (2013) Estimating density of ant nests using distance sampling. Insectes Soc 60:103–110. https://doi.org/10.1007/s00040-012-0274-2
Balfour NJ, Gandy S, Ratnieks FL (2015) Exploitative competition alters bee foraging and flower choice. Behav Ecol Sociobiol 69:1731–1738. https://doi.org/10.1007/s00265-015-1985-y
Chakravarti LJ, Cotton PA (2014) The effects of a competitor on the foraging behavior of the shore crab Carcinus maenas. PLoS ONE 9:e93546. https://doi.org/10.1371/journal.pone.0093546
Czaczkes TJ, Grüter C, Ellis L, Wood E, Ratnieks FL (2013) Ant foraging on complex trails: route learning and the role of trail pheromones in Lasius niger. J Exp Biol 216:188–197. https://doi.org/10.1242/jeb.076570
Dai R (2022) Seed-removal experiments indicate Aphaenogaster ants (Hymenoptera, Formicidae) as a potential keystone seed disperser of subtropical forest fragments and plantations in China. Asian Myrmecol 15:e015009. https://doi.org/10.20362/am.015009
David M, Gillingham MA, Salignon M, Laskowski KL, Giraldeau LA (2014) Speed–accuracy trade-off and its consequences in a scramble competition context. Anim Behav 90:255–262. https://doi.org/10.1016/j.anbehav.2014.02.009
Detrain C, Tasse O, Versaen M, Pasteels JM (2000) A field assessment of optimal foraging in ants: trail patterns and seed retrieval by the European harvester ant Messor barbarus. Insectes Soc 47:56–62. https://doi.org/10.1007/s000400050009
Dornhaus A, Powell S (2010) Foraging and defence strategies. In: Lach L et al. (ed) Ant ecology, 1st edn. Oxford University Press. Oxford. pp 210–230. https://doi.org/10.1093/acprof:oso/9780199544639.003.0012
Gordon DM (1991) Behavioral flexibility and the foraging ecology of seed-eating ants. Am Nat 138:379–411. https://doi.org/10.1086/285223
Higashi S, Hinomizu H, Nakano S, Ohtani T, Toda MJ, Yamamoto M (1987) Dispersion patterns of ant nests in a cool-temperate woodland of northern Japan. Res Bull Hokkaido Univ for 44:603–610
Hölldobler B (1976) Recruitment behavior, home range orientation and territoriality in harvester ants, Pogonomyrmex. Behav Ecol Sociobiol 1:3–44. https://doi.org/10.1007/BF00299951
Hölldobler B, Oldham NJ, Morgan ED, König WA (1995) Recruitment pheromones in the ants Aphaenogaster albisetosus and A. cockerelli (Hymenoptera: Formicidae). J Insect Physiol 41:739–744. https://doi.org/10.1016/0022-1910(95)00041-R
Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105:405–412. https://doi.org/10.1007/BF00328744
Mayo A, Benabib M (2009) Testing central place foraging theory in the ant genus Pogonomyrmex: a review of the literature from 1977 through 2008. Figshare. https://doi.org/10.6084/m9.figshare.769272.v2. Accessed 24 May 2024
Pyke GH (1984) Optimal foraging theory: a critical review. Annu Rev Ecol Syst 15:523–575. https://doi.org/10.1146/annurev.ecolsys.15.1.523
Pyke GH, Starr CK (2020) Optimal foraging theory. In: Starr CK (ed) Encyclopedia of social insects (Living edition). Springer Springer Nature, pp 1–9
Reznikova Z (2020) Spatial cognition in the context of foraging styles and information transfer in ants. Animal Cognition 23:1143–1159. https://doi.org/10.1007/s10071-020-01423-x
R Core Team (2024) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ryti RT, Case TJ (1984) Spatial arrangement and diet overlap between colonies of desert ants. Oecologia 62:401–404. https://doi.org/10.1007/BF00384274
Sotillo A, Baert JM, Müller W, Stienen EW, Soares AM, Lens L (2019) Time and energy costs of different foraging choices in an avian generalist species. Mov Ecol 7:1–1. https://doi.org/10.1186/s40462-019-0188-y
Wang H, Pandey S, Hu F, Xu P, Zhou J, Li J, Deng X, Feng L, Wen L, Li J, Li Y (2010) Farmers’ adoption of improved upland rice technologies for sustainable mountain development in Southern Yunnan. Mt Res Dev 30:373–380. https://doi.org/10.1659/MRD-JOURNAL-D-09-00012.1
Wetterer JK (1989) Central place foraging theory: when load size affects travel time. Theor Popul Biol 36:267–280. https://doi.org/10.1016/0040-5809(89)90034-8
Wills BD, Powell S, Rivera MD, Suarez AV (2018) Correlates and consequences of worker polymorphism in ants. Annu Rev Entomol 63:575–598. https://doi.org/10.1146/annurev-ento-020117-043357
World Weather Online. Kunming Historical Weather, YN, China. https://www.worldweatheronline.com/kunming-weather-history/yunnan/cn.aspx. 12 Feb 2024
Wright PJ, Bonser R, Chukwu UO (2000) The size–distance relationship in the wood ant Formica rufa. Ecol Entomol. 25:226–233. https://doi.org/10.13102/sociobiology.v66i4.4596
Yamaguchi T (1995) Intraspecific competition through food robbing in the harvester ant, Messor aciculatus (Fr. Smith), and its consequences on colony survival. Insectes Soc 42:89–101. https://doi.org/10.1007/BF01245701
Acknowledgements
We thank SK An and YS An for assisting with logistics and field data collection, XD Pan for coordinating the review process, and three reviewers for their valuable comments on this manuscript.
Author information
Authors and Affiliations
Contributions
RD collected data in the field. AA and RD analyzed the data. RD, AA and CW wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors claim that there are no competing interests involved in this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, R., Aslam, A. & Wang, C. Foraging time and neighborhood competition in Aphaenogaster ants: a field experiment. Insect. Soc. 71, 295–308 (2024). https://doi.org/10.1007/s00040-024-00976-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-024-00976-1