Abstract
Ants in the subtribe Attina belong to a monophyletic group, exclusive to the New World, that contains approximately 250 described species. All attine ants have a mutualistic relationship with the fungus they cultivate as food source. The present study provides a natural history and ecological account of five species of fungus-farming ants in the Brazilian Atlantic rainforest: Mycocepurus smithii, Mycetarotes parallelus, Mycetophylax morschi, Sericomyrmex parvulus, and Sericomyrmex saussurei. Specifically, we investigated nesting and foraging behavior and daily activity rhythms, and identified the substrates collected for fungiculture. Nests of all five ant species studied consist of chambers excavated in the soil, with variation on external appearance from inconspicuous holes in the ground to entrances surrounded by mounds of excavated soil pellets. S. saussurei was mainly nocturnal, M. morschi was active all day, and the other species presented diurnal activity. All species foraged exclusively on the ground and near their nests. All five species collected substrates of animal origin (mainly arthropod feces) and vegetable matter on which to cultivate their fungus gardens. Data on basic biological features of these ants, which represent phylogenetically diverse lineages, contribute to a better understanding of their mutualistic relationships with their fungal symbionts and of the evolutionary processes that produced the derived characteristics in the subtribe Attina. Additionally, this study adds to our knowledge of the natural history of fungus-farming ants in Atlantic rainforest and increases our understanding of their roles in this threatened biome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungus-farming ants (Formicidae, Myrmicinae, Attini, Attina; hereafter “attine” ants) represent a classical example of mutualism: inside their nests they cultivate fungi, which serve as food sources for the colony, and in return the ants provide the fungi with nourishment, dispersal to new locations, and an environment free of parasites and competition (Hölldobler and Wilson 2011). All attine ants are fungus growers and the domestication of fungi by these ants had a single origin in South American forests 55–65 million years ago (Schultz and Brady 2008; Branstetter et al. 2017; Li et al. 2018). This group is monophyletic, with approximately 250 species described in 17 valid genera (Bolton 2014; Ward et al. 2015; Li et al. 2018, Sosa-Calvo et al. 2018), and occurs exclusively in the New World (Mayhé-Nunes and Jaffé 1998; Fernández and Sendoya 2004; Schultz and Brady 2008).
Attines are divided into two clades: the Paleoattina and the Neoattina (Schultz and Brady 2008; Ješovnik et al. 2016; Branstetter et al. 2017; Sosa-Calvo et al. 2017; Li et al. 2018). The Neoattina includes, among other genera, the highly derived attine genera Acromyrmex and Atta, known as “leaf-cutter ants” due their behavior of cutting fresh leaves as the substrate on which they cultivate their fungus gardens (Rico-Gray and Oliveira 2007; Hölldobler and Wilson 2011). Leaf-cutters are the most studied group within the attine ants, mainly because they are considered the dominant herbivores of the Neotropics (Wirth et al. 2003; Herz et al. 2007; Rico-Gray and Oliveira 2007), one of the most important agricultural pests in Neotropical America (Verza et al. 2007; Della Lucia et al. 2013; Montoya-Lerma et al. 2012), important ecosystem engineers (Sternberg et al. 2007; Farji-Brener and Illes 2000; Corrêa et al. 2010; Leal et al. 2014), and secondary seed dispersers in forests and savannas (Leal and Oliveira 1998; Silva et al. 2007; Christianini and Oliveira 2009; Leal et al. 2014). Acromyrmex and Atta have the most derived characteristics within fungus-farming ants: densely populated nests with many chambers (e.g., Atta nests can have hundreds of subterranean chambers), worker polymorphism, and the use of fresh plant material as substrate for fungiculture (Wilson 1980; Mehdiabadi and Schultz 2009; Hölldobler and Wilson 2011).
The other attine genera, from both the Paleottina and Neottina clades, are far less studied probably due their small body sizes, discrete behaviors, and inconspicuous nests (Weber 1972; Fernández-Marín et al. 2005; Hölldobler and Wilson 2011). Differently from leaf-cutters, these ants usually are not polymorphic, have small to medium colonies (tens to hundreds of workers), and their nests have one or a few chambers (Weber 1937, 1969; Mueller and Wcislo 1998; Solomon et al. 2004, 2011; Klingenberg et al. 2007; Rabeling et al. 2007; Seal and Tschinkel 2008; Cardoso et al. 2011; Hölldobler and Wilson 2011; Sosa-Calvo et al. 2013, 2017; Ješovnik et al. 2018). In addition, the non-leaf-cutters generally use arthropods feces, plant detritus, insect corpses, and fresh plant parts (e.g., seeds, flowers, fruit pulp) to cultivate their symbiotic fungi (Oliveira et al. 1995; Leal and Oliveira 1998, 2000; Mehdiabadi and Schultz 2009; De Fine Licht and Boomsma 2010; Hölldobler and Wilson 2011; Ronque et al. 2018). Occasionally, Trachymyrmex and Sericomyrmex can also harvest fresh leaves and flowers (Weber 1972; Leal and Oliveira 2000; Seal and Tschinkel 2008).
Although non-leaf-cutters comprise most of the species of fungus-farming ants, the natural history and ecology of only a few species have been investigated in detail (Leal and Oliveira 2000; Solomon et al. 2004, 2011; Rabeling et al. 2007; Seal and Tschinkel 2008; Mehdiabadi and Schultz 2009; Sosa-Calvo et al. 2013, 2017; Ješovnik et al. 2018). Biological and ecological traits (i.e., colony size, nest structure, polymorphism, types of substrates used for fungus cultivation) are important for understanding the evolutionary processes that led to the derived characteristics within the group (Hölldobler and Wilson 2011), and these traits may vary across the ant genera (Mehdiabadi and Schultz 2009; Ness et al. 2010; Hölldobler and Wilson 2011). Additionally, basic data on field biology of non-leaf-cutter species can provide insights about the evolution of fungiculture in the attine ants (Mueller et al. 2001; Fernández-Marín et al. 2005). The present study provides a natural history and ecological account of five species of fungus-farming ants (non-leaf-cutters) in the Atlantic rainforest: Mycocepurus smithii (Forel, 1893), Mycetarotes parallelus (Emery, 1906), Mycetophylax morschi (Emery, 1888), Sericomyrmex parvulus Forel, 1912 and Sericomyrmex saussurei Emery, 1894. Specifically, we present qualitative and quantitative field data on these ants with respect to nesting and foraging behavior and daily activity rhythms, and identify the substrates collected for fungiculture.
Materials and methods
Study site
We carried out this study in the Atlantic rainforest at the Parque Estadual Serra do Mar - Núcleo Picinguaba, Ubatuba municipality, São Paulo State, Southeast Brazil. This area has several ecosystems such as sand dunes, sandy plain forest (“restinga” forest), lowland forest, and marine environment (Joly et al. 2008). The climate of the region has low thermal amplitude throughout the year and two periods: from October to April with frequent rains and maximum precipitation in January, and from May to September with less rains but without water deficit (San Martin-Gajardo and Morellato 2003). The mean annual rainfall is 2624 mm and the annual average temperature is 21.2 °C, with relative air humidity always exceeding 80%, and with rains throughout the year (San Martin-Gajardo and Morellato 2003).
Nests of fungus-farming ants were located in two different areas: (1) Lowland forest (− 23.364472, − 44.824583), characterized by clay-sandy soils, epiphytes, and trees reaching more than 20 m (Joly et al. 2008), and (2) “Restinga” forest (− 23.357889, − 44.850194), characterized by sandy soils and canopy up to 20 m in height (Joly et al. 2008). The observations on daily activity and foraging were performed between April and May 2015 for M. smithii, M. parallelus, M. morschi and S. parvulus; and in May 2016 for S. saussurei. The collection date of colonies for data on nest architecture and demography are indicated in Tables 1 and 2.
Nest architecture and demographic data
Nests of M. smithii, M. parallelus, and M. morschi were found in “restinga” forest, and nests of S. parvulus and S. saussurei were found in lowland forest. Nest entrances were located by active search on the ground in both forest sites, at the edge of the forest, and using baits of orange and oats to attract the ants. The nests were characterized according to their external (number of entrances and presence/absence of a mound) and internal architecture (chamber depth, number and size of the chambers). We collected colonies of all species for demographic data (see Table 1 for number of sampled nests), and counted the number of queens, winged females, males, workers, and brood. We attempted to excavate mature colonies of the five species studied; we chose those colonies with high numbers of foragers (based on previous observations) as an estimate of colony age.
Daily activity schedule
Ant activity rhythms were investigated by sampling three colonies of each species studied. We determined the activity pattern of the ants through censuses carried out over 24 h, in which we recorded the number of workers exiting and entering the nest every 2 h in sessions of 15 min. Temperature and humidity at nest entrances were also recorded at the beginning of each sampling session. To avoid disturbance of ant activity, nocturnal observations were performed using a red flashlight.
We used linear regression to access the influence of air temperature and humidity on colony foraging activity. A pseudo-R2 was calculated using the deviances of the final model as compared with the null model. This analysis was performed in R version 3.3.3 (R Core Team 2017).
Foraging behavior, collection of fungus-culturing material, and home ranges
The foraging behavior of the five species of fungus-farming ants was monitored intermittently for three colonies of each species for 10 h, totaling 30 h of observation per species. Except for S. saussurei that was sampled after sunset, all other species were sampled between 9:00 a.m. and 5:00 p.m., when ants were more active. When a worker went out to forage, we followed it and marked with a flag the position of the most distant point it reached before returning to its nest. At the end of the observations we measured the distance of each point marked in relation to the nest and determined its geographical direction with a compass. In addition, the resources collected by the ants for fungus cultivation were removed from their mandibles and preserved in 100% ethanol for further identification in the laboratory with a stereomicroscope (Leica M205C). We also noted if recruitment behavior and foraging trails occurred in these species.
We used R version 3.3.3 (R Core Team 2017) and the package adehabitatHR (Calenge 2006) to calculate through the minimum convex polygon method the area corresponding to the home range of each colony. A G test of independence with Williams correction (Gotelli and Ellison 2011) was employed for the proportion of types of culturing material collected by each ant species. In this analysis we considered four main categories of items collected: vegetable matter, arthropod feces, arthropod carcasses, and other. This analysis was performed in R version 3.3.3 (R Core Team 2017).
Ant voucher specimens are deposited at the “Museu de Zoologia da Universidade Estadual de Campinas”, São Paulo (ZUEC, Campinas, Brazil; registration numbers 4236 to 4240) and the Entomological Collection “Padre Jesus Santiago Moure” of the Universidade Federal do Paraná, Curitiba (DZUP).
Results
Nest architecture and demographic data
All nests found of the five species of fungus-farming ants occur in chambers excavated in the soil and their general characteristics are presented in Table 1 and Fig. 1. The external architecture differed among the species (Fig. 1). The nest entrances were surrounded by mounds of excavated soil pellets with irregular shape in S. parvulus and S. saussurei, in some nests of M. smithii, and in one nest of M. parallelus. Other nest entrances were inconspicuous holes in the ground, as in M. parallelus and some nests of M. smithii. Nests of M. morschi had a characteristic external architecture; the entrances were surrounded by a circular or semi-circular crater of soil pellets. With the exception of M. morschi that had some nests with two entrances, all nests of the other species had a single entrance.
External and internal appearance of nests of the five species of fungus-farming ants studied in an area of Atlantic rainforest at the Parque Estadual Serra do Mar, SE Brazil. Yellow arrows indicate the nest entrance. Note the fungus garden suspended from the ceiling in the M. smithii chamber (yellow arrow). (Color figure online)
Nests of M. parallelus consisted of a single chamber in the soil nearly 10 cm below the surface (Table 1), which contained the fungus garden and the queen. The only excavated nest of S. saussurei had two chambers (17 cm and 35 cm deep; Table 1), both of which contained fungus garden and workers, with the queen found in the deepest one. M. smithii, M. morschi, and S. parvulus had nests with one or more chambers, 15 to 27 cm below the ground surface (Table 1). In some excavated nests of M. smithii and M. morschi we found chambers (always the first chamber) with no fungus garden (with or without workers). Additionally, we observed waste present in the fungus chamber of M. morschi nests.
We observed that the location of the fungus garden in chambers differed among species. Whereas in M. smithii the fungus garden was suspended from the ceiling of the chamber (Fig. 1), in the other species the fungus garden was located on the chamber floor (Table 1). The fungus garden of M. parallelus was always amongst thin roots on the chamber floor (Table 1).
The demographic data of the five species of fungus-farming ants are presented in Table 2. S. saussurei presented the most populous colony among the five species studied (> 1000 individuals), whereas S. parvulus had smaller colonies of up to ≈ 580 individuals. M. smithii had the smallest colonies (≈ 50 to 170 workers), and M. parallelus and M. morschi had colonies averaging ≈ 170 and 210 individuals, respectively. We found colonies with more than one dealate queen in M. smithii and M. morschi. M. parallelus, S. parvulus, and S. saussurei, however, consistently had only one dealate queen in their colonies.
Daily activity schedule
The daily rhythm of M. smithii, M. parallelus, and S. parvulus was diurnal, with very few workers active after 6 p.m. (Fig. 2). On the other hand, the daily schedule of S. saussurei was mainly nocturnal, with the most intense activity beginning after sunset and continuing until sunrise (Fig. 2). M. morschi was the only species active throughout the whole day, without a well-marked peak of activity (Fig. 2).
Daily variation in the foraging activity of the five species of fungus-farming ants studied in an area of Atlantic rainforest at the Parque Estadual Serra do Mar, SE Brazil. Foraging activity is expressed as the sum of inbound and outbound workers (data are means ± SE). Air temperature and humidity were recorded simultaneously during each sampling of ant activity (data are means). The sunrise in the locality occurs at 6:10 a.m. and the sunset at 5:50 p.m.
The daily activity of M. smithii, M. parallelus, M. morschi, and S. parvulus was positively affected by temperature and negatively affected by humidity (Table 3). Inversely, the daily activity of S. saussurei was negatively affected by temperature and positively affected by humidity (Table 3).
Foraging behavior, collection of fungus-culturing material, and home ranges
All five ant species foraged exclusively on the ground to search for material on which to cultivate their fungus gardens. M. smithii, M. parallelus, M. morschi, and S. parvulus consistently used the same routes to search for culturing substrates. The two former species foraged alone or in pairs, whereas M. morschi and S. parvulus foraged in small groups (up to 10 workers). S. saussurei used foraging trails with up to 20 workers in the search for culturing material and on their way back to the nest. The trails of some S. saussurei colonies had subterranean portions up to 2.5 m long and 1 cm in diameter; we observed workers maintaining these trails by removing fragments of soil pellets. Additionally, we observed that all five species had timid behaviors: when disturbed they rely on crypsis, curling up the head and legs and remaining motionless.
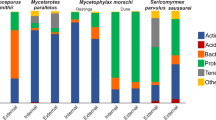
We did not observe ants harvesting fresh leaves on vegetation (as in Acromyrmex and Atta), although they collected dry or fresh fragments of plants on the ground. For instance, S. saussurei collected fallen fresh leaflets from leguminous plants (Anadenanthera L. and Enterolobium Mart.), and S. parvulus was seen cutting into small fragments a fallen fresh petal of Tibouchina Aubl. (Melastomataceae). We observed that the five species of fungus-farming ants collected substrates of animal origin and vegetable matter on which to cultivate their fungus gardens (Fig. 3). Arthropod feces, and to a lesser extent arthropod carcasses, comprised the items collected of animal origin (Fig. 3). Vegetable matter included dry or fresh fragments of flowers, leaves and grass, pieces of wood, leaflets, parts of flowers, and fruits (Fig. 4). The five species differed regarding the type of substrates collected for the fungus gardens (G = 111.19, df = 12, P < 0.01).
Material collected by each species of fungus-farming ant studied in an area of Atlantic rainforest at the Parque Estadual Serra do Mar, SE Brazil. The graph includes the four main categories of substrates collected: arthropod feces, arthropod carcass, vegetable matter, and “other” (material that could not be identified). Data based on 30 h of observation of foraging behavior for each species. Total number of collected items for each species: S. saussurei = 101; S. parvulus = 86; M. morschi = 111; M. smithii = 126; M. parallelus = 123. See also Fig. 4
Items collected by the five species of fungus-farming ants studied in Atlantic rainforest at the Parque Estadual Serra do Mar, SE Brazil. The figure includes all types of items collected by each species studied. Data based on 30 h of observation of foraging behavior for each species (“Undet. veg. material” = undetermined vegetable material)
The material collected for fungus-culturing by each species is shown in greater detail in Fig. 4. Parts of flowers were collected by S. parvulus (22%) and M. smithii (9%). Leaflets were collected by M. smithii (1%), S. parvulus (16%), and S. saussurei (1%). Except for M. parallelus, pieces of leaves were collected by all species. Wood was collected in small quantities (M. parallelus, M. morschi, S. parvulus, and S. saussurei), as were arthropod carcass (M. parallelus, M. morschi and S. saussurei), fruits (M. smithii and S. saussurei), and seeds (M. parallelus and M. morschi). Due to their very small sizes, some plant-derived items were categorized as undetermined vegetable material.
The home ranges used to collect substrates on which to cultivate the fungus gardens by workers of M. parallelus and S. saussurei were the largest among the five species (3.34 m2 and 3.42 m2, respectively; Table 4), and one colony of S. saussurei used 7.03 m2 as foraging ground. M. smithii, M. morschi, and S. parvulus used smaller areas (< 1.50 m2; Table 4). The maximum foraging distances traveled by workers of M. smithii, M. parallelus, M. morschi, and S. parvulus were less than 4 m, indicating that resources for fungus-culturing can be found near their nests (Table 4).
Discussion
The current study provides data on basic biological features for five species of fungus-farming ants in the Atlantic forest. Comparative results of the nesting habits of the five species revealed that all nests occur in a few shallow chambers excavated in the soil, although with variable external architecture. Additionally, we showed that these ants have small- to medium-sized colonies and forage exclusively on the ground near their nests, where they collect substrate of animal origin and vegetable matter on which to cultivate the fungus gardens.
Nest architecture in attines differs among species and could be an important evolutionary trait in this group (Weber 1982; Fernández-Marín et al. 2004; Mehdiabadi and Schultz 2009). Acromyrmex and Atta, the derived clades in attines, have nests with mounds and with many deep chambers (Moreira et al. 2004; Mehdiabadi and Schultz 2009; Hölldobler and Wilson 2011; Bollazzi et al. 2008). Some non-leafcutter species have nests with chambers between ≈ 5 to 50 cm below the ground surface (see Wheeler 1925; Solomon et al. 2004; Mayhé-Nunes and Brandão 2006; Klingenberg et al. 2007; Ješovnik et al. 2018), as reported in our study. Other non-leafcutters such as Mycetagroicus (M. cerradensis and M. inflatus), some Sericomyrmex, and Cyatta abscondita have nests with more than one chamber exceeding 1 m in depth (see Solomon et al. 2011; Ješovnik et al. 2013, 2018, Sosa-Calvo et al. 2013, 2015). Although the type of soil influences the depth of the chambers in attine ants (Ješovnik et al. 2018), and in this study the “restinga” forest grows on sandy soil and the lowland forest grows on clay-sandy soil, we observed that in general the nest chambers of the five species are shallow in these areas, indicating that these soil types may not affect chamber depth in the studied species. Indeed, we observed during excavations in both areas that the soil was very humid after ≈ 70 cm depth, which can be a limiting factor for deeper nests. Furthermore, external architecture was shown to be variable among the five attine species studied. As described here, nests of M. parallelus from Amazonia and Argentina also consisted of a single shallow chamber and one entrance ringed by old fungal substrate (Solomon et al. 2004; Mayhé-Nunes and Brandão 2006). Other species of Mycetophylax, such as M. conformis (Mayr, 1884) and M. simplex (Emery, 1888), showed the same pattern of nest architecture described here for M. morschi: one nest entrance surrounded by a circular or semi-circular crater of sand, most often containing only one chamber (Diehl-Fleig and Diehl 2007; Klingenberg et al. 2007). M. smithii nests from Amazonia had irregularly shaped mounds of fine grains of soil around the nest entrance, with on average seven chambers per nest and one nest with 15 chambers (Rabeling et al. 2007). Sericomyrmex species (including S. parvulus and S. saussurei) occurred in nests with 1–18 chambers and the external appearance in two main forms: with or without a soil mound (Ješovnik et al. 2018). We observed a smaller number of chambers for M. smithii, S. parvulus and S. saussurei than Rabeling et al. (2007) and Ješovnik et al. (2018). This could be due to climate factors, since our study area has low thermal amplitude and abundant rain throughout the year (San Martin-Gajardo and Morellato 2003). Indeed, the number of chambers and their depths are known to be affected by the local climate; Ješovnik et al. (2018) have shown that during the dry season fungus-growers move their chambers deeper in response to higher temperatures and lower humidity.
We observed that the species had fungus gardens in different locations inside the chamber (Table 1). The location of the fungus garden within the initial chamber excavated by the foundress queen differs among attine genera or even among species in the same genus (Fernández-Marín et al. 2004). For instance, Leal et al. (2011) report that Sericomyrmex ants place the fungus directly on the chamber floor, and Ješovnik et al. (2018) observed gardens of S. bondari Borgmeier 1937 and S. mayri Forel 1912 suspended from small rootlets in the roof of the chamber. Acromyrmex (e.g. A. echinatior Forel 1899, A. octospinosus Reich 1793, and A. cf. volcanus Wheeler 1937) uses rootlets as a platform for the garden (Fernández-Marín et al. 2004). Our results for M. smithii match those of Fernández-Marín et al. (2004, 2005) and Rabeling et al. (2007) with the same species, reporting that the ants suspend the fungus garden from the chamber ceiling and use their detached forewings as platform for incipient fungus. It has been hypothesized that suspension of the fungus from the ceiling or on rootlets could decrease contamination from pathogenic or competing soil microorganisms (Fernández-Marín et al. 2004).
Colony population sizes in fungus-farming ants are variable and could indicate an evolutionary trend in the group: in general, basally diverging clades have small colonies (between 50 and 1600 individuals; see Weber 1966; Solomon et al. 2004; Mayhé-Nunes and Brandão 2006; Klingenberg et al. 2007; Klingenberg and Brandão 2009; Rabeling et al. 2007), whereas derived clades (e.g., Trachymyrmex and Sericomyrmex) tend to have larger colonies with up to 6000 individuals (Ješovnik and Schultz 2017; Ješovnik et al. 2018), and the leaf-cutters (the derived genera Acromyrmex and Atta) have densely populated colonies that can reach millions of individuals (Hölldobler and Wilson 1990; Mehdiabadi and Schultz 2009; Hölldobler and Wilson 2011). Ješovnik et al. (2018) reported that S. saussurei have more populous colonies than S. parvulus, as observed in this study. Similar to what we found, Fernández-Marín et al. (2005) and Rabeling et al. (2007) reported that colonies of M. smithii had a minimum of 10 workers and a maximum of 170. Solomon et al. (2004) and Mayhé-Nunes and Brandão (2006) estimated that colonies of M. parallelus had ≈ 100 workers, and Klingenberg et al. 2007 reported that colony size in M. morschi ranged from 38 to 252 workers (see Table 2 for comparison). We observed that colonies of M. smithii and M. morschi have more than one queen (see Table 2), matching the results by Rabeling et al. (2007) for M. smithii and Mycocepurus goeldii Forel 1893 in Amazonia. However, occurrence of multiple queens in attine colonies does not necessarily indicate polygyny, since sometimes unmated daughter queens shed their wings and remain in the nest performing worker tasks (Weber 1941; Nehring et al. 2012). We observed these behaviors for M. parallelus, S. parvulus, and S. saussurei in captive colonies. However, DNA sequences of a fragment of the mitochondrial cytochrome c oxidase subunit 1 (COI) for M. morschi from ants from the same nest often represent two haplotypes, probably indicating the presence of more than one egg-laying queen in a colony (Ronque et al., in prep.).
The activity rhythm of fungus-farming ants ranged from diurnal (M. smithii, M. parallelus, and S. parvulus) to all-day-long (M. morschi), to mainly nocturnal (S. saussurei). Furthermore, temperature and humidity were shown to affect the activity of these species. The non-leaf-cutter attine ants include diurnal and nocturnal species, and foraging periods in the same species can alternate according to environmental conditions (Weber 1972). Leal (1998) also recorded diurnal activity for Mycocepurus and Mycetarotes species in Brazilian cerrado vegetation, and noted that this pattern was also influenced by temperature (as reported in this study), with the peak of the foraging activity differing among colony and across seasons. Despite the differences in vegetation and climate between cerrado and Atlantic rainforest, these non-leafcutter ants apparently exhibit a similar pattern of daily activity, suggesting that their foraging rhythm may be more endogenous than influenced by environmental factors (see Hölldobler and Wilson 1990). In leaf-cutter ants the foraging rhythm is well studied and is affected by temperature and humidity (Wetterer et al. 1998; Wirth et al. 2003; Caldato et al. 2016), light intensity (Lewis et al. 1974), nutritional requirements of the colony (Caldato et al. 2016), parasite pressure (Feener and Moss 1990; Orr 1992), and type of available resources (Bochynek et al. 2017).
Our data on foraging behavior showed that the ants forage exclusively on the ground and in general near their nests, corroborating previous studies on non-leaf-cutter attine ants (Waller 1989; Leal and Oliveira 2000; Mehdiabadi and Schultz 2009; Ješovnik et al. 2018). Although M. morschi and S. parvulus foraged in small groups (up to 10 workers), only S. saussurei used a well-marked foraging trail with up to 20 workers, including underground parts. The use of foraging trails is observed mainly in leaf-cutter ants, but Sericomyrmex and Trachymyrmex species can also form conspicuous foraging trails (Weber 1972). Some fungus-farming ants (e.g., Cyphomyrmex, Mycetarotes, Mycocepurus, Myrmicocrypta, Sericomyrmex, and Trachymyrmex) in Brazilian savanna forage in small groups or even alone, and the foraging distances range from 1 to 3 m (Leal and Oliveira 2000). We suggest that the behavior of making foraging trails is more frequent in genera more closely related to the leaf-cutter ants, whose trails may extend tens of meters from the colony and may contain hundreds of workers (Cherrett 1968; Wetterer 1995; Röschard and Roces 2003). In the current study, we observed that all species recruit nestmates to baits placed in the vicinity of their nests. In fact, Ješovnik et al. (2018) also observed that Sericomyrmex foragers recruit nestmates to newly discovered resources. The same has been reported for M. goeldii, which can recruit 20–50 nestmates after discovering a pulp-rich pod of Hymenaea courbaril L. on the ground (Oliveira et al. 1995).
In this study, some species such as S. saussurei and M. parallelus collected arthropod feces in much greater proportion than other types of substrate material (Fig. 3). The large foraging distances traveled by S. saussurei (up to 3.60 m) may be related with increased collection of frass in this species (Fig. 4e). Because feces pellets are usually scattered in the environment, these items may be hard to find and possibly require increased foraging effort (see Ronque et al. 2018). It is hypothesized that non-leaf-cutter ants are opportunistic regarding the substrates for fungiculture and collected resources may vary across seasons and also with their availability near the nest, or even with preference by fungus-farming species (Leal and Oliveira 2000; Mehdiabadi and Schultz 2009; Hölldobler and Wilson 2011). In general, we observed that arthropod feces and vegetable matter were the main resources collected by the ants. Previous studies have reported that organic material in the leaf litter, such as arthropod feces, seeds, pieces of wood, fragments of flowers and grass, flesh of fruits, and lichens, comprise the main material collected by non-leaf-cutter ants on which to cultivate their symbiotic fungi (Oliveira et al. 1995; Leal and Oliveira 1998, 2000; Mehdiabadi and Schultz 2009; De Fine Licht and Boomsma 2010; Sosa-Calvo et al. 2017; Ronque et al. 2018). Additionally, although it has been reported that some species of Trachymyrmex and Sericomyrmex also cut fresh vegetation (e.g., flower petals, leaflets, tender leaves), this behavior is not as common as seen in Acromyrmex and Atta, and neither was observed in the five species included in this study (Weber 1966; Leal and Oliveira 2000; Seal and Tschinkel 2008; Mehdiabadi and Schultz 2009).
Additionally, differences in the proportions of collected items by the five species do not seem to be related to the area (“restinga” or lowland forest) during the period we sampled the foraging activities. For instance, species in the same area such as S. parvulus and S. saussurei (lowland forest) collected mainly vegetable matter and arthropod feces, respectively, and some of their nests were only ≈ 1 m apart from one another. Similarly, in the “restinga” forest, M. parallelus and M. smithii collected mainly arthropod feces, whereas M. morschi collected mostly vegetable matter. Possibly, the types of items collected by the species in each area could differ in another period of the year depending on the availability of resources (such as flowers, fruits, and seeds) at the “restinga” and lowland forests.
In Brazilian cerrado savanna, where two well-marked seasons occur (warm/wet and cold/dry season), Leal and Oliveira (1998, 2000) reported that non-leafcutter species (genera Cyphomyrmex, Mycetarotes, Mycocepurus, Myrmicocrypta, Sericomyrmex, and Trachymyrmex) collect mainly flowers and fruits in the warm/wet season, whereas in the cold/dry season the ants forage for arthropod frass, seeds, and insect carcasses. These results suggest that non-leafcutters exhibit a seasonal variation in the material they collect for fungiculture. Although the investigation of seasonal foraging is beyond the scope of our study, it is possible that these ants collect more fruits and flowers when these become available in the leaflitter of the Atlantic rainforest, as seen in the studies cited above.
Our study revealed various aspects of the ecology and behavior of fungus-farming ants, including species of non-leaf-cutters from the Paleoattina (M. smithii) and Neoattina (M. parallelus, M. morschi, S. parvulus, and S. saussurei) clades. Our field account brought together qualitative and quantitative data on the natural history of these ants in Atlantic rainforest. In an ecological-evolutionary perspective, our data contribute to a better understanding of the mutualistic relationship of these ants with the fungus cultivated inside the nest and of the evolutionary processes that led to the derived characteristics in the subtribe Attina. In addition, this natural history account sheds light on the basic biological features of fungus-farming ants and their ecological roles in the threatened Atlantic rainforest.
References
Bochynek T, Tanner JL, Meyer B, Burd M (2017) Parallel foraging cycles for different resources in leaf-cutting ants: a clue to the mechanisms of rhythmic activity. Ecol Entomol 42:849–852
Bollazzi M, Kronenbitter J, Roces F (2008) Soil temperature, digging behaviour, and the adaptive value of nest depth in South American species of Acromyrmex leaf-cutting ants. Oecologia 158:165–175
Bolton B (2014) An online catalog of the ants of the World. http://antcat.org. Accessed 3 Sept 2018
Branstetter MG, Ješovnik A, Sosa-Calvo J, Lloyd MW, Faircloth BC, Brady SG, Schultz TR (2017) Dry habitats were crucibles of domestication in the evolution of agriculture in ants. Proc R Soc B 284:20170095
Caldato N, Forti LC, Bouchebti S, Lopes JFS, Fourcassié V (2016) Foraging activity pattern and herbivory rates of the grass-cutting ant Atta capiguara. Insectes Soc 63:421–428
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Cardoso DC, Cristiano MP, Tavares MG (2011) Methodological remarks on rearing basal Attini ants in the laboratory for biological and evolutionary studies: overview of the genus Mycetophylax. Insectes Soc 58:427–430
Cherrett JM (1968) The foraging behavior of Atta cephalotes. J Anim Ecol 37:387–403
Christianini AV, Oliveira PS (2009) The relevance of ants as seeds rescuers of a primarily Bird-dispersed tree in the Neotropical cerrado savanna. Oecologia 160:735–745
Corrêa MM, Silva PSD, Wirth R, Tabarelli M, Leal IR (2010) How leaf-cutting ants impact forests: drastic nest effects on light environment and plant assemblages. Oecologia 162:103–115
De Fine Licht HH, Boomsma JJ (2010) Forage collection, substrate preparation, and diet composition in fungus-farming ants. Ecol Entomol 35:259–269
Della Lucia TMC, Gandra LC, Guedes RNC (2013) Managing leaf-cutting ants: peculiarities trends and challenges. Pest Manag Sci 70:14–23
Diehl-Fleig E, Diehl E (2007) Nest architecture and colony size of the fungus-farming ant Mycetophylax simplex Emery, 1888 (Formicidae, Attini). Insectes Soc 54:242–247
Farji-Brener AG, Illes AE (2000) Do leaf-cutting ant nest make “bottom-up” gaps in Neotropical rain forests? A critical review of the evidence. Ecol Lett 3:219–227
Feener DH Jr, Moss KAG (1990) Defense against parasites by hitchhikers in leaf-cutting ants: a quantitative assessment. Behav Ecol Sociobiol 26:17–29
Fernández F, Sendoya S (2004) List of Neotropical ants (Hymenoptera: Formicidae). Biota Colomb 5:3–93
Fernández-Marín H, Zimmerman JK, Wcislo WT (2004) Ecological traits and evolutionary sequence of nest establishment in fungus-farming ants (Hymenoptera, Formicidae, Attini). Biol J Linn Soc 81:39–48
Fernández-Marín H, Zimmerman JK, Wcislo WT, Rehner SA (2005) Colony foundation, nest architecture and demography of a basal fungus-farming ant, Mycocepurus smithii (Hymenoptera, Formicidae). J Nat Hist 39:1735–1743
Gotelli NJ, Ellison AM (2011) Princípios de estatística em ecologia. Artmed, Porto Alegre
Herz H, Beyschlag W, Hölldobler B (2007) Herbivory rate of leaf-cutting ants in a tropical moist forest in Panama at the population and ecosystem scales. Biotropica 39:482–488
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Hölldobler B, Wilson EO (2011) The leafcutter ants: civilization by instinct. W.W Norton and Company, New York
Ješovnik A, Sosa-Calvo J, Lopes CT, Vasconcelos HL, Schultz TR (2013) Nest architecture, fungus gardens, queen, males and larvae of the fungus-growing ant Mycetagroicus inflatus Brandão & Mayhé-Nunes. Insectes Soc 60:531–542
Ješovnik A, González VL, Schultz TR (2016) Phylogenomics and divergence dating of fungus-farming ants (Hymenoptera: Formicidae) of the genera Sericomyrmex and Apterostigma. PLoS One 11:e0151059
Ješovnik A, Schultz TR (2017) Revision of the fungus-farming ant genus Sericomyrmex Mayr (Hymenoptera, Formicidae, Myrmicinae). ZooKeys 670:1–109
Ješovnik A, Chaul J, Schultz T (2018) Natural history and nest architecture of the fungus-farming ant genus Sericomyrmex (Hymenoptera: Formicidae). Myrmecol News 26:65–80
Joly CA, Martinelli LA, Alves LF, Vieira SA, Tamashiro JY, Aidar MPM, Camargo PB, Assis MA, Bernacci LC, Durigan G (2008) As parcelas permanentes do projeto temático Biota Gradiente Funcional: composição florística, estrutura e funcionamento da floresta ombrófila densa dos Núcleos Picinguaba e Santa Virgínia do Parque Estadual da Serra do Mar, Estado de São Paulo, Brasil. In: Sanquetta CR (ed) Experiências de monitoramento no Bioma Mata Atlântica com uso de parcelas permanentes. Funpar, Curitiba, pp 109–129
Klingenberg C, Brandão CRF, Engels W (2007) Primitive nest architecture and small monogynous colonies in basal Attini inhabiting sandy beaches of southern Brazil. Stud Neotrop Fauna Environ 42:121–126
Klingenberg C, Brandão CRF (2009) Revision of the fungus-growing ant genera Mycetophylax Emery and Paramycetophylax Kusnezov rev. stat., and description of Kalathomyrmex n. gen. (Formicidae: Myrmicinae: Attini). Zootaxa 2052:1–31
Leal IR (1998) Ecologia e história natural de formigas Attini em vegetação do Cerrado, PhD thesis. Instituto de Biologia, Universidade Estadual de Campinas, Campinas
Leal IR, Oliveira PS (1998) Interactions between fungus growing ants (Attini), fruits and seeds in Cerrado vegetation in Southeast Brazil. Biotropica 30:170–178
Leal IR, Oliveira PS (2000) Foraging ecology of attine ants in Neotropical savannah: seasonal use of fungal substrate in the Cerrado vegetation of Brazil. Insectes Soc 47:376–382
Leal IR, Silva PSD, Oliveira PS (2011) Natural history and ecological correlates of fungus-farming ants (Formicidae: Attini) in the Neotropical Cerrado Savanna. Ann Entomol Soc Am 104:901–908
Leal IR, Wirth R, Tabarelli M (2014) The multiple impacts of leaf-cutting ants and their novel ecological role in human-modified neotropical forests. Biotropica 46:516–528
Lewis T, Pollard GV, Dibley GC (1974) Micro-environmental factors affecting diel patterns of foraging in the leaf cutting ant Atta cephalotes (L.) (Formicidae: Attini). J Anim Ecol 43:143–153
Li H, Sosa-Calvo J, Horn HA, Pupo MT, Clardy J, Rabeling C, Schultz TR, Currie CR (2018) Convergent evolution of complex structures for ant–bacterial defensive symbiosis in fungus-farming ants. Proc Natl Acad Sci 115:10725
Mayhé-Nunes AJ, Jaffé K (1998) On the biogeography of Attini (Hymenoptera: Formicidae). Ecotropicus 11:45–54
Mayhé-Nunes AJ, Brandão CRF (2006) Revisionary notes on the fungus-farming ant genus Mycetarotes Emery (Hymenoptera, Formicidae). Rev Bras Entomol 50:463–472
Mehdiabadi NJ, Schultz TR (2009) Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol News 13:37–55
Montoya-Lerma J, Giraldo-Echeverri C, Armbrecht I, Farji-Brener A, Calle Z (2012) Leaf-cutting ants revisited: towards rational management and control. Int J Pest Manag 58:225–247
Moreira A, Forti LC, Andrade AP, Boaretto MAC, Lopes JFS (2004) Nest Architecture of Atta laevigata (F. Smith, 1858) (Hymenoptera: Formicidae). Stud Neotrop Fauna Environ 39:109–116
Mueller UG, Schultz TR, Currie CR, Adams RM, Malloch D (2001) The origin of the Attine ant-fungus mutualism. Q Rev Biol 76:169–197
Mueller UG, Wcislo WT (1998) Nesting biology of the fungus-farming ant Cyphomyrmex longiscapus Weber (Attini, Formicidae). Insectes Soc 45:181–189
Nehring V, Boomsma JJ, d’Ettorre P (2012) Wingless virgin queens assume helper roles in Acromyrmex leaf-cutting ants. Curr Biol 22:R671–R673
Ness J, Mooney K, Lach L (2010) Ants as mutualists. In: Lach L, Parr CL, Abbott KL (eds) Ant ecology. Oxford University Press, Oxford, pp 97–114
Oliveira PS, Galetti M, Pedroni F, Morellato LPC (1995) Seed cleaning by Mycocepurus goeldii ants (Attini) facilitates germination in Hymenaea courbaril (Caesalpiniaceae). Biotropica 27:518–522
Orr MR (1992) Parasitic flies (Diptera: Phoridae) influence foraging rhythms and caste division of labor in the leaf-cutter ant, Atta cephalotes (Hymenoptera. Formicidae). Behav Ecol Sociobiol 30:395–402
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 4 Sept 2018
Rabeling C, Verhaagh M, Engels W (2007) Comparative study of the nest architecture and colony structure of the fungus-farming ants, Mycocepurus goeldii and Mycocepurus smithii. J Insect Sci 7:1–13
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant-plant interactions. The University of Chicago Press, Chicago
Ronque MUV, Migliorini GH, Oliveira PS (2018) Thievery in rainforest fungus-growing ants: interspecific assault on culturing material at nest entrance. Insectes Soc 65:507–510
Röschard J, Roces F (2003) Cutters, carriers and transport chains: distance-dependent foraging strategies in the grass-cutting ant Atta vollenweideri. Insectes Soc 50:237–244
San Martin-Gajardo I, Morellato LP (2003) Fenologia de Rubiaceae de sub-bosque em floresta Atlântica no sudeste do Brasil. Rev Bras Bot 26:299–309
Schultz TR, Brady SG (2008) Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA 105:5435–5440
Silva PD, Leal IR, Wirth R, Tabarelli M (2007) Harvesting of Protium heptaphyllum (Aubl.) March. seeds (Burseraceae) by leaf-cutting ant Atta sexdens L. promotes seed aggregation and seedling mortality. Rev Bras Bot 30:553–560
Seal JN, Tschinkel WR (2008) Food limitation in the fungus-gardening ant, Trachymyrmex septentrionalis. Ecol Entomol 33:597–607
Solomon SE, Mueller UG, Schultz TR, Currie CR, Price SL, Oliveira da Silva-Pinhati AC, Bacci M Jr, Vasconcelos HL (2004) Nesting biology of the fungus growing ants Mycetarotes Emery (Attini, Formicidae). Insectes Soc 51:333–338
Solomon SE, Lopes CT, Mueller UG et al (2011) Nesting biology and fungiculture of the fungus-growing ant, Mycetagroicus cerradensis: new light on the origin of higher attine agriculture. J Insect Sci 11:1–14
Sosa-Calvo J, Schultz TR, Brandão CRF et al (2013) Cyatta abscondita: taxonomy, evolution, and natural history of a new fungus-farming ant genus from Brazil. PLoS One 8:e80498
Sosa-Calvo J, Ješovnik A, Okonski E, Schultz TR (2015) Locating, collecting, and maintaining colonies of fungus-farming ants (Hymenoptera: Myrmicinae: Attini). Sociobiology 62:300–320
Sosa-Calvo J, Ješovnik A, Vasconcelos HL, Bacci M Jr, Schultz TR (2017) Rediscovery of the enigmatic fungus-farming ant “Mycetosoritis” asper Mayr (Hymenoptera: Formicidae): implications for taxonomy, phylogeny, and the evolution of agriculture in ants. PLoS One 12:e0178498
Sosa-Calvo J, Schultz TR, Ješovnik A, Dahan RA, Rabeling C (2018) Evolution, systematics, and natural history of a new genus of cryptobiotic fungus-growing ants. Syst Entomol 43:549–567
Sternberg LD, Pinzon MC, Moreira MZ, Moutinho P, Rojas EI, Herre EA (2007) Plants use macronutrients accumulated in leaf-cutting ant nests. Proc R Soc B 274:315–321
Verza SS, Forti LC, Lopes JFS, Hughes WOH (2007) Nest architecture of the leaf-cutting ant Acromyrmex rugosus rugosus. Insectes Soc 54:303–309
Waller DA (1989) Foraging behaviour of Trachymyrmex turrifex Wheeler (Formicidae: Attini). Southwestern Nat 34:271–275
Ward PS, Brady SG, Fisher BL, Schultz TR (2015) The evolution of Myrmicinae ants: phylogeny and biogeography of hyperdiverse ant clade (Hymenoptera: Formicidae). Syst Entomol 40:61–81
Weber NA (1937) The biology of the fungus-growing ants Part 1. New forms. Rev Entomol 7:378–409
Weber NA (1941) The biology of the fungus-farming ants. Part VII. The Barro Colorado Island, Canal Zone, species. Rev Entomol 12:93–130
Weber NA (1966) Fungus-farming ants. Science 153:587–604
Weber NA (1969) A comparative study of the nests, gardens and fungi of the fungus-growing ants, Attini. VI Congress of IUSSI, Bern, pp 299–307
Weber NA (1972) Gardening ants, the attines. Memoirs of the American Philosophical Society, Philadelphia, 146 pp
Weber NA (1982) Fungus ants. In: Hermann HR (ed) Social insects, vol IV. Academic Press, New York, pp 255–363
Wetterer JK (1995) Forager size and ecology of Acromyrmex coronatus and other leaf-cutting ants in Costa Rica. Oecologia 104:409–415
Wetterer JK, Gruner DS, Lopez JE (1998) Foraging and nesting ecology of Acromyrmex octospinosus (Hymenoptera: Formicidae) in a Costa Rican tropical dry forest. Fla Entomol 81:61–67
Wheeler WM (1925) A new guest-ant and other new Formicidae from Barro Colorado Island, Panama. Biol Bull 49:150–184
Wilson EO (1980) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav Ecol Sociobiol 7:143–156
Wirth W, Herz H, Ryel RJ, Beyschlag W, Hölldobler B (2003) Herbivory of leaf-cutting ants: a case study on Atta colombica in the Tropical Rainforest of Panama. Ecol Stud 164:1–230
Acknowledgements
We thank Ted Schultz for valuable comments on the manuscript. The Parque Estadual Serra do Mar (Núcleo Picinguaba) and the Instituto Florestal do Estado de São Paulo provided logistic support, and Eliane Rodrigues gave permission to work at the Pousada Betânia. We thank G.H. Migliorini and A. Tacioli for helping during fieldwork, A. Ješovnik for identifying the Sericomyrmex species, H. Soares Jr. for helping with the photos; A.V. Christianini, F.B. Noll and M.S.C. Morini for comments at early stages of the study. M.U.V R. was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), R.M.F. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 302462/2016-3), and P.S.O. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 306115/2013-1, 302219/2017-0) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2014/23141-1, 2017/16645-1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ronque, M.U.V., Feitosa, R.M. & Oliveira, P.S. Natural history and ecology of fungus-farming ants: a field study in Atlantic rainforest. Insect. Soc. 66, 375–387 (2019). https://doi.org/10.1007/s00040-019-00695-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-019-00695-y