Abstract
Climate change, such as warming, is a threat to mountain ecosystems in the forest-line ecotone. This influence could seriously affect bryophytes, because they easily lose their internal water at high temperatures. We conducted experimental warming using open-top chambers (OTCs) in a forest-line ecotone in central Japan and examined its influence on bryophyte cover. Six years after the experiment was initiated, the total bryophyte cover was not significantly different between the control and OTC treatments. However, the two dominant bryophyte species (Pogonatum japonicum and Dicranum majus) responded differently to the OTC treatment. The cover of P. japonicum significantly increased under the OTC treatment, while that of D. majus decreased to approximately 14% of the initial cover under the OTC treatment. These results could be explained by D. majus being better adapted to high-elevation climates than P. japonicum. The decline of D. majus cover was potentially further enhanced by the decrease in rainfall and fog within the OTCs. These are important water sources for D. majus because the species lacks water-conducting systems that enable mosses to absorb water from their substrates. As the OTCs in this study were tall (210 cm high), they may have blocked slanting rain and fog from reaching the plants, increasing water stress in D. majus. In contrast, P. japonicum develops water-conducting systems and may be less susceptible to the decrease in rainfall and fog. These results can aid future experimental studies in the mountains to elucidate the mechanisms underlying bryophyte responses to warming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change, such as warming, is a major global driver of changes in vegetation (Sala et al. 2000; Gobiet et al. 2014). Shifts in vegetation due to global warming affect ecosystem processes, including net primary production (Xu et al. 2013), nutrient cycling (Wahren et al. 2005), biomass (Deane-Coe et al. 2015), and biological interactions (Roy et al. 2004). These changes are expected to be severe at high latitudes (Sala et al. 2000; Gobiet et al. 2014), because global warming substantially increases the temperature due to its feedback at the surface and the decreased albedo through melting snow and ice cover (Pithan and Mauritsen 2014). According to experimental warming studies in high-arctic and tundra ecosystems, plant groups respond differently to warming across time and space (Hollister et al. 2005; Walker et al. 2006; Hudson and Henry 2010; Elmendorf et al. 2012). In contrast to high-arctic and tundra ecosystems at high latitudes, the number of experimental warming studies conducted in mid-latitude regions is limited (Suzuki and Kudo 2000; Taguchi and Wada 2000; Wada 2000; Wada et al. 2002; Kudo and Suzuki 2003; Walker et al. 2006; Hoffmann et al. 2010; Kudo et al. 2010; Xu et al. 2012; Xu et al. 2012; Tanaka et al. 2013). This raises concerns when evaluating the influence of climate warming on vegetation in mid-latitude regions, because the responses of plant communities to warming depend on the initial conditions of their ecosystems and climates (Jónsdóttir et al. 2005).
Among the various types of mountain ecosystems at mid-latitudes, the forest-line ecotone (or subalpine–alpine ecotone) may be particularly vulnerable to climate warming. This is because both subalpine and alpine species coexist in this ecotone; thus, subalpine species can easily replace alpine species under elevated temperatures. For example, in central Japan, subalpine evergreen conifer trees (e.g., Abies mariesii) dominate the lower parts of the forest line, whereas Erman’s birch (Betula ermanii) and the Japanese dwarf pine (Pinus pumila) dominate the upper parts. Climate warming might cause the dominant species in the upper parts to be replaced by those from the lower parts (Fujiwara et al. 1999; Horikawa et al. 2009). Such a replacement in dominant species from high-elevation to low-elevation species might result in substantial vegetation changes in this ecotone. However, to date, experimental warming in the forest-line ecotone (Danby and Hik 2007; Hoffmann et al. 2010) has been scarce (Tanaka et al. 2013).
Plant groups sensitive to warming are useful for examining possible changes in vegetation in a forest line. Bryophytes may be a suitable plant group for this purpose because of their sensitivity to changes in environmental conditions caused by climate warming, such as enhanced drought stress (Gignac 2001) and timing of the disappearance of snow beds (Hohenwallner et al. 2011). The sensitivity of bryophytes to these changes is attributed to their poikilohydric nature; that is, they lack the ability to actively regulate their water content to achieve homeostasis (Proctor and Tuba 2002). The water status of bryophytes depends on external environmental conditions; therefore, they easily lose their internal water at high temperatures, which increases their evaporation rates (He et al. 2016). The water deficit leads to a shorter period of photosynthetic activity, which can significantly reduce bryophyte diversity at elevated temperatures (He et al. 2016).
Previous studies have reported that bryophyte cover is more susceptible to experimental warming than other indices such as species richness (Sun et al. 2017; Alatalo et al. 2020; van Zuijlen et al. 2021), diversity (Alatalo et al. 2020), and evenness (van Zuijlen et al. 2021). Sun et al. (2017) conducted experimental warming in an ecosystem above the tree line, which revealed that the cover of forest bryophytes was more vulnerable to increased temperature than that of shrubland bryophytes owing to the adaptation of the forest bryophytes to a cooler and damper environment. These results imply that climate warming can cause significant changes in the cover of dominant bryophytes. Contrary to the expectation that bryophytes are vulnerable to warming, their responses to the warming effect varied from negative to positive in high-arctic and tundra ecosystems. Analyses of plant responses to experimental warming in tundra ecosystems revealed that the bryophyte cover decreased (Hollister et al. 2005; Jónsdóttir et al. 2005; Walker et al. 2006; Alatalo et al. 2020). However, other studies show an increase in bryophyte cover under experimental warming (Hudson and Henry 2010; Shortlidge et al. 2017) or no change (Jägerbrand et al. 2009, 2012). These varied responses of bryophytes to experimental warming warrant further studies investigating the influence of warming on bryophytes in various habitats.
In this study, we performed experimental warming in a forest-line ecotone to examine the response of bryophytes to increased temperature in a mid-latitude region (Japan). We hypothesized that the cover of low-elevation species would replace that of high-elevation species, considering that the high-elevation species are more susceptible to warmer climates. This examination contributes to an understanding of climate change on the dynamics of bryophytes in the forest-line ecotone at mid-latitudes.
Methods
Study sites
The experimental warming study was conducted in a forest-line ecotone (altitude of approximately 2570–2590 m) of Mt. Shoginokashira in central Japan (Fig. 1). This experiment aimed to examine the responses of vegetation, including bryophytes, to a warming climate. The study site is located in the Nishikoma Station at the Education and Research Center of Alpine Field Science of Shinshu University. The site experiences heavy snowfall, and packed snow covers the ground from November to June. The mean monthly temperature at the closest meteorological station (altitude of 2600 m) ranged from − 11.5 °C in December to 13.0 °C in August 2016 (Kobayashi et al. 2018). The vegetation around the study site comprises a mixed sparse forest of distorted deciduous broadleaf trees (Betula ermanii and Sorbus matsumurana) and subalpine conifers (Abies mariesii and Abies veitchii). The upper boundary of the study site is covered by a dense stand of dwarf alpine pines (Pinus pumila), while the lower part is covered by dense subalpine conifer trees. The forest floor is covered with shrubs, herbs, and bryophytes. The major shrub species are Elliottia bracteata, Rhododendron pentandrum, and Vaccinium ovalifolium; and the major herb species are Maianthemum dilatatum, Solidago virgaurea subsp. leiocarpa, and Streptopus streptopoides subsp. japonicus.
Two bryophyte species widely distributed in the study site are Pogonatum japonicum and Dicranum majus. They often coexist in the forest-line of subalpine forests, but their elevational distributions differ. In central Japan, P. japonicum dominates the upper-temperate to subalpine zone (Osada 1965), whereas D. majus dominates the subalpine to alpine zone (Takaki 1964). According to the elevational distributions of these species, P. japonicum and D. majus are considered low- and high-elevation species, respectively. Other bryophytes in the study site include common subalpine species such as Brachythecium noesicum, Hylocomiastrum himalayanum, Hylocomium splendens, Pleurozium schreberi, and Rhytidiadelphus squarrosus.
Experimental warming and bryophyte monitoring
In the study site, we commenced experimental warming in October 2010 using 10 open-top chambers (OTCs; each 105 cm long × 105 cm wide × 210 cm high). The chambers were made of clear corrugated polycarbonate panels and steel frames. These OTCs increase the temperature inside, thereby simulating climate warming (Suzuki and Nagaoka 2017). Five of the OTCs were used for the year-round OTC treatment, in which clear panels were installed throughout the year. The other five OTCs were used for the summer-time OTC treatment, in which clear panels were installed to the OTCs only during the summer season (late July to early October). One quadrat (55 cm × 55 cm) was set within each OTC (i.e., ten quadrats were under OTC treatment), and six quadrats (55 cm × 55 cm) were set outside the OTCs for the control treatment. All OTC and control plots (16 plots in total) were randomly placed within the study site. However, we changed the experimental design, because some OTCs had been damaged severely by heavy snow by 2013. In July 2013, we excluded four OTCs (one year-round and three summer-time). We also changed the remaining 4-year-round OTCs to the summer-time OTC treatment to avoid damage to OTCs during the winter. Thus, our experimental warming study was continued in 6 OTCs (two summer-time OTCs during 2010–2016, and 4 year-round OTCs until July 2013 which changed thereafter to summer-time) and 6 controls (12 quadrats in total).
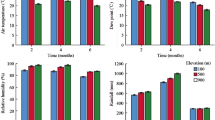
The influence of OTCs on abiotic factors (temperature, humidity, water content of the soil, and relative light intensity) was examined, and the effects of OTCs are summarized as follows. The temperature was measured during 2010–2016 using data loggers (iButton [DS1920]; Maxim Integrated, San Jose, CA, USA) at 30 cm above ground level. The OTCs increased the temperature by 0.32 ± 0.12 °C during the summers of 2010–2016. In contrast, the OTC treatment did not enhance the temperature (− 0.01 ± 0.44 °C) during the other times of the year in 2010–2012 because the OTCs were buried under heavy snow. Relative humidity was also measured using data loggers (iButton [DS1923]; Maxim Integrated, San Jose, CA, USA) at 30 cm above ground level during July–September in 2013. The relative humidity decreased from 94.1 to 93.2% in OTC quadrats. The water content of soil was measured once in August 2013. The content was calculated by subtracting the dry weight of 100 g soil from that of wet weight. The OTC treatment decreased soil water content from 45.1 g to 38.1 g water/100 g soil. The relative light intensity was measured at soil surface (LI-190SA, Li-Cor, Lincoln, NE, USA). The average relative light intensities were 4.12% and 2.91% in the control and OTC treatments, respectively. Thus, the OTC treatment increased the temperature (+ 0.32 °C), whereas it decreased the relative humidity (− 0.9%). water content of the soil (− 7.0 g/100 g soil), and relative light intensity (− 1.21%).
Bryophyte cover in those 12 (6 OTC and 6 control) quadrats was recorded during September 2010–2016. In each quadrat, three types of bryophyte cover (total, Pogonatum [P. japonicum], and Dicranum [D. majus]) were measured using grids of two sizes (normal: 5 × 5 cm, fine: 1 × 1 cm). Both or either of the targeted species grew in the surveyed quadrats. The total cover included all the terrestrial bryophytes that grow in the area (i.e., the sum of the cover of Pogonatum, Dicranum, and the other species). The cover of Rigodiadelphus robustus was not included in the calculation of total cover, because this species often falls from the trees to the ground. The bryophyte cover was visually estimated for each grid cell (5 × 5 cm and 1 × 1 cm) and then averaged per plot. The cover of herbs and ferns on the ground was also recorded in 2010 and 2016.
Statistical analysis
The changes in total, Pogonatum, and Dicranum cover were chronologically plotted in 2010, 2012, 2014, and 2016 for the control and OTC treatments. The differences between these treatments were then examined using Bayesian methods. These methods are suitable for handling small sample sizes such as those in this study, because they are based on a Markov chain Monte Carlo simulation (McNeish 2016). For this model, we assumed that (1) the change in bryophyte cover in the control is expressed by a constant, and (2) the influence of the OTC treatment on bryophyte cover is expressed by a constant term. Based on these assumptions, the state equation (Eq. 1) and the observation equation (Eq. 2) for change in bryophyte cover are expressed as follows:
where μ[i, t] is the true value of bryophyte cover in quadrat i in surveyed year t; α expresses the change in bryophyte cover in the controls; β is the effect of the OTC treatment on the change in bryophyte cover; W expresses the presence/absence of the OTC treatment (W = 1 for the OTC treatment, W = 0 for the control treatment); \(\upgamma\) is the effect of the initial OTC treatment during winter on the change in bryophyte cover; IW expresses the presence/absence of the initial OTC treatment in winter during 2010–2012 (IW = 1 for the OTC treatment, IW = 0 for the control treatment); εμ [i, t] is a process error in quadrat i reflecting the strength of the autocorrelation between the survey years; Y[i, t] is the observed bryophyte cover; and εγ [i, t] is the observational error in bryophyte cover in quadrat i in year t. In the model, εμ [i, t] and εγ [i, t] were assumed to follow a normal distribution with mean 0 and standard deviation σμ and σγ, expressed as Normal (0, σμ), and Normal (0, σγ), respectively.
We then constructed a likelihood function for the parameters and examined the differences in α, β, and \(\upgamma\) values based on a Bayesian 95% credible interval (quantiles between 2.5 and 97.5%). When the credible intervals of β and \(\upgamma\) deviated from zero, the difference in the changes in cover between the control and OTC treatments was regarded as significant. To avoid the arbitrary selection of prior distributions, we adapted weakly informative prior distributions: a half-Cauchy distribution with mean 0 and standard deviation 5 for σμ and σγ; a normal distribution, Normal (0, 100), for α, β, and \(\upgamma\); and a uniform distribution with minimum 0 and maximum 1404 (maximum cover observed in the quadrats) for μ[i, t]. Posterior estimates were visually examined for chain convergence with trace plots and a potential scale reduction factor (Gelman and Rubin 1992). The calculations were performed using Stan (Carpenter et al. 2017; Stan Development Team 2020) via the RStan interface of R version 4.0.3 (R Core Team 2021).
Results
Changes in vegetation cover
The changes in bryophyte cover during 2010–2016 are shown in Figs. 2 and 3 (Online Resource 1). The total bryophyte cover in the control and OTC quadrats in 2010 was 577 ± 199 cm2 (average ± SD) and 527 ± 391 cm2, respectively, and in 2016 was 747 ± 473 cm2 and 344 ± 408 cm2, respectively. The total cover after 6 years increased to 130% of its initial value in the control treatment, whereas it decreased to 65% of its initial value after the OTC treatment. The effect of OTC treatment (parameters β and \(\upgamma\)) was not significant in the Bayesian model (Table 1). In 2010, the total cover of herbs and ferns in the control and OTC quadrats was 35 ± 50 cm2 and 104 ± 245 cm2, respectively, and in 2016 was 91 ± 164 cm2 and 142 ± 256 cm2, respectively. The average cover of herbs and ferns increased to 262% of its initial value during 2010–2016 in the controls, whereas the increase in the OTC quadrats was 137% of its initial value during the same period (Online Resource 1).
Mean annual changes in the relative bryophyte cover per quadrat for 2010–2016. a Total cover, (b) Pogonatum cover, and (c) Dicranum cover. The bryophyte cover in 2010 was adjusted to 1.0. In the OTC quadrats, the Dicranum cover significantly decreased over time compared to that in the control quadrats, based on statistical models using a Bayesian framework
The two moss species exhibited different responses to the OTC treatment. The parameter α for Pogonatum cover was significant, but parameters β and \(\upgamma\) were not significant. Thus, the Pogonatum cover increased over the 6 years of the experiment despite the OTC treatments. Notably, the rate of increase in the Pogonatum cover (i.e., the ratio of the final to initial cover) in the OTC plots (414%) was nearly double that in the control plots (238%). In contrast, the Dicranum cover increased to 179% of its initial value in the control treatment but considerably decreased to 14% of its initial value in the OTCs. The β value for the Dicranum cover was negative between the 2.5–97.5% quantiles (Table 1), indicating that the Dicranum cover significantly decreased due to the OTC treatment.
Discussion
Responses of bryophytes to experimental warming
The total cover of bryophytes did not change during the OTC experiment because of the opposite responses of the two dominant species to the OTC treatment. Our results support the implication that bryophytes do not respond to climate warming as a single plant group (Sun et al. 2017), which can partially explain the varied responses of bryophyte cover to experimental warming in previous studies.
The Dicranum cover in the OTC treatment significantly decreased after 6 years of the experiment. In contrast, the Pogonatum cover increased both under the control and OTC treatments, and the increase was larger in the OTC quadrats than in the control quadrats. The different responses of these two moss species could be attributed to their optimal growth environments related to their elevational distributions. The OTC treatment may have negatively affected D. majus because of the adaptation of the species to the cooler climate at higher elevations, whereas the same treatment may have had a considerable positive effect on the low-elevation species P. japonicum. In particular, the forest line corresponds to almost the upper boundary for the P. japonicum distribution in central Japan (Osada 1965), whereas D. majus is distributed up to the alpine area (Takaki 1964). Based on the elevational distribution, the increased temperature might be beneficial for the growth of P. japonicum, resulting in the increase of its cover through the OTC treatment. These results correspond to our hypothesis that the cover of the high-elevation species (D. majus) would significantly decrease in the OTC quadrat, while that of the low-elevation species (P. japonicum) would increase.
Nevertheless, considering that the increase in temperature was only 0.3 °C in the OTCs, other factors could have also affected the decrease in the D. majus cover. A possible factor could be a blocking effect of the OTCs against rainfall and fog. Rainfall and fog are specifically crucial for the survival of certain mosses, known as ectohydric mosses, that lack water-conducting systems to absorb water from their substrates (Glime 2017). However, the height of the OTCs (210 cm) may have physically blocked the slanting rainfall and fog from entering the OTCs, thus reducing the amount of water reaching the plants. Because D. majus is classified as an ectohydric moss (van der Wal et al. 2005), the decrease in rainfall and fog could have negatively affected the growth of this species.
In contrast to D. majus, P. japonicum, an endohydric moss, develops water-conducting systems (Glime 2017; Oishi 2018); hence, this species may have been less affected by the decrease in rainfall and fog in the OTCs. In our experiment, the soil water content in the OTCs was lower compared to that of the controls. Nevertheless, the water content in the OTCs was sufficient for the growth of P. japonicum; that is, the decrease in soil water content did not negatively affect the Pogonatum cover in the OTC quadrats.
Our results indicate that differences in the development of water-conducting systems in mosses could be related to the responses of the bryophytes to the warming experiment. Similar results were obtained in a previous study (Alatalo et al. 2014), wherein the authors suggested that acrocarpous species with more advanced water-conducting systems than pleurocarpous species can be less sensitive to drier conditions caused by experimental warming (Alatalo et al. 2014). Moreover, Oishi (2018) revealed that endohydric mosses have a higher ability to retain water during dry periods than ectohydric species. Therefore, considering these results, the development of water-conducting systems might provide a valuable perspective for understanding bryophyte responses to increased drought stress caused by climate warming.
Interaction between bryophytes and other plants
In addition to abiotic factors (temperature, rainfall, and fog), the changes in plant–plant interactions may have had an effect on the decrease in the Dicranum cover. However, in contrast to previous studies (Walker et al. 2006; Alatalo et al. 2020), the influence of these interactions on bryophytes is marginal or insignificant based on the changes in plant cover in the OTC quadrats. This is because the decrease in the Dicranum cover did not correspond to the increase in other plant covers in the OTC quadrats (Plot Nos. 6, 9, and 14 in Online Resource 1). Furthermore, the differences in light intensity between the control and OTC treatments were small, implying that shading by tree canopy had a negligible impact on the results of the OTC experiment. These findings indicate that the low influence of plant-plant interactions on the bryophytes were potentially related to the characteristics of the vegetation at the study plots. The plots were located under the canopy of dwarf alpine pines and subalpine conifers, where the cover of ferns and herbs was low throughout the OTC experiment (Online Resource 1). Hence, the competition between bryophytes and other vascular plants was perhaps weakened in our OTC experiment.
Conclusions
The results of this study do not contradict our hypothesis that an increase in temperature can negatively affect high-elevation species. However, further studies are required to validate the hypothesis, because these results may also be attributed to the decrease in the amount of rainfall and fog that reached the plants owing to the height of the OTCs. This influence is potentially considerably high for ectohydric mosses, which lack systems for conducting water from substrates.
This study has important implications for OTC experiments at high elevations. First, the measurement of the changes in rainfall and fog is necessary for elucidating the effects of OTC treatment on bryophytes. These measurements are particularly important when tall OTCs are used. Second, the differences in water absorption strategies of bryophytes (ectohydric vs. endohydric species) can affect their responses to increased drought stress caused by climate warming. Finally, considering that few studies are available on experimental warming at the forest-line ecotone in mid-latitude regions, our results are an important reference for future warming studies in this ecosystem.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Alatalo JM, Jägerbrand AK, Molau U (2014) Climate change and climatic events: community-, functional- and species-level responses of bryophytes and lichens to constant, stepwise, and pulse experimental warming in an alpine tundra. Alp Bot 124:81–91. https://doi.org/10.1007/s00035-014-0133-z
Alatalo JM, Jägerbrand AK, Erfanian MB et al (2020) Bryophyte cover and richness decline after 18 years of experimental warming in alpine Sweden. AoB Plants 12:plaa061. https://doi.org/10.1093/aobpla/plaa061
Carpenter B, Gelman A, Hoffman MD et al (2017) Stan: a probabilistic programming language. J Stat Soft. https://doi.org/10.18637/jss.v076.i01
Danby RK, Hik DS (2007) Responses of white spruce (Picea glauca) to experimental warming at a subarctic alpine treeline. Glob Change Biol 13:437–451. https://doi.org/10.1111/j.1365-2486.2006.01302.x
Deane-Coe KK, Mauritz M, Celis G et al (2015) Experimental warming alters productivity and isotopic signatures of tundra mosses. Ecosystems 18:1070–1082. https://doi.org/10.1007/s10021-015-9884-7
Elmendorf SC, Henry GH, Hollister RD et al (2012) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15:164–175. https://doi.org/10.1111/j.1461-0248.2011.01716.x
Fujiwara T, Okada N, Yamashita K (1999) Comparison of growth response of Abies and Picea species to climate in Mt. Norikura, central Japan. J Wood Sci 45:92–97. https://doi.org/10.1007/BF01192324
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. https://doi.org/10.1214/ss/1177011136
Gignac LD (2001) Bryophytes as indicators of climate change. Bryologist 104:410–420. https://doi.org/10.1639/0007-2745(2001)104[0410:BAIOCC]2.0.CO;2
Glime JM (2017) Capter 7–2 water relations: movement. In: Glime JM (ed) Bryophyte ecology vol. 1. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. http://digitalcommons.mtu.edu/bryophyte-ecology/. Accessed 17 Feb 2022
Gobiet A, Kotlarski S, Beniston M et al (2014) 21st century climate change in the European Alps—a review. Sci Total Environ 493:1138–1151. https://doi.org/10.1016/j.scitotenv.2013.07.050
He X, He KS, Hyvönen J (2016) Will bryophytes survive in a warming world? Perspect Plant Ecol Evol Syst 19:49–60. https://doi.org/10.1016/j.ppees.2016.02.005
Hoffmann AA, Camac JS, Williams RJ et al (2010) Phenological changes in six Australian subalpine plants in response to experimental warming and year-to-year variation. J Ecol 98:927–937. https://doi.org/10.1111/j.1365-2745.2010.01667.x
Hohenwallner D, Zechmeister HG, Moser D et al (2011) Alpine bryophytes as indicators for climate change: a case study from the Austrian Alps. In: Tuba Z, Slack NG, Stark LD (eds) Bryophyte ecology and climate change. Cambridge University Press, Cambridge, pp 237–250
Hollister RD, Webber PJ, Tweedie CE (2005) The response of Alaskan arctic tundra to experimental warming: differences between short- and long-term responses. Glob Change Biol 11:525–536. https://doi.org/10.1111/j.1365-2486.2005.00926.x
Horikawa M, Tsuyama I, Matsui T et al (2009) Assessing the potential impacts of climate change on the alpine habitat suitability of Japanese stone pine (Pinus pumila). Lands Ecol 24:115–128. https://doi.org/10.1007/s10980-008-9289-5
Hudson JMG, Henry GHR (2010) High arctic plant community resists 15 years of experimental warming. J Ecol 98:1035–1041. https://doi.org/10.1111/j.1365-2745.2010.01690.x
Jägerbrand AK, Alatalo JM, Chrimes D, Molau U (2009) Plant community responses to 5 years of simulated climate change in meadow and heath ecosystems at a subarctic-alpine site. Oecologia 161:601–610. https://doi.org/10.1007/s00442-009-1392-z
Jägerbrand AK, Kudo G, Alatalo JM, Molau U (2012) Effects of neighboring vascular plants on the abundance of bryophytes in different vegetation types. Polar Sci 6:200–208. https://doi.org/10.1016/j.polar.2012.02.002
Jónsdóttir IS, Magnússon B, Gudmundsson J et al (2005) Variable sensitivity of plant communities in Iceland to experimental warming. Glob Change Biol 11:553–563. https://doi.org/10.1111/j.1365-2486.2005.00928.x
Kobayashi H, Nomizo Y, Kinoshita W et al (2018) Meteorological data of Nishikoma Station, AFC. Shinshu University, 2016. Bull Shinshu Univ Alpine Field Center 16:67–68 (in Japanese)
Kudo G, Suzuki S (2003) Warming effects on growth, production, and vegetation structure of alpine shrubs: a five-year experiment in northern Japan. Oecologia 135:280–287. https://doi.org/10.1007/s00442-003-1179-6
Kudo G, Kimura M, Kasagi T et al (2010) Habitat-specific responses of alpine plants to climatic amelioration: comparison of fellfield to snowbed communities. Arct Antarct Alp Res 42:438–448. https://doi.org/10.1657/1938-4246-42.4.438
McNeish D (2016) On using Bayesian methods to address small sample problems. Struct Equ Model 23:750–773. https://doi.org/10.1080/10705511.2016.1186549
Oishi Y (2018) Evaluation of the water-storage capacity of bryophytes along an altitudinal gradient from temperate forests to the alpine zone. Forests 9:433. https://doi.org/10.3390/f9070433
Osada T (1965) Japanese Polytrichaceae. Introduction and the genus Pogonatum. J Hattori Bot Lab 28:171–201
Pithan F, Mauritsen T (2014) Arctic amplification dominated by temperature feedbacks in contemporary climate models. Nat Geosci 7:181–184. https://doi.org/10.1038/ngeo2071
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytol 156:327–349. https://doi.org/10.1046/j.1469-8137.2002.00526.x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Roy BA, Güsewell S, Harte J (2004) Response of plant pathogens and herbivores to a warming experiment. Ecology 85:2570–2581. https://doi.org/10.1890/03-0182
Sala OE, Chapin FS, Armesto JJ et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. https://doi.org/10.1126/science.287.5459.1770
Shortlidge EE, Eppley SM, Kohler H et al (2017) Passive warming reduces stress and shifts reproductive effort in the Antarctic moss, Polytrichastrum alpinum. Ann Bot 119:27–38. https://doi.org/10.1093/aob/mcw201
Stan Development Team (2020) RStan: the R interface to Stan. R package version 2.21.2. http://mc-stan.org/. Accessed 22 Feb 2021
Sun S-Q, Wang G-X, Chang SX et al (2017) Warming and nitrogen addition effects on bryophytes are species- and plant community-specific on the eastern slope of the Tibetan Plateau. J Veg Sci 28:128–138. https://doi.org/10.1111/jvs.12467
Suzuki S, Kudo G (2000) Responses of alpine shrubs to simulated environmental change during three years in the mid-latitude mountain, northern Japan. Ecography 23:553–564. https://doi.org/10.1034/j.1600-0587.2000.230506.x
Suzuki RO, Nagaoka K (2017) Warming can enhance the detrimental effect of pathogens on a host plant, Miscanthus sinensis, in a cool-temperate montane grassland in Nagano, Japan. Écoscience 24:137–144. https://doi.org/10.1080/11956860.2017.1378963
Taguchi Y, Wada N (2000) Variations of leaf traits of an alpine shrub Sieversia pentapetala along an altitudinal gradient and under a simulated environmental change. Polar Biosci 14:79–87
Takaki T (1964) A revision of Japanese Dicranum. J Hattori Bot Lab 27:73–123
Tanaka K, Hirao A, Suzuki R et al (2013) Impact of global warming on mountain and polar ecosystems: what have artificial warming experiments told? J Geogr 122:628–637. in Japanese with English abstract. https://www.jstage.jst.go.jp/article/jgeography/122/4/122_122.628/_article/-char/ja/. https://doi.org/10.5026/jgeography.122.628
van der Wal R, Pearce IS, Brooker RW (2005) Mosses and the struggle for light in a nitrogen-polluted world. Oecologia 142:159–168. https://doi.org/10.1007/s00442-004-1706-0
van Zuijlen K, Asplund J, Sundsbø S et al (2021) Ambient and experimental warming effects on an alpine bryophyte community. Arct Sci. https://doi.org/10.1139/as-2020-0047
Wada N (2000) Responses of floral traits and increase in female reproductive effort to a simulated environmental amelioration in a hermaphrodite alpine dwarf shrub, Sieversia pentapetala (Rosaceae). Arct Antarct Alp Res 32:208–211. https://doi.org/10.1080/15230430.2000.12003357
Wada N, Shimono M, Miyamoto M, Kojima S (2002) Warming effects on shoot developmental growth and biomass production in sympatric evergreen alpine dwarf shrubs Empetrum nigrum and Loiseleuria procumbens. Ecol Res 17:125–132. https://doi.org/10.1046/j.1440-1703.2002.00469.x
Wahren C-HA, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Change Biol 11:537–552. https://doi.org/10.1111/j.1365-2486.2005.00927.x
Walker MD, Wahren CH, Hollister RD et al (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci U S A 103:1342–1346. https://doi.org/10.1073/pnas.0503198103
Xu Z, Hu T, Zhang Y (2012) Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. Eur J Forest Res 131:811–819. https://doi.org/10.1007/s10342-011-0554-9
Xu X, Sherry RA, Niu S et al (2013) Net primary productivity and rain-use efficiency as affected by warming, altered precipitation, and clipping in a mixed-grass prairie. Glob Chang Biol 19:2753–2764. https://doi.org/10.1111/gcb.12248
Acknowledgements
The authors are grateful to Kiyotaka Hori for his contribution to bryophyte identification in 2010–2011; to Keiko Furukawa, Mitsunari Yakubo, and Taki Yabuta for their assistance with the bryophyte cover surveys; and to the staff and students at the Education and Research Center of Alpine Field Science, Shinshu University and the Sugadaira Research Station, University of Tsukuba for their assistance with the management and performance of the experimental warming in 2010–2016.
Funding
This work was supported by Research and Education Funding for Japanese Alps Inter-Universities Cooperative Project, MEXT, Japan, and the Japan Society for the Promotion of Science—Grant-in-Aid for Young Scientists (B) (Grant Number 24710029).
Author information
Authors and Affiliations
Contributions
TK and HK conceived and designed the experiment; RK, DM, and TK designed the open-top chambers used in this study; TK, HK, SNS, RK, and DM managed and performed the experiment; YO and TK surveyed the bryophyte cover; YO and SNS conducted the statistical analysis; YO interpreted the results and wrote early drafts of the paper; and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oishi, Y., Kobayashi, H., Suzuki, S.N. et al. Bryophyte responses to experimental climate change in a mid-latitude forest-line ecotone. Alp Botany 132, 329–336 (2022). https://doi.org/10.1007/s00035-022-00280-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-022-00280-3