Abstract
Fluctuating asymmetry (FA) has been suggested as a useful indicator of elevation stress and, hence, distribution limits in plants. However, no plant studies have been carried out to test (i) whether FA shows a gradual increase towards the alpine distribution limit and (ii) whether FA responds to elevation stress independent of other stressors which is necessary for FA to be a useful indicator in this context. To test these two hypotheses, this 2-year field study investigated the dose–response relationship between elevation stress and FA in mountain birch (Betula pubescens) under contrasting levels of insect attack in northern Norway. The results showed that FA increased linearly from sea level towards the tree line in both years independent of insect attack, which had no observable effect on FA, i.e. insect attack did not appear to disturb the FA-elevation relation. Thus, in mountain birch, FA appeared to be a reliable indicator of elevation stress. Further investigation is now needed in order to develop this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inadequate buffering of development processes produces deviation in morphological traits. Such developmental instability is often quantified as fluctuating asymmetry (FA), i.e. random deviation from phenotypic symmetry (Van Valen 1962; Palmer and Strobeck 1986). A range of stressors in various taxa have been associated with increased FA (reviewed by Møller and Swaddle 1997), which is therefore often used in ecological studies as a measure of stress (Parsons 1990, 1992; Palmer 1996). Since the development of corresponding body sides are controlled by the same genes, FA presumably reflects stress during development. However, as yet, the causes of FA, in particular in plants, are poorly understood (see Polak 2003; Pélabon et al. 2004)
Forest dominated by mountain birch (Betula pubescens) is a distinct, but geographically restricted ecosystem in northern Europe that usually forms a narrow biogeographic zone between coniferous boreal forest and mountain tundra and thus sets the northern and alpine forest limit (Ahti et al. 1969; Wielgolaski 2001). Concerns have been raised to the future of the mountain birch forest, due to changes in the factors supposed to limit its distribution and productivity (Wielgolaski et al. 2004). Harsh climates and herbivory are believed to be the main determinants of the mountain birch forest’s tree-line dynamics (e.g. Sveinbjörnsson et al. 2002), although their relative importance is largely unknown, partly due to the slow dynamics of forest processes. In case of slow dynamics and potentially delayed response to environmental change, indicators tracking the effects of current levels of ambient stressors, such as FA, may be particularly useful.
In mountain birch, pollution (Kozlov et al. 1996; Zvereva et al. 1997), herbivory (Lempa et al. 2000), and hybridisation (Wilsey et al. 1998) have been associated with increased FA. As another example, Wilsey et al. (1998) found that leaf FA in mountain birch was higher in a tree-line common garden than in a lower elevation garden. As they readily pointed out, this could suggest that leaf FA may serve as a useful indicator of elevation stress and distribution limits in this tree species. However, nobody has investigated in detail how FA in mountain birch or any other plant species changes towards either the alpine or northern distribution limit. Also, to be useful as an indicator in this context, FA needs to respond to elevation stress independent of other stressors, which has never been investigated.
We measured FA in leaves of mountain birch along 10 altitudinal transects, from sea level to the tree limit, in northern Norway. In all transects, to test whether FA responds to elevation stress independent of other stressors, we recorded abundances of the geometrid moth species Epirrita autumnata and Operophtera brumata, which are key herbivores on mountain birch in Northern Fennoscandia (Tenow 1972). Both of them show cyclic outbreak dynamics with a period of approximately 10 years in this ecosystem (Tenow 1972). The outbreaks may have dramatic effect, with complete defoliation and, occasionally, death of the birch forest over hundreds or thousands of km2 (Tenow 1972). The impact of these insects has been suggested to increase towards the tree line (Tenow 1972), and it is possible that synergetic effects of harsh climates and herbivory by these insects may set the mountain birch forest limit (Skre et al. 2002; Sveinbjörnsson et al. 2002), which should be possible to detect as a statistical interaction between herbivory and elevation stress on FA. Because our study area contained a large spatial variation in local densities of these insects, the data provided an excellent opportunity to explore the effects of herbivory on the link between elevation stress and FA.
Methods
Study area and design
The study was conducted in 2001 and 2002 at Reinøya in northern Norway (70° 00′ N, 19° 49′ E) in a north-easterly oriented slope (mean slope 23.3°) where the mountain birch forest forms a fairly homogenous belt from sea level and up to a rather sharp forest limit at approximately 240–250 m elevation. Ten parallel altitudinal transects, spaced at 200 m intervals from sea level to the forest limit were established. Each transect had 4 stations at the following altitudes: 30, 100, 170 and 240 m (i.e. at the forest limit). The length of transects varied between 514 and 628 m. Forest structure and variation in environmental factors among the 40 sampling stations were explored by Principle Component Analysis in Mjaaseth et al. (2005). The most important structural forest variable was tree height, which did not change in monotonous fashion with altitude (Mjaaseth et al. 2005). The forest consisted of open stands of relatively small birch trees both at the lowest and the highest altitude, with denser stands composed of higher trees at the two intermediate altitudes (Mjaaseth et al. 2005). Mean snow depth was the most important temperature dependent environmental factor that was measured, which increased monotonously with altitude (Mjaaseth et al. 2005).
Sampling procedure
Air temperatures drop predictably by 0.5–0.65°C per 100 m elevation under normal atmospheric pressure (Tveito et al. 2001), and leaf growth is a directly temperature-dependent process (Junttila and Nilsen 1993; Karlsson et al. 2003). To test whether the altitudinal transects comprised a biologically relevant temperature gradient, we therefore compared leaf development (i.e. phenology) among altitudes. This was done by measuring leaf size at three dates simultaneously at all 40 transect stations at the onset of the growing season in both years. The first measurement date was selected so that the leaves had not started to grow yet at the highest altitude. The first date was May 29 in 2001 and May 15 in 2002, reflecting a clear difference both in the onset of the growth season (see Fig. 1) and in spring temperatures between the two years (see Fig. 2). The subsequent two measurements were made at weekly intervals. At each measurement date, nine undamaged leaves/buds from three different trees (i.e. 27 leaves in total) were measured at each sampling station (i.e. 270 leaves at each altitude). The size measurement was the length from the base to the tip of the bud/leaf with an accuracy of 1.0 mm.
Leaves for FA measurements were obtained at all 40 transect stations by collecting 10 undamaged leaves from 10 different trees each year in mid August. The leaves were randomly sampled from branches at about chest height, pointing in the same direction relative to the trunk (north or downhill). The FA was measured in the lab to the nearest 0.01 mm, by using an electronic calliper. The leaf dimensions measured were the width of the left and right half, at the midpoint between the base and the tip, perpendicular to the mid vein. Two independent repeats were obtained for each measurement, to separate FA and measurement error, by numbering each leaf with a water proof pen. All of the leaves were first measured once. Thereafter, a new workbook was established and all of the leaves were re-measured, to increase independence during the measurement campaign. The length of the leaves (from tip to basis) was also measured, to control for any effects of leaf size on FA.
The abundance of E. autumnata and O. brumata larvae at each sampling station was estimated in late June (2001) and early July (2002), by shaking 10 arm-length birch twigs, collected haphazardly from 10 different trees, over a large plastic box and then counting the larvae (Hagen et al. 2003a, b; Ims et al. 2004). Control checks of beaten branches revealed that the method was efficient for the purpose of obtaining an index of larval density. In the statistical analysis (see below), we used the sum of the two species as a station specific measure of herbivore pressure on mountain birch.
Statistical approach
To analyse the FA data, we used a linear mixed model with station, tree within station, leaf within tree, and measurement within leaf as random, nested factors. This was done to correctly specify the various levels of replication (i.e. station/tree/leaf), while simultaneously taking into account the various levels of within-replicate variation (i.e. 10 trees per station, 10 leaves per tree, and the two FA measurements taken per leaf, i.e. measurement error) (Pinheiro and Bates 2000), There was evidence (all P-values <0.05) of relationship between station, year, length and directional asymmetry (DA) (i.e. a consistently larger left that right side). Such a mixture of DA and FA is common and is probably due to genetics (Palmer and Strobeck 1986). Before proceeding with the main analysis (see below), this tendency of DA was statistically removed, because we wanted to focus on unbiased FA (i.e. random deviation) for stress estimation (Palmer and Strobeck 1986). The procedure used to remove DA involved using the residuals of a linear model of the difference Left–Right as a function of station, year, length and the interaction station × year. FA was defined as the absolute (unsigned) value of these signed residuals. The FA-values were then transformed to achieve approximate constancy of variance of residuals in the main analysis, described below. A square-root transformation was chosen (see Pélabon et al. 2004). Such an approach is similar to the use of absolute residuals to analyse variability, as recommended for example by Altman (1993). Sources of variation in FA were then determined by using model selection based on the maximum likelihood method (ML) and the Information Criteria AIC and BIC (Burnham and Anderson 1998; Miller 2002), the latter being more conservative. The predictor variables were year, altitude, larval density, and leaf size. Altitude was modelled both as a factor and as a continuous variable to assess non-linear effects. Since two different persons were involved in the collection of leaves, “collector” was modeled as a covariate to both test and control for sampling bias on FA. The “best model”, as indicated by model selection criteria AIC and BIC, was re-fitted using the restricted maximum likelihood method (REML) before any inference was made, because estimates of variance components based on ML are biased (Pinheiro and Bates 2000). The analysis was carried out in R (R Core Development Team 2004).
Results
The relative progress of leaf development in spring decreased gradually with increasing altitude in both of the years (Fig. 1), showing that the altitudinal transects comprised a biologically relevant temperature gradient. Figure 2 shows the daily mean temperatures for May and June during the same period for the two year of the study. While spring and early summer was generally cooler than the average in 2001, the next year (2002) was warmer than normal. This in turn influenced the relative progress of leaf development in spring in the two years; 2002 was about 14 days earlier than 2001 (Fig. 1).
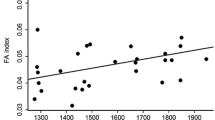
Using the material from both years, the most parsimonious statistical model for FA included altitude, year and leaf length (Table 1). Estimates obtained from this model showed that FA increased towards the tree limit in both years (b = 0.028/100 m, s.e. = 0.0070; 95% C.I. [0.013; 0.043]) (Fig. 3). Moreover, FA was significantly lower in the relatively warmer summer compared with the relatively colder summer (b = −0.063, s.e. = 0.011, 95% C.I. [−0.085, −0.042]). The magnitude of the estimated coefficients (b) showed that the difference in FA between the two years was slightly larger than the difference between the highest and lowest altitude (210 m; i.e. 2.1 × 0.028 = 0.059 compared to 0.063), which is consistent with the fact that the average temperature during the growth period of birch was more than 2°C warmer in 2002 than 2001 (Fig. 2). According to AIC and BIC, altitude explained more parsimoniously the variation in FA if modelled as a quantitative/continuous variable than when modelled as a factor. Also this latter result indicates a linear “dose–response” relationship on the scale considered (square-root).
The density of insects (E. autumnata + O. brumata) varied significantly, from low to intermediate densities, both within and between altitudes and years (Table 2). Although far from the extreme outbreak densities that are sometimes achieved by these insects, the intermediate densities constituted a conspicuously large abundance of caterpillars, which we expected would have a clear negative impact on the birch trees. Surprisingly, there was no evidence that FA was related to the density of insects (b = −0.000056, s.e. = 0.0028, 95% C.I. [−0.00053,0.00064]), nor was there any interaction effects selected according to the AIC-criterion. Thus, it could seem like harsh climates generally are a far more important source of stress towards the northern and alpine distribution limits of mountain birch than are insect herbivores, although this will need to be verified for higher densities of larvae than observed in this study (i.e. in outbreak years; see Ims et al. 2004).
Moreover, we found that leaf FA increased with leaf size (b = 0.0055, s.e. = 0.00062, 95% C.I. [0.0043; 0.0067]), which was controlled for by including size as a variable in the statistical analysis. Of other confounding factors is the potential effect of different persons in collecting the leaves. Such “observer variability” was, however, unimportant in explaining the variation in FA observed in this study (Table 1). Moreover, measurement error of FA was small compared to differences in FA among leaves (σMeasure/Leaf/Tree = 0.113;? σLeaf/Tree = 0.430; other components of variance were estimated as: σTree = 0.067 and σRes = 0.076).
Conclusion
This observational study, carrying out a detailed dose–response analysis of how FA in leaves of mountain birch responds to gradual increases in altitude under variable insect attack, has demonstrated that leaf FA in this tree species seems to be a robust indicator of elevation stress. Leaf FA as well as leaf phenology exhibited a clear linear relationship with altitude, and for both of these variables the year effect was consistent with the altitude effect. This point to spatial climatic variation (i.e. harsh climate at the tree-line) as the main determinant of FA in tree-line mountain birches. Moreover, leaf FA seems to be a useful and cost efficient monitoring tool for detecting immediate forest responses to climate impacts also in the temporal domain, which is consistent with previous findings based on much longer time series (e.g. Valkama and Kozlov 2001). For an indicator to be useful, it must be reliable as well as simple to use, and for deciduous trees it is possible to get virtually any number of within individual repeats (i.e. leaves). Furthermore, measurement error of FA will probably be relatively small for most deciduous trees, because the structures to be measured (i.e. leaves) are relatively large. Indeed, our results show that, at least in an extensive leaf material, the effect of measurement error is so small that it may not be necessary to obtain repeated measurements of individual leaves, as done here. Although our study was regional scale relative to other studies of FA in plants, there is a need for future studies evaluating whether the simple dose–response relationship in birch forests demonstrated here is valid for even more large-scale environmental gradients in which other confounding issues than different levels of herbivory will be present.
References
Ahti T, Hämet-Ahti L, Jalas J (1969) Vegetation zones and their sections in northwestern Europe. Ann Bot Fennici 5:169–211
Altman DG (1993) Construction of age-related reference centiles using absolute residuals. Stat Med 12:917–924
Burnham KP, Anderson DR (1998) Model selection and inference. A practical information theoretic approach. Springer-Verlag, New York
Chapin FS III, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth forms in an Alaskan tundra ecosystem. Implications for herbivory. J Ecol 68:189–209
Hagen SB, Folstad I, Jakobsen SW (2003a) Autumn colouration and herbivore resistance in mountain birch (Betula pubescens). Ecol Lett 6:807–811
Hagen SB, Ims RA, Yoccoz NG (2003b) Density-dependent melanism in sub-arctic populations of winter moth larvae (Operophtera brumata). Ecol Entomol 28:659–665
Hodkinson ID, Bird J (1998) Host-specific insect herbivores as sensors of climate change in arctic and Alpine environments. Arct Alp Res 30:78–83
Hoogesteger J, Carlson PS (1992) Effects of defoliation on radial stem growth and photosynthesis in the mountain birch (Betula pubescens ssp. tortuosa). Funct Ecol 6:317–323
Ims RA, Yoccoz NG, Hagen SB (2004) Do sub-Arctic winter moth populations in coastal birch forest exhibit spatially synchronous dynamics? J Anim Ecol 73:1129–1136
Junttila O, Nilsen J (1993) Growth and development of northern forest trees as affected by temperature and light. In: Alden J., Mastrantonio JL, Ødum S (eds), Forest development in cold climates. Plenum Press, New York, 43–57
Karlsson PS, Bylund H, Neuvonen S, Heino S, Tjus M (2003) Climatic response of budburst in the mountain birch at two areas in northern Fennoscandia and possible responses to global change. Ecography 26:617–625
Kozlov MV, Wilsey BJ, Koricheva J, Haukioja E (1996) Fluctuating asymmetry of birch leaves increases under pollution impact. J Appl Ecol 33:1489–1495
Lempa K, Martel J, Koricheva J, Haukioja E, Ossipov V, Ossipova S, Pihlaja K (2000) Covariation of fluctuating asymmetry, herbivory and chemistry during birch leaf expansion. Oecologia 122:354–360
Martel J, Lempa K, Haukioja E (1999) Effects of stress and rapid growth on fluctuating asymmetry and insect damage in birch leaves. Oikos 86:208–216
Miller AJ (2002) Subset selection in regression, IInd edn. Chapman and Hall/CRC, London
Mjaaseth RR, Hagen SB, Yoccoz NG, Ims RA (2005) Phenology and abndance in relation to climatic variation in a sub-arctic insect herbivore-mountain birch system. Oecologica 145:53–65
Møller AP, Swaddle JP (1997) Asymmetry, developmental stability, and evolution. Oxford Univ. Press
Neuvonen S, Niemelä P, Virtanen T (1999) Climatic change and insect outbreaks in boreal forests: the role of winter temperatures. Ecol Bull 47:63–67
Palmer AR (1996) Waltzing with asymmetry. BioScience 46:518–532
Palmer AR, Strobeck C (1986) Fluctuating asymmetry: measurement, analysis and patterns. Annu Rev Ecol Syst 17:391–421
Parsons PA (1990) Fluctuating asymmetry: an epigenetic measure of stress. Biol Rev Cambridge Philos Soc 65:131–145
Parsons PA (1992) Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity 68:361–364
Pélabon C, Carlson ML, Hansen TF, Yoccoz NG, Armbruster WS (2004) Consequences of inter-population crosses on developmental stability and canalization of floral traits in Dalechampia scandens (Euphorbiaceae). J Evol Biol 17:19–32
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-Plus. Springer, New York
Polak M (ed) (2003) Developmental instability: causes and consequences. Oxford University Press, Oxford, U.K
R_Development_Core_Team (2004) R: A language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna, Austria
Skre O, Baxter R, Crawford RMM, Callaghan TV, Fedorkov A (2002) How will the tundra-taiga interface respond to climate change? Ambio 12:37–46
Sonesson M, Osborne C, Sandberg G (1994) Epiphytic lichens as indicators of snow depth. Arct Alp Res 26:159–165
Sveinbjörnsson J, Hofgaard A, Lloyd A (2002) Natural causes of the tundra-taiga boundary. Ambio 12:23–29
Tenow O (1972) The outbreaks of Oporinia autumnata Bkh. and Operophtera spp. (Lep., Geomtridae) in the Scandinavian mountain chain and northern Finland 1862–1968 Zoologiska Bidrag från Uppsala 2:1–107
Tveito OE, Førland E, Alexandersson H, Drebs A, Jónsson T, Tuomenvirta H, Vaarby Laursen E (2001) Nordic climate maps. 06/01 Klima 06/01. Norwegian Meteorological Institute, Oslo
Valkama J, Kozlov MV (2001) Impact of climatic factors on the developmental stability of mountain birch growing in a contaminated area. J Appl Ecol 38:665–673
Van Dongen S, Lens L (2000) Symmetry, size and stress. Trends Ecol Evol 15:330–331
van Valen L (1962) A study of fluctuating asymmetry. Evolution 16:125–142
Wielgolaski FE (ed) (2005) Plant ecology, herbivory and human impact in nordic mountain birch forests. Ecol Stud 180 Springer, Berlin
Wielgolaski FE (2001) Nordic mountain birch ecosystems. UNESCO, Paris and Partenon, New York
Wilsey BJ, Haukioja E, Koricheva J, Sulkinoja M (1998) Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology 79:2092–2099
Zvereva EL, Kozlov MV, Niemela P, Haukioja E (1997) Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109:368–373
Acknowledgements
We thank Ragnhild R. Mjaaseth for assistance in the field. We also thank ACIA (Arctic Climate Impact Assessment) and The Research Counsel of Norway for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hagen, S.B., Ims, R.A., Yoccoz, N.G. et al. Fluctuating asymmetry as an indicator of elevation stress and distribution limits in mountain birch (Betula pubescens). Plant Ecol 195, 157–163 (2008). https://doi.org/10.1007/s11258-007-9312-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9312-y