Abstract
The tropical alpine flora in the northern Andes has caught the attention of evolutionary biologists and conservationists because of the extent of its diversity and its vulnerability. Although population genetics studies are essential to understand how diversity arises and how it can be maintained, plant populations occurring above 4100 m a.s.l. in the so-called super-páramo have rarely been studied at the molecular level. Here, we use 11 microsatellite DNA markers to examine genetic structure in populations of Lupinus alopecuroides, a long-lived semelparous giant rosette known from only 10 geographically isolated populations. Each population is located on a different mountain top, of which three are in Colombia and seven in Ecuador. We analysed 220 individuals from all the ten known populations. We find low genetic variation in all but one of the populations. Four populations are completely monomorphic, and another five show only one polymorphic locus each. On the other hand, we find extremely high genetic differentiation between populations. We discuss the mechanisms that might cause this pattern, and we suggest that it is related to founder effects, lack of gene flow, and autogamy. The genetic relationships among the populations, and the lack of correlation between the genetic and geographic distances also point to the importance of founder effects and colonization history in driving differentiation among the populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The highlands in the northern Andes host one of the World’s most species-rich tropical alpine floras (Smith and Cleef 1988; Sklenář et al. 2011), characterized by rapid and recent evolution (van der Hammen et al. 1973; Hughes and Eastwood 2006; Madriñán et al. 2013). This is the so-called páramo ecosystem that lies between 3000 and 4900 m a.s.l., and the highest tiers of it, above 4100 m, is called the super-páramo (Cuatrecasas 1968; van der Hammen and Cleef 1986). Given its restricted geographic distribution, páramo plant diversity is severely threatened by habitat destruction due to global warming and land-use changes (Balslev and Luteyn 1992; Morueta-Holme et al. 2015; Vásquez et al. 2015). Although the páramo flora has received significant attention from evolutionary biologists and conservationists, population genetic studies are scarce (Oleas et al. 2012). To our knowledge, super-páramo plant populations have never been studied at the molecular level.
Patterns of genetic variation across populations are determined by various interrelated factors, and population history plays an important role (Ellstrand and Elam 1993; Hewitt 2000). In the northern Andes, Pleistocene climatic oscillations produced elevational range shifts that enormously impacted páramo species. During glaciations, species expanded across broader landscapes at lower elevations, establishing new populations and gene flow between populations that had been isolated. During interglacials, species were again restricted to geographically isolated “islands in the sky” (van der Hammen et al. 1973; Hooghiemstra et al. 2006; Graham 2009; Sklenář et al. 2011). Another historical factor that impacted the páramo populations is volcanism (Oleas et al. 2012). Volcanic activity is a driver of population extinction and recolonization (Carson et al. 1990; Beheregaray et al. 2003), and thus influences evolutionary trajectories of the populations.
We examined the genetic structure of populations of Lupinus alopecuroides Desr.—a long-lived semelparous giant rosette restricted to the super-páramo in Colombia and Ecuador. Semelparous giant rosettes are a distinctive feature of tropical alpine floras, and a fascinating example of convergent adaptive evolution to harsh high-elevation environments (Rundel et al. 1994; Sgorbati et al. 2004). Marcescent dead leaves of the rosette protect the meristem from nocturnal temperatures, which often are below zero (Monasterio 1986; Rundel et al. 1994), and lanate hairs on the up-to 1 m high inflorescence provide insulation for reproductive organs (Miller 1986) (Fig. 1). Semelparous plants maximize reproduction by investing all resources in a single reproductive episode because, due to harsh environmental conditions, future survival and reproduction are less likely (Young and Augspurger 1991).
The genetic structure of populations is also determined by species-specific biological traits such as dispersal ability and breeding system. One of the most common seed dispersal modes observed in lupines is ballistichory—a mechanical process in which the walls of the mature pod twist in opposite directions, firing the seeds 1–3 m away from the parent plant. Long-distance dispersal of lupine seeds is usually afforded by waterways, human activities, and animals, mainly rodents (Milla and Iriondo 2011; Fagan et al. 2005; Maron and Kauffman 2006; Australian Department of Health and Ageing 2013). The reproductive biology of L. alopecuroides has remained unstudied. Other perennial species of Lupinus have asexual reproduction by vegetative regeneration, and there is no evidence of apomictic reproduction in the genus (Richards 1986). Most annual lupines are self-compatible while perennials are self-incompatible (Kittelson and Maron 2000). However, in the papilionoid legumes, to which Lupinus belongs, self-sterility is rare (East 1940; Kittelson and Maron 2000). For facultatively autogamous species, selfing may occur either as a result of insufficient pollinator visitation, or as a result of pollinator visits due to the close proximity of viable pollen to the receptive stigma (Pazy 1984; Karoly 1992), or due to transfer of pollen between flowers on the same individual (de Jong et al. 1993).
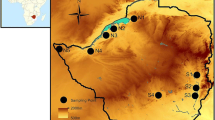
Another important factor influencing the genetic structure of natural populations is landscape (Slatkin 1987; Manel and Holderegger 2013). Populations of Lupinus alopecuroides are known from only ten geographically isolated mountain tops (Fig. 2). In geographically isolated populations, enhanced genetic drift and lack of gene flow result in genetic differentiation and loss of genetic diversity (Lesica and Allendorf 1995; Frankham et al. 2002; Allendorf and Luikart 2006). In these tropical “islands in the sky”, founder effects may also occur, as founders arrive over long distances from similar habitats, rather than from adjacent sites at lower elevations, which are ecologically different (Carson and Templeton 1984). Enhanced genetic drift, restricted gene flow, and founder events put the sky island populations of L. alopecuroides at risk of inbreeding depression, and potentially make them vulnerable to an extinction vortex (Frankham et al. 2002). In populations of Puya raimondii, which is another sky island long-lived semelparous giant rosette occurring around 4000 m a.s.l from Peru to northern Bolivia, low genetic variation was reported across 217 AFLP marker loci in 160 individuals from eight populations. Four populations were completely monomorphic, and each of the others displayed only one to three polymorphic loci. It was suggested that the causes of this pattern are high rates of selfing and repeated bottlenecks (Sgorbati et al. 2004). Phaedranassa tunguraguae, which is a long-lived, partially selfing plant, showed homozygote excess at 12 microsatellite loci. Its populations were restricted to a single valley in the Ecuadorian Andes, at 1500–2500 m a.s.l., and the most likely explanation suggested for this pattern was genetic drift due to small population size and restricted gene flow (Oleas et al. 2012).
It is well established that founding of a new population by a small number of individuals causes abrupt changes in allele frequencies that can lead to loss of genetic variation within populations and increased genetic differentiation among populations (Mayr 1954, Allendorf and Luikart 2006). However, founder effects are hard to detect, because it is difficult to disentangle their signatures from those of other evolutionary forces (Hoeck et al. 2010; Spurgin et al. 2014). In island populations of Berthelot’s pipit (Anthus berthelotii), Spurgin et al. (2014) detected genetic and phenotypic changes that resulted from founder events, and suggested that, under lack of gene flow and selection, founder effects can persist for evolutionary time scales. In populations of silvereyes (Zosterops lateralis), Clegg et al. (2002) demonstrated that sequential founder events produced divergence among populations. Finally, Prugnolle et al. (2005) have suggested that patterns of neutral genetic differentiation in human populations are the result of sequential founder events that occurred as small populations of modern humans migrated out of Africa.
Population genetics studies can help us understand how contemporary diversity arises and how it can be maintained (Frankham et al. 2002; Allendorf and Luikart 2006). Here, we use microsatellite markers to examine patterns of genetic variation in the sky island populations of Lupinus alopecuroides, and we discuss the factors underlying genetic variation and population structure. In particular, we address the following questions. (1) How high is genetic diversity within populations? (2) Are populations genetically differentiated, and how is genetic variation distributed within and among the populations? (3) Is there gene flow between the populations? (4) How are the different populations related, and is there a geographical correlation?
Materials and methods
The species
Lupinus alopecuroides Desr. is a long-lived semelparous giant rosette known from only ten geographically isolated populations. Each population is located on a different mountain top, of which three are in Colombia and seven in Ecuador (Fig. 2). Within the populations, individuals are found forming dense clusters (Fig. 1). This might be related to the species’ ballistic seed dispersal. We assume that long-distance dispersal of L. alopecuroides seeds, which weigh approx. 40 mg and measure 5 × 3 mm, is limited. Elsewhere rodents and human activity are important dispersers of Lupinus (Milla and Iriondo 2011; Fagan et al. 2005, Maron and Kauffman 2006; Australian Department of Health and Ageing 2013), but these agents are nearly absent in the super-páramo (Sklenář and Ramsay 2001, Rangel 2006). Anemochory or epizoochory are also unlikely in L. alopecuroides, since the seeds have a smooth surface without appendages or other adaptations that could facilitate external transport by animals. Some long-distance dispersal may be provided by waterways, white-tailed deer (Odocoileus virginianus), and domestic cattle, which have been seen to feed on L. alopecuroides pods (Vásquez, personal observation). The reproductive biology of the species has remained unstudied. Bumblebees are the most likely pollinator of L. alopecuroides, but their activity may be reduced by the harshness of the environment where this species grows (Dillon et al. 2006). During our fieldwork for this (10 weeks) and previous studies, we never observed insects visiting the flower, even on the sunniest days. We also observed that flowers remain closed for a long time after anthesis.
Population sampling
Exhaustive field and herbarium work in 2013–2014 permitted us to sample all ten known populations of L. alopecuroides, each of them located on a different mountain (Fig. 2). On the Cayambe and Sincholagua mountains, populations consist of three subpopulations clearly separated by 1–10 km (Table 1). Population size, elevation, and slope were recorded for each population and subpopulation (Table 1). Population size was estimated by counting all individuals or, when population size exceeded 50, by extrapolating it from the average number of individuals counted in sub-plots. Population area was estimated from the polygon obtained from GPS-delimited boundaries using Google Earth Pro. Leaf tissue was randomly collected from 8 to 67 clearly distinct individuals, and stored in silica gel.
Molecular methods
DNA was extracted from leaf tissue of 220 individuals. Nine loci (Luna1, Luna3, Luna4, Luna6, Luna8, Luna12, Luna15, Luna17, Luna20) developed for Lupinus nanus (Molecular Ecology Resources Primer Development Consortium et al. 2012), and two loci (AG55-20-22, AG55-26-16) developed for Lupinus microcarpus (Drummond and Hamilton 2005) were amplified in four separate multiplexes, using QIAGEN Multiplex PCR Maxter Mix (Qiagen, Valencia, CA, USA). PCR products were electrophoresed on an automated capillary sequencer (3130xl Genetic Analyzer, Applied Biosystems, Foster City, CA, USA) with Genescan-600 (LIZ) size standard (Applied Biosystems). Sizes of alleles (in base pairs) were determined using GeneMarker (Soft Genetics).
Statistical analysis
To estimate within-population genetic diversity, allelic richness as well as observed heterozygosity (Ho), and unbiased expected heterozygosity (He) according to Nei (1987) were calculated for each population. Genetic differentiation among all populations was estimated using Weir and Cockerham’s θ (1984), an estimator of F ST, and R ST, an F ST analogue based on the stepwise mutation model (Slatkin 1995). The program FSTAT 2.9.3 (Goudet 2001) was used for these analyses. Tests for significant deviation from Hardy–Weinberg equilibrium were conducted using exact tests implemented in Arlequin 3.5 (Excoffier and Lischer 2010). Genetic relationships among the populations were estimated using POPTREEW (Takezaki et al. 2014) to construct a neighbour-joining phenogram based on dmu2 distances (Goldstein et al. 1995). Bootstrap values across loci were based on 10,000 permutations by locus. Spatial genetic structure was further explored by testing for isolation by distance using a Mantel test between a matrix of dmu2 genetic distances (Goldstein et al. 1995) and a matrix of geographic distances as implemented in R package adegenet 2.0.0 (Jombart and Ahmed 2011).
Results
A total of 46 unambiguously scorable and reproducible alleles ranging from 75 to 346 bp were detected at 11 microsatellite loci across 220 individuals, representing 10 populations distributed throughout the species’ geographic range. Numbers of alleles per locus ranged from 2 to 6. Monomorphic loci were observed in all the populations except COCUY, where all eleven loci were polymorphic. Five populations showed only one polymorphic locus each. The other four populations (CHIMBORAZO, IMBABURA, CAYAMBE, SINCHOLAGUA), were completely monomorphic (Table 1). Consequently, there was no genetic variation between the subpopulations neither in CAYAMBE nor in SINCHOLAGUA. Moreover, CAYAMBE and SINCHOLAGUA were fixed for the same alleles (Online Resource 1). Therefore, all individuals within these populations were identical multilocus genotypes. Significant deviation from Hardy–Weinberg equilibrium was found across all non-monomorphic loci except Luna 3 (p value = 0.23) and Luna 17 (p value = 0.02) in the population COCUY. Genetic differentiation among all populations was high: R ST = 0.65 and θ = 0.84 (95 % CI 0.753–0.900).
The neighbour-joining phenogram (Fig. 3) constructed from dmu2 distances (Goldstein et al. 1995) grouped all Ecuadorian populations except ANTISANA in a well-supported group (65 % bootstrap value). COTACACHI, CAYAMBE and SINCHOLAGUA were also a group with high bootstrap value (65 %). Test of isolation by distance yielded a non-significant outcome (p value = 0.416).
Neighbour-joining dendrogram relating 10 Lupinus alopecuroides populations based on dmu2 distances (Goldstein et al. 1995). Bootstrap values across loci were based on 10,000 permutations by locus
Discussion
Genetic diversity and genetic differentiation
Our analyses revealed extremely low genetic diversity in populations of L. alopecuroides throughout its geographic range. From the total of ten known populations, four were completely monomorphic (all individuals within the population were identical multilocus genotypes) and another five showed only one polymorphic locus each (Online Resource 1). On the other hand, the high θ and R ST values show that the ten populations of L. alopecuroides are highly genetically differentiated. R ST incorporates molecular distances between alleles, and if R ST is substantially higher than θ, then this might indicate that mutations have contributed to differentiation, indicating a substantial phylogeographical signal (Slatkin 1995). In the present case, however, θ > R ST suggesting that drift is the predominant force underlying differentiation. Our results also show that populations of L. alopecuroides are not in Hardy–Weinberg equilibrium. We suggest that this disequilibrium is due to selfing and enhanced genetic drift rather than null alleles, as it seems unlikely that null alleles should occur at all loci and populations, whereas selfing would be expected to affect all loci. Finally, we suggest that low genetic diversity within populations and high genetic differentiation between populations are related to founder effects, lack of gene flow, and/or autogamy.
Founder effects—in tropical sky islands, populations are likely founded by individuals arriving from long distances, rather than from the warm tropical surroundings (Carson and Templeton 1984). Long-distance dispersal of L. alopecuroides seeds might be limited since rodents and human activity, important dispersers of Lupinus elsewhere (Milla and Iriondo 2011; Fagan et al. 2005, Maron and Kauffman 2006; Australian Department of Health and Ageing 2013), are nearly absent in the super-páramo (Sklenář and Ramsay 2001, Rangel 2006). Therefore, L. alopecuroides populations are likely founded by a small number of individuals. This produces severe bottlenecks that cause loss of genetic variation and increases genetic differentiation (Mayr 1954). Furthermore, Pleistocene climatic oscillation and intensive volcanic activity could also promote founder and bottleneck events by causing population expansions and contractions, and by affecting the populations’ extinction–recolonization rate (Carson and Templeton 1984; Carson et al. 1990; Beheregaray et al. 2003). Repeated founder events may have triggered genetic divergence among populations (Clegg et al. 2002), and loss of genetic variation through sequential bottlenecks, as has previously been invoked to explain low variation in other species (e.g. Hedrick 1996). Interestingly, COCUY is the only population that is not located on a volcanic mountain massif (Kroonenberg et al. 1990) and it showed the highest genetic diversity. However, further research is needed to test the influence of volcanic activity and Pleistocene climatic oscillations on within-population genetic variation.
Lack of gene flow—in isolated populations, genetic drift leads to fixation of random alleles, and hence to loss of genetic variation and high genetic differentiation due to the absence of gene flow (Lesica and Allendorf 1995; Frankham et al. 2002; Allendorf and Luikart 2006; Bech et al. 2009). The high θ value in our data indicates that populations of L. alopecuroides are fixed for different alleles, suggesting an absence of gene flow.
Autogamy—low within-population diversity and high differentiation among populations are often observed in selfing species (Hamrick and Godt 1996). In L. alopecuroides, high rates of selfing may be expected due to low availability of pollinators (Vásquez personal observation). Autogamous self-fertilization, which is common among islands plants (Carlquist 1974; Baker 1967), provides reproductive assurance when outcross pollination is limited by low availability of mates and/or pollinators (Karoly 1992). In the Venezuelan highlands, Berry and Calvo (1989) found near absence of pollinators and higher levels of selfing in four species of Espeletia (Asteraceae) growing above 4000 m a.s.l. Like lupines, Espeletia species are mainly pollinated by bumblebees. Significant decrease of diversity and activity levels of pollinators with elevation was also found in the Chilean Andes (Arroyo et al. 1985). Although, high inbreeding was indicated in the COCUY population and self-sterility is rare among lupines (East 1940; Kittelson and Maron 2000), further research is needed to demonstrate autogamy in L. alopecuroides and its role in determining the populations genetic structure.
Genetic relationships between populations
The neighbour-joining phenogram (Fig. 3) identified a well-supported group consisting of all Ecuadorian populations except ANTISANA. Within that group, CAYAMBE and SINCHOLAGUA, which were shown to be genetically indistinguishable (Online Resource 1), are the only populations located on the eastern Ecuadorian Cordillera. The population ANTISANA was shown to differ genetically from the rest of the populations. Interestingly, this population was also phenotypically different from the rest. In comparison with other populations, individuals have smaller leaves and inflorescence, and the overall size of the plants was also smaller (Vásquez, personal observation).
Finally, correlation between genetic and geographic distances of pair of populations was not significant, as was shown by the test for isolation by distance. This indicates that the genetic structure of L. alopecuroides populations is not the simple product of geographic spatial structure, pointing towards the importance of colonization history and founder effects in driving differentiation among populations. Although it has been heavily debated, the role of founder effects in evolution remains poorly understood (Clegg et al. 2002; Spurgin et al. 2014). It is unclear if founder effects can persist through evolutionary time when at the same time ongoing selection, mutation, gene flow and drift affect the genetic composition of populations (Hoeck et al. 2010; Spurgin et al. 2014). In this regard, it has been suggested that, depending on the severity and the continuality of the founder effects (Clegg et al. 2002), and on the extent of gene flow and selection, founder effects may be detectable in present populations, and may play an important role in the initial stages of speciation (Spurgin et al. 2014).
Possible effects of microsatellite cross-species amplification
Success of cross-species transfer of microsatellite markers depends on the evolutionary distance between the source and the target species (Rossetto 2001; Selkoe and Toonen 2006; Barbara et al. 2007). Lupinus alopecuroides, L. microcarpus, and L. nanus belong to a large western New world “super-radiation” (5.0–13.2 Ma) that comprises the western lupines from North America, Mexico and the Andes (Drummond et al. 2012). Low sequence divergence in adaptive radiations allows transfer of polymorphic microsatellite markers between species of the same subfamily and beyond (Barbara et al. 2007; Bezault et al. 2012). Otherwise, in plants, cross-species transfer of polymorphic markers is likely to be successful mainly within genera (Rossetto 2001; Barbara et al. 2007).
Cross-species transfer of microsatellites markers is often accompanied by a decrease in allelic diversity (Selkoe and Toonen 2006). However, if cross-species amplification underlies the low variation observed in the present study, then this should be a species-wide effect, whereas low variation within populations but higher variation in the species as a whole would suggest that low variation reflects demographic history. The latter was the case in our study; the total number of alleles for the species ranged from 2 to 6 across loci (Online Resource 1). One population (COCUY) in fact exhibited most of the observed alleles, whereas the other populations were mostly fixed for different alleles. We, therefore, conclude that the observed patterns of microsatellite DNA variation reflect genuine demographic processes and are not a result of reduced variation due to cross-species transfer.
Conclusions
Our study reveals extremely low genetic diversity within populations and high genetic differentiation between populations of L. alopecuroides across its range. We suggest that this pattern is related to founder effects, lack of gene flow, and possibly autogamy. However, further research is needed to provide evidence for autogamy in L. alopecuroides. The genetic relationships between the populations and the lack of correlation between genetic and geographic distances point to the importance of colonization history and founder effects in determining the populations’ genetic structure. Although based on a limited number of markers, our study gives insights into the evolution of the unexplored but fascinating sky island Andean flora. Moreover, L. alopecuroides provides an exceptional opportunity for understanding the role of founder effects in evolution. Future studies should focus on reconstructing the colonization history of L. alopecuroides, including the impact of volcanism and Pleistocene climatic oscillations on their population dynamics. Moreover, the low genetic variation and supposedly low adaptive potential in most populations suggest that the species could be particularly susceptible to anthropogenic disturbance. Formulation of a conservation strategy to protect L. alopecuroides is, therefore, strongly recommended.
References
Allendorf FW, Luikart G (2006) Conservation and the genetics of populations. Blackwell, London
Arroyo MTK, Armesto JJ, Primack RB (1985) Community studies in pollination ecology in the high temperate Andes of central Chile II. Effect of temperature on visitation rates and pollination possibilities. Plant Syst Evol 149:187–203
Australian Government. Department of Health and Ageing, Office of the Gene Technology Regulator (2013) The biology of Lupinus L. (lupin or lupine). http://ogtr.gov.au/internet/ogtr/publishing.nsf/Content/biologylupin2013-toc/$FILE/biologylupin20132.pdf. Accessed 5 March 2016
Baker HG (1967) Support for Baker’s law—as a rule. Evolution 21:853–856
Balslev H, Luteyn JL (1992) Páramo: an Andean ecosystem under human influence. Academic Press, London
Barbara T, Palma-Silva C, Paggi GM, Bered F, Fay MF, Lexer C (2007) Cross-species transfer of nuclear microsatellite markers: potential and limitations. Mol Ecol 16:3759–3767
Bech N, Boissier J, Drovetski S, Novoa C (2009) Population genetic structure of rock ptarmigan in the ‘sky islands’ of French Pyrenees: implications for conservation. Anim Conserv 12:138–146
Beheregaray LB, Ciofi C, Geist D, Gibbs JP, Caccone A, Powell JR (2003) Genes record a prehistoric volcano eruption in the Galápagos. Science 302:75
Berry PE, Calvo RN (1989) Wind pollination, self-incompatibility, and altitudinal shifts in pollination systems in the high Andean genus Espeletia (Asteraceae). Am J Bot 76:1602–1614
Bezault E, Rognon X, Gharbi K, Baroiller JF, Chevassus B (2012) Microsatellites cross-species amplification across some African cichlids. Int J Evolut Biol. doi:10.1155/2012/870935
Carlquist S (1974) Island biology. Columbia University Press, New York
Carson HL, Templeton AR (1984) Genetic revolutions in relation to speciation phenomena: the founding of new populations. Annu Rev Ecol Syst 15:97–131
Carson HL, Lockwood JP, Craddock EM (1990) Extinction and recolonization of local populations on a growing shield volcano. Proc Natl Acad Sci USA 87:7055–7057
Clegg SM, Degnan SM, Kikkawa J, Moritz C, Estoup A, Owens IP (2002) Genetic consequences of sequential founder events by an island-colonizing bird. Proc Natl Acad Sci USA 99:8127–8132
Cuatrecasas J (1968) Páramo vegetation and its life-forms. In: Troll C (ed) Geoecology of mountainous regions of the tropical America. Colloquium geographicum 9. Geographisches Institut, Bonn, pp 163–186
de Jong TJ, Waser NM, Klinkhamer PGL (1993) Geitonogamy: the neglected side of selfing. Trends Ecol Evol 8:321–325
Dillon ME, Frazier MR, Dudley R (2006) Into thin air: physiology and evolution of alpine insects. Integr Comp Biol 46:49–61
Drummond CS, Hamilton MB (2005) Isolation and characterization of nuclear microsatelliteloci in Lupinus group Microcarpi (Leguminosae). Mol Ecol Notes 5:510–513
Drummond CS, Eastwood RJ, Miotto ST, Hughes CE (2012) Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): testing for key innovation with incomplete taxon sampling. Syst Biol 61:443–460
East EM (1940) The distribution of self-sterility in the flowering plants. Proc Am Philos Soc 82:449–518
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Fagan WF, Lewis M, Neubert MG, Aumann C, Apple JL, Bishop JG (2005) When can herbivores slow or reverse the spread of an invading plant? A test case from Mount St. Helens. Am Nat 166:669–685
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Goldstein DB, Ruiz Linares A, Cavalli-Sforza LL, Feldman MW (1995) Genetic absolute dating based on microsatellites and the origin of modern humans. Proc Natl Acad Sci USA 92:6723–6727
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3)
Graham A (2009) The Andes: a geological overview from a biological perspective. Ann Missouri Bot Gard 96:371–385
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc B 351:1291–1298
Hedrick PW (1996) Bottleneck(s) or metapopulation in cheetahs. Conserv Biol 10:897–899
Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Hoeck PE, Bollmer JL, Parker PG, Keller LF (2010) Differentiation with drift: a spatio-temporal genetic analysis of Galápagos mockingbird populations (Mimus spp.). Philos Trans R Soc B 365:1127–1138
Hooghiemstra H, Wijninga VM, Cleef AM (2006) The paleobotanical record of Colombia: implications for biogeography and biodiversity. Ann Missouri Bot Gard 93:297–324
Hughes C, Eastwood R (2006) Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA 103:10334–10339
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27:3070–3071
Karoly K (1992) Pollinator limitation in the facultatively autogamousannual, Lupinus nanus (Leguminosae). Am J Bot 79:49–56
Kittelson PM, Maron JL (2000) Outcrossing rate and inbreeding depression in the perennial yellow bush lupine, Lupinus arboreus (Fabaceae). Am J Bot 87:652–660
Kroonenberg SB, Bakker JG, van der Wiel AM (1990) Late Cenozoic uplift and paleogeography of the Colombian Andes: constraints on the development of high-Andean biota. Geol Mijnbouw 6:279–290
Lesica P, Allendorf FW (1995) When peripheral populations are valuable for conservation. Conserv Biol 9:753–760
Madriñán S, Cortés AJ, Richardson JE (2013) Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front Genet 4:192
Manel S, Holderegger R (2013) Ten years of landscape genetics. Trends Ecol Evol 28:614–621
Maron JL, Kauffman MJ (2006) Habitat-specific impacts of multiple consumers on plant population dynamics. Ecology 87:113–124
Mayr E (1954) Change of genetic environment and evolution. In: Huxley J, Hardy AC, Ford EB (eds) Evolution as a process. Allen and Unwin, London, pp 157–180
Milla R, Iriondo AEM (2011) Congruence between geographic range distribution and local competitive ability of two Lupinus species. Am J Bot 98:1456–1464
Miller GA (1986) Pubescence, floral temperature and fecundity in species of Puya (Bromeliaceae) in the Ecuadorian Andes. Oecologia 70:1432–1939
Molecular Ecology Resources Primer Development Consortium, Abreu AG, Albaina A, Alpermann TJ, Apkenas VE et al (2012) Permanent genetic resources added to molecular ecology resources database 1 October 2011–30 November 2011. Mol Ecol Resour 12:374–376
Monasterio M (1986) Adaptive strategies of Espeletia in the Andean desert páramo. In: Vuilleumier F, Monasterio M (eds) High altitude tropical biogeography. Oxford University Press, New York, pp 49–80
Morueta-Holme N, Engemann K, Sandoval-Acuña P, Jonas JD, Segnitz RM, Svenning JC (2015) Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc Natl Acad Sci USA 112:12741–12745
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Oleas NH, Meerow AW, Francisco-Ortega J (2012) Population dynamics of the endangered plant, Phaedranassa tunguraguae, from the Tropical Andean Hotspot. J Hered 103:557–569
Pazy B (1984) Insect induced self-pollination. Plant Syst Evol 144:315–320
Prugnolle F, Manica A, Balloux F (2005) Geography predicts neutral genetic diversity of human populations. Curr Biol 15:R159–R160
Rangel O (2006) The biodiversity of the Colombian páramo and its relation to antropoghenic impact. In: Spehn EM et al (eds) Land use change and mountain biodiversity. CRC Press, Boca Raton, pp 108–109
Richards AJ (1986) Plant breeding systems. George Allen & Unwin, London
Rossetto M (2001) Sourcing of SSR markers from related plant species. In: Henry RJ (ed) Plant genotyping: the DNA fingerprinting of plants. CAB International, Oxford, pp 211–224
Rundel PW, Smith AP, Meinzer FC (1994) Tropical alpine environments: plant form and function. Cambridge University Press, Cambridge
Selkoe KA, Toonen RJ (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9:615–629
Sgorbati S, Labra M, Grugni E et al (2004) A survey of genetic diversity and reproductive biology of Puya raimondii (Bromeliaceae), the endangered queen of the Andes. Plant Biol 6:222–230
Sklenar P, Ramsay PM (2001) Diversity of zonal páramo plant communities in Ecuador. Divers Distrib 7:113–124
Sklenář P, Duškova E, Balslev H (2011) Tropical and temperate: evolutionary history of páramo flora. Bot Rev 2:71–108
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Smith JMB, Cleef AM (1988) Composition and origins of the world’s tropicalpine floras. J Biogeogr 15:631–645
Spurgin LG, Illera JC, Jorgensen TH, Dawson DA, Richardson DS (2014) Genetic and phenotypic divergence in an island bird: isolation by distance, by colonization or by adaptation? Mol Ecol 5:1028–1039
Takezaki N, Nei M, Tamura K (2014) POPTREEW : web version of POPTREE for constructing population trees from allele frequency data and computing other population statistics. Mol Biol Evol 31:1622–1624
van der Hammen T, Cleef AM (1986) Development of the high Andean páramo flora and vegetation. In: Vuilleumier F, Monasterio M (eds) High altitude tropical biogeography. Oxford University Press, Oxford, pp 153–201
van der Hammen T, Werner JH, van Dommelen H (1973) Palynological record of the upheaval of the Northern Andes: a study of the Pliocene and Lower Quaternary of the Colombian Eastern Cordillera and the early evolution of its high-Andean biota. Palaeogeogr Palaeocl 16:1–24
Vásquez DLA, Balslev H, Sklenář P (2015) Human impact on tropical-alpine plant diversity in the northern Andes. Biodivers Conserv 24:2673–2683
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Young TP, Augspurger CK (1991) Ecology and evolution of long-lived semelparous plants. Trends Ecol Evol 6:285
Acknowledgments
Thanks to Eva Dušková and Lenka Flašková for laboratory assistance, and to Jan Brotánek and Vojtěch Zeisek for help with fieldwork. We also thank Filip Kolář for helpful discussions and the Fond Mobility of Charles University in Prague for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
35_2016_165_MOESM1_ESM.docx
Additional supporting information in the online version of this article (see “Supplementary Material”) contains the following: ESM_1 – Allele frequencies
Rights and permissions
About this article
Cite this article
Vásquez, D.L.A., Balslev, H., Hansen, M.M. et al. Low genetic variation and high differentiation across sky island populations of Lupinus alopecuroides (Fabaceae) in the northern Andes. Alp Botany 126, 135–142 (2016). https://doi.org/10.1007/s00035-016-0165-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-016-0165-7