Abstract
Freshwater bivalves perform a range of ecological functions in both rivers and lakes. However, the effects of these functions in artificial ditches, one of the main habitats for bivalves, have not yet been examined. Among the burrowing bivalves, the shells of the Asian clam, genus Corbicula, decay slowly underwater and often dominate the bottom of natural rivers and artificial ditches. Here, a manipulative experiment was performed in a mesocosm ditch to determine the differences in the abundance and community composition of aquatic fauna in cages with and without Corbicula shells and to identify the relationship between shell size composition and fauna. Following the experiment, kick net sampling was performed to identify the fauna in the ditch. The results of the experiment indicated that macroinvertebrate species richness was higher in cages with a mix of small and large shells than in those without shells. The total number of macroinvertebrates was higher in cages with shells than in those without shells. However, both the species richness and total number of macroinvertebrates did not significantly differ among the treatments with different shell size compositions. Kick net sampling revealed macroinvertebrate fauna and the presence of a fish species in the mesocosm. These results suggested that Corbicula shells may be useful for the conservation of macroinvertebrates in artificial ditches with concrete-lined and simple structures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater-burrowing bivalves, including the genus Corbicula and order Unionoida, have important ecological functions in rivers and lakes (Sousa et al. 2009; Vaughn 2018; Vaughn and Hoellein 2018). The physical presence of living bivalves and their spent shells results in habitat complexity and heterogeneity (Vaughn and Hakenkamp 2001; Gutiérrez et al. 2003; Strayer et al. 2004; Bódis et al. 2014; Ilarri et al. 2018). Bivalve biofiltration can remove substantial quantities of particles from the water column and alter the water quality (Welker and Walz 1998; Vaughn et al. 2008; Pigneur et al. 2014; Choudhury et al. 2016). Bivalve excretion influences ecosystem processes by modifying nutrient cycling (Atkinson et al. 2013) as bivalves excrete a considerable amount of feces and pseudofeces (Strayer 2014; Vaughn 2018), and this deposition is partially responsible for the growths of benthic algae, macroinvertebrates, and lampreys (Howard and Cuffey 2006; Spooner and Vaughn 2006; Limm and Power 2011; Allen et al. 2012; Strayer 2014; Atkinson et al. 2021; Simeone et al. 2021). Bioturbation occurs when bivalves move and feed, altering the sediment water content, oxygen penetration, solute flux, and sediment nitrate, chloride, and calcium carbonate concentrations (Vaughn and Hakenkamp 2001).
Bivalves generally produce large amounts of shells, either individually or when aggregated in dense beds. These shells alter the benthic environments and are thus, important elements in a habitat structure, as they support the maintenance of high species richness, particularly in soft-bottomed environments (Gutiérrez et al. 2003; Sousa et al. 2009; Ilarri et al. 2019). Shells are substrata for the attachment of epibionts, thereby providing refuges from predation and disturbance and controlling the transport of resources in the benthic environment (Gutiérrez et al. 2003). They persist after the bivalve has died and retain their ecological function (Gutiérrez et al. 2003; Strayer and Malcom 2007; Ilarri et al. 2015b, 2019).
Bivalves inhabit and can often be found in abundance in artificial habitats, such as ditches, including canals and navigations, predominantly for agricultural use (Araujo and Ramos 2000; Gómez and Araujo 2008; Negishi et al. 2011; Sousa et al. 2019, 2021). However, their ecological functions in ditches have yet to be explored. Ditches frequently harbor uncommon biota and endangered species (Herzon and Helenius 2008; Chester and Robson 2013; Katayama et al. 2015). Diches often have high beta diversity of aquatic organisms, such as fish and invertebrates, which in turn contribute to gamma diversity at a landscape scale (Armitage et al. 2003; Williams et al. 2004; Verdonschot et al. 2011; Hill et al. 2016; Ishiyama et al. 2016). However, many traditional ditches with natural bottom and side structures are concrete-lined with simple structures, which are less suitable habitats for aquatic organisms (Katano et al. 2003; Gómez and Araujo 2008; Katayama et al. 2015; Sousa et al. 2021).
The Asian clam genus Corbicula originated in Asia, Australia, and Africa (Sousa et al. 2008). Corbicula has rapidly expanded its distribution worldwide (Crespo et al. 2015) and is considered an opportunistic species because of its short life cycle, rapid growth, early sexual maturity, and high fecundity (Sousa et al. 2008, 2014). The shell of Corbicula is thicker and harder than that of other freshwater bivalves, such as unionids. Therefore, it decays more slowly underwater (Strayer and Malcom 2007; Schmidlin et al. 2012; Ilarri et al. 2015b, 2019). Corbicula shells often dominate the shells that have accumulated in the beds of rivers and lakes, thereby providing a higher level of ecological function than other species (Strayer and Malcom 2007; Ilarri et al. 2019). Overall, Corbicula is widely distributed in artificial ditches (Sousa et al. 2021). Given the important role of shells in natural rivers and lakes, the presence of Corbicula shells may strengthen habitat complexity and heterogeneity in artificial ditches, thereby supporting aquatic fauna. However, to the best of my knowledge, this has not yet been demonstrated.
This study aimed to explore whether microhabitat complexity and heterogeneity created by Corbicula shells positively affect aquatic fauna in artificial ditches. An experiment was performed in a mesocosm ditch mimicking agricultural ditches to (i) determine the difference in aquatic fauna between microhabitat patches (cages) with and without shells and (ii) identify the relationship between shell size composition and fauna because the ecological effects may differ depending on shell size composition (Ilarri et al. 2015a, 2018).
Methods
Study species
Corbicula was selected because it is one of the most representative bivalve species in artificial ditches (Nakano and Morii 2019; Sousa et al. 2021). Corbicula fluminea (Müller 1774) is considered non-native to Japan and is currently distributed throughout the Japanese archipelago (Okawa et al. 2016). However, Corbicula fluminea and native C. leana Prime, 1864 cannot be distinguished based on their morphology and genetics. It is likely that C. leana is a population of C. fluminea (Komaru et al. 2012, 2013; Wang et al. 2014), and so all shells in the present study were allocated to the genus Corbicula.
Experiment
Intact shells (with both sides attached) of dead Corbicula were collected on April 25, 2021, from an artificial ditch (31°43′ N, 131°04′ E) in Miyakonojo City, Miyazaki Prefecture, southern Kyushu Island, Japan. The ditch had a live Corbicula density of 5350 individuals·m− 2 and an empty shell density of 3825 individuals·m− 2 on the sampling day. In the laboratory of Minami Kyushu University, Miyazaki Prefecture, shells were cleaned using brushes to remove any attached matter including the soft tissues of Corbicula. The samples were then dried at 50 °C for 63 h and weighed to the nearest 0.1 g. Their shell lengths were measured to the nearest 0.05 mm using a caliper.
The surface area of each shell was estimated in accordance with previous studies (Beckett et al. 1996; Werner and Rothhaupt 2007; Bódis et al. 2014). Thirty shells (shell length range 13.15–40.10 mm) collected from the ditch were wrapped and fitted with aluminum foil to estimate the shell surface area, with the outside and inside of each shell being fitted separately. The foil masses on both sides were measured to a precision of 0.0001 g using an electronic analytical balance (METTLER TOLEDO Inc., type: AG204). Subsequently, a foil mass-to-area regression curve was constructed (y = 2.1941 x − 23.782, R2 = 0.9817).
The manipulative experiment was performed in a mesocosm, which was an artificial ditch that was constructed in 2009 for study and education in an outside field at Minami Kyushu University (Fig. 1). The mesocosm was 30 m long, 0.8–4.0 m wide of water surface, and with up to 20 cm of water depth, and a flow velocity reaching up to 0.6 cm·s− 1. The base and sides of the mesocosm were lined with concrete, and sediment accumulated at the concrete base. The mesocosm was divided into five parts with four small waterfalls (8–30 cm in height) that mimicked the waterfalls in agricultural ditches (Fujimoto et al. 2008). The underground water supplied by a pump flowed continuously throughout the year. There were no bivalves inhabiting the ditch, and the only aquatic plant observed was the crystalwort Riccia. Insects, including dragonflies (order Odonata), and frog Glandirana rugosa (Temminck et Schlegel 1838) spawn annually in the mesocosm. Although there was no record of release history in the ditch before 2019, other aquatic organisms, such as gastropods, crustaceans, and fish, were believed to have been introduced into the mesocosm until 2019 after which they colonized (see results for detailed biota). Because the mesocosm was not connected to any other waterbodies, they could not invade on their own. Moreover, no organisms were released after 2020.
Photographs of the manipulative experiment. The mesocosm ditch considered for experimentation (a taken from upstream on September 30, 2022). The downstream section where the experiment was performed (b taken from downstream on September 30, 2022, c taken on May 23, 2021 during the experiment period). Stainless cage used in the experiment (d from the side, e from the top)
The experiment was performed between May 23 and June 20, 2021. This period was selected because aquatic animals, including dragonfly larvae and tadpoles, were abundant in previous years (Nakano, unpublished data). The water temperatures at the start and end of the experiment were 25.0 and 22.7 °C, respectively. A total of 16 stainless cages, each with 22.5 cm diameter, 11.2 cm height, 0.2 cm mesh size, and an open top were placed in the mud at the bottom of the downstream section(8.6 m long). The surface area of the mud in each cage was 0.0254 m2 and the cages were located approximately 50 cm apart. They were filled with mud to a depth of 5 cm and buried 5 cm into the mesocosm substrate so that the sediment in the cage was level with that of the mesocosm. This left the approximately upper 6 cm of the cage extending into the water column. This design allowed aquatic organisms to access the cages from the open tops but prevented the immigration/emigration of Corbicula shells to maintain constant shell densities over time.
The experimental design comprised four shell treatments, each with a constant shell weight across treatments, namely, large shells, small shells, and a mix of large and small shells, along with a shell-free control with only sediment, comprising four replications for each. Sixteen cages were used in the study, and each cage was assigned to one of the treatment groups. The shell lengths for the large and small shells were 28.3–40.4 and 13.2–20.6 mm, respectively. The number and surface area of shells per cage were determined using a constant dry weight for each cage (mean ± standard deviation:11.4 ± 0.8 g; 450.1 ± 32.8 g ∙ m− 2) (Table 1). The mean ± standard deviation of the area per cage were 193.8 ± 6.0 cm2 (7631.3 ± 235.8 cm2 ∙ m− 2) in the large, 253.5 ± 3.9 cm2 (9979.8 ± 153.8 cm2 ∙ m− 2) in the small, and 220.0 ± 14.1 cm2 (8662.5 ± 554.8 cm2 ∙ m− 2) in the mixed treatments. All cages were placed in the mesocosm ditch for 28 d (May 23–June 20). At the end of the experiment, the cage contents were collected and sieved using a 0.5 mm mesh sieve. The organisms were sorted, fixed in 99% ethanol, identified to the lowest practical taxonomic level using a binocular stereomicroscope, and counted in the laboratory.
To identify fauna in the mesocosm during the experimental period, samples were collected immediately after the end of the experiment on June 20. The aquatic organisms were collected by two people using kick nets with a 1 mm mesh size for 15 min in the sediment and water column in the downstream section of the mesocosm. Subsequently, the organisms were sorted, fixed in 99% ethanol, identified to the lowest practical taxonomic level using a binocular stereomicroscope, and counted in the laboratory.
Data analysis
To estimate the effect of shell treatments on aquatic fauna, generalized linear models (GLMs) were fitted to the data using Poisson distribution. A negative binomial distribution was not used because the R-squared values of the models using this distribution were lower than those of the models using Poisson distribution. Each model included one of the objective variables, namely species richness or the total number of individuals for all species, and the shell treatment as the categorical explanatory variable with four categories of large, small, mixed, and control treatments. The R-squared value for each GLM was calculated to determine whether shell presence was the main factor affecting the aquatic fauna in the cages. The number of individuals per species was not used as an objective variable because it was too low to indicate significant results, regardless of the species.
All analyses were performed using R software (version 4.0.0; R Core Team 2020). The glm function of the stats package was used to construct the GLMs, the r2 function of the performance package was used for calculating R2, and the confint function of the stats package was used to estimate the 95% confidence limits.
Results
A total of 337 individuals from 10 taxa were observed in the cages (Table 2). Seven macroinvertebrate taxa, namely the tadpole G. rugosa, isopod Asellus hilgendorfii Bovallius, 1886, chironomid Chironomidae larvae, crane fly Tipulidae larvae, dragonfly Sympetrum eroticum (Selys 1883) larvae, dragonfly Coenagrionidae larvae, and worm Oligochaeta, were only present in cages that contained Corbicula shells. Three taxa, namely the gastropod Semisulcospira libertina (Gould 1859), shrimp Neocaridina, and dragonfly Libellulidae larvae, were present in cages with and without Corbicula shells.
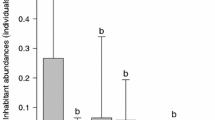
The model results indicated that the species richness and total number of macroinvertebrates differed significantly among the treatments. Specifically, the species richness was significantly higher in cages with mixed-sized shells than in those without shells (Table 3). The effects of homogenous large and small shell size treatments on species richness were not significant. The ranges of species richness partly overlapped among the large, small, and mixed shell size treatments (Fig. 2), and there was no clear difference in species richness among these treatments. The total number of macroinvertebrates was significantly higher in cages with shells than in those without shells. The effects of all the treatments with shells on the total number of macroinvertebrates were significant. The ranges of the total number of macroinvertebrates widely overlapped among these treatments; therefore, there was no clear difference in total number of macroinvertebrates among the treatments with shells.
Kick net sampling in the mesocosm resulted in the capture of 177 individuals from eight taxa (Table 2). One fish species, the Chinese minnow Rhynchocypris oxycephalus jouyi (Jordan et Snyder 1901), which was not present in any cage in the experiment, was captured using this method. The gastropod S. libertina was the most abundant species, accounting for 55% of the total (98 gastropods/177 all species), followed by the shrimp Neocaridina, which formed 26% of the total (46 shrimps/177 all species). These results along with the experimental results indicated that Chironomidae, Tipulidae, and Oligochaeta were present in cages with Corbicula shells. However, these organisms were not present in cages without Corbicula shells, and were not collected by kick net sampling in the mesocosm.
Discussion
Corbicula is frequently abundant in artificial ditches, and mainly agricultural ditches. To determine whether the microhabitat complexity and heterogeneity created by Corbicula shells affect the aquatic fauna in artificial ditches, a manipulative experiment was performed in a mesocosm ditch with a narrow width, shallow water depth, concrete-lined simple bottom, and side structures in the same way as modern agricultural ditches (Negishi et al. 2011; Katayama et al. 2015; Sousa et al. 2019). The aquatic fauna in agricultural ditches was well reproduced in the mesocosm because aquatic organisms, such as gastropods, tadpoles, shrimps, dragonfly larvae, chironomid larvae, and cyprinid minnows, inhabiting the mesocosm are widely distributed in agricultural ditches (Katano et al. 2003; Watson and Ormerod 2004; Herzon and Helenius 2008; Amano et al. 2010; Katayama et al. 2015). Therefore, the results of this study are potentially applicable to modern agricultural ditches and other artificial ditches under similar abiotic and biotic conditions.
The results of the manipulative experiment indicated that the species richness and total number of macroinvertebrates were significantly higher in cages with Corbicula shells than in those without the shells, with the exception of the species richness in the homogenous large and small shell size treatments. Chironomidae larvae, Tipulidae larvae, and Oligochaeta were only present in cages with Corbicula shells. The species richness and total macroinvertebrates did not clearly differ among the large, small, and mixed shell size treatments, indicating that the effects of size composition, surface area, and number of shells on macroinvertebrates were not evidently detected. Results from the kick net collection revealed macroinvertebrate fauna in the mesocosm as well as the presence of the Chinese minnow R. o. jouyi. To the best of my knowledge, no previous study has explored the ecological function of shells in artificial ditches. The results of the present study were then compared with those of previous studies performed in natural rivers and lakes. This comparison was required to clarify the differences in shell function between natural and artificial habitats.
The species richness and total number of macroinvertebrate individuals were mostly more abundant in cages with shells than in those without shells. This finding was consistent with the results of previous studies performed in natural habitats. Werner and Rothhaupt (2007) showed that the density of mayfly larvae increased in boxes with empty shells of C. fluminea compared to boxes containing live C. fluminea or only lake sand. Bódis et al. (2014) found that empty bivalve shells positively affected the presence of amphipods, caddis larvae, and isopods in a river, and highlighted that the effects of non-native the pond mussel Sinanodonta woodiana (Lea 1834) and C. fluminea shells on the habitat were particularly important for macroinvertebrate communities. The present study suggested that the presence of Corbicula shells positively affects the species richness and number of macroinvertebrates in both natural habitats and artificial ditches.
Although effects of shells on macroinvertebrates were detected, the main mechanisms were not determined in the present study. Shells generally provide resources and refuges to avoid physical and physiological disturbances and predation (Gutiérrez et al. 2003). Thus, the possible mechanisms responsible for the results of the present study may include providing refuges for macroinvertebrates to avoid predation because a potential predator is the omnivorous fish R. o. jouyi which inhabits the mesocosm. However, this remains speculative, and future studies are required to confirm this possibility and other potential mechanisms.
The species richness did not clearly differ among the treatments with different shell size compositions. This result was consistent with that of Ilarri et al. (2018), indicating that the species richness of macroinvertebrates in a river did not vary among shell treatments containing homogenous and heterogeneous bivalve species. In contrast, Ilarri et al. (2015a) found that macroinvertebrate species richness was higher with large unionid shells than with small C. fluminea shells or with both unionid and C. fluminea shells in a river. Therefore, the relationship between shell size composition and species richness is likely to differ among habitats with different biotic and abiotic conditions. Although Ilarri et al. (2015a) performed an interspecific comparison between C. fluminea and unionids, the present study performed an intraspecific comparison of Corbicula shells. This may be one of the reasons for the different results between the studies. Additional studies are needed to determine these, especially in artificial ditches, where studies on the ecological function of bivalves are scarce.
The total number of macroinvertebrates were more abundant in cages with shells than in those without shells. The dominant species was the gastropod S. libertina, which accounted for over 60% of all the species in each cage in all treatments. Corbicula shells are likely to provide an available surface for S. libertina attachment. Ilarri et al. (2018) suggested that the overall dominance of the faucet gastropod Bithynia tentaculata (Linnaeus 1758) was observed for all shell treatments. They highlighted that several physical and biological characteristics provided by the deposition of bivalve shells were positively related to the number of gastropods. This may also be applicable to libertina in artificial ditches.
Ilarri et al. (2018) showed that the substrate complexity created by bivalve shells affects the macroinvertebrate density in a river, with treatments containing heterogeneous shells from multiple species yielding significantly denser macroinvertebrate assemblages than homogeneous shells from the treatment with one species. However, the results of the present study differed from those of Ilarri et al. (2018). This is because there was no clear difference in macroinvertebrate density among the treatments with different shell size compositions. As was the case with the species richness results, this inconsistency between studies suggested that the ecological function of Corbicula shells may differ among habitats under different biotic and abiotic conditions and that the effect of the size composition of simple Corbicula species was weak (or nothing) compared with the effect of shell size composition of multiple species.
Artificial ditches suitable for aquatic organisms generally harbor uncommon biota and endangered species (Herzon and Helenius 2008; Chester and Robson 2013; Katayama et al. 2015). Diches often have high beta diversity of aquatic organisms, such as fish and invertebrates, which in turn contribute to gamma diversity at a landscape scale (Armitage et al. 2003; Williams et al. 2004; Verdonschot et al. 2011; Hill et al. 2016; Ishiyama et al. 2016). However, many traditional ditches with natural bottom and side structures have been reconstructed into modern ditches with concrete-lined and simple structures, which are less suitable habitats for aquatic organisms (Katano et al. 2003; Gómez and Araujo 2008; Katayama et al. 2015; Sousa et al. 2021). As suggested in the present study, Corbicula shells should be used for the conservation of macroinvertebrates in modern ditches. For example, cleaning and maintenance activities are undertaken in ditches without focusing on biodiversity, consequently, resulting in the removal of sediments and mussels from ditches (Sousa et al. 2019, 2021). When these activities are undertaken, the remaining Corbicula shells may be effective in conserving macroinvertebrates in ditches. However, Corbicula can rapidly expand its distribution (Crespo et al. 2015). The colonization of non-native Corbicula often reduces the population of native unionids, and causes socioeconomic damages (Yeager et al. 1999; Sousa et al. 2008, 2009; Ferreira-Rodríguez et al. 2018; Haag et al. 2020). Therefore, the introduction of live Corbicula in uncolonized areas should be avoided.
Because the experiment in the present study was performed in one mesocosm, it is important to explore the effect of shells on the aquatic fauna in artificial ditches under different biotic and abiotic conditions from the mesocosm ditch. Water depth, flow velocity, sediment, and vegetation often differ among ditches (Katano et al. 2003; Ohira et al. 2015; Nakano et al. 2017). Although the fauna and physical conditions of agricultural ditches were well reproduced in the mesocosm ditch, paddy drainage water, pesticides, and fertilizers generally inflow into agricultural diches (Katayama et al. 2015; Nakano 2017) and may affect shell function. However, this was not determined in the present study and should be explored in the future.
Conclusion
Corbicula shells are widely distributed in artificial ditches. Given the important role of shells in natural rivers and lakes, the presence of Corbicula shells may strengthen habitat complexity and heterogeneity in artificial ditches, thereby supporting aquatic fauna. However, this possibility has not been examined as yet. To determine whether the microhabitat complexity and heterogeneity created by Corbicula shells affect aquatic fauna in ditches, a manipulative experiment was performed in a mesocosm ditch with physical conditions and fauna similar to agricultural ditches. The results of the experiment indicated that the species richness and total number of macroinvertebrates were mostly higher in cages with Corbicula shells than in those without the shells. This study provided evidence for the first time that Corbicula shells can be used for the conservation of macroinvertebrates in ditches. For example, when cleaning and maintenance activities are undertaken in ditches, the remaining Corbicula shells could be effective in conserving macroinvertebrates. The ecological effect of Corbicula shells may be particularly important in modern ditches with concrete-lined and simple structures, which are unsuitable habitats for aquatic organisms.
Data availability
The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
Allen DC, Vaughn CC, Kelly JF, Cooper JT, Engel MH (2012) Bottom-up biodiversity effects increase resource subsidy flux between ecosystems. Ecology 93:2165–2174. https://doi.org/10.1890/11-1541.1
Amano K, Hamano T, Nakata K, Miwa J, Denda M (2010) Effects of habitat fragmentation on the amphidromous freshwater shrimp, Caridina leucosticta (Decapoda, Atyidae) in a rice paddy drainage channel. Crustaceana 83:1125–1133. https://doi.org/10.1163/001121610X521190
Araujo R, Ramos MA (2000) Status and conservation of the giant european freshwater pearl mussel (Margaritifera auricularia) (Spengler 1793) (Bivalvia: Unionoidea). Biol Conserv 96:233–239. https://doi.org/10.1016/S0006-3207(00)00075-6
Armitage PD, Szoszkiewicz K, Blackburn JH, Nesbitt I (2003) Ditch communities: a major contributor to floodplain biodiversity. Aquat Conserv Mar Freshwater Ecosyst 13:165–185. https://doi.org/10.1002/aqc.549
Atkinson CL, Vaughn CC, Forshay KJ, Cooper JT (2013) Aggregated filter-feeding consumers alter nutrient limitation: consequences for ecosystem and community dynamics. Ecology 94:1359–1369. https://doi.org/10.1890/12-1531.1
Atkinson CL, Halvorson HM, Kuehn KA, Winebarger M, Hamid A, Waters MN (2021) Filter-feeders have differential bottom-up impacts on green and brown food webs. Oecologia 195:187–198. https://doi.org/10.1007/s00442-020-04821-7
Beckett DC, Green BW, Thomas SA, Miller AC (1996) Epizoic invertebrate communities on upper Mississippi River unionid bivalves. Am Midl Nat 135:102–114. https://doi.org/10.2307/2426876
Bódis E, Tóth B, Szekeres J, Borza P, Sousa R (2014) Empty native and invasive bivalve shells as benthic habitat modifiers in a large river. Limnologica 49:1–9. https://doi.org/10.1016/j.limno.2014.07.002
Chester ET, Robson BJ (2013) Anthropogenic refuges for freshwater biodiversity: their ecological characteristics and management. Biol Conserv 166:64–75. https://doi.org/10.1016/j.biocon.2013.06.016
Chowdhury GW, Zieritz A, Aldridge DC (2016) Ecosystem engineering by mussels supports biodiversity and water clarity in a heavily polluted lake in Dhaka, Bangladesh. Freshw Sci 35:188–199. https://doi.org/10.1086/684169
Crespo D, Dolbeth M, Leston S, Sousa R, Pardal M (2015) Distribution of Corbicula fluminea (Müller 1774) in the invaded range: a geographic approach with notes on species traits variability. Biol Invasions 17:2087–2101. https://doi.org/10.1007/s10530-015-0862-y
Ferreira-Rodríguez N, Sousa R, Pardo I (2018) Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 810:85–95. https://doi.org/10.1007/s10750-016-3059-1
Fujimoto Y, Ouchi Y, Hakuba T, Chiba H, Iwata M (2008) Influence of modern irrigation, drainage system and water management on spawning migration of mud loach, Misgurnus anguillicaudatus C. Environ Biol Fishes 81:185–194. https://doi.org/10.1007/s10641-007-9188-7
Gómez I, Araujo R (2008) Channels and ditches as the last shelter for freshwater mussels: the case of Margaritifera auricularia and other naiads inhabiting the mid Ebro River Basin, Spain. Aquat Conserv Mar Freshwater Ecosyst 18:658–670. https://doi.org/10.1002/aqc.860
Gutiérrez JL, Jones CG, Strayer DL, Iribarne OO (2003) Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101:79–90. https://doi.org/10.1034/j.1600-0706.2003.12322.x
Haag WR, Culp J, Drayer AN, McGregor MA, White DE, Price SJ (2020) Abundance of an invasive bivalve, Corbicula fluminea, is negatively related to growth of freshwater mussels in the wild. Freshw Biol 66:447–457. https://doi.org/10.1111/fwb.13651
Herzon I, Helenius J (2008) Agricultural drainage ditches, their biological importance and functioning. Biol Conserv 141:1171–1183. https://doi.org/10.1016/j.biocon.2008.03.005
Hill MJ, Chadd RP, Morris N, Swaine JD, Wood PJ (2016) Aquatic macroinvertebrate biodiversity associated with artificial agricultural drainage ditches. Hydrobiologia 776:249–260. https://doi.org/10.1007/s10750-016-2757-z
Howard JK, Cuffey KM (2006) The functional role of native freshwater mussels in the fluvial benthic environment. Freshw Biol 51:460–474. https://doi.org/10.1111/j.1365-2427.2005.01507.x
Ilarri MI, Souza AT, Modesto V, Guilhermino L, Sousa R (2015a) Differences in the macrozoobenthic fauna colonising empty bivalve shells before and after invasion by Corbicula fluminea. Mar Freshw Res 66:549–558. https://doi.org/10.1071/MF14004
Ilarri MI, Souza AT, Sousa R (2015b) Contrasting decay rates of freshwater bivalves’ shells: aquatic versus terrestrial habitats. Limnologica 51:8–14. https://doi.org/10.1016/j.limno.2014.10.002
Ilarri MI, Amorim L, Souza AT, Sousa R (2018) Physical legacy of freshwater bivalves: Effects of habitat complexity on the taxonomical and functional diversity of invertebrates. Sci Total Environ 634:1398–1405. https://doi.org/10.1016/j.scitotenv.2018.04.070
Ilarri MI, Souza AT, Amorim L, Sousa R (2019) Decay and persistence of empty bivalve shells in a temperate riverine system. Sci Total Environ 683:185–192. https://doi.org/10.1016/j.scitotenv.2019.05.208
Ishiyama N, Sueyoshi M, Watanabe N, Nakamura F (2016) Biodiversity and rarity distributions of native freshwater fish in an agricultural landscape: the importance of β diversity between and within water-body types. Aquat Conserv Mar Freshwater Ecosyst 26:416–428. https://doi.org/10.1002/aqc.2583
Katano O, Hosoya K, Iguchi KI, Yamaguchi M, Aonuma Y, Kitano S (2003) Species diversity and abundance of freshwater fishes in irrigation ditches around rice fields. Environ Biol Fishes 66:107–121. https://doi.org/10.1023/A:1023678401886
Katayama N, Baba YG, Kusumoto Y, Tanaka K (2015) A review of post-war changes in rice farming and biodiversity in Japan. Agric Syst 132:73–84. https://doi.org/10.1016/j.agsy.2014.09.001
Komaru A, Houki S, Yamada M, Miyake T, Obata M, Kawamura K (2012) 28S rDNA haplotypes of males are distinct from those of androgenetic hermaphrodites in the clam Corbicula leana. Dev Genes Evol 222:181–187. https://doi.org/10.1007/s00427-012-0395-7
Komaru A, Yamada M, Houki S (2013) Relationship between two androgenetic clam species, Corbicula leana and Corbicula fluminea, inferred from mitochondrial cytochrome b and nuclear 28S rRNA markers. Zool Sci 30:360–365. https://doi.org/10.2108/zsj.30.360
Limm MP, Power ME (2011) Effect of the western pearlshell mussel Margaritifera falcata on Pacific lamprey Lampetra tridentata and ecosystem processes. Oikos 120:1076–1082. https://doi.org/10.1111/j.1600-0706.2010.18903.x
Nakano M (2017) The effect of paddy drainage water on the survival and growth of unionoid mussels. Agric Ecosyst Environ 247:189–194. https://doi.org/10.1016/j.agee.2017.06.035
Nakano M, Morii K (2019) Factors affecting the abundance of a clam (genus Corbicula) and distribution overlap between the clam and unionids in agricultural ditches. Jpn J Environ Entomol Zool 30:1–8. https://doi.org/10.11257/jjeez.30.1
Nakano M, Takakura K-I, Morii K, Urabe M (2017) Unionid mussel composition and ditch environments in floodplain and alluvial fan geomorphic types: a case study of a Lake Biwa river basin. Limnology 18:41–49. https://doi.org/10.2108/zs150001
Negishi JN, Katano I, Kayaba Y (2011) Seasonally tracking vertical and horizontal distribution of unionid mussels (Pronodularia japanensis): implications for agricultural drainage management. Aquat Conserv Mar Freshwater Ecosyst 21:49–56. https://doi.org/10.1002/aqc.1153
Ohira M, Tsunoda H, Nishida K, Mitsuo Y, Senga Y (2015) Niche processes and conservation implications of fish community assembly in a rice irrigation system. Aquat Conserv Mar Freshw Ecosyst 25:322–335. https://doi.org/10.1002/aqc.2558
Okawa T, Kurita Y, Kanno K, Koyama A, Onikura N (2016) Molecular analysis of the distributions of the invasive asian clam, Corbicula fluminea (OF Müller 1774), and threatened native clam, C. leana Prime, 1867, on Kyushu Island. Japan BioInvasions Rec 5:25–29. https://doi.org/10.3391/bir.2016.5.1.05
Pigneur LM, Falisse E, Roland K, Everbecq E, Deliège JF, Smitz JS, Doninck KV, Descy JP (2014) Impact of invasive asian clams, Corbicula spp., on a large river ecosystem. Freshw Biol 59:573–583. https://doi.org/10.1111/fwb.12286
R Core Team (2020) R: a language and environment for statistical computing, R Foundation for Statistical Computing. Vienna, Austria. https://www.r-project.org/. Accessed 10 June 2022
Schmidlin S, Schmera D, Baur B (2012) Alien molluscs affect the composition and diversity of native macroinvertebrates in a sandy flat of Lake Neuchâtel, Switzerland. Hydrobiologia 679:233–249. https://doi.org/10.1007/s10750-011-0889-8
Simeone D, Tagliaro CH, Beasley CR (2021) Filter and deposit: a potential role of freshwater mussels in ecosystem functioning associated with enhanced macroinvertebrate assemblage structure in a neotropical river. Hydrobiologia 848:4211–4223. https://doi.org/10.1007/s10750-021-04633-7
Sousa R, Antunes C, Guilhermino L (2008) Ecology of the invasive asian clam Corbicula fluminea (Müller 1774) in aquatic ecosystems: an overview. Ann Limnol Int J Limnol 44:85–94. https://doi.org/10.1051/limn:2008017
Sousa R, Gutiérrez JL, Aldridge DC (2009) Non-indigenous invasive bivalves as ecosystem engineers. Biol Invasions 11:2367–2385. https://doi.org/10.1007/s10530-009-9422-7
Sousa R, Novais A, Costa R, Strayer DL (2014) Invasive bivalves in fresh waters: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 735:233–251. https://doi.org/10.1007/s10750-012-1409-1
Sousa R, Teixeira A, Benaissa H, Varandas S, Ghamizi M, Lopes-Lima M (2019) Refuge in the sāqya: irrigation canals as habitat for one of the world’s 100 most threatened species. Biol Conserv 238:108209. https://doi.org/10.1016/j.biocon.2019.108209
Sousa R, Halabowski D, Labecka AM, Douda K, Aksenova O, Bespalaya Y, Bolotov I, Geist J, Jones HA, Konopleva E, Klunzinger MW, Lasso C, Lewin I, Liu X, Lopes-Lima M, Mageroy J, Mlambo M, Nakamura K, Nakano M, Osterling M, Pfeiffer J, Prié V, Paschoal LRP, Riccardi N, Santos R, Shumka S, Smith AK, Son M, Teixeira A, Thielen F, Torres S, Varandas S, Vikhrev IV, Wu X, Zieritz A, Nogueira JG (2021) The role of anthropogenic habitats in freshwater mussel conservation. Global Change Biol 27:2298–2314. https://doi.org/10.1111/gcb.15549
Spooner DE, Vaughn CC (2006) Context-dependent effects of freshwater mussels on stream benthic communities. Freshw Biol 51:1016–1024. https://doi.org/10.1111/j.1365-2427.2006.01547.x
Strayer DL (2014) Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 735:277–292. https://doi.org/10.1007/s10750-013-1461-5
Strayer DL, Malcom HM (2007) Shell decay rates of native and alien freshwater bivalves and implications for habitat engineering. Freshw Biol 52:1611–1617. https://doi.org/10.1111/j.1365-2427.2007.01792.x
Strayer DL, Downing JA, Haag WR, King TL, Layzer JB, Newton TJ, Nichols JS (2004) Changing perspectives on pearly mussels, North America’s most imperiled animals. Bioscience 54:429–439. https://doi.org/10.1641/0006-3568(2004)054[0429:CPOPMN]2.0.CO;2
Vaughn CC (2018) Ecosystem services provided by freshwater mussels. Hydrobiologia 810:15–27. https://doi.org/10.1007/s10750-017-3139-x
Vaughn CC, Hakenkamp CC (2001) The functional role of burrowing bivalves in freshwater ecosystems. Freshw Biol 46:1431–1446. https://doi.org/10.1046/j.1365-2427.2001.00771.x
Vaughn CC, Hoellein TJ (2018) Bivalve impacts in freshwater and marine ecosystems. Annu Rev Ecol Evol Syst 49:183–208. https://doi.org/10.1146/annurev-ecolsys-110617-062703
Vaughn CC, Nichols SJ, Spooner DE (2008) Community and foodweb ecology of freshwater mussels. J N Am Benthol Soc 27:409–423. https://doi.org/10.1899/07-058.1
Verdonschot RC, Keizer-vlek HE, Verdonschot PF (2011) Biodiversity value of agricultural drainage ditches: a comparative analysis of the aquatic invertebrate fauna of ditches and small lakes. Aquat Conserv Mar Freshw Ecosyst 21:715–727. https://doi.org/10.1002/aqc.1220
Wang GP, Zhang T, Zhang J, Li DL, Xiao TY (2014) Morphological and molecular differentiation of genus Corbicula suggests that two species are sympatrically distributed in Datong Lake in the Central Yangtze River Basin. Zool Stud 53:1–8. https://doi.org/10.1186/s40555-014-0064-9
Watson AM, Ormerod SJ (2004) The distribution of three uncommon freshwater gastropods in the drainage ditches of british grazing marshes. Biol Conserv 118:455–466. https://doi.org/10.1016/j.biocon.2003.09.021
Welker M, Walz N (1998) Can mussels control the plankton in rivers?—A planktological approach applying a lagrangian sampling strategy. Limnol Oceanogr 43:753–762. https://doi.org/10.4319/lo.1998.43.5.0753
Werner S, Rothhaupt KO (2007) Effects of the invasive bivalve Corbicula fluminea on settling juveniles and other benthic taxa. J N Am Benthol Soc 26:673–680. https://doi.org/10.1899/07-017R.1
Williams P, Whitfield M, Biggs J, Bray S, Fox G, Nicolet P, Sear D (2004) Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol Conserv 115:329–341. https://doi.org/10.1016/S0006-3207(03)00153-8
Yeager MM, Neves YR, Cherry DS (1999) Competitive interactions between early life stages of Villosa iris (Bivalvia: Unionidae) and adult Asian clams (Corbicula fluminea). Proceedings of the First Freshwater Mollusk Conservation Society Symposium, p 253–259
Acknowledgements
I thank the members of the Landscape Conservation Laboratory of Minami Kyushu University for their assistance with the experiments, and the two anonymous reviewers for their suggestions that helped improve my manuscript. This work was supported by the Japan Society for the Promotion of Science Scientific Research (grant number: 22K18054).
Funding
This work was partially supported by the Japan Society for the Promotion of Science Scientific Research (grant number: 22K18054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interests to declare that are relevant to the content of this article.
Ethical approval
All work conformed to the legal requirements of the country in which it was carried out.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakano, M. Effect of Asian clam shells on aquatic fauna in an artificial ditch. Aquat Sci 85, 18 (2023). https://doi.org/10.1007/s00027-022-00918-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00918-8