Abstract
Shelter availability is one of the key features governing crayfish habitat quality. It can directly influence crayfish’s individual survival of by lowering the risk of predation, but the ecosystem-wide impacts of sheltering on water quality are largely unknown. To test the effects of shelter availability for Procambarus clarkii in clear-water macrophyte-dominated lakes, we performed a 24-day mesocosm experiment in 20 tanks (4 with one crayfish with and without shelters, 4 with two crayfish with and without shelters and 4 controls). The bottom of each tank was almost completely covered by the eelgrass Vallisneria denseserrulata. Compared with the treatments with shelters, more broken leaves occurred in the treatments without shelters at both crayfish densities at equivalent crayfish numbers, and total phosphorus was higher in the treatments without shelters. Total suspended solids and total nitrogen concentrations were higher in the treatments with two crayfish without shelters than in those with shelters, whilst these variables did not differ between treatments in the mesocosms with one crayfish only. Our results suggest that shelter availability reduces the activity of crayfish (e.g. movement and burrowing) and agonistic behaviour, thereby decreasing the negative effect of the invasive P. clarkii on water quality in V. denseserrulata-dominated clear-water lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Red Swamp Crayfish Procambarus clarkii (Girard, 1852) is one of the most widespread invasive species in Chinese subtropical and tropical freshwater ecosystems (Zhan et al., 2016). According to Penn (1954), 100 specimens of Red Swamp Crayfish were carried from New Orleans to Japan in 1927, of which only 20 arrived alive and were introduced to a pond near Tokyo (Penn, 1954; Kawai & Kobayashi, 2005). Two years later, Red Swamp Crayfish were translocated from Japan to Nanjing, China (Dai, 1983) and rapidly spread to most provinces of China where they established dense populations (Wang, 1999). This non-native crayfish has contributed to a decline in submerged macrophyte biomass in aquatic ecosystems in Asia (Jiang et al., 2007; Zeng et al., 2013) and decreased the aquatic insect diversity of invaded wetlands (Watanabe & Ohba, 2022).
As ecosystem engineers, Red Swamp Crayfish impact the aquatic nutrient dynamics, community composition and ecosystem processes through bioturbation. Their feeding, movement and burrowing activities increase sediment resuspension (Angeler et al., 2001; Geiger et al., 2005; Matsuzaki et al., 2009). Red Swamp Crayfish alter the physical habitat of benthic sediments as they forage and/or build shelters (Ottolenghi et al., 2002; Statzner et al., 2000, 2003; Creed & Reed, 2004; Albertson & Daniels, 2016). They reduce the standing stock of macrophytes through direct consumption (Rodríguez et al., 2003; Alcorlo et al., 2004) or by increasing water turbidity through sediment resuspension (Haubrock et al., 2019). They can also diminish the macrophyte biomass through non-consumptive plant shredding (Nyström et al., 2001; Gao et al., 2021). The omnivorous Red Swamp Crayfish also feeds on animals such as amphibians, fish and other invertebrates (Gherardi et al., 2001; Gherardi, 2006). Accordingly, their invasion can lead to a severe increase in phytoplankton abundance, shifting lakes from a macrophyte-dominated clearwater to a turbid state (Rodríguez et al., 2003; Matsuzaki et al., 2009; van der Wal et al., 2013; Oficialdegui et al., 2020).
Agonistic behaviour, i.e. aggressive encounters with conspecifics, of Red Swamp Crayfish can also alter the physical features of benthic habitats (Moore, 2007). Red Swamp Crayfish compete for shelters as a critical resource as they offer protection from predators, conspecifics and environmental changes (Figler et al., 1999; Martin III & Moore, 2010). An increasing number of artificial shelters reduces intraspecific competition and cannibalism amongst Red Swamp Crayfish (Mason, 1979; Matsuzaki et al., 2009). Although the agonistic behaviour of crayfish has been studied extensively in laboratory settings and the field (Issa et al., 1999; Gherardi & Cioni, 2004; Moore, 2007), the impact of shelter presence on water quality is largely unknown.
We conducted a mesocosm experiment to elucidate the effects of the invasive Red Swamp Crayfish on a submerged macrophyte, the eelgrass Vallisneria denseserrulata (Makino) Makino, which is widespread in Asia, and the physical and chemical properties of lake water in the presence and absence of shelters. V. denseserrulata is distributed mainly in southern China (Chen et al., 2008; Wang et al., 2010). Vallisneria species also play important roles in the maintaining and stabilising of freshwater ecosystems, such as providing food for waterfowl, nursery habitats for fishes and substrates for invertebrates. They also contribute to self-purification and water quality (Li et al., 2005) and are therefore used frequently to restore freshwater ecosystems (Korschgen et al., 1997; Liu et al., 2018; Zhang et al., 2021). Nowadays, V. denseserrulata is frequently planted to recover Chinese eutrophic shallow lakes because (1) it is green all the year round and (2) it is not growing to the surface, which otherwise might create problems to use the lake for recreation purposes (Liu et al., 2018). We hypothesised that shelter availability would reduce the negative effects of crayfish on V. denseserrulata and the water quality in lakes with a clear-water macrophyte-dominated state. Examining the behaviour of Red Swamp Crayfish may increase our understanding of the environmental impacts of this invasive species, which may potentially be important to lake management.
Materials and methods
Experimental mesocosms
The mesocosm experiment was performed in 20 circular plastic tanks containing sediment and water (60 cm upper diameter, 50 cm bottom diameter, 70 cm height, 15 cm sediment depth, 50 cm water depth). The tanks were placed in a transparent organic glass-covered outdoor experimental house without walls. Ground sediment (0.61 mg g−1 total nitrogen [TN], 0.65 mg g−1 total phosphorus [TP]) was obtained from the Xunsi River, an outlet channel of a shallow lake in Wuhan City. The sediment was air-dried, and coarse debris was removed. Then, it was mixed using a 10.0 mm × 10.0 mm mesh sieve. The characteristics of this sediment were similar to most lakes with Red Swamp Crayfish invasions. An approximately 15-cm-thick layer of homogenised sediment was added to each tank, and the tanks were then filled with tap water (1.08 mg l−1 TN, 0.11 mg l−1 TP), exposed to natural sunlight and equilibrated for 2 days. Subsequently, 35 V. denseserrulata (height≈45 cm) were planted in each tank. After 5 weeks, V. denseserrulata covered most of the tank bottom and the experiments began. The experiments lasted from 7 October to 1 November 2019, and the tanks were exposed to natural sunlight for the entire experimental duration. At approximately 10 am every three days, the water temperature was measured using an YSI meter (YSI ProPlus, Yellow Springs, OH, USA). The water temperatures were 19.7 ± 0.2 °C (mean value ± SD), 19.9 ± 0.4 °C, 22.3 ± 0.2 °C, 20.2 ± 0.2 °C,19.4.0 ± 0.3 °C, 19.9 ± 0.3 °C, 19.8 ± 0.2 °C, 19.7 ± 0.2 °C and 19.9 ± 0.2 °C in the nine samplings.
To investigate the effects of shelter availability, four three-way plastic pipes (5 cm diameter, 9 cm length) were added as shelters. The number of plastic pipes was thus greater than the number of crayfish. One adult male Red Swamp Crayfish (≈5 ind. m−2) was randomly added to each of four mesocosms without (1-CF) and with plastic pipes (1-CF + S). Two adult male Red Swamp Crayfish (≈10 ind. m−2) were randomly added to each of another four tanks without (2-CF) and with plastic pipes (2-CF + S). The remaining four tanks held no crayfish and served as a control treatment (CK treatment). The Red Swamp Crayfish were directly collected by cage from a crayfish culture pond and then were maintained in 80 l tanks with V. denseserrulata for two weeks before being added to the tanks. We did not feed the crayfish during the experimental period. However, some naturally hatched invertebrates such as snails, zooplankton and dragonfly larvae were observed in the tanks during the experiment period.
Sampling and analysis
At approximately 10 am every three days, water samples were collected from 10 cm below the water surface in the middle of the tank for nutrient and chlorophyll a (Chl a) analyses. The samples were analysed according to Chinese standard methods (China EPA, 2009), which correspond to US standards (APHA, 1998). TN and TP concentrations were determined spectrophotometrically after digestion with persulphate, TP following the ammonium molybdate method and TN with the hydrochloric acid method. Chl a concentrations were determined spectrophotometrically after sample filtration through cellulose acetate filters and extraction of the filtered material with 90% acetone. Suspended material was filtered onto GF/C (pore size 1.2 μm) filters to measure total suspended solids (TSS) after drying at 105 °C for 24 h.
The number of broken leaves floating on the water surface of each mesocosm was recorded every three days. Broken leaves were defined as the opposite of intact leaves, i.e. leaves with an intact leaf tip or a leaf length ≥ 5 cm. At the end of the experiment, all V. denseserrulata were harvested by hand after emptying the tank. The biomass (wet weight), mean leaf length and number of V. denseserrulata individual plants per tank were recorded.
Statistical analyses
All statistical analyses were performed using SPSS 19.0 (Statistical Product and Service Solutions, USA), and the significance level was P < 0.05.
Time series data, including broken leaves, TSS, TN and TP, were analysed by repeated-measures ANOVA (RM-ANOVA) with time as the repeated factor. If a significant difference was observed, a Bonferroni post-hoc test was used to detect which treatments differed. All data sets were examined for homogeneity of variances using Levene's tests. One-way ANOVA was performed to detect differences amongst pairwise comparisons on each sampling occasion. If a significant difference was observed, the Bonferroni test was used to detect differing treatments. For one-way ANOVAs, all data sets were examined for normality. In some cases, the variance was not equally distributed, and Tamhane's T2 test was used to assess the differences amongst groups.
We used Pearson's correlation to test for relationships between the number of broken leaves of V. denseserrulata and water quality parameters. Results indicated that the radiance data were significantly correlated with water quality parameters if the significance level was P < 0.05.
Results
Effects of crayfish on V. denseserrulata
The effects of the various treatments on the number of broken leaves of V. denseserrulata differed (RM-ANOVAs, treatment effect, d.f. = 4, F = 280.0, P < 0.01) (Fig. 1). No broken leaves were observed in the controls during the experimental period. More broken leaves occurred in the 2-CF than in the 2-CF + S, 1-CF, 1-CF + S and treatments (Bonferroni's Multiple Comparison, P < 0.01; Fig. 1). The number of broken leaves diminished in the following order: 2-CF > 2-CF + S > 1-CF > 1-CF + S > controls (Bonferroni's Multiple Comparison, P < 0.01; Fig. 1).
Mean (± SD, n = 4) number of broken leaves of Vallisneria denseserrulata in the different treatments. 1-CF one crayfish, 2-CF two crayfish, 1-CF + S one crayfish with shelters, 2-CF + S two crayfish with shelters and CK control. Treatment labels sharing a lowercase letter indicate no significant differences between treatments at P > 0.05
Effects on TSS and phytoplankton biomass
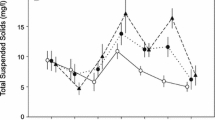
The effects of the various treatments on TSS concentrations (TSS) differed from each other (RM-ANOVAs, treatment effect, d.f. = 4, F = 432.1, P < 0.01; Fig. 2). Compared with the treatments, TSS were lowest in the controls during the experimental period (Bonferroni's Multiple Comparison, P < 0.01; Fig. 2). Higher TSS occurred in the 2-CF treatment than in the 2-CF + S, 1-CF, 1-CF + S treatments, as well as in the 2-CF + S treatment than in the 1-CF and 1-CF + S treatments (Bonferroni's Multiple Comparison, P < 0.01; Fig. 2). TSS did not differ amongst the 1-CF + S and the 1-CF treatments (Fig. 2).
Mean (± SD, n = 4) total suspended solid (TSS) concentrations in the different treatments. 1-CF one crayfish, 2-CF two crayfish, 1-CF + S one crayfish with shelters, 2-CF + S two crayfish with shelters and CK control. Treatment labels sharing a lowercase letter indicate no significant differences between treatments at P > 0.05
The effects of the various treatments on Chl a concentrations (Chl a) differed from each other (RM-ANOVAs, treatment effect, d.f. = 4, F = 61.4; Fig. 3). Higher Chl a concentrations occurred in the 2-CF than in the other treatments, and it was also higher in the 2-CF + S than in the 1-CF + S treatments (Bonferroni's Multiple Comparison, P < 0.01; Fig. 3). Chl a did not differ between the control and 1-CF + S treatments, or between 1-CF and 1or 2-CF + S treatments.
Mean (± SD, n = 4) chlorophyll a (Chl a) in the different treatments. 1-CF one crayfish, 2-CF two crayfish, 1-CF + S one crayfish with shelters, 2-CF + S two crayfish with shelters and CK control. Treatment labels sharing a lowercase letter indicate no significant differences between treatments at P > 0.05
Physical and chemical characteristics
The effects of the various treatments on TN concentrations (TN) differed from each other (RM-ANOVAs, treatment effect, d.f.TN = 4, FTN = 59.6, P < 0.01) (Fig. 4). The effects of the various treatments on TP concentrations (TP) also differed from each other (RM-ANOVAs, treatment effect, d.f.TP = 4, FTP = 208.2, P < 0.01) (Fig. 5). Compared with the crayfish treatments, TN and TP were lower in the controls during the experimental period (Bonferroni's Multiple Comparisons, P < 0.01; Figs. 4 and 5). Higher TN and TP occurred in 2-CF than in 1-CF and 2-CF + S (Bonferroni's Multiple Comparisons, P < 0.01; Figs. 4 and 5). TN did not differ between 1-CF + S and 2-CF + S or between 1-CF and 1-CF + S (Fig. 4). Higher TP occurred in 1-CF than in 1-CF + S as well as in 2-CF + S than in 1-CF + S (Bonferroni's Multiple Comparisons, P < 0.01; Fig. 5).
Mean (± SD, n = 4) water column total nitrogen (TN) in the different treatments. 1-CF one crayfish, 2-CF two crayfish, 1-CF + S one crayfish with shelters, 2-CF + S two crayfish with shelters and CK control. Treatment labels sharing a lowercase letter indicate no significant differences between treatments at P > 0.05
Mean (± SD, n = 4) water column total phosphorus (TP) in the different treatments. 1-CF one crayfish, 2-CF two crayfish, 1-CF + S one crayfish with shelters, 2-CF + S two crayfish with shelters and CK control. Treatment labels sharing a lowercase letter indicate no significant differences between treatments at P > 0.05
Relationships between number of broken leaves and environmental factors
Significant positive correlations were observed between environmental factors (TSS or Chl a or TN or TP, respective) and the number of broken leaves (Fig. 6).
Discussion
We found that Red Swamp Crayfish degraded water quality by increasing TSS, TN and TP concentrations. The number of broken plant leaves increased at higher crayfish densities, and water quality decreased further. Compared with treatments without shelters, fewer broken leaves occurred in crayfish treatments with shelters at equivalent crayfish numbers, and water quality increased. Our results indicated that shelter availability reduces the negative effects of the invasive Red Swamp Crayfish at equivalent crayfish numbers on clear-water lakes, likely by decreasing their activity (e.g. number of encounters, movement, burrowing, destruction of submerged macrophytes).
The introduction of Red Swamp Crayfish has been shown to decrease submerged macrophyte biomass (van der Wal et al., 2013; Souty-Grosset et al., 2016). Red Swamp Crayfish can induce a significant decline in macrophyte abundance through direct consumption of seedlings (Alcorlo et al., 2004; Cirujano et al., 2004), by diminishing macrophyte biomass through non-consumptive plant shredding (van der Wal et al., 2013; Gao et al., 2021) and by increasing water turbidity through sediment resuspension (Angeler et al., 2001; Rodríguez et al., 2003). In our mesocosm experiment, TN, TP, TSS and Chl a concentrations, as well as the numbers of broken leaves, were significantly higher after adding Red Swamp Crayfish. Previous research has also shown that water quality could decrease at high crayfish densities (van der Wal et al., 2013; Roessink et al., 2017). Macrophyte destruction under nutrient-rich conditions, particularly in eutrophic shallow lakes, may be followed by a shift from a clearwater to a turbid state dominated by planktonic microalgae, such as Microcystis (Rodríguez et al., 2003). In turn, this may further decrease the production of macrophytes and periphyton due to reduced light penetration (Gherardi, 2007). TSS, Chl a, TN and TP concentrations increased with the increase of number of broken leaves (Fig. 6), likely reflecting increased release and less uptake of nutrients with increasing number of broken leaves.
We found severe effects occurred at the high density of crayfish (two Red Swamp Crayfish per mesocosm ≈10 ind. m−2), but also major effects at half this density (one Red Swamp Crayfish per mesocosm ≈5 ind. m−2). The natural density range of Red Swamp Crayfish varies depending on geographic range and habitats (Matsuzaki et al., 2009). Lowery and Mendes (1977) showed that the population density of marketable-sized individuals of Red Swamp Crayfish was about 1–3 ind. m2 in tropical Lake Naivasha, Kenya. Harper et al. (2002) found Red Swamp Crayfish at densities of 6 to 77 ind. m−2 in littoral floating vegetation (often comprised mainly of floating water hyacinth) in Lake Naivasha in 1987–1988, and most specimens were juveniles. In the mesotrophic lake Lago della Doccia, Red Swamp Crayfish was first recorded in 2001 and had reached only a relatively low mean density of 0.2 ind. m−2 in summer 2003 (Gherardi & Acquistapace, 2007). The population density of crayfish has been suggested as a key factor impacting the magnitude of their ecosystem engineering (Chambers et al., 1990; Statzner & Peltret, 2006; van der Wal et al., 2011), including negative effects on submerged macrophyte abundance and water quality at higher densities (Chambers et al., 1990; van der Wal et al., 2011). Parkyn et al. (1997) showed that fine sediment removal from stream gravel substrates increased linearly with increasing densities of the New Zealand crayfish. However, most of the observed relationships between response variables and crayfish biomass were non-linear, indicating a saturation of the engineering effects at relatively low animal biomasses (Lodge & Lorman, 1987; Gherardi & Acquistapace, 2007; Matsuzaki et al., 2009).
The effect of crayfish may, however, depend on shelter availability. Rice et al. (2012) found that two similarly sized crayfish spent only slightly more time digging sediment than a single crayfish, and they suggested that crayfish spent time interacting, leading to little additional effect on bed topography or grain entrainment rates than when alone. Our results showed that crayfish behaviour changed markedly when shelters were available, creating weaker negative effects of crayfish on the water quality. Shelters may provide refugia that modify aggressive encounters amongst conspecifics (Hill & Lodge, 1994) and hyperactive behaviour (Statzner & Peltret, 2006). In our study, Red Swamp Crayfish were often found staying alone in the shelters, resulting in less time spent moving, burrowing and foraging (personal observation). Lower movement levels have previously been observed for other crayfish species due to sheltering behaviour when provided with shelters (Statzner et al., 2000). We found support for our hypothesis that shelter availability reduces crayfish-induced water quality changes and macrophyte destruction. Previous studies on the behaviour of other animals have revealed that individuals reared without shelter tended to be more active when placed in a new environment than those reared with shelter (Petrović et al., 2020).
Our results are relevant for invader management and freshwater restoration. Based on natural sheltering behaviour, our study showed that the presence of shelters could reduce crayfish activities (e.g. movement and burrowing) and agonistic behaviour, decreasing the adverse effects of Red Swamp Crayfish on affected aquatic ecosystems. The results indicate that shelter availability should be included as one of the crucial factors in lake management after invasion. For example, large woody debris additions and the restoration of the natural riparian vegetation of lakes could provide additional shelter for crayfish, thereby potentially diminishing their environmental impacts, but could also positively affect the overall crayfish densities due to less interference and loss by predation with a potentially negative effect on the environment on the long term. Therefore, it remains to be clarified how shelter availability affects the stress, behaviour, densities and environmental impacts of crayfish in natural freshwater ecosystems in a longer-term perspective.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
References
Albertson, L. K. & M. D. Daniels, 2016. Effects of invasive crayfish on fine sediment accumulation, gravel movement, and macroinvertebrate communities. Freshwater Science 35(2): 644–653.
Alcorlo, P., M. Otero & W. Geiger, 2004. Feeding preferences and food selection of the red swamp crayfish, Procambarus clarkii, in habitats differing in food item diversity. Crustaceana 77: 435–453.
Angeler, D. G., S. Sanchez-Carrillo, G. Garcia & M. Alvarez-Cobelas, 2001. The influence of Procambarus clarkii (Cambaridae, Decapoda) on water quality and sediment characteristics in a Spanish floodplain wetland. Hydrobiologia 464: 89–98.
APHA, 1998. Standard methods for the examination of water and wastewater, 20th edn. Americ Public Health Association, Washington DC.
Chambers, P. A., J. M. Hanson, J. M. Burke & E. E. Prepas, 1990. The impact of the crayfish Orconectes virilis on aquatic macrophytes. Freshwater Biology 24: 81–91.
Chen, L., Q. G. Ye, L. Z. Pan, L. M. Xu & W. Huang, 2008. Vallisneria species in lakes of the middle-lower reaches of the Yangtze River of China (in Chinese). Journal of Plant Ecology 32: 106–113.
China EPA, 2009. Water and wastewater monitoring and analysis methods, 4th ed. Chinese Environmental Science Press.
Cirujano, S., J. A. Camargo & C. Gómez-Cordovés, 2004. Feeding preference of the red swamp crayfish Procambarus clarkii (Girard) on living macrophytes in a Spanish wetland. Journal of Freshwater Ecology 19(2): 219–226.
Creed, R. P. & J. M. Reed, 2004. Ecosystem engineering by crayfish in a headwater stream community. Journal of the North American Benthological Society 23: 224–236.
Dai, A., 1983. Introduction of a kind of aquatic resource—Crayfish (in Chinese). Chinese Journal of Zoology 3: 51–53.
Figler, M. H., H. M. Cheverton & G. S. Blank, 1999. Shelter competition in juvenile red swamp crayfish (Procambarus clarkii): the influences of sex differences, relative size, and prior residence. Aquaculture 178: 63–75.
Gao, J., C. Yang, Z. Zhang, Z. Liu & E. Jeppesen, 2021. Effects of co-occurrence of invading Procambarus clarkii and Pomacea canaliculata on Vallisneria denseserrulata-dominated clear-water ecosystems: a mesocosm approach. Knowledge & Management of Aquatic Ecosystems 422: 29.
Geiger, W., P. Alcorlo, A. Baltanas & C. Montes, 2005. Impact of an introduced crustacean on the trophic webs of Mediterranean wetlands. Biological Invasions 7: 49–73.
Gherardi, F., 2006. Crayfish invading Europe: the case study of Procambarus clarkii. Marine and Freshwater Behaviour and Physiology 39: 175–191.
Gherardi, F., 2007. Understanding the impact of invasive crayfish. In Gherardi, F. (ed), Biological Invaders in Inland Waters: Profiles, Distribution, and Threats Springer, Dordrecht: 507–542.
Gherardi, F. & A. Cioni, 2004. Agonism and interference competition in freshwater decapods. Behaviour 141: 1297–1324.
Gherardi, F. & P. Acquistapace, 2007. Invasive crayfish in Europe: the impact of Procambarus clarkii on the littoral community of a Mediterranean lake. Freshwater Biology 52: 1249–1259.
Gherardi, F., B. Renai & C. Corti, 2001. Crayfish predation on tadpoles: a comparison between a native (Austropotamobius pallipes) and an alien species (Procambarus clarkii). Knowledge Management of Aquatic Ecosystems 361: 659–668.
Harper, D. M., A. C. Smart, S. Coley, S. Schmitz, A. G. de Beauregard, R. North, C. Adams, P. Obade & M. Kamau, 2002. Distribution and abundance of the Louisiana red swamp crayfish Procambarus clarkii Girard at Lake Naivasha, Kenya between 1987 and 1999. Hydrobiologia 488: 143–151.
Haubrock, P. J., A. F. Inghilesi, G. Mazza, M. Bendoni, L. Solari & E. Tricarico, 2019. Burrowing activity of Procambarus clarkii on levees: analysing behaviour and burrow structure. Wetlands Ecology and Management 27: 497–511.
Hill, A. M. & D. M. Lodge, 1994. Diel changes in resource demand: competition and predation in species replacement among crayfishes. Ecology 75(7): 2118–2126.
Issa, F. A., D. J. Adamson & D. H. Edwards, 1999. Dominance hierarchy formation in juvenile crayfish Procambarus clarkii. Journal of Experimental Biology 202(24): 3497–3506.
Jiang, S., L. Pang & C. Huang, 2007. Hazards and control of exotic species, Procambarus clarkii (in Chinese). Bulletin of Biology 42(5): 15–16.
Kawai, T. & Y. Kobayashi, 2005. Origin and current distribution of the alien crayfish Procambarus clarkii (Girard, 1852) in Japan. Crustaceana 78(9): 1143–1149.
Korschgen, C. E., W. L. Green & K. P. Kenow, 1997. Effects of irradiance on growth and winter bud production by Vallisneria americana and consequences to its abundance and distribution. Aquatic Botany 58: 1–9.
Li, Z. Q., Y. Dan & M. H. Tu, 2005. Seed germination of three species of Vallisneria (Hydrocharitaceae), and the effects of freshwater microalgae. Hydrobiologia 544: 11–18.
Liu, Z. W., J. R. Hu, P. Zhong, X. F. Zhang, J. J. Ning, S. E. Larsen, D. Y. Chen, Y. M. Gao, H. Hu & E. Jeppesen, 2018. Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. Water Research 146: 88–97.
Lodge, D. M. & J. G. Lorman, 1987. Reductions in submersed macrophyte biomass and species richness by the crayfish Orconectes rusticus. Canadian Journal of Fisheries and Aquatic Sciences 44: 591–597.
Lowery, R. S. & A. J. Mendes, 1977. Procambarus clarkii in Lake Naivasha, Kenya, and its effects on established and potential fisheries. Aquaculture 11(2): 111–121.
Martin, A. L., III. & P. A. Moore, 2010. Field observations of agonism in the crayfish, Orconectes rusticus: Shelter Use in a Natural Environment. Ethology 113(12): 1192–1201.
Mason, J. C., 1979. Effects of temperature, photoperiod, substrate and shelter on survival, growth and biomass accumulation of juvenile Pacifastacus leniusculus culture. Freshwater Crayfish 4: 73–82.
Matsuzaki, S. S., N. Usio, N. Takamura & I. Washitani, 2009. Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia 158(4): 673–686.
Moore, P. A., 2007. Agonistic behavior in freshwater crayfish: the influence of intrinsic and extrinsic factors on aggressive behavior and dominance. In Duffy, J. E. & M. Thiel (eds), Evolutionary Ecology of Social and Sexual Systems: Crustacea as Model Organisms Oxford University Press, Oxford: 90–114.
Nyström, P., O. Svensson, B. Lardner, C. Brönmark & W. Granéli, 2001. The influence of multiple introduced predators on a littoral pond community. Ecology 82: 1023–1039.
Oficialdegui, F. J., M. I. Sánchez & M. Clavero, 2020. One century away from home: how the red swamp crayfish took over the world. Reviews in Fish Biology and Fisheries 30: 121–135.
Ottolenghi, F., J. G. Qin & L. Mittiga, 2002. Enhancement of phosphorus release from lake sediments by aeration and crayfish activity. Journal of Freshwater Ecology 17(4): 635–640.
Parkyn, S. M., C. F. Rabeni & K. J. Collier, 1997. Effects of crayfish (Paranephrops planifrons parastacidae) on in-stream processes and benthic faunas: a density manipulation experiment. New Zealand Journal of Marine Freshwater Research 31: 685–692.
Penn, G. H., Jr., 1954. Introduction of American crawfishes into foreign lands. Ecology 35(2): 296.
Petrović, T. G., T. Z. Vŭcić, S. Z. Nikolić, J. P. Gavrić, S. G. Despotović, B. R. Gavrilović, T. B. Radovanović, C. Faggio & M. D. Prokić, 2020. The effect of shelter on oxidative stress and aggressive behavior in crested newt larvae (Triturus spp.). Animals 10(4): 603.
Rice, S. P., M. F. Johnson & I. Reid, 2012. Animals and the geomorphology of gravel-bed rivers. In Church, M., P. Biron & A. G. Roy (eds), Gravel-Bed Rivers: Processes, Tools, Environments Wiley, Chichester: 225–241.
Rodríguez, C. F., E. Bécares & M. Fernández-Alὰez, 2003. Shift from clear to turbid phase in Lake Chozas (NW Spain) due to the introduction of American red swamp crayfish (Procambarus clarkii). Hydrobiologia 506: 421–426.
Roessink, I., R. Gylstra, P. Heuts & B. Specken, 2017. Impact of invasive crayfish on water quality and aquatic macrophytes in the Netherlands. Aquatic Invasions 12(3): 397–404.
Souty-Grosset, C., P. M. Anastacio, L. Aquiloni, F. Banha, J. Choquer, C. Chucholl & E. Tricarico, 2016. The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica 58: 78–93.
Statzner, B. & O. Peltret, 2006. Assessing potential abiotic and biotic complications of crayfish-induced gravel transport in experimental streams. Geomorphology 74: 245–256.
Statzner, B., E. Fièvet, J.-Y. Champagne, R. Morel & E. Herouin, 2000. Crayfish as geomorphic agents and ecosystem engineers: biological behavior affects sand and gravel erosion in experimental streams. Limnology and Oceanography 45(5): 1030–1040.
Statzner, B., O. Peltret & S. Tomanova, 2003. Crayfish as geomorphic agents and ecosystem engineers: effect of a biomass gradient on baseflow and flood-induced transport of gravel and sand in experimental streams. Freshwater Biology 48: 147–163.
Van der Wal, J. E. M., 2011. Effects of crayfish on the establishment of macrophytes in a shallow peat lake. Wageningen UR.
Van der Wal, J. E. M., M. Dorenbosch, A. K. Immers, C. V. Forteza, J. J. M. Geurts, E. T. H. M. Peeters, B. Koese & E. S. Bakker, 2013. Invasive crayfish threaten the development of submerged macrophytes in lake restoration. PLoS ONE 8: e78579.
Wang, W. M., 1999. The exploitation and utilization of red swamp crayfish in China (in Chinese). Acta Hydrobiologica Sinica 23(4): 375–381.
Wang, Q. F., Y. H. Guo, R. R. Haynes & C. B. Hellquist, 2010. Hydrocharitaceae. In Wu, Z. Y. & P. Raven (eds), Flora of China, Vol. 23. Science Press, Beijing: 91–102.
Watanabe, R. & S. Ohba, 2022. Comparison of the community composition of aquatic insects between wetlands with and without the presence of Procambarus clarkii: a case study from Japanese wetlands. Biological Invasions 24(4): 1033–1047.
Zeng, Z., H. Wu, Y. Jiang & G. Peng, 2013. Dynamics and impacts of invasion by exotic species to Poyang Lake national nature reserve (in Chinese). Energy Research and Management 4: 15–18.
Zhan, A., P. Ni, W. Xiong, Y. Chen, Y. Lin & X. Huang, 2016. Biological invasions in aquatic ecosystems in China. Chapter 4. In Wan, F. et al. (eds) Biological Invasions and its Management in China, Invading Nature Springer Series in Invasion Ecology, Vol. 11: 67–96.
Zhang, X. M., W. Zhen, H. S. Jensen, K. Reitzel, E. Jeppesen & Z. W. Liu, 2021. The combined effects of macrophytes (Vallisneria denseserrulata) and a lanthanum-modified bentonite on water quality of shallow eutrophic lakes: A mesocosm study. Environmental Pollution 277: 116720.
Acknowledgements
The authors thank Anne Mette Poulsen and EditSprings for language assistance. This study was supported by the Natural Science Foundation of Hubei Province (2020CFB537), the National Natural Science Foundation of China (Grant No. 32170383), key scientific research project funding from the Hubei Provincial Water Resources Bureau (HBSLKY202014) and preliminary hydraulic research and consulting work of Hubei Water Resources Research Institute (P21800600002). EJ was supported by AQUACOSMplus (Network of Leading European AQUAtic MesoCOSM Facilities Connecting Mountains to Oceans from the Arctic to the Mediterranean), AnaEE Denmark (anaee.dk) and the TÜBITAK program BIDEB 2232 (project 118C250).
Funding
Jian Gao was supported by the Natural Science Foundation of Hubei Province (2020CFB537), the National Natural Science Foundation of China (Grant No. 32170383), key scientific research project funding from the Hubei Provincial Water Resources Bureau (HBSLKY202014) and preliminary hydraulic research and consulting work of Hubei Water Resources Research Institute (P21800600002). Erik Jeppesen was supported by AQUACOSMplus (Network of Leading European AQUAtic MesoCOSM Facilities Connecting Mountains to Oceans from the Arctic to the Mediterranean), AnaEE Denmark (anaee.dk) and the TÜBITAK program BIDEB 2232 (project 118C250).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling Editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Hu, S., Yang, C. et al. Shelter availability reduces the effects of the invasive Red Swamp Crayfish (Procambarus clarkii) on eelgrass-dominated clear-water lakes: a mesocosm approach. Hydrobiologia 849, 3597–3606 (2022). https://doi.org/10.1007/s10750-022-04969-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04969-8