Abstract
Sex-specific nutritional requirements, mating behavior, and parental care can potentially affect food selection and consumption in animals. However, relatively little is known about how sex and reproduction influence food use by aquatic herbivores. We measure male:female ratios in the field and studied sexual differences in algal food choice and feeding rates for the amphipod Gammarus aequicauda from a desert saline lake, where it is the most abundant mesograzer during the colder months. We also assessed the effects of precopulatory pairing and female egg development on feeding behavior. Males were more numerous than females over two sampling periods and significantly preferred one algal species in each of three pairwise food-choice combinations tested. Females were moderately less selective, expressing a preference in only two of these experiments, but showed significantly higher total consumption and feeding rates than males in most assays. When males and females expressed similar preferences, the magnitude of these preferences (% differences in consumption between the algae offered) was statistically equivalent for both sexes. Patterns of compensatory feeding on less nutritious algae were significant and similar for both sexes. Mated pairs had reduced feeding rates compared to unpaired females, but not unpaired males. In contrast, no short-term differences in feeding rates were detected for males and females separated from precopula or between females at two stages of egg development. Variations in population sex ratios and reproductive cycles can potentially modulate interactions between aquatic consumers and their food sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measuring intraspecific variation in feeding behavior is important for understanding resource utilization in nature and the evolution of diets. For herbivores, much of what is known about intraspecific variation in food selection has focused on insect specialization on food plants at local, regional, and latitudinal scales (Fox and Morrow 1981; Jaenike 1990; Drès and Mallet 2002). In marine systems, differences in feeding behavior have also been measured for populations separated geographically (Stachowicz and Hay 2000; Sotka and Hay 2002; Sanford et al. 2003; Sotka 2005; McCarty and Sotka 2013). The role of sexual differences in herbivore feeding has received less attention (Slansky 1993; Du Toit 2006; Ballhorn et al. 2013), although studies of sexual differences in behavior abound for other trophic groups (Shine 1989; Wearmouth and Sims 2008; Maklakov et al. 2008). Despite hundreds of studies on foraging, food choice, and animal nutrition, surprisingly little is known about these mechanisms of intraspecific variation in marine or freshwater consumers (Jormalainen et al. 2001; Wearmouth and Sims 2008; Cruz-Rivera et al. 2017).
Males and females generally allocate internal resources differently into reproductive processes, which can result in distinct nutrient profiles and turnovers for each sex (Morehouse et al. 2010). For example, females may have higher requirements for lipids and protein than males because of larger investments of these molecules into gonad maturation and in packing embryos with adequate nutrients for egg development (Izquierdo et al. 2001; Wouters et al. 2001; Williams 2005). This can result in increased consumption of certain foods by females as preparation for the reproductive season (Kyomo 1992; Barboza and Bowyer 2001; Lewis and Kappeler 2005) or right after mating (Tsukamoto et al. 2014). For species that engage in parental care, tending the young can result in higher energetic demands concomitantly reflected in altered feeding patterns for the sex engaged in rearing the offspring (Trivers 1972; Gautier-Hion 1980; Shine 1989), and even if both sexes contribute, this contribution may be asymmetric, thus affecting food use (Weimerskirch et al. 1997). These sex-specific nutritional demands can potentially modulate foraging strategy, territory use, food selection, consumption rates, and nutrient absorption efficiencies.
Maximizing reproductive success can also influence food utilization for species depending on secondary sexual traits affected by nutrition to attract females or competitively mate (Molleman et al. 2005; Morehouse et al. 2010; Walker et al. 2014; Bravo et al. 2014; Mitra et al. 2016). In fact, differential allocation of nutrients to secondary sexual structures has been shown at stoichiometric levels for sexually dimorphic species (Goos et al. 2016, 2017). Resulting differences in consumption between males and females could be dynamic and transient (e. g., only during mating season). For example, mate guarding may alter feeding behavior, leading to trade-offs between feeding and mating (Westneat 1994; Alberts et al. 1996; Saeki et al. 2005). For females, reproduction may carry additional costs that may impose further, sometimes progressive, demands for resources. Females carrying eggs (or young) may experience higher associated costs of movement. Gravid females move more slowly in copepods (Winfield and Townsend 1983; Svensson 1992; Mahjoub et al. 2011), amphipods (Lewis and Loch-Mally 2010), insects (Ercit et al. 2014), snakes (Seigel et al. 1987), and birds (Kullberg et al. 2005; Guillemette and Ouellet 2005). These studies have often detected higher predation rates on these reproductive females than on males or non-ovigerous conspecifics, however, almost nothing is known about how these indirect costs of brooding affect food selection and acquisition.
Amphipods in the family Gammaridae provide excellent models to study sexual differences in feeding as related to maintenance and reproductive processes. Most species are sexually dimorphic with larger males that engage in mate guarding. During this precopula, a male will grab a female with its gnathopods and swim until the female molts and is inseminated (Conlan 1991; Sutcliffe 1992; Jormalainen 1998). Males do not engage in parental care, but fertilized eggs are carried by the females in a ventral brood pouch until the embryos develop directly into juveniles. During this period, females exhibit various grooming and oxygenating behaviors to enhance hatching success (Dick et al. 1998; Tarutis et al. 2005). These differences suggest that females may need to adjust their feeding during the reproductive stage to cope with energetic demands of producing and maintaining offspring. Precopulatory behavior also has the potential to affect feeding by either reducing or stopping consumption during the period of mating. Thus, intraspecific variation in feeding behavior could arise in Gammarus as a function of both sex and sexual activity.
In this research, we focus on the feeding behavior of the amphipod Gammarus aequicauda (Martynov, 1931), a broadly distributed species along the Mediterranean and Black Sea. We studied this species from an Egyptian lake in which it is primarily herbivorous and its distribution follows closely that of the filamentous green algae it consumes (Cruz-Rivera et al. 2017), rather than patterns of zooplankton or other prey (Shadrin et al. 2016), although we acknowledge that this amphipod can be primarily omnivorous and even predatory in other ecosystems (Shadrin et al. 2020, 2021a, b). We address how sexual differences in requirements and reproductive behavior could affect patterns of herbivory in this species by asking: (1) Are there differences in the proportion of G. aequicauda males and females in the field? (2) Do male and female G. aequicauda express differences in food choice and feeding rates on naturally occurring algal foods? (3) If any, do these differences relate to basic nutritional traits of algae? (4) Is feeding behavior affected by precopula, and if so, how? and (5) Does reproductive state of ovigerous females affect feeding for this amphipod?
Materials and methods
Study site and amphipod sex ratios
Amphipods and algae used in this study were collected from Lake Qarun, in the Fayum Depression of Egypt (29°29′02.4"N 30°39′16.2"E) from February to April 2013. Gammarus aequicauda was first reported from this lake in the 1970s (El-Shabrawy and Dumont 2009; Shadrin et al. 2016), but populations of this amphipod may have occurred since the 1950s (Naguib 1961). The organisms were likely introduced during stocking activities that started populating the lake with Mediterranean fish and shrimp in the late 1920s to support local fisheries, as the lake became salinized losing most of its original freshwater biota (Holdich and Tolba 1985; El-Shabrawy and Dumont 2009; Cruz-Rivera and Malaquias 2016).
To quantify the densities and sex ratio of amphipods in algae, surveys were conducted in February and March. Mats of filamentous green algae, predominantly Ulva spp. (N = 10), were collected by placing re-sealable bags over algal thalli and dislodging them quickly from the substrate. In the lab, algae and amphipods were separated carefully and algae were centrifuged in a salad spinner to eliminate excess water before weighing. Gammarus aequicauda individuals were fixed in 10% formaldehyde and sorted to males or females under a dissection microscope. Amphipod densities were standardized by algal mass and differences between sexes and months were analyzed by two-way ANOVA after using squared root transformations to normalize data distributions and homogenize variances. We also compared sex ratios between these two months (unpaired t test).
Algal organic content
The organic content of the algae, which can influence herbivore feeding behavior, was quantified on individual algal thalli (N = 14). These samples included subsamples from the algae used during our feeding assays. Ash-free dry mass (AFDM), a measure of digestible organic matter, was obtained by drying algae at 65 °C for five days and burning the dry algae overnight at 450 °C. The remaining ash mass was subtracted from the dry mass and this number was expressed as a percentage of the original sample wet mass. Previous studies have shown that this approximation of algal nutrient content is highly predictive of feeding rates in amphipods (Cruz-Rivera and Hay 2001; Cruz-Rivera and Friedlander 2013) and other mesograzers (Stachowicz and Hay 1999).
Feeding experiments
Feeding experiments were conducted to assess differences in food choice and feeding rates as a function of sex and reproductive behavior. Amphipods and algae (two Ulva spp. and Cladophora glomerata) were collected from the southern margin of Lake Qarun and carried in large plastic containers to the American University in Cairo, where experiments were conducted. Lake water for experiments was collected in large carboys. Silt and suspended particulates were allowed to settle by gravity prior to filling up experimental replicates, but the water was unaltered otherwise. Amphipods were never starved before assays and were kept in aerated tanks with a mixture of all three algae tested and accompanying detritus as collected from the field. Salinity of collected water was ≈ 36 psu and experiments were kept at 23 °C.
Choice and no-choice feeding experiments were conducted based on widely used methods for studying small aquatic consumers (Cruz-Rivera et al. 2017). Pieces of algal thalli were spun in a salad spinner to remove excess water, weighed, and placed in replicate containers with Lake Qarun water. Amphipods were placed in some of the containers and allowed to feed for a limited time. A similar number of containers without consumers were interspersed to serve as controls for autogenic changes in algal mass. Upon terminating an assay, algal pieces were removed, spun, and weighed, and the eaten mass was calculated after correcting for autogenic changes using the controls (Cruz-Rivera and Malaquias 2016; Cruz-Rivera et al. 2017). We tested for allometric scaling of feeding rates using Pearson correlations (log algal consumption vs. log consumer mass). If allometric scaling of consumption could not be shown, we weighed results from feeding experiments as algae consumed per amphipod mass because standardizing consumption by replicate or number of consumers could obscure differences in feeding between sexes (Cruz-Rivera et al. 2017). Amphipod mass was obtained by gently padding animals with absorbent paper and weighing them to the closest mg immediately after each experiment was terminated.
Pairwise choice experiments were run in 900 ml replicate containers by simultaneously offering the amphipods pieces of two of the three dominant green filamentous macroalgae in the lake. All three possible pairwise food combinations (Ulva flexuosa vs. Cladophora glomerata, U. prolifera vs. C. glomerata, and U. flexuosa vs. U. prolifera), were assessed (N = 10–11). Because our interest was to detect potential differences in food choice between the sexes, groups of 2–4 males or females (depending on size) were placed in single-sex groups within replicates. The same number of replicates was simultaneously used for males, females, and control containers without consumers. Within each experiment, these three groups of containers were haphazardly interspersed to avoid pseudoreplication. To keep algal thalli from floating and to keep algal species clearly separated within replicates (especially the similar-looking Ulva spp.), a glass pipette was placed over the center of the algal clumps in the containers. Amphipods were allowed to feed for a maximum of three days. Paired t tests were used to analyze these experiments when data showed normal distributions and homogeneous variances (Shapiro–Wilk and Levene tests, respectively). When data could not be transformed to fulfil these assumptions, Wilcoxon signed-rank tests were used.
Total consumption of males and females in these choice assays were compared by adding the mass of all consumed food per replicate (U. flexuosa + C. glomerata, U. prolifera + C. glomerata, and U. prolifera + U. flexuosa), and then contrasting these totals for each male–female experimental set. We also calculated the percent of difference in consumption between the two algal choices offered to males and females in each of the three comparisons to assess the relative strength of preference between sexes for the same foods. These analyses detect potential differences in the way males and females assess their choices even when both sexes have similar preference patterns (Cruz-Rivera et al. 2017). Total consumption and percent differences within replicates for each male–female set were compared with two-tailed unpaired t tests.
We measured feeding rates of males and females on each of the three food algae using no-choice assays. These experiments provide information on consumer behavior by assessing flexibility in consumption in the absence of alternative foods and, when complemented with food nutritional information, on the capacity for compensatory feeding (Stachowicz and Hay 1999; Cruz-Rivera and Hay 2000, 2001; Cruz-Rivera and Friedlander 2013). For these experiments, 2–3 males or females per replicate were placed in 530 ml containers and confined to feeding on each alga alone. The three treatments (N = 12) were interspersed and run simultaneously, along with an equal number of containers without consumers that served as controls for autogenic changes in algal mass. Amphipods in these no-choice experiments were allowed to feed for approximately 2.5 days. Data were analyzed by two-way ANOVA, using diet and amphipod sex as factors, after log transformations to normalize distributions and homogenize variances. Compensatory feeding behavior was assessed by analyzing associations between feeding rates and algal organic content (AFDM WM−1), using linear regression analyzes. Matching replicates with algal organic content was possible using our methods (see above). If amphipods were using compensatory feeding, inverse relationships between these two variables were expected. Regression slopes for males and females were compared by analysis of covariance (ANCOVA) to discern potential differences in the magnitude of nutritional compensation between sexes.
Mate guarding and food consumption
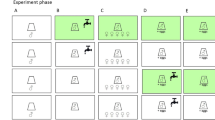
We analyzed the effect of G. aequicauda precopula on feeding rates using no-choice experiments as explained above. In these assays all amphipods were fed on U. flexuosa alone because it was the most abundant seaweed at that time. Replicates received two gravid females, two males, or a pair in precopula (N = 15), and amphipods were allowed to feed for two days. For the temperatures at which experiments were run (≈22 °C) precopula lasts between one and two days in this species (Janssen et al. 1979; Prato et al. 2006). Treatments were interspersed with controls lacking amphipods, as explained above, and numbers of precopulating pairs at the end of the experiment were recorded. Feeding rates for the three experimental groups were analyzed with one-way ANOVA, followed by Tukey–Kramer post hoc tests. We also compared the feeding rates of animals for which precopula was interrupted. The purpose of this experiment was to evaluate if amphipods required more food immediately after terminating precopula to compensate for lower consumption during mating. Precopulating pairs were collected and separated by gently drying them with paper towels, which often caused males to release females. Thus, for this experiment only, amphipod masses were obtained before placing the animals in the experimental containers. Two or three males or females per replicate were used (N = 10). These were either animals obtained from mated pairs or individuals not collected in precopula (which were also blot-dried), for a total of four experimental groups. Because our goal was to measure possible short-term responses in feeding, amphipods were allowed to feed on U. flexuosa for only one day. Data were analyzed with two-way ANOVA using precopula status and sex as factors.
Egg developmental stage and food consumption
Finally, we evaluated if reproductive stage affected female consumption. Females of G. aequicauda (Grèze 1977; Janssen et al. 1979; Kevrekidis and Koukouras 1989; Prato et al. 2006; Kevrekidis et al. 2009) and other amphipods (Sainte-Marie 1991; Sutcliffe 1992; Cruz-Rivera and Hay 2000) produce more than one egg clutch sequentially. The eggs also increase in size as the embryos develop inside them (Janssen et al. 1979; Sutcliffe 1992; Prato et al. 2006), potentially increasing drag and the energy expenditure needed to move around. We hypothesized that females with recently produced smaller eggs potentially consumed less food than females close to hatching eggs, which would be preparing to produce the next clutch and would experience more difficulty moving. It was only possible to compare these two groups in late March, when this experiment was performed, because no free-swimming females without eggs could be obtained from field collections (see also Janssen et al. 1979). Experimental containers (N = 14) received 2–3 microscope-sorted females carrying either recently laid eggs in their brood pouch (compact solid dark) or late-stage eggs (larger translucent eggs with visibly well-developed embryos close to hatching). Females were fed on U. flexuosa for 2 days, while interspersed among controls for autogenic changes in algal mass. Data were analyzed by unpaired t tests after normality and variance homogeneity were confirmed.
Results
We were able to determine the sex of all amphipods in our samples due to the lack of small immature juveniles. Overall, amphipod densities (Fig. 1) showed no significant differences between February than March (PMonth = 0.162), but males were significantly more abundant than females during both months (PSex = 0.001). No interaction between sampling month and prevalence of sexes was found (PMxS = 0.871). Although male:female ratios changed from 2.78 to 4.88 from February to March, variance was high, and these ratios were not significantly different between the two sampling dates (P = 0.705, unpaired t test). No other mesograzers were observed in the samples, although specimens of the isopod Sphaeroma serratum, small shrimp (possibly Palaemon elegans), and the cephalaspidean snail Haminoea orbignyana were seen occasionally in the area where the algae grew (Holdich and Tolba 1985; El-Shabrawy and Dumont 2009; Cruz-Rivera and Malaquias 2016).
There was no relationship between amount eaten and amphipod body mass for either diet or sex (Table S1). Given the lack of evidence for allometry in feeding, the use of covariance analyses was not justified, and we standardized all results by consumer mass, which essentially weighed the data against size discrepancies (Cruz-Rivera et al. 2017).
Differences in food choice between males and females were observed in one of the three pairwise comparisons (Fig. 2), but less obvious differences in total consumption were also detected (Table 1). Both sexes preferred U. flexuosa over C. glomerata (P = 0.025 for males, Wilcoxon signed-rank test, P = 0.004 for females, paired t test), and U. prolifera over U. flexuosa (P < 0.001 for both sexes, paired t tests). However, males significantly preferred U. prolifera over C. glomerata (P = 0.005 paired t test), whereas females did not exhibit a preference for either of these algae (P = 0.405, paired t test). Females in these choice experiments consumed significantly more algae than males overall only in the U. prolifera vs. C. glomerata (P = 0.002, unpaired t test), and U. prolifera vs. U. flexuosa (P < 0.001, unpaired t test) assays (Table 1). The strength of preference between male and female choices was not significantly different in any instance, despite males and females showing different in feeding patterns on U. prolifera vs. C. glomerata (P = 0.076, Table 1).
No-choice experiments (Fig. 3) detected significant effects of both food alga (PDiet < 0.001) and amphipod sex (PSex = 0.022), without a significant interaction between the two (PDxS = 0.358). On average, males consumed 77% more U. prolifera and 82% more U. flexuosa than C. glomerata, whereas females consumed 78% more of both U. prolifera and U. flexuosa than C. glomerata. However, females consumed ca. 28% more algae in total than males.
Significant negative relationships between feeding rates and algal AFDM WM−1 were found for both males and females (P < 0.001 for both), suggesting compensatory feeding (Fig. 4). However, algal organic content explained < 40% of the variance in feeding rates for males or females, suggesting that this compensatory behavior is highly variable (Fig. 4). Despite mean differences in patterns, the relationship between feeding and algal organic content was statistically equivalent for both sexes (P = 0.128, ANCOVA).
Feeding rate was affected, but not arrested, by mating behavior of G. aequicauda (P = 0.030, Fig. 5). Non-precopulating females had significantly higher feeding rates than pairs in precopula, but male feeding rates were intermediate, and statistically equivalent, to those in the other two groups (Fig. 5). We considered the possibility that our measured feeding rates were an artifact from individuals finishing mating and resuming normal feeding. However, our data showed that the highest ingestion rate in paired amphipods (8.47 mg alga mg amphipod−1 day−1) corresponded to a pair that was still in precopula at the moment the experiment was finished. Most individuals, however, ceased precopulation between 12 and 22 h. Contrary to these results, there were no immediate increases in feeding from animals released from precopula, compared to those collected as free-moving individuals, regardless of sex (PPrecopula = 0.924, PSex = 0.686, and PPxS = 0.568, Fig. 6). Feeding rates of early and late ovigerous females were also similar (P = 0.507, Fig. 7). No cannibalism was observed in replicates, likely because size differences were not marked within replicates and animals did not run out of food during experiments.
Discussion
In this study, G. aequicauda males numerically dominated a lake population during two consecutive samplings were somewhat more selective for certain algae than females, but ingested less food per mass in most of our comparisons. Variation in sex ratio can modify top-down control by predators on aquatic food webs (Fryxell et al. 2015), but similar studies on herbivores are lacking. The male bias of the Lake Qarun amphipod population could result in lesser grazing pressure overall, but particularly on C. glomerata, which was more readily consumed by females, especially in the presence of U. prolifera (Fig. 2). However, sex ratio in this amphipod and others can vary seasonally, with males or females dominating at different times of the year (Grèze 1977; Janssen et al. 1979; Prato and Biandolino 2003). The strength of top-down impacts could fluctuate accordingly. The lack of juveniles in our samples, along with the difficulty finding non-ovulating females for one experiment, could indicate that our research occurred during the end of the reproductive season for this amphipod population. Sharp declines in juveniles during the first months of the year have been documented for this amphipod before (Janssen et al. 1979; Casagranda et al. 2006) but no equivalent information is available for Lake Qarun. Nonetheless, anecdotal observations over three years suggest that both amphipods and green algae are noticeable during a 4–6-month period and decline strongly in abundance during the warmer summer. As such, our sampling covered approximately 1/3 of the normal population cycle of the species.
While males always significantly preferred one of the algae offered in paired-choice assays, females did not show a preference when U. prolifera and C. glomerata were simultaneously available (Fig. 2). Because the only nutritional trait of food algae we measured was total organic content, we are limited in our ability to explain this difference between sexes. Nevertheless, the results agree with behavioral observations of G. aequicauda from a hypersaline lake showing that males preferred animal prey, whereas females were less selective, combining animal and plant foods (Shadrin et al. 2021b). A lower food selectivity in females, compared to males, has been observed in isopods (Jormalainen et al. 2001), crabs (Mchenga and Tsuchiya 2011), beetles (Ballhorn et al. 2013), fish (Delbeek and Williams 1987), and birds (Bravo et al. 2014) but these cases do not follow a common underlying mechanism. For example, in insects, favoring certain foods by males has been related to the use of dietary chemicals to produce pheromones (South et al. 2011). Males could also be more selective (or have broader diets) if they have secondary traits that make them more efficient than females at handling certain prey (Buck et al. 2003; Kolts et al. 2013; Hübneṙ et al. 2015). Self-medication by male birds could also result in selective diets if ingested chemicals from prey decrease parasite loads and increase mating chances (Bravo et al. 2014). For females, food selectivity could be affected by their larger reproductive allocation compared to males. As such, females may be less selective by mixing foods to balance nutrition (Morehouse et al. 2010) or actively select high quality foods (Henry 1997; Beck et al. 2007; Lodberg-Holm et al. 2021). Even differences in visual acuity (Melin et al. 2010) or neurological integration (Fukushima et al. 2015) between sexes could lead to differences in diet choice.
In contrast with the slight difference in food choice, females consumed more food per body mass in almost all the experiments (Fig. 3, Table 1). This concurs with other crustacean (Webb et al. 1987; Salemaa 1987; Gaudy et al. 1996; Zhou et al. 1998; Ólafsson et al. 2002; Schuwerack et al. 2006; Cruz-Rivera et al. 2017) and vertebrate studies (Pandian 1970; Kunz 1974; Rothman et al. 2008). A generally invoked explanation for this is that females must compensate for the higher metabolic demands of making eggs (Williams 2005; Partridge et al. 2005; Morehouse et al. 2010). If so, reproductive periods are of limited duration for most species and such differences in feeding rates may only happen seasonally (Kyomo 1999; Paulo-Martins et al. 2011). Reproductive output is clearly seasonal in these amphipods (Grèze 1977; Janssen et al. 1979; Kevrekidis and Koukouras 1989; Kevrekidis et al. 2009), but no data on how it relates to feeding variability are available, and longer-term studies on feeding rates of other Gammarus species have not conclusively assessed differences between sexes or reproductive stages (Marchant and Hynes 1981). Interestingly, this latter study suggests that differences in gut evacuation rates and nutrient absorption efficiency could also serve as strategies allowing Gammarus females to gain the required nutrients to invest in reproduction. However, if females retain food in the guts longer to extract more nutrients per ingested mass, lower feeding rates than those of males would be expected. We found the opposite.
Differences in consumption in sexually dimorphic species, such as G. aequicauda, could also arise from allometric scaling of metabolism and concomitant energetic demands. Physiological models predict a decrease in mass-specific metabolic rate with increasing size (Brown et al. 2004; DeLong et al. 2010). Thus, the smaller females could consume more food per body mass based on allometry alone. Few studies assessing feeding rates of aquatic species have measured consumer mass, making it difficult to test this prediction broadly (Rall et al. 2012). Zhou et al. (1998) observed that red king crab females consumed significantly more food per body mass than males, but that was true for ovigerous females alone, not juvenile females. Shadrin et al. (2021b) recently showed that male G. aequicauda consumed more animal prey on average than females. Although they point out females were smaller, consumption was never standardized by mass, precluding a direct comparison with our results. No consistent relationship between size and consumption for males or females was observed for a tropical crab and this amphipod in a prior study (Cruz-Rivera et al. 2017). Despite being a cornerstone of ecological theory, the relationship between allometric scaling of feeding in relation to consumer mass has been poorly studied in aquatic species. Reviews and studies alike have found more complexity than the universally predicted ¾ power scaling of metabolic rate with size (Glazier 2005; Seibel and Drazen 2007; DeLong et al. 2010; Rall et al. 2012; Alcaraz 2016). While standardizing by mass has been criticized by physiologists before (Packard and Boardman 1988, 1999), the alternative recommended use of linear models like ANCOVA relies on a significant relationship between variables, which was clearly not observed here.
Our data show that mating may modulate, rather than arrest, the per capita effect of a consumer on its food. Animals ranging from insects to humans show mate guarding (Jormalainen 1998; Brotherton and Komers 2003; Martens et al. 2012), but little is known about how this phenomenon affects feeding in aquatic systems. For G. aequicauda, precopula reduced feeding on algae compared to females but not males (Fig. 5). This was unexpected because studies on vertebrates (Westneat 1994; Alberts et al. 1996; Komdeur 2001) and invertebrates (Saeki et al. 2005; Scharf et al. 2013) often interpreted or showed that mate guarding and food acquisition were mutually exclusive processes, with guarding males not feeding. Our results suggested that males either had better access to food while guarding or they quickly increased consumption after uncoupling. Studies on G. lawrencianus showed that males reduced feeding during precopula but did not stop eating altogether (Robinson and Doyle 1985), while a recent study on G. aequicauda reported that males released females before feeding after copulation (Shadrin et al. 2021b). Although the latter study strongly suggests a mechanism, we could not detect differences in consumption between free-moving and formerly mated individuals of either sex (Fig. 6). We interpret data from this experiment cautiously because of the necessary manipulation of the amphipods to break precopulatory pairs. This was likely a stressful process for the animals that could have altered normal feeding patterns for all individuals in the experiment (Cruz-Rivera et al. 2017).
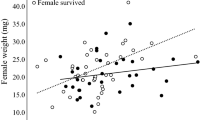
Females consumed algae at similar rates, regardless of the developmental stage of the eggs (Fig. 7). We had hypothesized that females closer to releasing offspring (i. e., with fully developed larger eggs) could be ingesting more food as a result of increased energy expenditure in moving (Lewis and Loch-Mally 2010) and as preparation to produce the next clutch (Kyomo 1999; Williams 2005; Tsukamoto et al. 2014). Mean volume of G. aequicauda eggs in the brood pouch increases over time from water absorption and the formation of body structures in the directly developing offspring (Sutcliffe 1992; Prato et al. 2006). This increase in egg volume, however, may be highly variable for G. aequicauda (Prato et al. 2006) and our assumption of higher energetic costs from ovigerous females having to move with a progressively larger clutch might have been unrealistic. We, unfortunately, were unable to compare ovigerous against non-ovigerous females (Zhou et al. 1998). Studies show that G. aequicauda females produce 2–15 broods with intervals of 7–15 days between them (Grèze 1977; Janssen et al. 1979; Kevrekidis and Koukouras 1989; Prato et al. 2006; Kevrekidis et al. 2009). Links between ovulation cycles and feeding variation have not been assessed.
The current macrofauna of Lake Qarun is dominated by carnivorous fishes and omnivorous shrimp that have been introduced to support local fisheries (El-Shabrawy and Dumont 2009). Mesograzers are the principal herbivores, including amphipods, isopods, and cephalaspidean gastropods (Holdich and Tolba 1985; El-Shabrawy and Dumont 2009; Cruz-Rivera and Malaquias 2016). During the colder months G. aequicauda dominated the algal epifauna, with few other species observed at all in the field. Our data suggest that the impact of these mesograzers on their foods could be modulated not only by environmental and population fluctuations, but also by varying sex ratios and mating behavior in the field.
Data availability
Data are available through figshare < https://doi.org/10.6084/m9.figshare.4810654.v1 >
References
Alberts SC, Altmann J, Wilson ML (1996) Mate guarding constrains foraging activity of male baboons. Anim Behav 51:1269–1277. https://doi.org/10.1006/anbe.1996.0131
Alcaraz M (2016) Marine zooplankton and the metabolic theory of ecology: is it a predictive tool? J Plankton Res 38:762–770. https://doi.org/10.1093/plankt/fbw012
Ballhorn DJ, Kautz S, Heil M (2013) Distance and sex determine host plant choice by herbivorous beetles. PLoS ONE 8:e55602. https://doi.org/10.1371/journal.pone.0055602
Barboza PS, Bowyer RT (2001) Seasonality of sexual segregation in dimorphic deer: extending the gastrocentric model. Alces 37:275–292
Beck CA, Iverson SJ, Bowen WD, Blanchard W (2007) Sex differences in grey seal diet reflect seasonal variation in foraging behaviour and reproductive expenditure: evidence from quantitative fatty acid signature analysis. J Anim Ecol 76:490–502. https://doi.org/10.1111/j.1365-2656.2007.01215.x
Bravo C, Bautista LM, García-París M et al (2014) Males of a strongly polygynous species consume more poisonous food than females. PLoS ONE 9:e111057. https://doi.org/10.1371/journal.pone.0111057
Brotherton PNM, Komers PE (2003) Mate guarding and the evolution of social monogamy in mammals. In: Boesch C, Reichard UH (eds) Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press, Cambridge, pp 42–58. https://doi.org/10.1017/CBO9781139087247.003
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Buck TL, Breed GA, Pennings SC et al (2003) Diet choice in an omnivorous salt-marsh crab: different food types, body size, and habitat complexity. J Exp Mar Biol Ecol 292:103–116. https://doi.org/10.1016/S0022-0981(03)00146-1
Casagranda C, Dridi MS, Boudouresque CF (2006) Abundance, population structure and production of macro-invertebrate shredders in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Estuar Coast Shelf Sci 66:437–446. https://doi.org/10.1016/j.ecss.2005.10.005
Conlan KE (1991) Precopulatory mating behavior and sexual dimorphism in the amphipod Crustacea. In: Watling L (ed) VIIth International Colloquium on Amphipoda: Proceedings of the VIIth International Colloquium on Amphipoda held in Walpole, Maine, USA, 14–16 September 1990. Springer, Dordrecht, pp 255–282. https://doi.org/10.1007/BF00047644
Cruz-Rivera E, Friedlander M (2013) Effects of algal phenotype on mesograzer feeding. Mar Ecol Prog Ser 490:69–78. https://doi.org/10.3354/meps10429
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219. https://doi.org/10.1890/0012-9658(2000)081[0201:CQRQFC]2.0.CO;2
Cruz-Rivera E, Hay ME (2001) Macroalgal traits and the feeding and fitness of an herbivorous amphipod: the roles of selectivity, mixing, and compensation. Mar Ecol Prog Ser 218:249–266. https://doi.org/10.3354/meps218249
Cruz-Rivera E, Malaquias MAE (2016) Ecosystem alterations and species range shifts: an Atlantic-Mediterranean cephalaspidean gastropod in an inland Egyptian lake. PLoS ONE 11:e0156760. https://doi.org/10.1371/journal.pone.0156760
Cruz-Rivera E, Petsche C, Hafez T (2017) Detecting sex-related differences in mesograzer feeding experiments: An often overlooked source of intraspecific variation in herbivory. Limnol Oceanogr Methods 15:542–553. https://doi.org/10.1002/lom3.10179
Delbeek JC, Williams DD (1987) Food resource partitioning between sympatric populations of brackishwater sticklebacks. J Anim Ecol 56:949. https://doi.org/10.2307/4959
DeLong JP, Okie JG, Moses ME et al (2010) Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc Nat Acad Sci 107:12941–12945. https://doi.org/10.1073/pnas.1007783107
Dick JTA, Montgomery I, Elwood RW (1993) Replacement of the indigenous amphipod Gammarus duebeni celticus by the introduced G. pulex: differential cannibalism and mutual predation. J Anim Ecol 62:79. https://doi.org/10.2307/5484
Dick JTA, Faloon SE, Elwood RW (1998) Active brood care in an amphipod: influences of embryonic development, temperature and oxygen. Anim Behav 56:663–672. https://doi.org/10.1006/anbe.1998.0797
Drès M, Mallet J (2002) Host races in plant–feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond, b, Biol Sci 357:471–492. https://doi.org/10.1098/rstb.2002.1059
Du Toit JT (2006) Sex differences in the foraging ecology of large mammalian herbivores. In: Ruckstuhl K, Neuhaus P (eds) Sexual segregation in VerTebrates. Cambridge University Press, Cambridge, pp 35–52. https://doi.org/10.1017/CBO9780511525629.004
El-Shabrawy GM, Dumont HJ (2009) The Fayum depression and its lakes. In: Dumont HJ (ed) The Nile: origin, environments, limnology and human use. Springer, Dordrecht, pp 95–124. https://doi.org/10.1007/978-1-4020-9726-3_6
Ercit K, Martinez-Novoa A, Gwynne DT (2014) Egg load decreases mobility and increases predation risk in female black-horned tree crickets (Oecanthus nigricornis). PLoS ONE 9:e110298. https://doi.org/10.1371/journal.pone.0110298
Fox LR, Morrow PA (1981) Specialization: species property or local phenomenon? Science 211:887–893. https://doi.org/10.1126/science.211.4485.887
Fryxell DC, Arnett HA, Apgar TM et al (2015) Sex ratio variation shapes the ecological effects of a globally introduced freshwater fish. Proc R Soc b: Biol Sci 282:20151970. https://doi.org/10.1098/rspb.2015.1970
Fukushima A, Hagiwara H, Fujioka H et al (2015) Sex differences in feeding behavior in rats: the relationship with neuronal activation in the hypothalamus. Front Neurosci 9:88. https://doi.org/10.3389/fnins.2015.00088
Gaudy R, Pagano M, Cervetto G et al (1996) Short term variations in feeding and metabolism of Acartia tonsa (pelagic copepod) in the Berre lagoon (France). Oceanol Acta 19:635–644
Gautier-Hion A (1980) Seasonal variations of diet related to species and sex in a community of Cercopithecus monkeys. J Anim Ecol 49:237. https://doi.org/10.2307/4287
Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev 80:611–662. https://doi.org/10.1017/S1464793105006834
Goos JM, Cothran RD, Jeyasingh PD (2016) Sex-specific nutrient use and preferential allocation of resources to a sexually selected trait in Hyalella amphipods. J Exp Biol 219:649–657. https://doi.org/10.1242/jeb.132498
Goos JM, Cothran RD, Jeyasingh PD (2017) Within-population variation in the chemistry of life: the stoichiometry of sexual dimorphism in multiple dimensions. Evol Ecol 31:635–651. https://doi.org/10.1007/s10682-017-9900-9
Grèze II (1977) Life cycle of Gammarus aequicauda (Martynov, 1931) in the Black Sea. Crustaceana Suppl 4:88–90
Guillemette M, Ouellet J-F (2005) Temporary flightlessness as a potential cost of reproduction in pre-laying common eiders Somateria mollissima. Ibis 147:301–306. https://doi.org/10.1111/j.1474-919x.2005.00402.x
Henry O (1997) The influence of sex and reproductive state on diet preference in four terrestrial mammals of the French Guianan rain forest. Can J Zool 75:929–935. https://doi.org/10.1139/z97-111
Holdich DM, Tolba MR (1985) On the cccurrence of Sphaeroma serratum (Isopoda, Sphaeromatidae) in an Egyptian inland salt lake. Crustaceana 49:211–214. https://doi.org/10.1163/156854085X00486
Hübneṙ L, Pennings SC, Zimmer M (2015) Sex- and habitat-specific movement of an omnivorous semi-terrestrial crab controls habitat connectivity and subsidies: a multi-parameter approach. Oecologia 178:999–1015. https://doi.org/10.1007/s00442-015-3271-0
Izquierdo MS, Fernández-Palacios H, Tacon AGJ (2001) Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197:25–42. https://doi.org/10.1016/S0044-8486(01)00581-6
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273. https://doi.org/10.1146/annurev.es.21.110190.001331
Janssen H, Scheepmaker M, van Couwelaar M, Pinkster S (1979) Biology and distribution of Gammarus aequicauda and G. insensibilis (Crustacea, Amphipoda) in the lagoon system of Bages-Sigean (France). Bijdr Dierkd 49:42–71. https://doi.org/10.1163/26660644-04901004
Jormalainen V (1998) Precopulatory mate guarding in crustaceans: male competitive strategy and intersexual conflict. Q Rev Biol 73:275–304. https://doi.org/10.1086/420306
Jormalainen V, Honkanen T, Mäkinen A et al (2001) Why does herbivore sex matter? Sexual differences in utilization of Fucus vesiculosus by the isopod Idotea baltica. Oikos 93:77–86. https://doi.org/10.1034/j.1600-0706.2001.930108.x
Kevrekidis TH, Koukouras ATH (1989) Seasonal variation of abundance of Gammarus aequicauda (Crustacea: Amphipoda) in the Evros Delta (NE Greece). Israel J Ecol Evol 36:113–123. https://doi.org/10.1080/00212210.1989.10688629
Kevrekidis T, Kourakos G, Boubonari T (2009) Life history, reproduction, growth, population dynamics and production of Gammarus aequicauda (Crustacea: Amphipoda) at extremely low salinities in a Mediterranean lagoon. Int Rev Hydrobiol 94:308–325. https://doi.org/10.1002/iroh.200811097
Kolts JM, Lovvorn JR, North CA et al (2013) Effects of body size, gender, and prey availability on diets of snow crabs in the northern Bering Sea. Mar Ecol Prog Ser 483:209–220
Komdeur J (2001) Mate guarding in the Seychelles warbler is energetically costly and adjusted to paternity risk. Proc R Soc Lond B Biol Sci 268:2103–2111. https://doi.org/10.1098/rspb.2001.1750
Kullberg C, Jakobsson S, Kaby U, Lind J (2005) Impaired flight ability prior to egg-laying: a cost of being a capital breeder. Funct Ecol 19:98–101. https://doi.org/10.1111/j.0269-8463.2005.00932.x
Kunz TH (1974) Feeding ecology of a temperate insectivorous bat (Myotis velifer). Ecology 55:693–711. https://doi.org/10.2307/1934408
Kyomo J (1992) Variations in the feeding habits of males and females of the crab Sesarma intermedia. Mar Ecol Prog Ser 83:151–155
Kyomo J (1999) Feeding patterns, habits and food storage in Pilumnus vespertilio (Brachyura: Xanthidae). Bull Mar Sci 65:381–389
Lewis RJ, Kappeler PM (2005) Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest. Am J Primatol 67:347–364. https://doi.org/10.1002/ajp.20187
Lewis SE, Loch-Mally AM (2010) Ovigerous female amphipods (Gammarus pseudolimnaeus) face increased risks from vertebrate and invertebrate predators. J Freshw Ecol 25:395–402. https://doi.org/10.1080/02705060.2010.9664382
Lodberg-Holm HK, Steyaert SMJG, Reinhardt S, Rosell F (2021) Size is not everything: differing activity and foraging patterns between the sexes in a monomorphic mammal. Behav Ecol Sociobiol 75:76. https://doi.org/10.1007/s00265-021-03010-7
Mahjoub M-S, Souissi S, Michalec F-G et al (2011) Swimming kinematics of Eurytemora affinis (Copepoda, Calanoida) reproductive stages and differential vulnerability to predation of larval Dicentrarchus labrax (Teleostei, Perciformes). J Plankton Res 33:1095–1103. https://doi.org/10.1093/plankt/fbr013
Maklakov AA, Simpson SJ, Zajitschek F et al (2008) Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol 18:1062–1066. https://doi.org/10.1016/j.cub.2008.06.059
Marchant R, Hynes HBN (1981) Field estimates of feeding rate for Gammarus pseudolimnaeus (Crustacea: Amphipoda) in the Credit River, Ontario. Freshw Biol 11:27–36. https://doi.org/10.1111/j.1365-2427.1981.tb01240.x
Martens A, Günther A, Suhling F (2012) Diversity in mate-guarding types within the genus Anax (Odonata: Aeshnidae). Libellula Suppl 12:113–122
McCarty AT, Sotka EE (2013) Geographic variation in feeding preference of a generalist herbivore: the importance of seaweed chemical defenses. Oecologia 172:1071–1083. https://doi.org/10.1007/s00442-012-2559-6
Mchenga ISS, Tsuchiya M (2011) Feeding choice and the cate of organic materials consumed by sesarma crabs Perisesarma bidens (De Haan) when offered different diets. J Mar Biol 2010:201932. https://doi.org/10.1155/2010/201932
Melin AD, Fedigan LM, Young HC, Kawamura S (2010) Can color vision variation explain sex differences in invertebrate foraging by capuchin monkeys? Current Zoology 56:300–312. https://doi.org/10.1093/czoolo/56.3.300
Mitra C, Reynoso E, Davidowitz G, Papaj D (2016) Effects of sodium puddling on male mating success, courtship and flight in a swallowtail butterfly. Anim Behav 114:203–210. https://doi.org/10.1016/j.anbehav.2016.01.028
Molleman F, Grunsven RHA, Liefting M et al (2005) Is male puddling behaviour of tropical butterflies targeted at sodium for nuptial gifts or activity? Biol J Linnean Soc 86:345–361. https://doi.org/10.1111/j.1095-8312.2005.00539.x
Morehouse NI, Nakazawa T, Booher CM et al (2010) Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119:766–778. https://doi.org/10.1111/j.1600-0706.2009.18569.x
Naguib M (1961) Studies on the ecology of Lake Qarûn (Faiyum-Egypt), part II. Kieler Meeresforsch 17:94–131
Ólafsson E, Buchmayer S, Skov MW (2002) The East African decapod crab Neosarmatium meinerti (de Man) sweeps mangrove floors clean of leaf litter. Ambio 31:569–573. https://doi.org/10.1579/0044-7447-31.7.569
Packard GC, Boardman TJ (1988) The misuse of ratios, indices, and percentages in ecophysiological research. Physiol Zool 61:1–9. https://doi.org/10.1086/physzool.61.1.30163730
Packard GC, Boardman TJ (1999) The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol Part A Mol Integr Physiol 122:37–44. https://doi.org/10.1016/S1095-6433(98)10170-8
Pandian TJ (1970) Intake and conversion of food in the fish Limanda limanda exposed to different temperatures. Mar Biol 5:1–17. https://doi.org/10.1007/BF00352487
Partridge L, Gems D, Withers DJ (2005) Sex and death: what Is the connection? Cell 120:461–472. https://doi.org/10.1016/j.cell.2005.01.026
Paulo-Martins C, Vinagre C, Silva A, Cabral H (2011) Variation of diet and food consumption of the scaldfish Arnoglossus laterna (Walbaum, 1792). J Appl Ichthyol 27:977–983. https://doi.org/10.1111/j.1439-0426.2011.01742.x
Prato E, Biandolino F (2003) Seasonal changes in population of the amphipod Gammarus aequicauda (Martynov, 1931). Medit Mar Sci. 4:49–56. https://doi.org/10.12681/mms.240
Prato E, Biandolino F, Scardicchio C (2006) Postembryonic growth, development and reproduction of Gammarus aequicauda (Martynov, 1931) (Gammaridae) in laboratory culture. Zool Stud 45:503–509
Rall BC, Brose U, Hartvig M et al (2012) Universal temperature and body-mass scaling of feeding rates. Philos Trans R Soc b, Biol Sci 367:2923–2934. https://doi.org/10.1098/rstb.2012.0242
Robinson BW, Doyle TW (1985) Trade-off between male reproduction (amplexus) and growth in the amphipod Gammarus lawrencianus. Biol Bull 168:482–488. https://doi.org/10.2307/1541528
Rothman JM, Dierenfeld ES, Hintz HF, Pell AN (2008) Nutritional quality of gorilla diets: consequences of age, sex, and season. Oecologia 155:111–122. https://doi.org/10.1007/s00442-007-0901-1
Saeki Y, Kruse KC, Switzer PV (2005) Physiological costs of mate guarding in the Japanese beetle (Popillia japonica Newman). Ethology 111:863–877. https://doi.org/10.1111/j.1439-0310.2005.01106.x
Sainte-Marie B (1991) A review of the reproductive bionomics of aquatic gammaridean amphipods: variation of life history traits with latitude, depth, salinity and superfamily. Hydrobiologia 223:189–227. https://doi.org/10.1007/BF00047641
Salemaa H (1987) Herbivory and microhabitat preferences of Idotea spp. (Isopoda) in the northern Baltic Sea. Ophelia 27:1–15. https://doi.org/10.1080/00785236.1987.10422007
Sanford E, Roth MS, Johns GC et al (2003) Local selection and latitudinal variation in a marine predator-prey interaction. Science 300:1135–1137. https://doi.org/10.1126/science.1083437
Scharf I, Peter F, Martin OY (2013) Reproductive trade-offs and direct costs for males in arthropods. Evol Biol 40:169–184. https://doi.org/10.1007/s11692-012-9213-4
Schuwerack P-MM, Barnes RSK, Underwood GJC, Jones PW (2006) Gender and species differences in sentinel crabs (Macrophthalmus) feeding on an Indonesian mudflat. J Crust Biol 26:119–123. https://doi.org/10.1651/C-2612.1
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond, b, Biol Sci 362:2061–2078. https://doi.org/10.1098/rstb.2007.2101
Seigel RA, Huggins MM, Ford NB (1987) Reduction in locomotor ability as a cost of reproduction in gravid snakes. Oecologia 73:481–485. https://doi.org/10.1007/BF00379404
Shadrin NV, EL-Shabrawy GM, Anufriieva EV, et al (2016) Long-term changes of physicochemical parameters and benthos in Lake Qarun (Egypt): Can we make a correct forecast of ecosystem future? Knowl Manag Aquat Ecosyst 417:18. https://doi.org/10.1051/kmae/2016005
Shadrin N, Yakovenko V, Anufriieva E (2020) Gammarus aequicauda and Moina salina in the Crimean saline waters: New experimental and field data on their trophic relation. Aquac Res 51:3091–3099. https://doi.org/10.1111/are.14643
Shadrin N, Yakovenko V, Anufriieva E (2021a) Can Gammarus aequicauda (Amphipoda) suppress a population of Baeotendipes noctivagus (Chironomidae) in a hypersaline lake? A case of Lake Moynaki (Crimea). Aquac Res 52:1705–1714. https://doi.org/10.1111/are.15024
Shadrin N, Yakovenko V, Anufriieva E (2021b) The behavior of Gammarus aequicauda (Crustacea, Amphipoda) during predation on chironomid larvae: sex differences and changes in precopulatory mate-guarding state. J Exp Zool A Ecol Integr Physiol 335:572–582. https://doi.org/10.1002/jez.2500
Shine R (1989) Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol 64:419–461. https://doi.org/10.1086/416458
Slansky F (1993) Nutritional ecology: the fundamental quests for nutrients. In: Stamp NE, Casey TE (eds) Caterpillars : ecological and evolutionary constraints on foraging. Chapman & Hall, New York, pp 29–91
Sotka EE (2005) Local adaptation in host use among marine invertebrates. Ecol Lett 8:448–459. https://doi.org/10.1111/j.1461-0248.2004.00719.x
Sotka EE, Hay ME (2002) Geographic variation among herbivore populations in tolerance for a chemically rich seaweed. Ecology 83:2721–2735. https://doi.org/10.1890/0012-9658(2002)083[2721:GVAHPI]2.0.CO;2
South SH, House CM, Moore AJ et al (2011) Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: Implications for the study of condition dependence. Evolution 65:1594–1606. https://doi.org/10.1111/j.1558-5646.2011.01233.x
Stachowicz JJ, Hay M (1999) Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Prog Ser 188:169–178
Stachowicz JJ, Hay ME (2000) Geographic variation in camouflage specialization by a decorator crab. Am Nat 156:59–71. https://doi.org/10.1086/303366
Sutcliffe DW (1992) Reproduction in Gammarus (Crustacea, Amphipoda): basic processes. Freshw Forum 2:102–128
Svensson J-E (1992) The influence of visibility and escape ability on sex-specific susceptibility to fish predation in Eudiaptomus gracilis (Copepoda, Crustacea). Hydrobiologia 234:143–150. https://doi.org/10.1007/BF00014246
Tarutis J, Lewis S, Dyke M (2005) Active parental care in a freshwater amphipod (Crustacea: Gammarus pseudolimnaeus): Effects of environmental factors. Am Midl Nat 153:276–283. https://doi.org/10.1674/0003-0031(2005)153[0276:APCIAF]2.0.CO;2
Trivers RL (1972) Parental investment and sexual selection. In: Campbell BG (ed) Sexual selection and the descent of man: 1871–1971, 1st edn. Aldine, Chicago, pp 136–179
Tsukamoto Y, Kataoka H, Nagasawa H, Nagata S (2014) Mating changes the female dietary preference in the two-spotted cricket Gryllus bimaculatus. Front Physiol 5:95
Walker LK, Thorogood R, Karadas F et al (2014) Foraging for carotenoids: do colorful male hihi target carotenoid-rich foods in the wild? Behav Ecol 25:1048–1057. https://doi.org/10.1093/beheco/aru076
Wearmouth VJ, Sims DW (2008) Sexual segregation in marine fish, reptiles, birds and mammals: Behaviour patterns, mechanisms and conservation implications. Adv Mar Biol 54:107–170. https://doi.org/10.1016/S0065-2881(08)00002-3
Webb P, Perissinotto R, Wooldridge T (1987) Feeding of Mesopodopsis slabberi (Crustacea, Mysidacea) on naturally occurring phytoplankton. Mar Ecol Prog Ser 38:115–123. https://doi.org/10.3354/meps038115
Weimerskirch H, Cherel Y, Cuenot-Chaillet F, Ridoux V (1997) Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78:2051–2063. https://doi.org/10.1890/0012-9658(1997)078[2051:AFSARA]2.0.CO;2
Westneat DF (1994) To guard mates or go forage: conflicting demands affect the paternity of male red-winged blackbirds. Am Nat 144:343–354. https://doi.org/10.1086/285679
Williams TD (2005) Mechanisms underlying the costs of egg production. Bioscience 55:39–48. https://doi.org/10.1641/0006-3568(2005)055[0039:MUTCOE]2.0.CO;2
Winfield IJ, Townsend CR (1983) The cost of copepod reproduction: increased susceptibility to fish predation. Oecologia 60:406–411. https://doi.org/10.1007/BF00376860
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001) Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture 202:1–21. https://doi.org/10.1016/S0044-8486(01)00570-1
Zhou S, Shirley TC, Kruse GH (1998) Feeding and growth of the red king crab Paralithodes camtschaticus under laboratory conditions. J Crust Biol 18:337–345. https://doi.org/10.2307/1549328
Acknowledgements
Work herein was conducted in partial fulfilment of an undergraduate senior thesis (by TH) at the American University in Cairo (AUC), Egypt. We thank the Panorama Resort in Lake Qarun for access to some of our collection sites. Comments from two reviewers greatly improved this article.
Funding
Funding and facilities were provided by the American University in Cairo. Internal funding from the Department of Biology (TH) and a Faculty Improvement Grant (EC-R) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
EC-R: developed the study concept, figures, and original draft; TH and EC-R: conducted experiments, collected, and analyzed data, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests that could influence the contents of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cruz-Rivera, E., Hafez, T. Sex, mate guarding, and reproductive state as potential modulators of herbivory in an aquatic consumer. Aquat Sci 85, 12 (2023). https://doi.org/10.1007/s00027-022-00911-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00911-1