Abstract
A comprehensive understanding of how interactions between catchments and downstream lakes affect fish growth rate is lacking for many species and systems, yet is necessary for predicting impacts of environmental change on productivity of freshwater fish populations. We investigated among-lake variability in growth rate of Northern Pike (Esox lucius), a fish species of widespread subsistence and commercial importance. Northern Pike were captured from 11 subarctic lakes that span 60,000 km2 and four ecoregions in the Dehcho Region of the Northwest Territories, Canada. Growth rates were related to stable isotope ratios and to lake and catchment physicochemistry. Growth, modelled using increment widths (n = 2953) measured on cleithra (n = 432), was significantly slower (p < 0.001, adj. r2 = 0.78) in lakes subject to greater inferred catchment influence, which was quantified using a combination of lake and catchment characteristics. While Northern Pike growth rate was not related to δ15N, it was positively related to δ13C (p < 0.001, adj. r2 = 0.75). Further analyses revealed that benthic invertebrates in lakes subject to greater inferred catchment influence had more depleted δ13C ratios, and we posit that Northern Pike growth is slower in these lakes because terrestrially derived organic matter has relatively lower nutritional value and bioaccessibility, but further research is necessary. By linking current among-lake variability in Northern Pike growth to trophic ecology and to both lake and catchment physicochemical data, results inform predictions of how future changes to subarctic lakes and catchments may affect fish growth and productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growth rates and growth-associated traits in freshwater fishes can be influenced by a range of biotic and abiotic factors that vary among lake ecosystems (Wilson et al. 2019; Höhne et al. 2020). As growth rates regulate accumulation of contaminants (e.g., mercury) in fish tissues, as well as fish population dynamics and ultimately fisheries sustainability (King 2007; Sharma et al. 2008), understanding drivers of variability in fish growth rates among systems is of toxicological, conservational, and managerial value, particularly in an era of rapid environmental change.
Northern Pike (Esox lucius) is a cool-water, lie-in-wait, visual hunter that often occupies an apex predator position in freshwater food webs (Scott and Crossman 1973; Vøllestad et al. 1986; Craig 1996). Northern Pike support important commercial, subsistence, and recreational fisheries throughout the northern hemisphere (Scott and Crossman 1973), and are increasingly used as a model organism in studies of ecology and evolution due to their wide geographic distribution (Forsman et al. 2015). Growth rates and population productivity of Northern Pike have been documented to vary in response to a number of environmental and biological factors, including water temperature (Casselman 1978; Margenau et al. 1998), water clarity (Craig and Babaluk 1989; Margenau et al. 1998), lake primary production (Diana 1987), prey quality and availability (Diana 1987; Navarro and Johnson 1992; Margenau et al. 1998; Venturelli and Tonn 2006), and habitat suitability (Vøllestad et al. 1986; Casselman and Lewis 1996). Several of these factors are mediated by inputs from the catchment, including terrestrially derived nutrients and dissolved organic matter (DOM).
While studies specific to Northern Pike are lacking, Finstad et al. (2014) found that increases in terrestrially derived dissolved organic carbon (DOC) resulted in a unimodal response in biomass of Brown Trout (Salmo trutta) in southern Norway. Fish biomass increased with DOC concentrations to reach a maximum at 3 mg/L; above this concentration of DOC, fish biomass decreased. An initial increase in fish biomass in response to increasing DOC concentrations (up to 4 mg/L) was also observed for Yellow Perch (Perca flavescens) in several boreal lakes in north-central Ontario, Canada (Tanentzap et al. 2014). When concentrations of DOC are relatively low (< 4 mg/L), positive relationships between DOC and fish biomass and growth rate have been attributed to energy subsidization, screening of UV-radiation, and increased loading of co-varying nutrients (e.g., phosphorus and nitrogen), which stimulate in-lake primary production (Finstad et al. 2014; Tanentzap et al. 2014). When DOC exceeds 3–4 mg/L (up to 22 mg/L), declines in fish biomass and growth rates have been documented in several freshwater fish species, including Pikeperch (Sander lucioperca), Walleye (Sander vitreus), Lake Trout (Salvelinus namaycush), and European Perch (Perca fluviatilis). These declines have been largely attributed to light attenuation and consequent impairing effects on water clarity, primary production, predator–prey interactions, metabolism, habitat suitability, foraging area, and feeding efficiency (e.g., Karlsson et al. 2009, 2015; Stasko et al. 2012; Finstad et al. 2014; Ranåker et al. 2014; Craig et al. 2017; van Dorst et al. 2019).
Effects of DOC concentrations on lake productivity are affected by the structural and chemical properties of DOM, which regulate its availability for biota at the base of food chains (see Nebbioso and Piccolo 2013; Creed et al. 2018). Terrestrial DOM that is delivered to freshwaters in runoff is predominantly derived from soil organic matter, which in turn is mainly composed of decomposition products of terrestrial vegetation. Soil-derived DOM exported from catchments tends to be aromatic in character, and higher molecular weight (e.g., tannins, phenols), as lower molecular weight compounds have already been metabolized in soils at earlier stages of decomposition (Kalbitz et al. 2003; Yang et al. 2021). Thus, terrestrially derived DOM tends to be less bioaccessible and less energy-rich, whereas DOM derived from in-lake primary production tends to be fresher in character, with more short-chain, low molecular weight compounds (proteins, sugars, amino acids) that may be quickly metabolized (Taipale et al. 2014; Brett et al. 2017). Relative proportions of catchment—versus lake—derived organic matter can, therefore, affect lake productivity and, ultimately, fish growth. As quantity and quality of DOM exported from terrestrial environments to downstream lakes is influenced by a myriad of catchment attributes, including area, elevation, slope, and land cover composition (e.g., Williams et al. 2010; Dranga et al. 2017; Kritzberg et al. 2020; Toming et al. 2020), gaining a fulsome understanding of among-system variability in fish growth rates requires comprehensive data on catchment and lake physicochemistry, in addition to data on in-lake biota. There is a general paucity of studies that link catchment characteristics with water chemistry and fish growth rates, and this is particularly true in remote northern regions, where food security and local economies are often reliant on healthy fisheries (Islam and Berkes 2016; Martin et al. 2020), and where climate-induced change to land cover is particularly rapid (e.g., Wang et al. 2020).
In the Dehcho Region, Northwest Territories (NT), Canada, Northern Pike are locally harvested as a subsistence food source for First Nations (Stewart and Low 2000). Along with other species (e.g., Walleye, Sander vitreus, and Lake Whitefish, Coregonus clupeaformis), Northern Pike also used to support commercial fisheries in some regional lakes during the second half of the twentieth century (Stewart and Low 2000; DFO 2010). Commercial harvest has been suspended since the early-2000s due to stock collapses (Kennedy 1962; DFO 2010), but local subsistence harvests of Northern Pike continue. Harvests of Northern Pike and associated human consumption have been affected by high mercury concentrations in some lakes in the Dehcho Region, and among-lake variability in mercury concentrations has been partially linked to fish size-at-age or growth rates (e.g., Evans et al. 2005; Lockhart et al. 2005). Despite local dietary significance and the known role of growth rate in regulating mercury concentrations in Northern Pike (e.g., Sharma et al. 2008), little is known about what drives variability in growth rates in Northern Pike in this large subarctic region. Local Indigenous fishers have suggested that changes to the land and to inflowing waters are affecting growth and feeding of several fish species in lakes, including Northern Pike. Interactions among fish ecology and lake and catchment physicochemistry thus deserve further investigation.
The objectives of this study were to quantify variability in Northern Pike growth rate among eleven lakes located within a 60,000 km2 area in the Dehcho Region, NT, and to investigate factors in-lake waters and catchments that may explain this variability. Indigenous knowledge regarding long-term changes in lake catchments and inflows, water color (tannins), amount of algae in nearshore lake waters, and fish numbers and quality, led to several predictions. We tested the prediction that growth rate in Northern Pike would be lower in lakes subject to more catchment influence, which we quantified using measures of water chemistry, lake area, catchment area, lake area to catchment area ratios, catchment elevation, catchment slope, and land cover composition of catchments. We also investigated whether growth rate in Northern Pike was higher in lakes with more in-lake primary production. Finally, relationships between growth and trophic ecology of Northern Pike were investigated by relating growth rates to stable Nitrogen and Carbon isotope ratios (used to infer trophic position and carbon source, respectively).
Methods
Field sampling
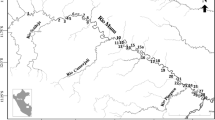
As part of a large, collaborative, and community-driven study on fish from the Dehcho Region, NT, eleven lakes (Fig. 1) were comprehensively sampled over a period of 7 years, from 2013 to 2019. Sampling was conducted by a joint University-Indigenous guardian crew, and took place each year in mid-August to mid-September. Between one and three lakes were sampled each year, depending on access (e.g., float plane and remote camp vs road access). Some lakes were sampled in more than 1 year to reflect community priorities and/or results from interim analyses. A full schedule of sampling efforts is presented in Table S1 (Supplementary Information). Four ecoregions were represented by the study lakes, including the Horn Plateau (Willow, Big Island, Mustard lakes), Northern Alberta Upland (Trout Lake), Hay River Lowland (Ekali, Gargan, Sanguez, McGill, Kakisa, Tathlina lakes), and Norman Range (Fish Lake).
Location of the study lakes in the Dehcho Region, NT, Canada. Willow, Big Island, and Mustard lakes are located in the Horn Plateau ecoregion, Trout Lake is located in the Northern Alberta Upland ecoregion, Fish Lake is located in the Norman Range ecoregion, and all other lakes are located in the Hay River Lowland ecoregion. Base data
In each lake, Northern Pike were caught using multi-mesh gill nets, with stretched mesh size ranging from 20 to 140 mm. Captured Northern Pike were measured on-site for fork length (FL). Northern Pike > 120 mm FL were then weighed (to the nearest g) and processed in the field, whereas individuals < 120 mm FL were frozen whole before being shipped to the laboratory for further processing. Dorsal muscle tissue (skinless) samples were extracted from each large fish for stable isotope analysis, and cleithra were extracted to estimate age, and subsequently growth rate (Casselman 1990; Faust et al. 2013). All tissues were frozen at − 20 °C in the field. Care was taken to ensure that large fish were maintained in a condition that was suitable for human consumption after sampling, and were supplied to band members of partner First Nations communities after sampling was complete.
Benthic invertebrates were collected from each lake to facilitate among-lake comparisons of stable isotope ratios in fish (i.e., baseline correction; Post 2002). Because fractionation can vary between littoral and pelagic primary producers during DIC uptake (France 1995), and because Northern Pike primarily feed in nearshore habitats (Vøllestad et al. 1986; Craig 1996), benthic invertebrates were collected from littoral, nearshore areas using dip nets (250 μm mesh size) to represent the isotopic baseline. Benthic invertebrates were sorted and identified to Family in the field, and frozen in the field at − 20 °C.
Lake water samples were collected near the surface (approximately 30 cm below the surface) from each lake. The remote nature and challenging logistics associated with comprehensive sampling of the study lakes prevented sampling of all lakes within 1 year. We were, however, particularly concerned with among-lake variation in water chemistry parameters being confounded with among-year variation. To address this, all study lakes were sampled for water chemistry in two dedicated campaigns in both 2018 and 2019. Samples were collected from each lake via floatplane within a period of 1 week in late August in each of 2018 and 2019. Water samples were collected from the approximate center of lakes. Known volumes of surface water were grab-collected in new, sterile amber Nalgene bottles, and were filtered on-site through ethanol-rinsed 0.42 μm Whatman® GF/F filters before being frozen for analysis of chlorophyll-a (Chl-a), a proxy for primary production. 60 ml of surface water was pumped directly from the lake through muffled quartz QMA filters using a peristaltic pump, stored in amber glass bottles, and kept cool for analyses of DOC, DIC, UV Absorbance at 254 nm (abs254), Freshness Index, and Specific UV Absorbance (SUVA). Approximately 500 mL of surface water was collected and kept cool for analyses of concentrations of nutrients and ions, including total phosphorus (TP), total nitrogen (TN), sodium (Na), calcium (Ca), manganese (Mg), chloride (Cl), alkalinity (measured as CaCO3), bicarbonate (measured as HCO3), and conductivity. At the time of water sampling, water clarity was characterized using a Secchi disk.
Laboratory processes
At the University of Waterloo (ON, Canada), frozen Northern Pike < 120 mm FL were thawed to room temperature, blotted to remove excess moisture, and then measured, weighed, and processed. Skinless dorsal muscle tissue samples and cleithra were removed and frozen at − 20 °C. All frozen Northern Pike and benthic invertebrate tissues (shells were removed, where applicable) were freeze-dried at − 54 °C and 0.014 mBar for 48 h using a LabConco® FreeZone freeze-drier (USA). Freeze-dried samples were homogenized using ultra-fine scissors and glass rods inside borosilicate scintillation vials, before being weighed (0.3–0.35 mg) into tin cups using a Mettler-Toledo® Analytical Microbalance model XP05DR (Switzerland). Prepared samples were analyzed for δ13C and δ15N using a Thermo Fisher Scientific® Delta XL continuous-flow isotope ratio mass spectrometer (USA) connected to a Fisons Instruments® 1108 Elemental Analyzer (UK) at the University of Waterloo Environmental Isotope Laboratory. Values of δ13C and δ15N are expressed in parts per mil (‰) relative to international standards of Vienna Pee-Dee Belemnite and N2 gas, respectively. Precision was estimated using duplicate samples that were completed for approximately 9% (n = 38) and 28% (n = 160) of Northern Pike and benthic invertebrate samples, respectively. Mean absolute difference (± one standard deviation) in δ15N and δ13C between duplicate samples was 0.001 (± 0.105) ‰ and 0.079 (± 0.229) ‰, respectively, for Northern Pike, and 0.038 (± 0.438) ‰ and 0.013 (± 0.437) ‰, respectively, for benthic invertebrates.
Whole cleithra were hand-cleaned to remove soft tissue after being immersed in warm water for 10–20 s. While placed beside a fixed ruler on a lab bench, air-dried cleithra were then photographed using a Nikon D7500 DSLR digital camera (Japan). Widths of annuli (i.e., yearly growth increments interpreted as alternating translucent and opaque zones under reflected light) were measured (in mm) from the origin to the outermost edge of cleithra along the anterior length (Craig 1996), with age calculated as the number of annuli. The outer limit of each translucent zone was considered as the formation point of the next annulus. For quality control, images were examined blind by an experienced reader twice, with a 3-week time interval between reads. Samples were examined for a third time if age estimates differed between the first two reads, and samples were not used in further analyses if the third reading did not agree with one of the first two.
Analyses of Chl-a, TP, and TN were conducted at the University of Alberta Biogeochemical Analytical Service Laboratory in Edmonton (AB, Canada). Briefly, Chl-a was extracted with 95% ethanol on a filter overnight and measured using a Shimadzu® RF-1501 Spectrofluorophotometer (North America Analytical & Measuring Instruments), and TP and TN analyses were performed by Flow Injection Analysis using a Lachat® QuikChem 8500 FIA automated analyzer (Canada). Analyses of DIC, DOC, abs254, Freshness Index, SUVA, Na, Ca, Mg, Cl, alkalinity, bicarbonate, and conductivity were conducted at the Western University Biotron Center for Experimental Climate Change Research in London (ON, Canada). Concentrations of DIC and DOC were determined using phosphoric acid digestion followed by persulphate oxidation on an Aurora® 1030 W total organic carbon analyzer (USA). Fluorescence and absorbance measures were made with appropriate corrections on a Horiba Aqualog® spectrofluorometer (Japan). Absorbance at 254 nm (abs254) is reported directly as an absolute measure of total absorbance characteristics of higher molecular weight DOM. Freshness Index (a measure of the ratio of more recently produced to more decomposed DOM; Parlanti et al. 2000) was calculated as the ratio of emission at 380 nm and the emission maximum between 420 and 235 nm at an excitation wavelength of 310 nm. Specific absorbance at 254 nm (SUVA), a measure of the degree of aromaticity of DOM, was calculated as abs254 divided by DOC concentrations (Weishaar et al. 2003). Analyses of Na, Ca, Mg, Cl, alkalinity, bicarbonate, and conductivity were performed using a Dionex ICS-1600 reagent-free ion chromatography system (USA) and a AS-50 autosampler (USA).
Lake trophic states
Trophic state was quantified for each lake using Carlson’s Trophic State Index (CTSI; Carlson 1977) and measures of Chl-a, TP, and Secchi depth (Secchi) with the following equations:
where ln was natural logarithm. Following Fuller and Jodoin (2016), lakes were then classified into one of four categories: oligotrophic (i.e., low productivity) for CTSI < 38, mesotrophic (i.e., moderate productivity) for 38 < CTSI > 48, eutrophic (i.e., high productivity) for 48 < CTSI > 61, and hypereutrophic (i.e., extreme productivity) for CTSI > 61. The ratio of TN to TP (TN:TP) was used to assess whether nitrogen or phosphorus limited primary production in each lake (e.g., Hecky et al. 1993).
Geospatial analysis
We used publicly available geospatial data on the Government of Canada portal to identify ecoregions and quantify lake and catchment characteristics. Data were accessed in May 2019 and analyzed using the coordinate system ESPG:3581 NAD83(CSRS)/NWT Lambert. For each lake, we calculated total catchment area (CA) based on the HRDEM v1.3 (HRDEM Natural Resource Canada 2021). This same source was used to delineate watershed divides, and obtain mean elevation and mean slope in most catchments, but the headwaters of Kakisa and Tathlina lakes are not included in this data layer; thus, the Canadian Digital Elevation Model 1945–2011 (CDEM Natural Resource Canada 2021) was used for these lakes. Lake surface areas (LA) and land cover compositions for each catchment were calculated based on the 30-m resolution 2015 Land Cover of Canada data layer (LCC Natural Resource Canada 2015). Land cover in our study area included ten categories: Wetland, Water, Temperate or Subpolar Shrubland, Temperate or Subpolar Needleleaf Forest, Temperate or Subpolar Broadleaf Deciduous Forest, Subpolar Taiga Needleleaf Forest, Subpolar or Polar Shrubland, Subpolar or Polar Grassland, Mixed Forest, and Barren Land. Detailed information about each category is available in Natural Resources Canada (2019). Land cover data were expressed as percentages of CA. Finally, the ratio of lake area to catchment area (LA:CA) was calculated by dividing LA to CA. Analyses of lake and catchment characteristics were done using QGIS v3.12.2 (QGIS Development Team 2020), GDAL/OGR (GDAL/OGR Contributors 2020), PostgreSQL/PostGIS v2.5.4 (The PostGIS Development Group 2020), and WhiteboxTools (Lindsay 2016), as well as with R packages “raster” v3.3-13 (Hijmans 2020) and “rgdal” v1.5-16 (Bivand 2020).
Data analysis
Statistical analyses were completed using RStudio v1.3.959 (RStudio Team 2020) integrated with R v4.0.1 (R Core Team 2020) and core packages, unless otherwise noted. Residuals of all linear models were visually assessed using residual plots, and tested for normality using Shapiro–Wilk tests. Data were log10-transformed where necessary to meet assumptions.
Lake-specific growth rates in Northern Pike were quantified using the following equation proposed by Gallucci and Quinn (1979):
where k is the growth coefficient parameter of the von Bertalanffy growth equation (von Bertalanffy 1938), and ω is an estimate of early growth rate, in a length-per-time unit. The ω growth parameter was chosen for among-lake comparison of growth because: (i) it is statistically robust, and its use in comparisons avoids problems of analyzing and interpreting the interdependent growth coefficient and asymptotic length parameters estimated by the von Bertalanffy growth equation (Gallucci and Quinn 1979); (ii) it facilitates comparisons with similar studies (e.g., Benoît et al. 2016); and, (iii) ω provides an estimate of growth rate in early life, when environmental conditions are particularly influential on fish growth rates (Pankhurst and Munday 2011). To fit growth models, we used incremental width data, which were measurements of individual annuli (in mm) made on images of cleithra using the package RFishBC v0.2.3 (Ogle 2019). This avoided the bias that can be introduced when fish size at previous ages is estimated through back-calculation (Francis 1990; Höhne et al. 2020). Fish size at a given age was represented by the size of cleithra at a given annulus (i.e., distance from the origin to ith annulus of cleithra along the anterior length). To check the assumption that size of cleithra represented the size of fish, distance from the origin to the outermost edge of cleithra along the anterior length was regressed against FL of corresponding individuals. A significant and positive linear relationship was found (y = 24.292 + 9.83x, adjusted r2 = 0.983). Incremental data did not appear to suffer from the Rosa Lee phenomenon (i.e., smaller estimated sizes for a given age in older fish compared to younger fish), according to inspection following Duncan (1980). Age 0+ Northern Pike were excluded from growth analyses, as were annuli that represented growth (since winter annulus formation) in the year of capture. Lake-specific growth models were fit with the package FSA v0.8.30 (Ogle et al. 2020) using incremental data for all fish in a given lake.
Relationships among the physiochemical variables for all lakes and their catchments were investigated using two principal component analyses (PCA) that were performed in the package vegan v2.5-6 (Oksanen et al. 2019). First, a PCA was completed on land cover data. A second PCA was performed using lake chemistry variables, including Chl-a, DOC, DIC, abs254, Freshness Index, SUVA, TP, TN, Na, Ca, Mg, Cl, alkalinity, bicarbonate, and conductivity. Lake area to catchment area ratios, mean catchment elevation, mean catchment slope, and lake-specific PC1 and PC2 scores of the land cover PCA were also included as variables in this second PCA. All data were z-transformed (i.e., mean-subtracted and divided by standard deviation) before PCAs were completed.
To investigate whether among-lake variation in growth rate of Northern Pike could be explained by variability among lakes in primary productivity, water clarity, inferred terrestrial influence, or fish trophic ecology, lake-specific estimates of growth rate (ω) in Northern Pike were related to Chl-a, Secchi depth, DOC concentrations, PC1 and PC2 scores from the second PCA (inferred catchment influence and in-lake productivity, respectively; see Results), and Northern Pike trophic level (δ15Nadj) and dietary carbon source (δ13C). Simple linear regressions (LR) were used, and alpha was set at 0.05. Each lake was treated as a replicate (n = 11). Delta13C and δ15Nadj ratios for Northern Pike were estimated at 560 mm FL, which was accomplished using an analysis of covariance (i.e., ANCOVA) in the package lsmeans v2.30-0 (Lenth 2016); mean FL of all Northern Pike sampled was 565.5 mm (median = 562.0 mm), and no extrapolation was necessary to estimate lake-specific isotope ratios at 560 mm. Delta15N values for individual Northern Pike were first adjusted for among-lake variation in baseline (δ15Nbase) (Post 2002) using the following equation:
Delta15N ratios of samples from the Sphaeriidae family were used for baseline adjustments (Post 2002). Following initial analyses focused on Northern Pike, linear regressions were also used to relate δ13C ratios in benthic invertebrates to PC1 and PC2 scores from the second PCA.
Results and discussion
Characteristics of lakes and inferred catchment influence
There was considerable variability in physical and chemical characteristics among the studied lakes and catchments (Table 1; Fig. 2). Lake and catchment areas varied by more than two orders of magnitude; LA ranged from 1 to 565 km2, and CA ranged from 113 to 15,809 km2 (Table 1). The LA:CA varied from 0.002 to 0.161, with extremes represented by McGill Lake (small lake with large catchment) and Big Island Lake (medium lake with small catchment). Lakes located in the Horn Plateau ecoregion were an order of magnitude larger relative to their catchments than lakes located in other ecoregions (Table 1; Fig. 2).
Lakes located on the Horn Plateau, as well as Fish Lake, located in the Norman Range ecoregion, had different catchment land cover compared to lakes located in the other ecoregions (Fig. 2). These higher-elevation/latitude lakes (i.e., Big Island, Mustard, Willow, and Fish) were clearly separated from lakes located in the Hay River Lowland (i.e., Ekali, Sanguez, Gargan, and McGill, Trout, Kakisa, and Tathlina) along the horizontal axis (i.e., PC1) of the land cover PCA (Fig. 3a). Subpolar/polar land cover types were negatively associated with PC1 whereas temperate/subpolar land cover types were positively associated with PC1, and this axis explained 65% of total variance in land cover composition (Fig. 3a). For example, while subpolar taiga Needleleaf forest covered 10–20% of catchment areas of lakes located at higher elevations or at higher latitudes (Big Island, Willow, Mustard, Fish), this forest type covered only 0.01–1% of the catchment areas for lakes located in the Hay River Lowland (i.e., Ekali, Sanguez, Gargan, and McGill, Trout, Kakisa, and Tathlina) (Fig. 2). Variability in the proportion of wetland area primarily drove separation along PC2, which explained another 20% of variance in catchment land cover (Fig. 3a). Lake-specific PC1 and PC2 scores of the land cover PCA were extracted and renamed “region” and “wetland”, respectively (Fig. 3a), for use in the second PCA (Fig. 3b).
Biplots of Principal Component Analyses (PCA). Left (a) depicts PCA performed using proportional land cover data (i.e., Fig. 2), and right (b) represents PCA performed using all variables presented in Table 1, as well as PC1 and PC2 scores from the analyses depicted in (a). Axes of the land cover PCA (a) were inferred to represent variability driven by ecoregion (PC1) and proportional cover of wetlands (PC2). Axes of the PCA that included all variables (b) were inferred to represent a gradient of catchment influence (PC1) and primary productivity (PC2)
Patterns among lakes and ecoregions in catchment size and composition were reflected in water chemistry, and results from the second PCA reflect a gradient of catchment influence along PC1 (Fig. 3b). Lakes located in larger catchments (relative to lake area) and with steeper-sloped catchments were characterized by higher levels of tracers that reflect terrestrial inputs, including higher levels of ions, DOC, DIC, alkalinity, conductivity, and abs254 (Table 1; Fig. 3b). Variables such as conductivity, alkalinity, bicarbonate, Ca, Mg, and Cl are reflective of mineral weathering in the catchment (e.g., Kamenik et al. 2001; Singh et al. 2008), and have previously been used as indicators of catchment influence on lake ecosystems in studies of fish ecology (e.g., Burke et al. 2020). The horizontal axis (PC1) of the second PCA explained 62% of total variance in the dataset, and while elevation and LA:CA were negatively associated with this axis, mean catchment slope, TN, DOC, DIC, abs254, alkalinity, bicarbonate, conductivity, Na, Ca, Mg, and Cl were all positively associated with this axis (Fig. 3b). Higher-elevation/latitude lakes (i.e., Big Island, Mustard, Willow, and Fish) were negatively associated with PC1 whereas all lakes located in the Hay River Lowland (i.e., Ekali, Sanguez, Gargan, and McGill, Kakisa, and Tathlina) were positively associated with PC1. We thus infer that PC1 reflects a gradient of catchment influence from low (negative scores) for lakes in the Horn Plateau and Norman Range ecoregions, to high (positive scores) for lakes in the Hay River Lowland. Trout Lake, located in the Northern Alberta Upland ecoregion, grouped with the higher elevation/latitude lakes, which likely reflects its relatively large LA to CA ratio (Table 1; Fig. 3b).
The vertical axis (PC2) of the second PCA explained 17% of variation in this analysis, and was driven by proxies and indicators of in-lake primary production, including Chl-a and Freshness Index (Fig. 3b). Primary production varied independently of the patterns observed among ecoregions for catchment influence and LA:CA; both the lowest and highest Chl-a values were observed for lakes in the Hay River Lowland (McGill and Kakisa, respectively). Positive and close associations of TP, Chl-a, and Freshness along PC2 (Fig. 3b) suggested that primary productivity in most of the studied lakes was limited by phosphorus. With TN:TP > 20, ten of eleven studied lakes were found to be phosphorus-limited (Hecky et al. 1993), and mesotrophic or moderately productive (Table S2). Kakisa Lake, however, was nitrogen-limited (i.e., TN:TP < 20; Hecky et al. 1993), and eutrophic or highly productive (Table S2).
Northern Pike growth rates
A total number of 2953 annuli widths were measured on Northern Pike cleithra (n = 432) and used to model growth rates in Northern Pike. Detailed descriptive statistics of incremental width data for annuli are presented in Table S3 (Supplementary Information). Northern Pike growth rate (i.e., ω) varied twofold among lakes, and was lowest (10.3) in Tathlina Lake and highest (20.9) in Mustard Lake (Table 2).
Among-lake variability in Northern Pike growth rates was best explained by inferred catchment influence, whereas there was no clear relationship between Northern Pike growth rates and either in-lake primary production or water clarity. Log10-transformed Northern Pike growth rate was significantly and negatively (LR: F1,9 = 36.80, p < 0.001, adj. r2 = 0.78; Fig. 4a) related to PC1 scores from the second PCA (Fig. 3b), which we inferred to reflect catchment influence; growth rates in Northern Pike were higher in lakes with lower inferred catchment influences. Consistent with this, we also found a significant negative relationship (F1,9 = 18.44, p = 0.002, adj. r2 = 0.64) between ω (log10) and DOC (log10) concentrations (Fig. 4b). While previous researchers have found that catchment influence or DOC concentrations have affected growth rates in other fish species, such as Pikeperch (Ranåker et al. 2014), Walleye (Stasko et al. 2015; Benoît et al. 2016), Lake Trout (Benoît et al. 2016), Bluegill (Lepomis macrochirus) (Craig et al. 2017), and European Perch (van Dorst et al. 2019) through effects on primary productivity and/or water clarity, our results suggest that this was not the case in our study. There were no significant relationships between Northern Pike growth rates (log10) and Chl-a (log10), Carlson’s trophic state index, PC2 (Chl-a, Freshness Index, TP, SUVA; Fig. 3b), or secchi depth (LR, F1,9 = ≤ 0.08, p ≥ 0.269, adj. r2 ≤ 0.04), and there were also no significant relationships between DOC (log10) and Chl-a (log10) (LR: F1,9 = 0.20, p = 0.663), or between DOC (log10) and water clarity (i.e., Secchi) (LR: F1,9 = 1.64, p = 0.232). In these generally well-mixed and relatively shallow lakes, it is likely that water clarity is influenced more by suspended sediments than DOC concentrations, although data that span more of the open-water season would be required to test this assertion.
Linear regressions between lake-specific estimates of Northern Pike growth rate (ω) and (a) inferred catchment influence (PC1 scores in Fig. 3b), and (b) concentrations of dissolved organic carbon
One possible explanation for the negative relationship between Northern Pike growth rates and inferred catchment influence is the quality of terrestrially derived basal food resources. There was a strong positive correlation (Pearson r = 0.93, p < 0.001) between DOC (log10) and abs254 (log10), and between DOC and other variables indicative of catchment weathering (e.g., conductivity, alkalinity, bicarbonate, Ca, Mg, and Cl; Kamenik et al. 2001; Singh et al. 2008) along PC1 (Fig. 3b), which indicates that the greater the catchment influence, the greater the inputs of more aromatic, high molecular weight terrestrial organic matter. Organic matter derived from terrestrial environments is often of relatively lower nutritional quality compared to DOM derived from internal production, which provide relatively higher levels of essential amino acids and macronutrients (Taipale et al. 2014; Brett et al. 2017). Further, elevated quantities of DOC from terrestrially derived sources do not compensate for the poorer quality of these resources (Hiltunen et al. 2019).
Our proposed mechanism for the negative relationship between Northern Pike growth rates and inferred catchment influence (i.e., lower-quality DOM from the catchment) is consistent with results of our analysis on Northern Pike growth rates and trophic ecology. Lake-specific growth rate in Northern Pike (i.e., log10 ω) was not related to lake-specific least squares mean δ15Nadj (LR: F1,9 = 0.79, p = 0.398) nor to lake-specific least squares mean raw (unadjusted) δ15N (LR: F1,9 = 0.074, p = 0.792); lake-specific least squares mean δ15Nadj (and δ15N) in Northern Pike varied less than three ‰ (~ one trophic level), indicating that Northern Pike feed at approximately the same trophic level in all studied lakes. There was, however, a significant and positive relationship between lake-specific log10 ω and least squares mean δ13C (LR: F1,9 = 31.08, p < 0.001, adj. r2 = 0.75; Fig. 5a). To determine if the positive relationship between Northern Pike growth and δ13C reflected among-lake differences in habitat use of Northern Pike (i.e., the more conventional interpretation) or among-lake differences in sources of DIC, we related δ13C of a representative baseline benthic invertebrate family (lake-specific arithmetic mean δ13C of Sphaeriidae) to inferred catchment influence (i.e., PC1 scores of the second PCA), with which DIC was strongly and positively associated (Fig. 3b). There was a significant negative relationship between δ13C of Sphaeriidae and inferred catchment influence (LR: F1,9 = 33.19, p < 0.001, adj. r2 = 0.77; Fig. 5b). Negative relationships between inferred catchment influence and δ13C were also observed when this analysis was conducted on other benthic invertebrate taxa that were captured in most or all lakes (Chironomidae, Dytiscidae, Corixidae, Lymnaeidae), as well as when all benthic invertebrate taxa were combined (Tables S4 and S5 in Supplementary Information). Relationships were stronger when analyses were restricted to individual taxonomic families, which is not surprising given the diversity of feeding tactics among analyzed taxa, and the fact that fractionation of carbon isotopes (13C and 12C) can vary among functional feeding groups (Woodland et al. 2012; Anas et al. 2019b). Overall, the results indicate that benthic invertebrates had more depleted δ13C ratios in lakes subject to more catchment influence, which is consistent with the findings of Anas et al. (2019a). Given that δ13C ratios of benthic invertebrates varied among lakes in a pattern similar to that of Northern Pike, and that a large proportion of this variability was explained by inferred catchment influence (e.g., 77% for Sphaeriidae), we infer that among-lake variability in δ13C ratios in Northern Pike reflected variability in the sources of DIC, rather than variability in habitat use. This inference is supported by a significant, strong, and positive (Pearson correlation, r = 0.93, df = 9, p < 0.001) relationship between lake-specific δ13C ratios in Northern Pike and lake-specific δ13C ratios in littoral benthic invertebrates (all taxa combined).
Results of linear regressions between (a) Northern Pike growth rate and least squares mean δ13C (calculated at 560 mm FL), and (b) mean δ13C of baseline benthic invertebrate family and inferred catchment influence (PC1 scores from in Fig. 3b)
Benthic consumers reliant on terrestrially derived organic matter tend to be relatively less nutritious (in terms of fatty acids and other macro nutrients) than those assimilating internally produced resources (e.g., Lau et al. 2009), although terrestrially derived organic matter can support consumer growth and production to some extent (e.g., Kelly et al. 2014). Given the low nutritional value and depleted δ13C ratios previously observed in benthic consumers that rely more on terrestrial resources (Lau et al. 2009; Anas et al. 2019a), we suggest that our observation of slower growth of Northern Pike in lakes with greater inferred catchment influence reflects relatively lower food quality that propagates through trophic levels. Further research, ideally studies that include fatty acid concentrations, would provide an independent test of our inference of lower nutritional quality in basal resources of lakes that are more influenced by their catchments. Further, we suggest that future research into relationships between terrestrial influence and δ13C ratios in fishes should ideally include species that are known to feed mostly in littoral habitats, (e.g., pike), mostly in pelagic habitats (e.g., adult cisco), and in both pelagic and littoral habitats (e.g., lake trout); as pike are known to occupy and feed mostly in littoral habitats and have relatively small home ranges, effects of whole-lake drivers (such as terrestrial influence)—especially those that affect nutrient cycling in the littoral zone—may be easier to detect.
As a lie-in-wait predator, feeding success and growth of Northern Pike are believed to depend most strongly on population density, prey-predator interactions, and the presence of submerged aquatic plants (Vøllestad et al. 1986; Diana 1987; Casselman and Lewis 1996; Craig 1996; Margenau et al. 1998). Our sampling relied heavily on subsistence harvest practices, and was not designed to allow quantification of fish community composition or relative abundance. Qualitative information retrieved from our gill nets (Table S6), however, indicate that Lake Whitefish are present in the majority of study lakes (n lake = 10 of 11); juvenile Lake Whitefish are a preferred prey item for Northern Pike (Scott and Crossman 1973). Other commonly observed small-bodied fish species (potential prey items for Northern Pike) include Cisco (Coregonus artedi) and Ninespine Stickleback (Pungitius pungitius). Diversity of forage fish species appears to vary among lakes (Table S6), however, and this likely affects growth of Northern Pike in a way that we cannot currently quantify. The presence of potential competitors also varied among lakes; we observed Lake Trout in lakes where Northern Pike grew relatively faster (Big Island, Willow, and Mustard lakes), and Walleye in lakes where Northern grew relatively slower (e.g., Sanguez, Tathlina, Ekali, and McGill lakes). Future research on the presence/absence and relative abundance of competitor fishes and prey fishes, as well as coverage of submerged aquatic vegetation, could yield additional insights into among-lake variability that we cannot yet explain in Northern Pike growth rates in these remote and understudied lakes that support food security and overall well-being of northerners in the region.
Conclusion
The Dehcho Region is located at subarctic latitudes, where climate warming is having profound effects on lake-catchment interactions via accelerated permafrost thaw, and changes in fire regimes and hydrology (Prowse et al. 2006; Hugelius et al. 2020). To our knowledge, this is the first study that integrates food web, water, lake, and catchment data to explore causes of among-lake variability in growth of Northern Pike, an important subsistence and commercial species across northern latitudes. Our results revealed that growth rate in Northern Pike decreased with increasing catchment influence on lake water chemistry. This may be driven by the lower nutritional value and bioaccessibility of terrestrially derived organic matter compared to more readily bioaccessible organic matter that is produced internally. Further research that included fatty acid concentrations and prey-predator interactions could lend further insight into among-lake variability in growth rates of Northern Pike. As many lake and catchment attributes included in our study can be quantified and monitored remotely (e.g., remote sensing), our findings may accelerate development of models for growth rate, especially in remote regions where field data are where fish are a primary subsistence food source. Our findings also aid in identifying and predicting faster and slower growing populations of Northern Pike in subarctic lakes, which is of significance for toxicologists and fisheries managers, given the vital regulatory role of growth rate in fish population dynamics, fisheries sustainability, and contaminant accumulation in fish tissues.
Data availability
Raw data used in this research is available on Polar Data Catalogue (CCIN Reference No: 13080) and in the online Supplementary Information.
References
Anas MUM, Scott KA, Wissel B (2019a) Water chemistry, landscape, and spatial controls of δ13C and δ15N of zooplankton taxa in boreal lakes: one size does not fit all. Freshw Biol 64:2006–2025. https://doi.org/10.1111/fwb.13389
Anas MUM, Simpson GL, Leavitt PR et al (2019b) Taxon-specific variation in δ13C and δ15N of subfossil invertebrate remains: insights into historical trophodynamics in lake food-webs. Ecol Indic 102:834–847. https://doi.org/10.1016/j.ecolind.2019.03.026
Benoît P-O, Beisner BE, Solomon CT (2016) Growth rate and abundance of common fishes is negatively related to dissolved organic carbon concentration in lakes. Can J Fish Aquat Sci 73:1230–1236. https://doi.org/10.1139/cjfas-2015-0340
Bivand R (2020) rgdal: Bindings for the “Geospatial” Data Abstraction Library. R package version 1.5-16. https://CRAN.R-project.org/package=rgdal
Brett MT, Bunn SE, Chandra S et al (2017) How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw Biol 62:833–853. https://doi.org/10.1111/fwb.12909
Burke SM, Zimmerman CE, Laske SM et al (2020) Fish growth rates and lake sulphate explain variation in mercury levels in ninespine stickleback (Ungitius pungitius) on the Arctic Coastal Plain of Alaska. Sci Total Environ 743:140564. https://doi.org/10.1016/j.scitotenv.2020.140564
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369. https://doi.org/10.4319/lo.1977.22.2.0361
Casselman JM (1978) Effects of environmental factors on growth, survival, activity, and exploitation of northern pike. Am Fish Soc Spec Publ 11:114–128
Casselman JM (1990) Growth and relative size of calcified structures of fish. Trans Am Fish Soc 119:673–688. https://doi.org/10.1577/1548-8659(1990)119%3c0673:GARSOC%3e2.3.CO;2
Casselman JM, Lewis CA (1996) Habitat requirements of northern pike (Esox lucius). Can J Fish Aquat Sci 53:161–174. https://doi.org/10.1139/f96-019
CDEM (Natural Resource Canada) Canadian Digital Elevation Model 1945-2011, Natural Resource Canada. https://open.canada.ca/data/en/dataset/7f245e4d-76c2-4caa-951a-45d1d2051333. Accessed 22 Jan 2021
Craig J (ed) (1996) Pike: biology and exploitation. Springer, Netherlands
Craig JF, Babaluk JA (1989) Relationship of condition of walleye (Stizostedion vitreum) and northern pike (Esox lucius) to water clarity, with special reference to Dauphin Lake, Manitoba. Can J Fish Aquat Sci 46:1581–1586. https://doi.org/10.1139/f89-201
Craig N, Jones SE, Weidel BC, Solomon CT (2017) Life history constraints explain negative relationship between fish productivity and dissolved organic carbon in lakes. Ecol Evol 7:6201–6209. https://doi.org/10.1002/ece3.3108
Creed IF, Bergström A-K, Trick CG et al (2018) Global change-driven effects on dissolved organic matter composition: implications for food webs of northern lakes. Glob Change Biol 24:3692–3714. https://doi.org/10.1111/gcb.14129
DFO (2010) Assessment of walleye (Sander vitreus) from Tathlina Lake, Northwest Territories. Canadian Science Advisory Secretariat, 2010/035. https://publications.gc.ca/site/eng/396897/publication.html
Diana JS (1987) Simulation of mechanisms causing stunting in northern pike populations. Trans Am Fish Soc 116:612–617. https://doi.org/10.1577/1548-8659(1987)116%3c612:SOMCSI%3e2.0.CO;2
Dranga SA, Hayles S, Gajewski K (2017) Synthesis of limnological data from lakes and ponds across Arctic and Boreal Canada. Arct Sci 4:167–185. https://doi.org/10.1139/as-2017-0039
Duncan KW (1980) On the back-calculation of fish lengths; modifications and extensions to the Fraser-Lee equation. J Fish Biol 16:725–730. https://doi.org/10.1111/j.1095-8649.1980.tb03751.x
Evans MS, Lockhart WL, Doetzel L et al (2005) Elevated mercury concentrations in fish in lakes in the Mackenzie River Basin: the role of physical, chemical, and biological factors. Sci Total Environ 351–352:479–500. https://doi.org/10.1016/j.scitotenv.2004.12.086
Faust MD, Breeggemann JJ, Bahr S, Graeb BDS (2013) Precision and bias of cleithra and sagittal otoliths used to estimate ages of northern pike. J Fish Wildl Manag 4:332–341. https://doi.org/10.3996/062013-JFWM-041
Finstad AG, Helland IP, Ugedal O et al (2014) Unimodal response of fish yield to dissolved organic carbon. Ecol Lett 17:36–43. https://doi.org/10.1111/ele.12201
Forsman A, Tibblin P, Berggren H et al (2015) Pike Esox lucius as an emerging model organism for studies in ecology and evolutionary biology: a review. J Fish Biol 87:472–479. https://doi.org/10.1111/jfb.12712
France RL (1995) Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol Oceanogr 40:1310–1313. https://doi.org/10.4319/lo.1995.40.7.1310
Francis RICC (1990) Back-calculation of fish length: a critical review. J Fish Biol 36:883–902. https://doi.org/10.1111/j.1095-8649.1990.tb05636.x
Fuller LM, Jodoin RS (2016) Estimation of a Trophic State Index for selected inland lakes in Michigan, 1999–2013. U.S. Geological Survey. https://pubs.usgs.gov/sir/2016/5023/sir20165023.pdf
Gallucci VF, Quinn TJ (1979) Reparameterizing, fitting, and testing a simple growth model. Trans Am Fish Soc 108:14–25. https://doi.org/10.1577/1548-8659(1979)108%3c14:RFATAS%3e2.0.CO;2
GDAL/OGR Contributors (2020) GDAL/OGR geospatial data abstraction software library. Open Source Geospatial Foundation
HRDEM (Natural Resource Canada) High Resolution Digital Elevation Model v1.3, Natural Resource Canada. https://open.canada.ca/data/en/dataset/957782bf-847c-4644-a757-e383c0057995. Accessed 22 Jan 2021
Hecky RE, Campbell P, Hendzel LL (1993) The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogr 38:709–724. https://doi.org/10.4319/lo.1993.38.4.0709
Hijmans RJ (2020) raster: geographic data analysis and modeling. R package version 3.3-13. https://CRAN.R-project.org/package=raster
Hiltunen M, Peltomaa E, Brett MT et al (2019) Terrestrial organic matter quantity or decomposition state does not compensate for its poor nutritional quality for Daphnia. Freshw Biol 64:1769–1786. https://doi.org/10.1111/fwb.13368
Höhne L, Palmer M, Monk CT et al (2020) Environmental determinants of perch (Perca fluviatilis) growth in gravel pit lakes and the relative performance of simple versus complex ecological predictors. Ecol Freshw Fish. https://doi.org/10.1111/eff.12532
Hugelius G, Loisel J, Chadburn S et al (2020) Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw. Proc Natl Acad Sci 117:20438–20446. https://doi.org/10.1073/pnas.1916387117
Islam D, Berkes F (2016) Indigenous peoples’ fisheries and food security: a case from northern Canada. Food Secur 8:815–826. https://doi.org/10.1007/s12571-016-0594-6
Kalbitz K, Schwesig D, Schmerwitz J et al (2003) Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol Biochem 35:1129–1142. https://doi.org/10.1016/S0038-0717(03)00165-2
Kamenik C, Schmidt R, Kum G, Psenner R (2001) The influence of catchment characteristics on the water chemistry of mountain lakes. Arct Antarct Alp Res 33:404–409. https://doi.org/10.1080/15230430.2001.12003448
Karlsson J, Bergström A-K, Byström P et al (2015) Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology 96:2870–2876. https://doi.org/10.1890/15-0515.1
Karlsson J, Byström P, Ask J et al (2009) Light limitation of nutrient-poor lake ecosystems. Nature 460:506–509. https://doi.org/10.1038/nature08179
Kelly PT, Solomon CT, Weidel BC, Jones SE (2014) Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology 95:1236–1242. https://doi.org/10.1890/13-1586.1
Kennedy WA (1962) A report on Tathlina and Kakisa Lakes. Fish Res Board Can Manuscr. https://waves-vagues.dfo-mpo.gc.ca/Library/59286.pdf
King M (2007) Fisheries biology, assessment and management, 2nd edn. John Wiley & Sons, Ltd
Kritzberg ES, Hasselquist EM, Škerlep M et al (2020) Browning of freshwaters: consequences to ecosystem services, underlying drivers, and potential mitigation measures. Ambio 49:375–390. https://doi.org/10.1007/s13280-019-01227-5
Lau DCP, Leung KMY, Dudgeon D (2009) Are autochthonous foods more important than allochthonous resources to benthic consumers in tropical headwater streams? J North Am Benthol Soc 28:426–439. https://doi.org/10.1899/07-079.1
LCC 2015 (Natural Resource Canada) 2015 Land Cover of Canada, Natural Resource Canada. https://open.canada.ca/data/en/dataset/4e615eae-b90c-420b-adee-2ca35896caf6. Accessed 22 Jan 2021
Lenth RV (2016) Least-squares means: the R package lsmeans version 2.30-0. https://CRAN.R-project.org/package=lsmeans
Lindsay JB (2016) Whitebox GAT: a case study in geomorphometric analysis. Comput Geosci 95:75–84. https://doi.org/10.1016/j.cageo.2016.07.003
Lockhart WL, Stern GA, Low G et al (2005) A history of total mercury in edible muscle of fish from lakes in northern Canada. Sci Total Environ 351–352:427–463. https://doi.org/10.1016/j.scitotenv.2004.11.027
Margenau TL, Rasmussen PW, Kampa JM (1998) Factors affecting growth of northern pike in small northern wisconsin lakes. North Am J Fish Manag 18:625–639. https://doi.org/10.1577/1548-8675(1998)018%3c0625:FAGONP%3e2.0.CO;2
Martin C, Parlee B, Neyelle M (2020) Fishing livelihoods in the Mackenzie river basin: stories of the Délįne Got’ine. Sustainability 12:7888. https://doi.org/10.3390/su12197888
Natural Resources Canada (2019) General information product. Natural Resources Canada, p 119e. https://doi.org/10.4095/315659
Navarro JE, Johnson DL (1992) Ecology of stocked northern pike in two Lake Erie controlled wetlands. Wetlands 12:171–177. https://doi.org/10.1007/BF03160606
Nebbioso A, Piccolo A (2013) Molecular characterization of dissolved organic matter (DOM): a critical review. Anal Bioanal Chem 405:109–124. https://doi.org/10.1007/s00216-012-6363-2
Ogle DH (2019) RFishBC. R package version 0.2.3. https://CRAN.R-project.org/package=RFishBC
Ogle DH, Wheeler P, Dinno A (2020) FSA: fisheries stock analysis. R package version 0.8.30. https://CRAN.R-project.org/package=FSA
Oksanen J, Blanchet FG, Friendly M et al (2019) vegan: community ecology package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan
Pankhurst NW, Munday PL (2011) Effects of climate change on fish reproduction and early life history stages. Mar Freshw Res 62:1015–1026. https://doi.org/10.1071/MF10269
Parlanti E, Wörz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31:1765–1781. https://doi.org/10.1016/S0146-6380(00)00124-8
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Prowse TD, Wrona FJ, Reist JD et al (2006) Climate change effects on hydroecology of arctic freshwater ecosystems. Ambio 35:347–358. https://doi.org/10.1579/0044-7447(2006)35[347:CCEOHO]2.0.CO;2
QGIS Development Team (2020) QGIS geographic information system. Open source geospatial foundation project. QGIS Development Team
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ranåker L, Persson J, Jönsson M et al (2014) Piscivore-prey fish interactions: mechanisms behind diurnal patterns in prey selectivity in brown and clear water. PLoS ONE 9:e102002. https://doi.org/10.1371/journal.pone.0102002
RStudio Team (2020) RStudio: integrated development environment for R. RSudio, Boston
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada. Bulletin 184. Fisheries Research Board of Canada, Ottawa
Sharma CM, Borgstrøm R, Huitfeldt JS, Rosseland BO (2008) Selective exploitation of large pike Esox lucius—effects on mercury concentrations in fish populations. Sci Total Environ 399:33–40. https://doi.org/10.1016/j.scitotenv.2008.03.026
Singh AK, Mondal GC, Kumar S et al (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54:745–758. https://doi.org/10.1007/s00254-007-0860-1
Stasko AD, Gunn JM, Johnston TA (2012) Role of ambient light in structuring north-temperate fish communities: potential effects of increasing dissolved organic carbon concentration with a changing climate. Environ Rev 20:173–190. https://doi.org/10.1139/a2012-010
Stasko AD, Johnston TA, Gunn JM (2015) Effects of water clarity and other environmental factors on trophic niches of two sympatric piscivores. Freshw Biol 60:1459–1474. https://doi.org/10.1111/fwb.12581
Stewart DB, Low G (2000) A review of information on fish stocks and harvests in the Deh Cho area, Northwest Territories. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2549: iv + 73 p. https://www.researchgate.net/publication/281667122_A_review_of_information_on_fish_stocks_and_harvests_in_the_Deh_Cho_area_Northwest_Territories
Taipale SJ, Brett MT, Hahn MW et al (2014) Differing Daphnia magna assimilation efficiencies for terrestrial, bacterial, and algal carbon and fatty acids. Ecology 95:563–576. https://doi.org/10.1890/13-0650.1
Tanentzap AJ, Szkokan-Emilson EJ, Kielstra BW et al (2014) Forests fuel fish growth in freshwater deltas. Nat Commun 5:4077. https://doi.org/10.1038/ncomms5077
The PostGIS Development Group (2020) PostGIS: an extension to the PostgreSQL object-relational database system. The PostGIS Development Group
Toming K, Kotta J, Uuemaa E et al (2020) Predicting lake dissolved organic carbon at a global scale. Sci Rep 10:8471. https://doi.org/10.1038/s41598-020-65010-3
van Dorst RM, Gårdmark A, Svanbäck R et al (2019) Warmer and browner waters decrease fish biomass production. Glob Change Biol 25:1395–1408. https://doi.org/10.1111/gcb.14551
Venturelli PA, Tonn WM (2006) Diet and growth of northern pike in the absence of prey fishes: initial consequences for persisting in disturbance-prone Lakes. Trans Am Fish Soc 135:1512–1522. https://doi.org/10.1577/T05-228.1
Vøllestad LA, Skurdal J, Qvenild T (1986) Habitat use, growth, and feeding of pike (Esox lucius L.) in four Norwegian lakes. Fundam Appl Limnol Arch Hydrobiol 108:107–117
von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws II). Hum Biol 10:181–213
Wang JA, Sulla-Menashe D, Woodcock CE et al (2020) Extensive land cover change across Arctic-Boreal Northwestern North America from disturbance and climate forcing. Glob Change Biol 26:807–822. https://doi.org/10.1111/gcb.14804
Weishaar JL, Aiken GR, Bergamaschi BA et al (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708. https://doi.org/10.1021/es030360x
Williams CJ, Yamashita Y, Wilson HF et al (2010) Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol Oceanogr 55:1159–1171. https://doi.org/10.4319/lo.2010.55.3.1159
Wilson KL, Gisi JD, Cahill CL et al (2019) Life-history variation along environmental and harvest clines of a northern freshwater fish: plasticity and adaptation. J Anim Ecol 88:717–733. https://doi.org/10.1111/1365-2656.12965
Woodland RJ, Magnan P, Glémet H et al (2012) Variability and directionality of temporal changes in δ13C and δ15N of aquatic invertebrate primary consumers. Oecologia 169:199–209. https://doi.org/10.1007/s00442-011-2178-7
Yang Y, Liu H, Dai Y et al (2021) Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci Total Environ 750:141719. https://doi.org/10.1016/j.scitotenv.2020.141719
Acknowledgements
We are grateful to the Environmental Isotope Laboratory of the University of Waterloo, the University of Alberta Biogeochemical Analytical Service Laboratory, and the Western University Biotron Center for Experimental Climate Change Research for laboratory analyses, to Shelley Lundvall, Leanne Baker, and Amy Nguyen for field and laboratory assistance, to Leander Höhne for statistical assistance during the earlier versions of this work, and to First Nations in the Dehcho Region, especially Líídlii Kúé First Nation (Fort Simpson), Tthets’éhk’edéli First Nation (Jean Marie River), Sambaa K’e First Nation (Trout Lake), Ka’a’gee Tu First Nation (Kakisa), Deh Gáh Got’ie First Nation (Fort Providence), and Pehdzeh Ki First Nation (Wrigley) for their support throughout the project. We also thank the editors and anonymous reviewers whose comments and suggestions improved and clarified our work.
Funding
This research was supported by Canada First Excellence Research Fund (Global Water Futures; Northern Water Futures project), the Cumulative Impacts Monitoring Program (Government of the Northwest Territories), the Northern Contaminants Program, Dehcho First Nations, and Dehcho Aboriginal Aquatic Resources and Oceans Management program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Ethical approval
This research was conducted under Aurora Research Institute scientific research license #16046 and University of Waterloo Animal Care Committee Animal Use Protocol A-18-04.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moslemi-Aqdam, M., Low, G., Low, M. et al. Catchments affect growth rate of Northern Pike, Esox lucius, in subarctic lakes. Aquat Sci 83, 59 (2021). https://doi.org/10.1007/s00027-021-00817-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-021-00817-4