Abstract
Leucine-rich, glioma inactivated 1 (LGI1) is a secreted glycoprotein, mainly expressed in the brain, and involved in central nervous system development and physiology. Mutations of LGI1 have been linked to autosomal dominant lateral temporal lobe epilepsy (ADLTE). Recently auto-antibodies against LGI1 have been described as the basis for an autoimmune encephalitis, associated with specific motor and limbic epileptic seizures. It is the second most common cause of autoimmune encephalitis. This review presents details on the molecular structure, expression and physiological functions of LGI1, and examines how their disruption underlies human pathologies. Knock-down of LGI1 in rodents reveals that this protein is necessary for normal brain development. In mature brains, LGI1 is associated with Kv1 channels and AMPA receptors, via domain-specific interaction with membrane anchoring proteins and contributes to regulation of the expression and function of these channels. Loss of function, due to mutations or autoantibodies, of this key protein in the control of neuronal activity is a common feature in the genesis of epileptic seizures in ADLTE and anti-LGI1 autoimmune encephalitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The epilepsies affect more than 1% of the world population and have diverse causes and prognoses. While mutations of genes encoding ion channels are the most frequent cause of inherited epilepsies, other mechanisms may be involved. For instance leucine-rich, glioma inactivated 1 protein (LGI1) is a secreted synaptic protein and mutations of LGI1 are associated with autosomal dominant lateral temporal lobe epilepsy (ADLTE) [1,2,3]. In addition, auto-antibodies against LGI1 are linked to an autoimmune encephalitis (AE). It is becoming clear that such auto-immune disorders are responsible for many unexplained drug-resistant epilepsies [4,5,6]. AE with anti-LGI1 antibodies is the second most frequent type of AE, and is characterized by limbic encephalitis associated with pathognomonic tonic–dystonic seizures (TDS) [7,8,9,10].
LGI1 is a member of the LGI protein family. This family consists of secreted glycoproteins with a leucine-rich repeat (LRR) domain and an epitempin (EPTP) domain, which influence several aspects of nervous system development and physiology [11]. LGI1 was initially described as a putative tumor suppressor, since it was found at a chromosomal translocation breakpoint in a glioma cell line [12]. LGI1 was also underexpressed in glioblastoma cells [12,13,14] and reduced tumor cell proliferation and migration [15,16,17]. However, the link to cancer remains controversial. LGI1 is expressed at low levels in glial cells [18], and tumors are not associated with either ADLTE [19] or with the AE linked to LGI1 auto-antibodies [20].
This review first examines the molecular structure, expression and physiological functions of LGI1. We then discuss how disruption of LGI1 functions, due to either genetic mutations or auto-antibodies, contributes to genetic or autoimmune epileptic syndromes.
Structure, expression of LGI1 and molecular partners
LGI1 structure and domain organization
The human LGI1 gene, located in the chromosomal region 10q24, is 39.6 kb long and consists of eight exons and seven introns. It encodes a 2.2 kb transcript. The mouse LGI1 gene shares 91% of its nucleotide sequence with its human orthologue, resulting in 97% amino acid sequence identity [12, 21]. LGI1 is a protein of 557 amino acids with a molecular weight of 64 kDa [12]. It includes an N-terminal leucine-rich repeat (LRR) domain and a C-terminal epitempin repeat (EPTP) domain, also called epilepsy-associated repeat (EAR) domain (Fig. 1). LGI1 has no transmembrane domain, and cleavage of the signal peptide at the N-terminal extremity produces a mature protein of 60 kDa. LGI1 is a secreted glycoprotein [1,2,3, 22, 23]. Before secretion, LGI1 is N-glycosylated on the N192, N127 and N422 residues in the endoplasmic reticulum [3, 24].
Domain organization of LGI1 and localization of mutations linked to ADLTE. Mutations are distributed along the whole gene sequence, without domain-preference. The list of mutations was compiled from Boillot and Baulac, 2016 [95] and Yamagata and Fukai, 2020 [96]. SP: signal peptide (cleaved in mature protein); C: cysteine cluster
The LRR domain is composed of four leucine-rich patterns, each of 24 amino acids, and has a cysteine cluster on each side. It is coded by exons 1 to 6 [21, 25, 26]. The EPTP domain is coded by the exons 7 and 8. It contains a seven-bladed beta-propeller, with each blade composed of four-stranded antiparallel β-sheets of around 45 amino acids [26,27,28,29]. LRR and EPTP domains are both thought to mediate interactions with other proteins [28, 30].
LGI1 expression profile
Various commercial antibodies directed against LGI1 have been shown to cross-react with other members of the LGI protein family [1, 24, 31, 32]. This lack of specificity might seem to preclude analysis of LGI1 protein distribution by immunohistochemistry. However, anti-LGI1 antibodies purified from auto-immune encephalitis patients are known to be specific [33, 34].
In mouse and human tissue, LGI1 transcripts are expressed at high levels in the brain, at moderate levels in spinal cord, and are not detected in heart, liver, lungs, placenta, or pancreas [12, 25, 35,36,37]. In mouse brain, LGI1 expression is higher in neurons, than in glial cells [24, 35, 38]. LGI1 is expressed by both excitatory and inhibitory neurons. Ohkawa et al. [33] used dual hybridization in situ to show that in mouse hippocampus, LGI1 mRNA was coexpressed with vGluT1, the vesicular glutamate transporter 1, specific to excitatory neurons, and also with GAD67, glutamate decarboxylase 67kD, an inhibitory cell marker.
In adult mice, LGI1 mRNA levels are higher in the hippocampus, other limbic structures and neocortex. Hippocampal mRNA signals are most strongly associated with CA3 pyramidal cells and granule cells of the dentate gyrus, with lower expression in the CA1 subfield [1, 39,40,41]. The application of serum and cerebrospinal fluid (CSF) IgG from anti-LGI1 AE patients to mouse brain sections, revealed strong LGI1 labeling in hippocampal neuropil [7, 33]. In slices from LGI1−/− mice hippocampus, viral re-expression of LGI1 showed expression in both axons and dendrites [42]. A spatially restricted expression suggested LGI1 is secreted in paracrine fashion. LGI1 has been shown to be enriched at synapses [43, 44] and the axon initial segment of hippocampal neurons [45, 46].
LGI1 mRNA is expressed in diverse neocortical areas, with some variability between layers: cortical layers V–VI have higher expression levels than layers I–IV. In adult mice, LGI1 transcripts are detected in the amygdala, piriform cortex, entorhinal cortex and cerebellum. In the cerebellum, anti-LGI1 antibodies from AE patients induce labeling at basket cell synapses but not on the soma of Purkinje cells. LGI1 transcripts are absent or expressed at low levels in the olfactory bulb, diencephalon and brainstem [2, 7, 36, 39,40,41].
A developmental time profile of LGI1 expression has been resolved using Western blot analysis of mouse brain lysates. It shows LGI1 protein first appears during late embryonic development (E15) and increases until adulthood [2, 37, 38, 47,48,49]. During embryogenesis, LGI1 expression is not restricted to differentiated neurons but is rather diffuse [31, 49]. This diffuse expression seems to be regulated in a spatial and temporal manner [31]. LGI1 is associated with certain immature migrating neurons [31]. This orchestrated time profile sustains the idea that LGI1 plays a significant role in normal brain development (cf. paragraph 3.1).
LGI1 interactors
LGI1 protein network
Protein partners of LGI1 have been identified by affinity purification in knock-in (KI) mice expressing an epitope-tagged LGI1 protein. Partner proteins from the precipitated complex linked to LGI1 were identified by mass spectrometry and Western Blot [50]. The most abundant proteins identified in this way were ADAM22 (A Disintegrin and Metalloproteinase 22) and ADAM23. Less abundant proteins included ADAM11, postsynaptic membrane-associated guanylate kinase proteins (MAGUKs: PSD95, PSD93 and SAP97), Kv1 channels, presynaptic scaffolding proteins (CASK and Lin7), and the 14–3–3 protein, a known partner of ADAM22 [50, 51]. Some of the identified proteins share some functional attributes with LGI1. As we will describe, genetic disruptions of LGI1 produce animals which exhibit generalized epileptic seizures during early postnatal life [37, 50, 52]. Similar seizures were seen in animals in which ADAM11 [53], ADAM22 [54], ADAM23 [55] and KCNA1 and KCNA2—which code for Kv1 channel subunits [56, 57]—genes are inactivated.
Membrane receptors for LGI1: ADAM11, ADAM22, ADAM23 and Nogo receptor 1
ADAM proteins
Members of the ADAM protein family are components of multi-molecular protein complexes which include LGI1 [50]. The extracellular domain of ADAM proteins contains a metalloproteinase-like domain, a disintegrin domain, a cysteine-rich domain and an epidermal growth factor (EGF)-like domain. Three ADAM proteins (ADAM22, ADAM23 and ADAM11) bind to LGI1 and are exclusively expressed in the CNS [58]. They all lack a zinc-binding motif resulting in a catalytically inactive metalloproteinase domain due to the absence of critical amino acids at this site [58,59,60].
Further insight into the nature of the multi-molecular protein complex was obtained by immunoprecipitation based on tagged ADAM22 rather than LGI1 [44]. Affinity purification showed that partner proteins included LGI family members (LGI1 to LGI4), ADAM proteins (ADAM11, ADAM22 and ADAM23), pre and postsynaptic MAGUKs and scaffold proteins, presynaptic Kv1 channels, and the 14–3–3 protein partner of ADAM22. Close similarities of protein complexes based on tagged LGI1 or ADAM22 suggest they form part of the same pathway.
One isoform of ADAM22 contains an intracellular C-terminal PDZ binding motif specifically interacting with the third PDZ domain of the MAGUK family protein PSD95 [2]. LGI1 and ADAM22 are the two main components of the protein complex associated with PSD95, identified by immunoprecipitation [2]. PSD95 acts as a postsynaptic anchor for proteins at excitatory synapses. It contributes to synaptogenesis and synaptic plasticity among other processes [61]. ADAM11 and ADAM23 lack the PDZ domain binding motif and, therefore, do not directly interact with PSD95, suggesting other partners [55]. Binding to PSD95, suggests ADAM22 and LGI1 are localized at the postsynaptic side of the synaptic cleft. Time-lapse imaging of mCherry tagged ADAM22 and ADAM23 in cell culture, provides further support for a postsynaptic location of ADAM22 and suggests that ADAM23 may be located presynaptically. Axonal transport of ADAM23 was largely anterograde, while ADAM22 was predominantly transported in a retrograde sense [46]. However, this assumed localization may not be completely resolved. ADAM22 is also enriched presynaptically in the axonal compartment at the axon initial segment [46]. Further studies may bring clarification.
ADAM proteins act as chaperone-like proteins for LGI1 as well their role in anchoring LGI1 to the cell membrane. They participate in LGI1 maturation and N-glycosylation processes [46]. Furthermore, ADAM22 and ADAM23 colocalize with LGI1 in axonal transport vesicles [46], suggesting possible interdependent interactions for their transport and subcellular distribution. Membrane expression of LGI1 is strongly decreased in ADAM22−/− and ADAM23−/− mice [62], as is ADAM22 and ADAM23 expression in LGI1−/− animals [50, 62], providing further evidence for interactions between ADAM family proteins and LGI1.
Nogo receptor 1 (NgR1)
The Nogo receptor 1, NgR1, is coded by the RTN4R gene. It is a 473 amino acid protein, which contains a signal peptide, eight LRR domains, an LRR C-terminal flanking domain cysteine rich, a unique region and a glycosylphosphatidylinositol membrane anchorage site [63]. NgR1 interacts directly with LGI1 [48, 64]. It co-immunoprecipitates with ADAM22 from rat brain lysate suggesting functional interactions exist [48]. However, it is not present in the multi-molecular protein complex recovered by LGI1 purification, nor in those purified by immunoprecipitation based on tagged ADAM22, Kv1, or PSD95 [2, 44, 50, 65]. The absence of NgR1 in these protein complexes is hard to explain. We speculate that NgR1 binding to LGI1 may be weaker than that to the ADAM receptors and associated proteins. This hypothesis could be usefully tested.
One study showed that the NgR1–ADAM22 complex could facilitate binding of LGI1 to ADAM22 [48]. However, LGI1 also bound NgR1 independently in HEK293T cells [48]. In this way LGI1 seems to act as a competitive inhibitor of NgR1, antagonizing the effects of growth inhibition mediated by the ligands Nogo66, myelin-associated glycoprotein (MAG) and oligodendrocyte myelin glycoprotein (OMPG) [48].
Trans-synaptic assemblies of LGI1
Immunoprecipitation studies suggest that LGI1 interacts with both pre and postsynaptic molecules. It is co-immunoprecipitated with presynaptic anti-Kv1.1 antibodies [65], and with postsynaptic anti-PSD95 antibodies [2]. In addition, both pre and postsynaptic proteins are present in the LGI1-purified multi-molecular complex [50]. Moreover, ADAM22 and ADAM23 co-immunoprecipitated from wild-type mice brain lysates but not those derived from LGI1−/− mice [50]. Based on this evidence, the LGI1 protein has been suggested to form a trans-synaptic bridge between the ADAM23 and ADAM22 proteins [50].
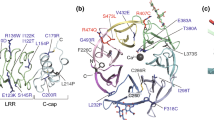
Superresolution imaging has recently provided more details on interactions between LGI1 and ADAM22 using an epitope-tagged ADAM22 KI animal [26]. Cryo-electron microscopy demonstrated 1:1, 2:2 and 3:3 ADAM22-LGI1 complexes in solution, in a ratio of approximately 6:3:1. Crystallization of the 2:2 ADAM22–LGI1–LGI1–ADAM22 hetero-tetramer [26] showed that LGI1 binds to the metalloproteinase-like domain of ADAM22 via its EPTP domain. This LGI1–EPTP domain alone is sufficient to mediate ADAM22 binding. This binding is mediated by interactions between the amino acids Trp398, Tyr408 and Tyr409 of ADAM22 and a hydrophobic pocket in the EPTP beta propeller of LGI1. The LGI1–ADAM22 complex dimerizes through binding between LRR domains of pairs of LGI1 molecules, thus forming 2:2 dimer-of-dimer heterotetramers [26] (Fig. 2A).
Synaptic organization of LGI1 and disruption by mutations and autoantibodies. A Organization of LGI1 in a trans-synaptic complex with protein partners, and indirect functional interactions with presynaptic Kv1 channels and postsynaptic AMPA-Rs. B Pathogenic disruptions of LGI1, either by loss-of-function mutation or by autoantibodies. LGI1 alterations were shown to disrupt both Kv1 channels and AMPA-R expression and function. This could result from (1) internalization of Kv1 channels with their protein complex [133], (2) a decrease in Kv1 current, through fast-inactivation mediated by Kvβ1 [65, 86], (3) removal of Kv1 channels and AMPA-R from the synapse, or decreased expression [45, 85, 133, 138]. The loss of Kv1 currents enhances neurotransmitter release and so increases synaptic glutamate levels. Similarly, internalization, loss of channel function or changes in synaptic expression might be involved for AMPA-R channels. These may be the most promising candidate mechanisms that link LGI1 disruption to seizures. We used Servier Medical Art to create the figure
This dimerization is consistent with a trans-synaptic role for 2:2 ADAM22–LGI1 assemblies. The length of the 2:2 heterotetramer is estimated at 19 nm [26], which is close to the size of the synaptic cleft [66]. Different LGI1–ADAM complexes could be possible, since LGI1 may similarly bind to ADAM22, ADAM23 and ADAM11 [26, 29]. Experimental evidences for the existence of this trans-synaptic complex in vivo, and of the association with different ADAM proteins, remain to be conclusively demonstrated.
In addition to the trans-synaptic complex, LGI1 is also enriched at the axon initial segment, where it colocalizes with ADAM22 and Kv1 channels [45, 46]. Complexes at this site may involve 1:1 or 3:3 ADAM–LGI1 assemblies.
Physiological functions of LGI1
Role of LGI1 during development
LGI1 is required for a normal brain development
LGI1 is expressed during late development in the mouse and expression increases to reach a peak during the first two postnatal weeks (cf. paragraph 2.2). A role for LGI1 in brain development was inferred from structural abnormalities in animals from which LGI1 is genetically deleted. The cortex of early postnatal LGI1−/− mice (P7–P20) shows a diffuse dysplasia together with an abnormal neuronal layer organization [67]. Cortical dysplasia is also induced in mice by conditional inactivation of LGI1 in nestin immunopositive cells, but not in GFAP cells, CAMK2a cells or parvalbumin cells [68]. This data suggests that cortical malformations may result from a loss of LGI1 function in neuronal precursor cells during development. In a different LGI1−/− mouse, cortical malformations were not observed but neuronal loss occurred in the CA1 and CA3 areas, and the thickness of the granule cell layer was enhanced with neuronal dispersion [37]. Defects in cerebellar foliation have been detected in P2–P4 LGI1−/− animals together with a reduction in thickness of the external granule cell layer [69].
LGI1 enhances neurite growth and synapse formation and maturation
Several studies suggest that LGI1 controls synapse numbers and activity. In LGI1−/− mice, cortical and hippocampal neurons receive fewer synapses and synaptic signaling is reduced [64, 67]. Conversely, application of LGI1 to cultured hippocampal neurons over 6 days increased synapse numbers [64]. LGI1 stimulates dendritic growth of neurons in culture, and enhances axonal growth by suppressing myelin-induced growth cone collapse [48, 55].
Interactions between LGI1 and the Nogo receptor 1, NgR1, (cf. paragraph 2.3.2) are suggested to mediate these effects on axonal and dendritic growth and also on the formation and maturation of synapses [64]. When occupied by its myelin-derived agonist ligands, NgR1 activates RhoA signaling. This small GTPase acts to limit synapse numbers in development [70], and is crucial for normal neuronal morphogenesis [71]. Hippocampal neurons of NgR1−/− mice receive more synapses than control mice [64]. Work on LGI1−/− mice suggests that LGI1 inhibits RhoA activation via NgR1 [64]. Thus, the NgR1–LGI1 balance would regulate RhoA activity during development, and in this way affect synaptic number and function.
LGI1 may also affect neuronal maturation through its interactions with proteins of the ADAM family. Indeed, LGI1–ADAM22 and LGI1–ADAM23 interactions have been shown to influence neuronal development [55]. Furthermore, NgR1 and ADAM22 interact directly, although it is not clear whether ADAM proteins and NgR1 participate in independent LGI1-associated pathways, or function as co-receptors for LGI1. Both arrangements may coexist.
Distinct LGI1−/− mouse strains have provided opposite evidence on LGI1 influences on neuronal process growth and synaptogenesis [43, 62]. Electron microscopy provided no evidence that dendritic growth or synaptogenesis were altered in different LGI1−/− mice. The reasons for these inconsistencies are unknown, and further work is needed to understand differences between animals with LGI1 deletions.
LGI1 regulates neuronal myelination
Electron microscopy examination of LGI1−/− mice has suggested LGI1 facilitates myelination in the central and peripheral nervous system [72]. In addition, recent data has shown that LGI1 may control the differentiation of oligodendrocyte precursor cells [38]. In LGI1−/− animals the expression of TSC1 (tuberous sclerosis complex 1) was reduced, which could be responsible for an upregulating activity of the mTORC1 complex in oligodendrocytes. Since mTORC activity is known to affect CNS myelination [73], over-activation in LGI1−/− mice could tend to reduce myelination [38].
Roles of LGI1 in the control of neuronal ion channels
LGI1 regulates presynaptic voltage-dependant K+ current through Kv1 channels
Kv1 channels are a family of K+ channels, which are expressed as protein tetramers of 4 alpha subunits, each with 6 transmembrane domains. Eight alpha subunits have been described so far (Kv1.1–Kv1.8). They can assemble into homotetramers or heterotetramers. Different combinations control the biophysics, pharmacology and dynamics of K+ currents and also sites of membrane insertion and channel mobility [74]. Alpha subunits may be associated with accessory intracellular beta subunits which modify channel properties and currents [75]. Kv1 channels are expressed ubiquitously and exhibit great diversity in expression, topography, and associated subunits [74, 76]. Expression levels are high in axons and terminal boutons as well as somata and dendrites. Kv1 channels are enriched and colocalize with LGI1 in axon initial segments [45, 46].
K+ currents mediated by Kv1 channels activate at membrane potentials near neuronal firing threshold [77] thus operating to prevent hyperexcitability by limiting and delaying neuronal firing [78, 79]. Kv1 channels control action potential shape, timing and frequency and transmitter release from axonal terminals [79,80,81,82]. Kv1.1 subunits tend to promote fast activating, slowly inactivating outward K+ currents in response to depolarization [74], which are blocked by the specific inhibitor dendrotoxin-K [83, 84].
Interactions between Kv1 and LGI1 suggest that LGI1 affects Kv1 expression and functional properties. Co-immunoprecipitation studies with anti-LGI1 antibodies precipitate Kv1 channels, and reciprocally LGI1 protein was isolated in co-immunoprecipitation work based on anti-Kv1.1 antibodies [50, 65]. However, LGI1 does not bind directly to Kv1, but interactions are mediated indirectly via proteins of the ADAM family and PSD95-like MAGUKs [44]. LGI1 does not interact in the same way with all different Kv1 subunits. In LGI1−/− mice, Kv1.1 and Kv1.2 expression, but not that of Kv1.4 and Kv1.6, was considerably reduced [45, 85].
Functional interactions between LGI1 and Kv1.1 were first described by Schulte et al. in a heterologous expression system [65]. LGI1 potentiated K+ currents by blocking fast-inactivation of Kv1.1 channels, which is mediated by the beta subunit Kvβ1. Similarly, LGI1, associated with ADAM22, potentiates K+ currents in HEK293 cells transfected with Kv1.1/Kv1.4 [86]. Furthermore in LGI1−/− mice, axonal expression of Kv1.1 channels in CA3 pyramidal cells is strongly decreased, as well as Kv1.1 currents, which increases neuronal excitability [45]. Dendrotoxin-K, the specific Kv1.1 antagonist, increases neuronal excitability and transmitter release in wild-type neurons, but not in neurons lacking LGI1 [45], confirming that LGI1 acts on Kv1.1 channels.
LGI1 regulates postsynaptic AMPA-R channels
Glutamate-activated ionotropic AMPA receptors (AMPA-R) underlie fast, excitatory synaptic transmission in the mammalian brain. They are heterotetramers formed by diverse assemblies of AMPA-receptor subunits GluR1 to GluR4. When activated by synaptically released glutamate, ion flux through a membrane pore opened in the receptor mediates a postsynaptic excitatory current [87].
While the multi-molecular protein complex derived by immunoprecipitation of tagged LGI1 did not contain AMPA-R, it did contain PSD95 and SAP97 [50], which both interact with AMPA-Rs. PSD95 is a scaffolding protein, which stabilizes AMPA-R at the postsynaptic membrane [61]. It binds, through its two first PDZ domains, to stargazin protein which in turn binds to AMPA-R subunits [88]. SAP97 is another anchoring protein of the MAGUK family. It binds directly through its second PDZ domain, to a PDZ ligand motif on the C-terminus of the GluR1 subunit [89]. An alternatively spliced version of ADAM22 also possesses a C-terminal cytoplasmic PDZ ligand motif which binds to the third PDZ motif of PSD95 [2].
PSD95 overexpression increases the number of AMPA-R expressed at postsynaptic sites and so increases the amplitude of synaptic events [61, 88]. This effect was abolished in LGI1−/− mice and in KI mice expressing a mutated version of ADAM22 without its PDZ binding site to PSD95 [42, 44]. These data suggest that both LGI1 and ADAM22 are needed for PSD95 membrane anchoring of AMPA-R. Postsynaptic stabilization of AMPA-R is thus indirectly linked to LGI1, via complexes of either ADAM22–PSD95–stargazin or ADAM22–PSD95–SAP97.
LGI1 may also regulate AMPA-R mediated synaptic currents. Application of LGI1 protein to acute slices of rat hippocampus enhanced EPSC amplitudes [2]. Inversely, AMPA-R currents decreased in slices from LGI1−/− mice or from animals expressing mutated ADAM22 [42, 44, 50]. Moreover, AMPA-R currents were restored by LGI1-expressing lentivirus injections in hippocampal slice culture from LGI1−/− mice [42]. Thus LGI1 directly enhances postsynaptic events presumably by recruiting AMPA-Rs to postsynaptic sites. AMPA-R decrease in LGI1−/− mice was not a consequence of seizures in these animals, as the study of Lovero et al. [42] reported AMPA-R alteration in P8 mice, before the emergence of spontaneous seizures (cf. paragraph 4.1.2).
Some of these findings have been disputed in work with other strains of LGI1−/− mice. Other studies reported no differences in AMPA-R expression or in EPSC properties compared to control mice [43, 52]. The reasons for those discrepancies remain unclear. However, a number of different approaches, including LGI1−/− mice, ADAM22 KI mice expressing a non-functional ADAM22, as well as PSD95 overexpression in LGI1−/− and ADAM22-mutated mice, tend to conclude that LGI1 acts on molecules that stabilize AMPA-Rs at post-synaptic sites and so enhances the number of receptors expressed. These findings are congruent with data from protein interaction studies.
Human pathologies related to LGI1 disruption
Genetic disruption of LGI1
Autosomal dominant lateral temporal lobe epilepsy (ADLTE)
In 1995, Ottman et al. reported a familial focal epilepsy with autosomal dominant inheritance linked to chromosome 10q [90]. Predominant auditory features were suggestive of a lateral temporal lobe onset. LGI1 mutations were identified a few years later in several families with autosomal dominant lateral temporal lobe epilepsy [25, 35, 39]. Although heterozygous mutations in reelin, RELN, have also been reported in ADLTE families [91], LGI1 mutations remain predominant and are identified in about one-half of cases [35, 39, 92]. A reduced penetrance around 70% is described [25, 35, 39, 92,93,94].
The first symptoms of ADLTE occur during adolescence or early adulthood. Focal aware seizures—formerly known as ‘auras’—with auditory features are the most suggestive manifestations. Ictal auditory symptoms are reported in 62% of patients and seizures are reportedly triggered by sudden sounds, such as a phone ringing for about 20% of them [93]. Initial ictal symptoms include simple sounds, such as buzzing, ringing and humming, or more complex hallucinations, such as voices or music. Positive or negative auditory illusions include altered sound volumes or frequencies. Patients may experience other less common types of focal aware seizures: visual or olfactory hallucinations, dysmnesic features (déjà-vu), or vertigo. 20% of patients describe ictal aphasia [93, 94] as a receptive aphasia, formerly called Wernicke’s aphasia. Focal to bilateral tonic–clonic seizures affect about two thirds of patients [93]. Brain MRI scans are frequently normal, while EEG records may reveal temporal epileptiform activity. ADLTE is generally well controlled by antiepileptic drugs. However, drug treatment must be maintained for life, since relapses are frequent when drugs are withdrawn.
LGI1 mutations: a loss-of-function pathophysiology
At least 45 distinct mutations of LGI1 have been identified in ADLTE patients. Lists of mutations were published in 2016 and 2020 [95, 96], and no new mutations of LGI1 have been reported since 2020. Of the 45 mutations, there are 30 missense (67%), 7 frameshift (16%), 4 in splicing donor or acceptor sites (9%), 2 nonsense (4.5%) and 2 microdeletions (4.5%). The mutations are homogeneously distributed throughout the LGI1 sequence, with no specific domain preference (Fig. 1).
Three lines of LGI1−/− mice have been generated to model pathophysiological effects of a genetic loss of LGI1 function [37, 50, 52]. They exhibit similar behavioral phenotypes. Spontaneous generalized epileptic seizures appeared during the second postnatal week at a frequency of approximately one per hour. Ictal EEG events originated earlier in the hippocampus than in the cortex [37]. Weight gain in these young animals was strongly reduced when seizures emerged and LGI1−/− mice died prematurely during the third postnatal week [37, 50, 52]. No spontaneous epileptic seizures have been observed in LGI1+/− mice. Their survival was normal, as was their fertility [37, 50, 52]. However, at one month, they were more sensitive than LGI1+/+ mice to the induction of seizures by the convulsant pentylenetetrazole (PTZ) [50] and by sound stimulation [37]. Thus, LGI1 loss of functions leads to an epileptic phenotype, in a gene-dose dependent manner.
The function of LGI1 mutations associated with ADLTE has been assessed. Frameshift, splice-site, nonsense and deletion mutations seem likely to induce loss of function, since protein sequences are strongly altered. Most missense mutations tested (21/24) show defects in secretion, while 3/24 are secretion competent [96]. The loss of secretion may result from abnormal protein conformations which would lead to premature degradation by quality control machinery. This hypothesis was tested for the E383A mutation, with a transgenic LGI1−/− model made to express the mutated protein [62], and also for the L385R mutation, in a transgenic rat model [97]. Both animal models exhibited generalized seizures and died prematurely between the second and fourth week of life. Both mutated proteins were unstable and prematurely degraded. The chemical corrector, 4-phenylbutyric acid, which acts to stabilize misfolded proteins, improved secretion of the mutated LGI1 and reduced seizure susceptibility in LGI1–E383A mice, suggesting possible conformational alterations in the LGI1–E383A protein [62].
The three secretion-competent LGI1 mutations were also functionally assessed. The S473L mutation impairs LGI1 binding to ADAM22 but not to ADAM23 [26, 62]. The R474Q mutation did not alter LGI1–ADAM22 or LGI1–ADAM23 binding, but did suppress LGI1 dimerization [26]. The third secretion-competent LGI1 mutation, R407C, did not affect LGI1–ADAM binding, or LGI1–LGI1 binding. Lethal epilepsy was not detected in LGI1−/− mice crossed with transgenic mice to overexpress wild-type LGI1 [50]. Expression of LGI1–R407C in LGI1−/− mice rescued the phenotype, as observed with re-expression of wild-type LGI1. Such a rescue did not occur with the S473L and R474Q mutations [26, 62]. These data suggest that the R407C mutation is non-pathogenic. In contrast, for the pathogenic but secretion-competent mutations S473L and R474Q, function is lost but with no major effects on protein conformation. If the R407C mutation is not pathogenic, could its detection in one ADLTE family [98] be a false-positive? Possibly mutation of a different gene, such as reelin [91], might be involved in this family.
In summary, most LGI1 mutations induce a loss of function which is independent of the type of mutation and its localization in the gene, leading to the epileptic disease. Genotype–phenotype correlations are, therefore, absent. No differences were found between truncating and missense mutations, or between the mutations from different sites along the gene. No correlation has been established between different mutations and their respective penetrance [95, 99, 100]. In addition, the behavioral phenotype of LGI1+/− mice overexpressing LGI1–S473L or LGI1–R474Q was similar to that of LGI1+/− mice, suggesting the absence of dominant-negative effects [62].
Autoimmune disruption of LGI1
Autoimmune encephalitis associated with antibodies directed against LGI1
An AE with antibodies targeting the LGI1 protein was reported in 2010 [7, 9]. Before this report, a group of AE syndromes involving central and peripheral nervous systems had been identified and related to antibodies directed against the voltage-gated potassium channel complex, or VGKC [101]. Anti-VGKC antibodies were later shown to target either LGI1 or contactin-associated protein-like2 (CASPR2), which are two protein members of the VGKC complex [7, 9]. The term ‘anti-VGKC encephalitis’ has now been redefined as two distinct subgroups—anti-LGI1 encephalitis and anti-CASPR2 encephalitis—with distinct symptoms and prognosis.
Anti-LGI1 encephalitis is the second most common subtype of AE-related to anti-neuropil antibodies—after anti-NMDA (N-methyl-d-aspartate) receptor encephalitis. It represents about 25% (range 17.6–34.6%) of all AE subtypes [102,103,104,105,106], and is the most frequently observed subtype in epilepsy units [107].
Demographic characteristics
Patients with anti-LGI1 AE have a median age of 65 in the largest cohort (n = 196) [20] and from 56 to 60 in other significant cohorts [108,109,110,111]—which could explain an association with Alzheimer’s disease. Patients are more often males than females in a ratio of 2:1 [8, 10, 20, 108,109,110,111,112,113]. Data from several cohorts suggests a genetic predisposition exists with a strong association to Human Leukocyte Antigen (HLA) class II alleles. HLA-DRB1*07:01 was found in 88–91% of patients [114,115,116,117]. In a large cohort of anti-LGI1 AE patients, non-carriers of this allele were younger (median age: 46), more frequently female, with fewer psychiatric symptoms and less frontal lobe dysfunction [117].
Clinical phenotypes
Two brain regions—motor cortex and the mesial temporal lobe—are mainly affected by anti-LGI1 AE and clinical phenotypes differ accordingly (Fig. 3) [10]. When the motor cortex is involved, tonic–dystonic seizures (TDS) are generated. They have also been termed faciobrachial dystonic seizures (FBDS) and are pathognomonic of anti-LGI1 encephalitis. When the mesial temporal lobe is involved, clinical symptoms are indicative of a limbic encephalitis.
Two main targets of anti-LGI1 encephalitis: mesial temporal lobe and motor cortex. Left, Mesial temporal lobe involvement. Hippocampal hyper-intensity and edema can be found on the brain MRI (top left, coronal T2 image), as well as focal fronto-temporal lobe seizures (middle left; arrows indicate the EEG channels involved in the seizure) on EEG, and hippocampal hypermetabolism on F18–FDG–PET (bottom left; coronal view, superimposed on brain MRI). A striking bilateral striatal hypermetabolism is often detected in anti-LGI1 AE. Right, Motor cortex involvement. Tonic–dystonic seizures (TDS), also called faciobrachial dystonic seizures (FBDS), occur as in the top right (tonic contraction of the right hemiface and arm). Concomitant scalp EEG shows a fronto-central slow wave (middle right, black arrows) before the contralateral muscle contraction (white arrow, right arm EMG). F18–FDG–PET (bottom right) shows a hypermetabolism located on the primary motor cortex. EEG, MRI, and F18–FDG–PET figures are reproduced with permission from Navarro et al. 2016 [10]
Clinical characteristics of TDS are described in Table 1 and supported by a video recording in the Online Resource 1. We tend to prefer the term tonic–dystonic seizure (TDS) to faciobrachial dystonic seizure (FBDS), since face involvement may be variable or absent, although both terms are used. TDS events in video-EEG recordings are preceded by a specific and highly reproducible slow wave [10, 118, 119]. This wave, which can be easily missed, lasts around 500 ms and arises from fronto-central electrodes contralateral to the TDS. EEG analysis using average montage and co-recording of EMG activity of the corresponding muscles can help better identify this slow wave (Fig. 3, right). In EMG records, the tonic component of the TDS is characterized by a ‘rhombus’-like shape of duration 1–2 s. Polymyographic records revealed two different EMG profiles associated with TDS [10]. In 2/5 patients, EMG activity was continuous, corresponding to a purely tonic spasm. In 3/5 patients, EMG activity consisted of short rhythmic bursts corresponding to a tonic spasm with superimposed short duration (20–40 ms) myoclonus. The temporal and spatial organization of the jerks was compatible with rapid pyramidal conduction along the corticospinal pathway, as for cortical myoclonus. Taken together, these features support a cortical origin for TDS. Finally, 18F-fluoro-deoxy-glucose (18F-FDG) positron emission tomography (PET) showed a strong increase of metabolism in contralateral primary motor cortex (Fig. 3, right) [10]. These observations suggest TDS originates in the motor cortex. MRI unilateral signal abnormalities and PET strong bilateral hypermetabolism in basal ganglia—in particular the striatum—may be detected in anti-LGI1 AE with TDS [120, 121]. MRI changes (T1 and/or T2 hyperintensities) have also been found in basal ganglia in some patients [120, 121]. While the motor cortex activation may generate the tonic phase of TDS [10], the striatum involvement might cause the dystonic posture [122].
Limbic encephalitis (Fig. 3, left) is often characterized by mesial temporal lobe seizures and cognitive disorders of subacute onset (typically less than 3 months). Patients with anti-LGI1 AE may initially experience focal mesial temporal lobe seizures (MTLS) with no loss of consciousness, mainly including vegetative symptoms (ascending epigastric feeling, hot and cold feeling, pilo-erection), as well as sudden fear or anxiety, which may be misdiagnosed as psychiatric symptoms. Dysmnesic symptoms (déjà-vu, déjà-vécu) can also occur, but rather in patients suffering from epilepsy in relation to a hippocampal sclerosis as late sequelae, without any active dysimmune encephalitis. The duration of seizures in patients with limbic encephalitis is typically a few seconds, and their frequency may reach 30–40 per day. Focal seizures with impaired awareness and automatisms, and focal to bilateral tonic–clonic seizures and status epilepticus may occur thereafter or as the first manifestation. Cognitive disorders associated with limbic encephalitis center on memory impairment, largely involving episodic verbal and visuospatial memories, due to hippocampal involvement. Confusion and disorientation can also be identified. Mean Mini-Mental State Examination (MMSE) score is 20–23.5 points, indicating a mild cognitive impairment [10, 108, 123, 124]. MRI scan can be normal at the early beginning of the disease, especially when limbic symptoms are discrete. Hippocampal edema can be seen thereafter (Fig. 3, left). At later stage, after the disease is controlled by immunomodulatory drugs, hippocampal edema, defined by an increased volume, disappears. In later stages, signs of hippocampal sclerosis may be seen, including T2/FLAIR hypersignal associated with atrophy and loss of internal structure. 18F-FDG PET highlights hippocampal hypermetabolism in active limbic encephalitis. Frontal and temporal lobe hypometabolism is typical in patients with cognitive impairment.
Hyponatremia is reported in about two thirds of AE patients (39–69%) [7, 9, 10, 20, 108,109,110, 112]. It is thought to result from a syndrome of inappropriate antidiuretic hormone secretion (SIADH), which may originate from a hypothalamic involvement. Accordingly, a link between hyponatremia and mesial temporal involvement with major cognitive disorders was reported [8, 10]. Peripheral nervous system may also be involved. Peripheral neuropathy and peripheral nerve hyperexcitability have been reported in approximately 5–10% of patients with anti-LGI1 AE [20, 125]. Neuropathic pain usually displays a length-dependent pattern and responds to immunotherapy [125].
Clinical evolution of the disease
Anti-LGI1 AE originates either in the motor cortex or the mesial temporal structures. Later, both regions can be affected (Fig. 4). In some patients TDS is initially unilateral and may become bilateral with time. Similarly, the mesial temporal lobe may first be affected unilaterally before the encephalitis may affect bilateral cortical structures and severe cognitive impairment ensues. As knowledge on the syndrome improves, the delay between symptom onset and diagnosis has decreased, from about 12 months a decade ago to 1–3 months at present. The disease usually follows a monophasic time course. Relapses appear to occur in 25–41% of patients, usually in the first year after recovery [126,127,128]. Sequels of an initial encephalitis usually stem from a delayed diagnosis, associated with a temporal lobe involvement. For instance an early hippocampal edema can evolve into hippocampal sclerosis and induce MTLE. Epileptic seizures persist in about 20% of patients after treatment and recovery from encephalitis. Administration of corticosteroids may protect against this evolution [109, 128]. In older patients, interaction with Alzheimer’s disease processes can aggravate cognitive dysfunction. CSF biomarkers should be explored to understand such interactions.
Natural clinical history of anti-LGI1 encephalitis. At disease onset unilateral motor cortex (unilateral TDS, top left) or mesial temporal lobe (isolated mesial temporal lobe seizure, bottom left) may be equally affected structures. The further evolution is characterized by bilateral involvement, leading to either bilateral TDS, or cognitive disorders and MTLS. Finally, both structures, motor cortex and mesial temporal lobe, may be involved. The figure is reproduced with permission from Navarro et al. 2016 [10]
Biological findings
Antibodies against LGI1 are mainly IgG4 subtypes [129,130,131]. They can be identified in both CSF and blood. Antibodies can sometimes be detected only in blood. IgG4 are not pro-inflammatory antibodies, but rather mediate inhibitory protein–protein interactions with their targets [132]. CSF may be normal, and in contrast to anti-NMDAR AE, pleocytosis is rare and moderate. To exert their pathological effects on their antigens, autoantibodies have to access the CNS. Entry of autoantibodies into the CNS can occur via diffusion of IgG, or via the passage of B cells, from the periphery to the CNS, across the blood brain barrier. On study reported B cell IgG synthesis in the CNS of 3 anti-LGI1 AE patients [34]. It is not known if this mechanism could coexist with peripheral IgG diffusion to CNS, which is suggested by the approximately 100 times more concentrated antibodies in the serum compared to the CSF [133]. The role of inflammation in anti-LGI1 AE remains to be clarified. Possibly ‘encephalopathy’ could be a better description since there may be no direct inflammation, even if hippocampal edema is observed probably resulting from inflammation due to excitotoxic processes [134] (Fig. 3, left).
Treatments
TDS and MTLE are poorly controlled by standard antiepileptic drugs [102, 130, 135, 136]. Immunomodulatory and immunosuppressive treatments acting on B lymphocytes and immunoglobulins are more efficient. High doses of corticosteroids with intravenous polyclonal immunoglobulins are often used as a first-line therapy. Alternatively plasma exchange can be used; rituximab has also been used. Other drugs have also been used, such as cyclophosphamide. Only one double-blind placebo-controlled study was reported in anti-LGI1 AE and suggested the efficacy of intravenous polyclonal immunoglobulins in reducing seizure frequency [137]. However, there is still no class 1 evidence for the use of specific drugs.
Anti-LGI1 antibodies inhibit LGI1
When an antibody has been linked to an autoimmune disease, it is crucial to determine whether it plays an active role in the pathophysiology or whether it is just an effective biomarker. For anti-LGI1 AE, the absence of major inflammatory reactions in the CSF, and IgG4 subtype of most autoantibodies in patients (cf. paragraph 4.2.1) tend to favor a humoral rather than a cellular dysimmune reaction. The reversal of symptoms by immunotherapy also suggests that antibodies mediate AE, and not cytotoxic T lymphocytes which would induce irreversible tissue lesions.
The effects of human anti-LGI1 antibodies have been studied on neurons, in vitro and in vivo. Anti-LGI1 antibodies are polyclonal and recognize epitopes of both the LRR and EPTP domains of LGI1 [33, 34, 133] which have recently been shown to initiate distinct pathophysiological effects [34, 133]. A recent study showed that binding to the EPTP domain impairs interactions between LGI1 and ADAM22 and also ADAM23. This disruption is similar to that induced by the secretion-competent mutation S473L (cf. paragraph 4.1.2). In contrast antibody binding to the LRR domain induces internalization of LGI1 and its membrane receptors.
Antibody injections into mouse hippocampus or cerebral ventricles, reduce expression of Kv1 and AMPA-R channels after 7–14 days [133, 138], reproducing effects observed in LGI1−/− mice (cf. paragraph 4.1.2). AMPA-R expression was also decreased after adding anti-LGI1 antibodies to rat hippocampal neurons in culture medium [33]. Anti-LGI1 antibodies consistently increased neuronal excitability, regardless of the domain targeted, in vitro [34] and in vivo [133, 138]. In vivo infusion of antibodies in mice, reduces synaptic long-term potentiation [133, 138], and induced reversible memory deficits [138].
Surprisingly seizures did not emerge, even if intracerebral anti-LGI1 antibody injection in vivo decreased expression of Kv1 and AMPA-R channels and increased neuronal excitability [133, 138]. AE evolves over weeks or months, so perhaps a 2 week exposure to antibodies, which decreased channel expression by ~ 15%, did not suffice to trigger seizures [138]. For comparison, Kv1.1 expression was reduced by ~ 50% from control in LGI1 KO mice that exhibited frequent generalized seizures [45, 85]. Thus, antibody infusion, critical to demonstrate pathogenicity, may not be the optimal method to explore human epileptic phenotypes.
In summary, LGI1 autoantibodies change neuronal and synaptic function so as to increase neuronal excitability. This loss of LGI1 function in AE is a pathophysiological common point with changes induced by LGI1 mutations in ADLTE patients (Fig. 2B). Seizures represent a common pathological point for AE and ADLTE, even if many clinical symptoms differ sharply.
Conclusion
This review has explored consequences of the loss of function of LGI1, a feature common to ADLTE, a genetic syndrome and AE, an autoimmune disease. Reduced Kv1 currents due to loss of LGI1 function led to an increased neuronal excitability and activity, in both genetic and autoimmune animal models, which seem likely to contribute to epileptic disorders linked to LGI1.
LGI1 also controls AMPA-R expression. Precise details on mechanisms have added support to this previously controversial hypothesis. However, it remains unclear how a reduced efficacy of fast synaptic excitation could favor the emergence of seizures in the genetic syndrome ADLTE or in the immune related AE. The same question remains to be answered for anti-AMPA-R AE patients, who may also exhibit seizures in estimated 28% of patients [139]. One proposed mechanism involves a specific decrease of AMPA-R activity in inhibitory interneurons [33]. However, conditional inactivation of LGI1 in either glutamatergic cells or in parvalbumin-containing interneurons [32] did not support this hypothesis. Conditional inactivation in glutamatergic cells sufficed to trigger seizures, but deletion of LGI1 limited to parvalbumin cells did not induce seizures. Thus seizures in LGI−/− mice may depend on an increased efficacy of excitatory cells rather than a disinhibitory reduction in interneuron efficacy. A reduced AMPA-R expression could also be involved in some non-epileptic symptoms of ADLTE and anti-LGI1 AE patients.
Evidence that LGI1 mutations induce an aberrant brain organization, with abnormal neuronal and synaptic morphologies, suggests that subtle developmental abnormalities may also contribute to seizures resulting from LGI1 mutations in ADLTE. More severe epileptic phenotypes result when LGI1 is deleted during embryogenesis than when deletions are made during adult life [32]. Developmental effects might partly explain different clinical profiles of ADLTE patients and anti-LGI1 AE patients, for whom LGI1 is inhibited in a fully mature brain.
LGI1 has emerged as a major molecular influence on the regulation of brain development, neuronal excitability and synaptic transmission and plasticity. Comprehension of this physiology is crucial to understand normal brain function as well as the pathophysiological syndromes reviewed here. Better understanding of the physiology, pharmacology and biochemistry will open new therapeutic approaches, such as targeting LGI1 molecular partners, or designing anti-epileptic drugs to target the altered cellular and synaptic currents.
Availability of data and materials
Not applicable.
References
Senechal KR, Thaller C, Noebels JL (2005) ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum Mol Genet 14:1613–1620. https://doi.org/10.1093/hmg/ddi169
Fukata Y, Adesnik H, Iwanaga T et al (2006) Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313:1792–1795. https://doi.org/10.1126/science.1129947
Sirerol-Piquer MS, Ayerdi-Izquierdo A, Morante-Redolat JM et al (2006) The epilepsy gene LGI1 encodes a secreted glycoprotein that binds to the cell surface. Hum Mol Genet 15:3436–3445. https://doi.org/10.1093/hmg/ddl421
Dalmau J, Geis C, Graus F (2017) Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev 97:839–887. https://doi.org/10.1152/physrev.00010.2016
Goodfellow JA, Mackay GA (2019) Autoimmune encephalitis. J R Coll Physicians Edinb 49:287–294. https://doi.org/10.4997/JRCPE.2019.407
Husari KS, Dubey D (2019) Autoimmune epilepsy. Neurotherapeutics 16:685–702. https://doi.org/10.1007/s13311-019-00750-3
Irani SR, Alexander S, Waters P et al (2010) Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133:2734–2748. https://doi.org/10.1093/brain/awq213
Irani SR, Michell AW, Lang B et al (2011) Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 69:892–900. https://doi.org/10.1002/ana.22307
Lai M, Huijbers MG, Lancaster E et al (2010) Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 9:776–785. https://doi.org/10.1016/S1474-4422(10)70137-X
Navarro V, Kas A, Apartis E et al (2016) Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 139:1079–1093. https://doi.org/10.1093/brain/aww012
Pakozdy A, Patzl M, Zimmermann L et al (2015) LGI proteins and epilepsy in human and animals. J Vet Intern Med 29:997–1005. https://doi.org/10.1111/jvim.12610
Chernova OB, Somerville RP, Cowell JK (1998) A novel gene, LGI1, from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene 17:2873–2881. https://doi.org/10.1038/sj.onc.1202481
Krex D, Hauses M, Appelt H et al (2002) Physical and functional characterization of the human LGI1 gene and its possible role in glioma development. Acta Neuropathol 103:255–266. https://doi.org/10.1007/s004010100463
Besleaga R, Montesinos-Rongen M, Perez-Tur J et al (2003) Expression of the LGI1 gene product in astrocytic gliomas: downregulation with malignant progression. Virchows Arch 443:561–564. https://doi.org/10.1007/s00428-003-0874-3
Kunapuli P, Chitta KS, Cowell JK (2003) Suppression of the cell proliferation and invasion phenotypes in glioma cells by the LGI1 gene. Oncogene 22:3985–3991. https://doi.org/10.1038/sj.onc.1206584
Kunapuli P, Kasyapa CS, Hawthorn L, Cowell JK (2004) LGI1, a putative tumor metastasis suppressor gene, controls in vitro invasiveness and expression of matrix metalloproteinases in glioma cells through the ERK1/2 pathway. J Biol Chem 279:23151–23157. https://doi.org/10.1074/jbc.M314192200
Nadia G, Masola V, Quartesan S et al (2006) Increased expression ofLGI1 gene triggers growth inhibition and apoptosis of neuroblastoma cells. J Cell Physiol 207:711–721. https://doi.org/10.1002/jcp.20627
Piepoli T, Jakupoglu C, Gu W et al (2006) Expression studies in gliomas and glial cells do not support a tumor suppressor role for LGI11. Neuro Oncol 8:96–108. https://doi.org/10.1215/15228517-2005-006
Gu W, Brodtkorb E, Piepoli T et al (2005) LGI1: a gene involved in epileptogenesis and glioma progression? Neurogenetics 6:59–66. https://doi.org/10.1007/s10048-005-0216-5
Gadoth A, Pittock SJ, Dubey D et al (2017) Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol 82:79–92. https://doi.org/10.1002/ana.24979
Somerville RPT, Chernova O, Liu S et al (2000) Identification of the promoter, genomic structure, and mouse ortholog of LGI1. Mamm Genome 11:622–627. https://doi.org/10.1007/s0033500101280
Chabrol E, Gourfinkel-An I, Scheffer IE et al (2007) Absence of mutations in the LGI1 receptor ADAM22 gene in autosomal dominant lateral temporal epilepsy. Epilepsy Res 76:41–48. https://doi.org/10.1016/j.eplepsyres.2007.06.014
de Bellescize J, Boutry N, Chabrol E et al (2009) A novel three base-pair LGI1 deletion leading to loss of function in a family with autosomal dominant lateral temporal epilepsy and migraine-like episodes. Epilepsy Res 85:118–122. https://doi.org/10.1016/j.eplepsyres.2009.02.007
Head K, Gong S, Joseph S et al (2007) Defining the expression pattern of the LGI1 gene in BAC transgenic mice. Mamm Genome 18:328–337. https://doi.org/10.1007/s00335-007-9024-6
Gu W, Wevers A, Schröder H et al (2002) The LGI1 gene involved in lateral temporal lobe epilepsy belongs to a new subfamily of leucine-rich repeat proteins. FEBS Lett 519:71–76. https://doi.org/10.1016/S0014-5793(02)02713-8
Yamagata A, Miyazaki Y, Yokoi N et al (2018) Structural basis of epilepsy-related ligand–receptor complex LGI1–ADAM22. Nat Commun 9:1546. https://doi.org/10.1038/s41467-018-03947-w
Scheel H (2002) A common protein interaction domain links two recently identified epilepsy genes. Hum Mol Genet 11:1757–1762. https://doi.org/10.1093/hmg/11.15.1757
Staub E, Pérez-Tur J, Siebert R et al (2002) The novel EPTP repeat defines a superfamily of proteins implicated in epileptic disorders. Trends Biochem Sci 27:441–444. https://doi.org/10.1016/S0968-0004(02)02163-1
Leonardi E, Andreazza S, Vanin S et al (2011) A computational model of the LGI1 protein suggests a common binding site for ADAM proteins. PLoS ONE 6:e18142. https://doi.org/10.1371/journal.pone.0018142
Kobe B, Kajava AV (2001) The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11:725–732. https://doi.org/10.1016/s0959-440x(01)00266-4
Silva J, Wang G, Cowell JK (2011) The temporal and spatial expression pattern of the LGI1 epilepsy predisposition gene during mouse embryonic cranial development. BMC Neurosci 12:43. https://doi.org/10.1186/1471-2202-12-43
Boillot M, Huneau C, Marsan E et al (2014) Glutamatergic neuron-targeted loss of LGI1 epilepsy gene results in seizures. Brain 137:2984–2996. https://doi.org/10.1093/brain/awu259
Ohkawa T, Fukata Y, Yamasaki M et al (2013) Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 33:18161–18174. https://doi.org/10.1523/JNEUROSCI.3506-13.2013
Kornau H, Kreye J, Stumpf A et al (2020) Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol 87:405–418. https://doi.org/10.1002/ana.25666
Morante-Redolat JM (2002) Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet 11:1119–1128. https://doi.org/10.1093/hmg/11.9.1119
Furlan S, Roncaroli F, Forner F et al (2006) The LGI1/epitempin gene encodes two protein isoforms differentially expressed in human brain. J Neurochem 98:985–991. https://doi.org/10.1111/j.1471-4159.2006.03939.x
Chabrol E, Navarro V, Provenzano G et al (2010) Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain 133:2749–2762. https://doi.org/10.1093/brain/awq171
Xie Y-J, Zhou L, Wang Y et al (2018) Leucine-rich glioma inactivated 1 promotes oligodendrocyte differentiation and myelination via TSC-mTOR signaling. Front Mol Neurosci 11:231. https://doi.org/10.3389/fnmol.2018.00231
Kalachikov S, Evgrafov O, Ross B et al (2002) Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet 30:335–341. https://doi.org/10.1038/ng832
Herranz-Pérez V, Olucha-Bordonau FE, Morante-Redolat JM, Pérez-Tur J (2010) Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res 1307:177–194. https://doi.org/10.1016/j.brainres.2009.10.013
Smedfors G, Olson L, Karlsson TE (2018) A Nogo-like signaling perspective from birth to adulthood and in old age: brain expression patterns of ligands, receptors and modulators. Front Mol Neurosci 11:42. https://doi.org/10.3389/fnmol.2018.00042
Lovero KL, Fukata Y, Granger AJ et al (2015) The LGI1–ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function. Proc Natl Acad Sci USA 112:E4129–E4137. https://doi.org/10.1073/pnas.1511910112
Boillot M, Lee C-Y, Allene C et al (2016) LGI1 acts presynaptically to regulate excitatory synaptic transmission during early postnatal development. Sci Rep 6:21769. https://doi.org/10.1038/srep21769
Fukata Y, Chen X, Chiken S et al (2021) LGI1–ADAM22–MAGUK configures transsynaptic nanoalignment for synaptic transmission and epilepsy prevention. Proc Natl Acad Sci USA 118:e2022580118. https://doi.org/10.1073/pnas.2022580118
Seagar M, Russier M, Caillard O et al (2017) LGI1 tunes intrinsic excitability by regulating the density of axonal Kv1 channels. Proc Natl Acad Sci USA 114:7719–7724. https://doi.org/10.1073/pnas.1618656114
Hivert B, Marien L, Agbam KN, Faivre-Sarrailh C (2019) ADAM22 and ADAM23 modulate the targeting of the Kv1 channel-associated protein LGI1 to the axon initial segment. J Cell Sci. https://doi.org/10.1242/jcs.219774
Ribeiro PAO, Sbragia L, Gilioli R et al (2008) Expression profile of Lgi1 gene in mouse brain during development. J Mol Neurosci 35:323–329. https://doi.org/10.1007/s12031-008-9096-0
Thomas R, Favell K, Morante-Redolat J et al (2010) LGI1 Is a nogo receptor 1 ligand that antagonizes myelin-based growth inhibition. J Neurosci 30:6607–6612. https://doi.org/10.1523/JNEUROSCI.5147-09.2010
Kusuzawa S, Honda T, Fukata Y et al (2012) Leucine-rich glioma inactivated 1 (Lgi1), an epilepsy-related secreted protein, has a nuclear localization signal and localizes to both the cytoplasm and the nucleus of the caudal ganglionic eminence neurons: nuclear translocation of Lgi1. Eur J Neurosci 36:2284–2292. https://doi.org/10.1111/j.1460-9568.2012.08129.x
Fukata Y, Lovero KL, Iwanaga T et al (2010) Disruption of LGI1–linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA 107:3799–3804. https://doi.org/10.1073/pnas.0914537107
Gödde NJ, D’Abaco GM, Paradiso L, Novak U (2006) Efficient ADAM22 surface expression is mediated by phosphorylation-dependent interaction with 14–3-3 protein family members. J Cell Sci 119:3296–3305. https://doi.org/10.1242/jcs.03065
Yu YE, Wen L, Silva J et al (2010) Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum Mol Genet 19:1702–1711. https://doi.org/10.1093/hmg/ddq047
Kole MJ, Qian J, Waase MP et al (2015) Selective loss of presynaptic potassium channel clusters at the cerebellar basket cell terminal pinceau in adam11 mutants reveals their role in ephaptic control of Purkinje cell firing. J Neurosci 35:11433–11444. https://doi.org/10.1523/JNEUROSCI.1346-15.2015
Sagane K, Hayakawa K, Kai J et al (2005) Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci 6:33. https://doi.org/10.1186/1471-2202-6-33
Owuor K, Harel NY, Englot DJ et al (2009) LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci 42:448–457. https://doi.org/10.1016/j.mcn.2009.09.008
Smart SL, Lopantsev V, Zhang CL et al (1998) Deletion of the KV1.1 potassium channel causes epilepsy in mice. Neuron 20:809–819. https://doi.org/10.1016/S0896-6273(00)81018-1
Brew HM, Gittelman JX, Silverstein RS et al (2007) Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 98:1501–1525. https://doi.org/10.1152/jn.00640.2006
Edwards D, Handsley M, Pennington C (2008) The ADAM metalloproteinases. Mol Aspects Med 29:258–289. https://doi.org/10.1016/j.mam.2008.08.001
Sagane K, Yamazaki K, Mizui Y, Tanaka I (1999) Cloning and chromosomal mapping of mouse ADAM11, ADAM22 and ADAM23. Gene 236:79–86. https://doi.org/10.1016/s0378-1119(99)00253-x
Liu H, Shim AHR, He X (2009) Structural characterization of the ectodomain of a disintegrin and metalloproteinase-22 (ADAM22), a neural adhesion receptor instead of metalloproteinase. J Biol Chem 284:29077–29086. https://doi.org/10.1074/jbc.M109.014258
El-Husseini AE, Schnell E, Chetkovich DM et al (2000) PSD-95 involvement in maturation of excitatory synapses. Science 290:1364–1368
Yokoi N, Fukata Y, Kase D et al (2015) Chemical corrector treatment ameliorates increased seizure susceptibility in a mouse model of familial epilepsy. Nat Med 21:19–26. https://doi.org/10.1038/nm.3759
Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409:341–346. https://doi.org/10.1038/35053072
Thomas RA, Gibon J, Chen CXQ et al (2018) The Nogo receptor ligand LGI1 regulates synapse number and synaptic activity in hippocampal and cortical neurons. eNeuro. https://doi.org/10.1523/ENEURO.0185-18.2018
Schulte U, Thumfart J-O, Klöcker N et al (2006) The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron 49:697–706. https://doi.org/10.1016/j.neuron.2006.01.033
Savtchenko LP, Rusakov DA (2007) The optimal height of the synaptic cleft. Proc Natl Acad Sci U S A 104:1823–1828. https://doi.org/10.1073/pnas.0606636104
Silva J, Sharma S, Cowell JK (2015) Homozygous deletion of the LGI1 gene in mice leads to developmental abnormalities resulting in cortical dysplasia: loss of LGI1 leads to cortical dysplasia. Brain Pathol 25:587–597. https://doi.org/10.1111/bpa.12225
Silva J, Qin H, Cowell JK (2019) Selective inactivation of LGI1 in neuronal precursor cells leads to cortical dysplasia in mice. Genesis 57:e23268. https://doi.org/10.1002/dvg.23268
Xie Y-J, Zhou L, Jiang N et al (2015) Essential roles of leucine-rich glioma inactivated 1 in the development of embryonic and postnatal cerebellum. Sci Rep 5:7827. https://doi.org/10.1038/srep07827
Wills ZP, Mandel-Brehm C, Mardinly AR et al (2012) The Nogo receptor family restricts synapse number in the developing hippocampus. Neuron 73:466–481. https://doi.org/10.1016/j.neuron.2011.11.029
Luo L (2000) Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 1:173–180. https://doi.org/10.1038/35044547
Silva J, Sharma S, Hughes B et al (2010) Homozygous inactivation of the LGI1 gene results in hypomyelination in the peripheral and central nervous systems: hypomyelination in LGI1 null mice. J Neurosci Res 88:3328–3336. https://doi.org/10.1002/jnr.22496
Lebrun-Julien F, Bachmann L, Norrmén C et al (2014) Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J Neurosci 34:8432–8448. https://doi.org/10.1523/JNEUROSCI.1105-14.2014
Ovsepian SV, LeBerre M, Steuber V et al (2016) Distinctive role of KV1.1 subunit in the biology and functions of low threshold K+ channels with implications for neurological disease. Pharmacol Ther 159:93–101. https://doi.org/10.1016/j.pharmthera.2016.01.005
Dodson PD, Forsythe ID (2004) Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci 27:210–217. https://doi.org/10.1016/j.tins.2004.02.012
Trimmer JS (2015) Subcellular localization of K+ channels in mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron 85:238–256. https://doi.org/10.1016/j.neuron.2014.12.042
Guan D, Lee JCF, Tkatch T et al (2006) Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones. J Physiol 571:371–389. https://doi.org/10.1113/jphysiol.2005.097006
Guan D, Lee JCF, Higgs MH et al (2007) Functional roles of Kv1 channels in neocortical pyramidal neurons. J Neurophysiol 97:1931–1940. https://doi.org/10.1152/jn.00933.2006
Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D (2010) Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci 30:12885–12895. https://doi.org/10.1523/JNEUROSCI.0740-10.2010
Yellen G (2002) The voltage-gated potassium channels and their relatives. Nature 419:35–42. https://doi.org/10.1038/nature00978
Ovsepian SV, Steuber V, Le Berre M et al (2013) A defined heteromeric K V 1 channel stabilizes the intrinsic pacemaking and regulates the output of deep cerebellar nuclear neurons to thalamic targets: K V 1 channel governs cerebellar output to thalamus. J Physiol 591:1771–1791. https://doi.org/10.1113/jphysiol.2012.249706
Guan D, Pathak D, Foehring RC (2018) Functional roles of Kv1-mediated currents in genetically identified subtypes of pyramidal neurons in layer 5 of mouse somatosensory cortex. J Neurophysiol 120:394–408. https://doi.org/10.1152/jn.00691.2017
Wang FC, Bell N, Reid P et al (1999) Identification of residues in dendrotoxin K responsible for its discrimination between neuronal K+ channels containing Kv1.1 and 1.2 alpha subunits. Eur J Biochem 263:222–229. https://doi.org/10.1046/j.1432-1327.1999.00494.x
Harvey AL, Robertson B (2004) Dendrotoxins: structure-activity relationships and effects on potassium ion channels. CMC 11:3065–3072. https://doi.org/10.2174/0929867043363820
Zhou L, Zhou L, Su L et al (2018) Celecoxib ameliorates seizure susceptibility in autosomal dominant lateral temporal epilepsy. J Neurosci 38:3346–3357. https://doi.org/10.1523/JNEUROSCI.3245-17.2018
Lancaster E, Burnor E, Zhang J, Lancaster E (2019) ADAM23 is a negative regulator of Kv1.1/Kv1.4 potassium currents. Neurosci Lett 704:159–163. https://doi.org/10.1016/j.neulet.2019.04.012
Henley JM, Nair JD, Seager R et al (2021) Kainate and AMPA receptors in epilepsy: cell biology, signalling pathways and possible crosstalk. Neuropharmacology 195:108569. https://doi.org/10.1016/j.neuropharm.2021.108569
Schnell E, Sizemore M, Karimzadegan S et al (2002) Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A 99:13902–13907. https://doi.org/10.1073/pnas.172511199
von Ossowski L, Tossavainen H, von Ossowski I et al (2006) Peptide binding and NMR analysis of the interaction between SAP97 PDZ2 and GluR-A: potential involvement of a disulfide bond. Biochemistry 45:5567–5575. https://doi.org/10.1021/bi0511989
Ottman R, Risch N, Hauser WA et al (1995) Localization of a gene for partial epilepsy to chromosome 10q. Nat Genet 10:56–60. https://doi.org/10.1038/ng0595-56
Dazzo E, Fanciulli M, Serioli E et al (2015) Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy. Am J Hum Genet 96:992–1000. https://doi.org/10.1016/j.ajhg.2015.04.020
Michelucci R, Poza JJ, Sofia V et al (2003) Autosomal dominant lateral temporal epilepsy: clinical spectrum, new epitempin mutations, and genetic heterogeneity in seven European families: familial lateral temporal epilepsy. Epilepsia 44:1289–1297. https://doi.org/10.1046/j.1528-1157.2003.20003.x
Michelucci R, Pasini E, Malacrida S et al (2013) Low penetrance of autosomal dominant lateral temporal epilepsy in Italian families without LGI1 mutations. Epilepsia 54:1288–1297. https://doi.org/10.1111/epi.12194
Bisulli F, Naldi I, Baldassari S et al (2014) Autosomal dominant partial epilepsy with auditory features: a new locus on chromosome 19q13.11-q13.31. Epilepsia 55:841–848. https://doi.org/10.1111/epi.12560
Boillot M, Baulac S (2016) Genetic models of focal epilepsies. J Neurosci Methods 260:132–143. https://doi.org/10.1016/j.jneumeth.2015.06.003
Yamagata A, Fukai S (2020) Insights into the mechanisms of epilepsy from structural biology of LGI1–ADAM22. Cell Mol Life Sci 77:267–274. https://doi.org/10.1007/s00018-019-03269-0
Baulac S, Ishida S, Mashimo T et al (2012) A rat model for LGI1-related epilepsies. Hum Mol Genet 21:3546–3557. https://doi.org/10.1093/hmg/dds184
Striano P, Busolin G, Santulli L et al (2011) Familial temporal lobe epilepsy with psychic auras associated with a novel LGI1 mutation. Neurology 76:1173–1176. https://doi.org/10.1212/WNL.0b013e318212ab2e
Rosanoff MJ, Ottman R (2008) Penetrance of LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology 71:567–571. https://doi.org/10.1212/01.wnl.0000323926.77565.ee
Nobile C, Michelucci R, Andreazza S et al (2009) LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat 30:530–536. https://doi.org/10.1002/humu.20925
Vincent A, Buckley C, Schott JM et al (2004) Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127:701–712. https://doi.org/10.1093/brain/awh077
de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH et al (2019) Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABA B R encephalitis. Neurology 92:e2185–e2196. https://doi.org/10.1212/WNL.0000000000007475
Shen C, Fang G, Yang F et al (2020) Seizures and risk of epilepsy in anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis. Ann Clin Transl Neurol 7:1392–1399. https://doi.org/10.1002/acn3.51137
Ilyas-Feldmann M, Prüß H, Holtkamp M (2021) Long-term seizure outcome and antiseizure medication use in autoimmune encephalitis. Seizure 86:138–143. https://doi.org/10.1016/j.seizure.2021.02.010
Qiao S, Wu H-K, Liu L-L et al (2021) Characteristics and prognosis of autoimmune encephalitis in the East of China: a multi-center study. Front Neurol 12:642078. https://doi.org/10.3389/fneur.2021.642078
Shan W, Yang H, Wang Q (2021) Neuronal surface antibody-medicated autoimmune encephalitis (limbic encephalitis) in China: a multiple-center retrospective study. Front Immunol 12:621599. https://doi.org/10.3389/fimmu.2021.621599
Cousyn L, Lambrecq V, Houot M et al (2021) Seizures in autoimmune encephalitis: specific features from a systematic comparative study. Epileptic Disord. https://doi.org/10.1684/epd.2021.1355
Qiao S, Wu H, Liu L et al (2021) Clinical features and long-term outcomes of anti-leucine-rich glioma-inactivated 1 encephalitis: a multi-center study. NDT 17:203–212. https://doi.org/10.2147/NDT.S292343
Lin N, Liu Q, Chen J et al (2021) Long-term seizure outcomes in patients with anti-leucine-rich glioma-inactivated 1 encephalitis. Epilepsy Behav 122:108159. https://doi.org/10.1016/j.yebeh.2021.108159
Li T-R, Zhang Y-D, Wang Q et al (2021) Clinical characteristics and long-term prognosis of anti-LGI1 encephalitis: a single-center cohort study in Beijing. China Front Neurol 12:674368. https://doi.org/10.3389/fneur.2021.674368
Zhao Q, Sun L, Zhao D et al (2021) Clinical features of anti-leucine-rich glioma-inactivated 1 encephalitis in northeast China. Clin Neurol Neurosurg 203:106542. https://doi.org/10.1016/j.clineuro.2021.106542
van Sonderen A, Schreurs MWJ, de Bruijn MAAM et al (2016) The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 86:1692–1699. https://doi.org/10.1212/WNL.0000000000002637
Binks SNM, Veldsman M, Easton A et al (2021) Residual fatigue and cognitive deficits in patients after leucine-rich glioma-inactivated 1 antibody encephalitis. JAMA Neurol 78:617. https://doi.org/10.1001/jamaneurol.2021.0477
Kim T-J, Lee S-T, Moon J et al (2017) Anti-LGI1 encephalitis is associated with unique HLA subtypes: HLA subtypes in anti-LGI1 encephalitis. Ann Neurol 81:183–192. https://doi.org/10.1002/ana.24860
van Sonderen A, Roelen DL, Stoop JA et al (2017) Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4: anti-LGI1 encephalitis. Ann Neurol 81:193–198. https://doi.org/10.1002/ana.24858
Binks S, Varley J, Lee W et al (2018) Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 141:2263–2271. https://doi.org/10.1093/brain/awy109
Muñiz-Castrillo S, Haesebaert J, Thomas L et al (2021) Clinical and prognostic value of immunogenetic characteristics in anti-LGI1 encephalitis. Neurol Neuroimmunol Neuroinflamm 8:e974. https://doi.org/10.1212/NXI.0000000000000974
Wennberg R, Steriade C, Chen R, Andrade D (2018) Frontal infraslow activity marks the motor spasms of anti-LGI1 encephalitis. Clin Neurophysiol 129:59–68. https://doi.org/10.1016/j.clinph.2017.10.014
Liu X, Han Y, Yang L et al (2020) The exploration of the spectrum of motor manifestations of anti-LGI1 encephalitis beyond FBDS. Seizure 76:22–27. https://doi.org/10.1016/j.seizure.2019.12.023
Flanagan EP, Kotsenas AL, Britton JW et al (2015) Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol Neuroimmunol Neuroinflamm 2:e161. https://doi.org/10.1212/NXI.0000000000000161
Liu X, Shan W, Zhao X et al (2020) The clinical value of 18F-FDG-PET in autoimmune encephalitis associated with LGI1 antibody. Front Neurol 11:418. https://doi.org/10.3389/fneur.2020.00418
Brüggemann N (2021) Contemporary functional neuroanatomy and pathophysiology of dystonia. J Neural Transm (Vienna) 128:499–508. https://doi.org/10.1007/s00702-021-02299-y
Celicanin M, Blaabjerg M, Maersk-Moller C et al (2017) Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies - a national cohort study. Eur J Neurol 24:999–1005. https://doi.org/10.1111/ene.13324
Hang H, Zhang J, Chen D et al (2020) Clinical characteristics of cognitive impairment and 1-year outcome in patients with anti-LGI1 antibody encephalitis. Front Neurol 11:852. https://doi.org/10.3389/fneur.2020.00852
Ramanathan S, Tseng M, Davies AJ et al (2021) LGI1- versus CASPR2-antibody neuropathic pain: clinical and biological comparisons. Ann Neurol. https://doi.org/10.1002/ana.26189
van Sonderen A, Thijs RD, Coenders EC et al (2016) Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87:1449–1456. https://doi.org/10.1212/WNL.0000000000003173
Chen W, Wang M, Gao L et al (2021) Neurofunctional outcomes in patients with anti-leucine-rich glioma inactivated 1 encephalitis. Acta Neurol Scand. https://doi.org/10.1111/ane.13503
Smith KM, Dubey D, Liebo GB et al (2021) Clinical course and features of seizures associated with LGI1-antibody encephalitis. Neurology. https://doi.org/10.1212/WNL.0000000000012465
Ariño H, Armangué T, Petit-Pedrol M et al (2016) Anti-LGI1–associated cognitive impairment: presentation and long-term outcome. Neurology 87:759–765. https://doi.org/10.1212/WNL.0000000000003009
Thompson J, Bi M, Murchison AG et al (2018) The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141:348–356. https://doi.org/10.1093/brain/awx323
Bien CG, Bien CI, Dogan Onugoren M et al (2020) Routine diagnostics for neural antibodies, clinical correlates, treatment and functional outcome. J Neurol 267:2101–2114. https://doi.org/10.1007/s00415-020-09814-3
Koneczny I (2018) A new classification system for IgG4 autoantibodies. Front Immunol 9:97. https://doi.org/10.3389/fimmu.2018.00097
Ramberger M, Berretta A, Tan JMM et al (2020) Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 143:1731–1745. https://doi.org/10.1093/brain/awaa104
González-Burgos I, Velázquez-Zamora DA, Beas-Zárate C (2009) Damage and plasticity in adult rat hippocampal trisynaptic circuit neurons after neonatal exposure to glutamate excitotoxicity. Int J Dev Neurosci 27:741–745. https://doi.org/10.1016/j.ijdevneu.2009.08.016
Irani SR, Stagg CJ, Schott JM et al (2013) Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 136:3151–3162. https://doi.org/10.1093/brain/awt212
Feyissa AM, Lamb C, Pittock SJ et al (2018) Antiepileptic drug therapy in autoimmune epilepsy associated with antibodies targeting the leucine-rich glioma-inactivated protein 1. Epilepsia Open 3:348–356. https://doi.org/10.1002/epi4.12226
Dubey D, Britton J, McKeon A et al (2020) Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann Neurol 87:313–323. https://doi.org/10.1002/ana.25655
Petit-Pedrol M, Sell J, Planagumà J et al (2018) LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain. https://doi.org/10.1093/brain/awy253
Zhang T-Y, Cai M-T, Zheng Y et al (2021) Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor encephalitis: a review. Front Immunol 12:652820. https://doi.org/10.3389/fimmu.2021.652820
Acknowledgements
We thank Richard Miles, for critical reading of the manuscript.
Funding
Agence nationale de la recherche, ‘Investissements d’avenir’ program ANR-10–IAIHU-06. Fondation pour la Recherche Médicale, grant FDT202012010523. Fondation Assitance Publique-Hôpitaux de Paris (EPIRES, Marie Laure PLV Merchandising).
Author information
Authors and Affiliations
Contributions
Original draft preparation and editing: PB, LC, VN.
Corresponding author
Ethics declarations
Conflict of interest
V.N. reports personal fees from UCB, Liva Nova, and EISAI, outside the submitted work. The other authors declare that they have no competing interests.
Informed consent
The authors affirm that patients provided informed consent for publication of the images in Fig. 3 and in Online Resource 1.
Consent for publication
All authors gave their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Online Resource 1 Video recordings of TDS (or FBDS) in a patient with anti-LGI1 AE. The patient presented both right and left TDS, as well as some ‘à bascule’ TDS affecting one side then the other one after a few seconds. (AVI 9926 KB)
Rights and permissions
About this article
Cite this article
Baudin, P., Cousyn, L. & Navarro, V. The LGI1 protein: molecular structure, physiological functions and disruption-related seizures. Cell. Mol. Life Sci. 79, 16 (2022). https://doi.org/10.1007/s00018-021-04088-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-021-04088-y