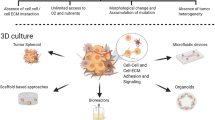
Abstract
Despite numerous advances in tumor screening, diagnosis, and treatment, to date, tumors remain one of the leading causes of death, principally due to metastasis and the physiological damage produced by tumor growth. Among the main limits related to the study of tumor physiology there is the complex and heterogeneity nature of its environment and the absence of relevant, simple and inexpensive models able to mimic the biological processes occurring in patients allowing the correct clinical translation of results. To enhance the understanding of the mechanisms of tumors and to develop and evaluate new therapeutic approaches the set-up of advanced and alternative models is mandatory. One of the more translational approaches seems to be the use of humanized three-dimensional (3D) tissue culture. This model allows to accurately mimic tumor morphology and biology, maintaining the native microenvironment without any manipulation. However, little is still known on the real clinical relevance of these models for the study of tumor mechanisms and for the screening of new therapy. The aim of this descriptive systematic literature review was to evaluate and summarize the current knowledge on human 3D tumor tissue culture models. We reviewed the strategies employed by researchers to set-up these systems, also considering the different approaches and culture conditions used. All these aspects greatly contribute to the existing knowledge on tumors, providing a specific link to clinical scenarios and making the humanized 3D tumor tissue models a more attractive tool both for researchers and clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumors represent a type of multifaceted pathological condition, where normal cells begin to be hyper-proliferative and start the expression of a number of factors, i.e., cytokines, chemokines and growth factors, involved in cell proliferation, invasion and metastasis [1]. To date, surgery associated to standard chemotherapeutic protocols and radiotherapy represents the first-line treatment for most tumors, with a significant increase in survival rates [2, 3]. Immunotherapy, i.e., immune checkpoint blockade, cancer vaccines, and adoptive cell therapy, also allows an increase in anti-tumor immune responses and an improvement of the patient’s clinical outcomes with minimal toxicity [4]. However, tumors still continue to represent one of the main causes of mortality [5, 6] principally due to metastasis and the physiological damage produced by tumor growth. The most frequent sites for metastatic disease are lung, liver, thyroid, bone and brain [7, 8]. To date, the therapeutic strategies used for metastasis are mostly palliative, with very limited opportunities for complete eradication [6]. Thus, the development and evaluation of new and advanced treatments for both tumor and metastasis are mandatory. Nevertheless, this issue requires not only a great understanding of the cellular and molecular mechanisms leading to tumor and/or metastasis but also the use of clinically relevant models able to strictly recapitulate and mimic the real complexity of the in vivo physiology.
In the last years, to investigate the mechanisms underlying tumor pathogenesis, progression and resistance to treatments, two-dimensional (2D) in vitro models have been widely employed [9,10,11,12]. These models are easy to handle and quite inexpensive to set-up and have led to a greater understanding in the ability of tumor cells to grow [13]. However, 2D models are for some aspects reductive and do not provide information about the real biological mechanisms; moreover, they are not able to mimic tumor and metastasis macrostructure, cellular heterogeneity and microenvironment complexity [14,15,16]. In addition, super imposed spatial cues, including substrate depth and cell connectivity, limit the applicability of these models for testing new therapies [17]. These restrictions provide unreliable data for translating results into clinical applications. In vivo animal models overcome many of the limits linked to 2D models, trying to mimic the native microenvironment in which tumors and metastasis reside [9]. There are several established in vivo models that differ in the species used, type of cancer/metastasis and method of cancer/metastasis induction [18]. Generally, xenograft animal models are extensively used to study tumorigenesis process and new therapies, showing several advantageous features, such as short reproductive cycle and ease manipulation [9, 19,20,21]. Patient derived xenografts, established from small fragments of human tumor tissue samples directly implanted into immunocompromised mice, are also frequently used as preclinical models able to closely resemble tumor/metastasis phenotype and human intra-tumor heterogeneity [21,22,23,24]. However, these models are expensive, also in terms of ethical issues, and challenging in the set-up as they show difficulties in tracking tumor growth and in drug screening studies. Moreover, the length of time necessary for implantation, propagation, and drug screening makes this model unsuitable for direct clinical use in patients [25, 26]. Recent studies tried to overcome several of the limits linked to 2D and in vivo animal models with the development of advanced three-dimensional (3D) in vitro models [9, 27,28,29,30]. Several types of 3D systems were designed to resemble in vivo tumors, considering both tumor heterogeneity and tumor–stroma interactions [31, 32]. In vitro 3D models include tumor-derived organoids and spheroids that tried to reproduce the tumor microenvironment [33]. However, these approaches mimic the tumor complexity only partially following a mechanical dissociation and enzymatic treatments of the tumor tissue [34,35,36]. Although the selective growth of tumor cells in an artificial environment can be studied and used in 3D, they recapitulate only few aspects of the tumor complexity, and remain essentially reductionist models [14]. A more translational approach seems to be the use of 3D tissue culture, in particular culture of patient-derived tumor tissue [37,38,39,40]. These models allow to accurately mimic tumor morphology and biology, thus maintaining the native microenvironment [41,42,43] without any manipulation [44,45,46,47,48]. However, little is still known on the possible clinical relevance of these 3D tissue models for the study of tumor mechanisms, but also for the evaluation of novel and advanced therapeutic strategies.
This descriptive systematic literature review considered and evaluated humanized 3D tumor tissue models and the strategies employed by researchers to set-up these systems, as well as taking into consideration their advantages and/or disadvantages, thus to understand if these models can be used as clinically relevant, advanced and translational systems for the study of tumor mechanisms and for the evaluation of novel therapeutic strategies.
Methods
Descriptive systematic literature review
Our descriptive literature review involved a systematic search that was carried out, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, in three databases (www.pubmed.org, www.scopus.com, www.webofknowledge.com). The keywords were: “(tumor OR cancer) AND (tissue culture) AND (ex vivo model OR ex vivo explant)”. We sought to identify studies, where 3D models of tumor tissue were employed. Publications from 2009 to 2019 (original articles in English and full text) were included. Exclusion criteria were articles not written in English, reviews and articles in which cell cultures, spheroids, organoids, cancer-on-chip, bioreactors, microfluidic devices and in vivo models were used. Additional studies that were not found by our initial search were identified analyzing the reference lists from the included articles. A public reference manager (www.mendeley.com) was used to delete duplicate articles.
Results
An initial literature search retrieved 1782 articles: 372 articles were identified using PubMed, 1061 articles using Scopus and 349 were found in ISI Web of Knowledge (Fig. 1). Articles were submitted to a public reference manager (Mendeley 1.14, www.mendeley.com) to eliminate duplicate articles (n = 107). The resulting articles (n = 1675) were evaluated by two independent researchers (DC and FS) by reviewing titles and abstracts. Subsequently, 1607 complete articles were reviewed to establish whether the publication met the inclusion criteria and 59 articles were recognized eligible for this review.
3D tumor tissue models setup
As reported in Table 1, from the 59 articles on 3D human tumor tissue model analyzed in this review 16 articles used tumor tissue from breast [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64], 14 from prostate [60, 63, 65,66,67,68,69,70,71,72,73,74,75,76], six from brain [64, 77,78,79,80,81], seven from lung [40, 63, 64, 82,83,84,85], eight from colorectal [55, 63, 64, 86,87,88,89,90], five from pancreas [63, 64, 91,92,93], five from ovary [63, 64, 94,95,96], one from testicle [97], two from head and neck (oral, oropharyngeal and nasal origin) [98, 99], two from liver [63, 100], two from skin [64, 101], three from uterus [63, 64, 102], two from stomach [63, 103], four from bladder/ureter [63, 64, 76, 104], three from kidney [63, 64, 105], and one from dental apparatus (mandible) [106] (Fig. 2).
Human tissue withdrawal and resection techniques
The analyzed studies retrieved from this review showed that human tumor tissue for the set-up of 3D models is usually obtained and employed within minutes or hours from surgical resection, thus minimizing tissue deterioration and loss of cellular viability [50, 54, 59, 60, 97, 103, 104]. Several studies specifically demonstrated that tumor tissue resection does not interfere with morphology and functional activity of the tissue, ensuring the preservation of cell phenotypes and the heterogeneity of cancer sub-populations [54, 61, 92]. However, it was observed that, though manual manipulation of tumor tissue samples, using choppers, scissors and scalpels, is technically simple and easy to perform it does present drawbacks, specifically the development of isolating fragments of various shapes and thicknesses, obtained from tumor regions of heterogeneous composition [40, 52, 54, 64, 66, 76, 84, 106]. Therefore, several studies showed a further refinement of the cut method involving the use of microtomes and vibratomes to prepare tumor samples with standardized and reproducible characteristics [53, 54, 58, 59, 61,62,63, 71, 79,80,81, 83, 87, 92, 94, 95, 98, 102, 105].
Culture media
Concerning the experimental set-up of these models, different culture media were used in the analyzed studies. Generally, commercially available media, i.e., Dulbecco's Modified Eagle Medium (DMEM), DMEM/F12, RPMI, supplemented with fetal bovine serum (FBS) and antibiotics (penicillin/streptomycin) were employed. For some tumors the basic culture medium was added with nutrients and growth factors to optimize the culture conditions [50, 54, 58, 60, 61, 65, 68, 70, 73, 75, 79, 80]. For instance, in breast and prostate cancer tissue culture the medium was often supplemented with insulin [50, 54, 58, 60, 61, 65, 68, 70, 73, 75], while in glioblastoma tissue culture with B-27 factor [79, 80]. Some authors supplemented the culture medium also with estrogens or androgens to further maintain the endocrine signaling, as in breast and prostate cancer tissue cultures [50, 56, 59, 60, 71, 74].
Microenvironment
Another important issue for 3D tumor tissue models set-up is the microenvironment, since hypoxia is typically present in solid tumors and is known to enhance tumor progression and therapy resistance. Results from the reviewed studies showed that 4/59 studies [79, 84, 92, 102] used a hypoxic environment to culture tumor tissue (oxygen 1–2%), but none of them found clear advantage in the hypoxic microenvironment over the normoxic ones (oxygen 20%) [92, 102]. In fact, despite different oxygen levels, the tissues apoptosis rates were comparable among normoxic and hypoxic cultures, suggesting the adaptation of tumor tissue to the microenvironment [84]. Leithner et al. [84] also observed that tissues cultured under hypoxia were entirely hypoxic, while only a core of hypoxia was found in tissues cultured under normoxia. However, it was observed that major hypoxia-markers were significantly increased in hypoxic tissues culture, i.e., hypoxia-inducible factor 1-alpha (HIF-1α) and carbonic anhydrase IX (CA IX) [84]. It was also observed that HIF-2α, which is known to be stabilized by hypoxia, was expressed only at low levels, both in normoxia and hypoxia, and was not elevated in hypoxic tissues. This indicates that the difference in oxygen concentration was preserved inside the tumor tissues [84]. Contrarily, Parker et al. [79] demonstrated that by culturing the tissues under hypoxia, they showed a rapid physiologic response, inducing the release of vascular endothelial growth factor (VEGF) that has not been observed under normoxia [79].
Culture methods
In addition to different techniques for tumor tissue resection, culture medium and microenvironment (normoxic and hypoxic), to set-up the 3D tumor tissue models also different culture methods were used. In most of the examined studies tumor tissues were cultured on the bottom of the plates and submerged in medium [50, 52,53,54,55, 58, 61, 62, 64, 70, 81,82,83,84,85,86,87,88,89, 91, 94,95,96,97,98,99,100]. In these experimental setups, to avoid loss of tissue integrity and viability, the incorporation of a continuous rotational movement seems to be critical for the perfusion of oxygen to the tumor tissue and for the nutrient exchange [54, 61, 62, 71, 88, 91, 100]. In fact, Naipal et al. [54] showed that breast cancer tissues cultured under dynamic conditions, i.e., subjected to rotation using a mini orbital shaker placed in the incubator, maintained its viability for 7 days and showed more proliferating cells compared to the same tissue cultured under static conditions [54]. Several studies also cultured the tumor tissue on different types of scaffolds [40, 49, 51, 56, 57, 59, 60, 63, 65,66,67,68,69, 71,72,73,74,75,76,77,78,79,80, 90, 92, 93, 101,102,103,104,105,106]. Some authors used a lens paper supported by a mesh grid made of titanium or stainless steel for prostate tumor tissues culture, showing the maintain of their viability for up to 5–7 days [68, 71, 72, 75], while uterine leiomyoma tissues cultured on metal grids preserved their viability and proliferation for 48 h [102]. As alternative to titanium grid, many authors utilized also media pre-soaked gelatin sponge, collagen type I matrix, tissue support (VTEs) or different type of inserts (PTFE, porous filter membranes or moisturized) as alternative scaffold for tumor tissue culture [40, 49, 51, 56, 57, 59, 60, 63, 65,66,67, 69, 73, 74, 76,77,78,79,80, 90, 92, 93, 101, 103,104,105,106]. In some studies the tumor tissue was cultured on the top of the sponge, matrix or inserted at the air/liquid interface, acting as point of exchange for nutrients, thus allowing the preservation of the 3D tissue structure and giving an efficient oxygenation and a good viability to the tumor tissues [40, 49, 51, 56, 57, 59, 60, 63, 65,66,67, 69, 73, 74, 76,77,78,79,80, 90, 92, 93, 101, 103,104,105,106]. Bastos et al. [106] cultured ectomesenchymal odontogenic tumor tissue on type I collagen scaffolds obtaining a good reproduction of the growth pattern including cell proliferation and migration into the collagen matrix, preservation of the tissue architecture and maintenance of cell viability for more than 30 days [106]. Similarly, also urothelial carcinoma tissues cultured on gelatine matrices preserved its morphology and cell vitality over 20 day culture [104]. Pancreatic cancer tissues cultured on a supporting tissue bed containing stromal cells, matrix and vasculature (VTEs) maintained its histo-architecture, viability and genomic status of the primary tumor up to 10 days when compared to tumor tissues cultured without support [93]. Finally, some studies showed that breast and prostate cancer tissues, cultured on gelatine sponge, maintained tissue morphology and viability up to 6 days also showed the capacity for de novo cells proliferation [60, 74]. In addition to the roles of matrices in the maintenance of structure and morphology of tumor tissues, it was seen that they also improve the expression of steroid receptors during culture, thus delaying the loss of stromal cells [56, 59, 74].
Evaluation measurement tools
3D tumor tissue systems, with and/or without scaffolds, also allows performing histological, biochemical and molecular analyses directly on the tissue to measure tumor cell proliferation, detect the occurrence of genomic lesions and cell death and to examine the activation of oncogenic signal transduction cascades. Histopathological analysis allowed detecting and/or confirm the absence of significant change in tissue morphology and cell density; cells showed complete integrity and no areas of degeneration and/or necrosis [40, 52,53,54, 57, 60, 61, 63, 67, 71, 74, 76, 79, 91,92,93, 97, 103, 104, 106]. Immunohistochemical analyses (Ki-67, cleaved caspase-3, CK8, brdU uptake, TUNEL) revealed significant levels of ongoing proliferation of tumor cells [40, 53, 54, 57, 60, 61, 63, 71, 74, 76, 84, 92, 97, 102,103,104], confirming the ability of tumor tissues to maintain 80–90% of original viability [53, 54, 57, 58, 60, 63, 71, 74, 76, 79, 84, 92, 97, 102,103,104] without significant increase in cell apoptosis [40, 63, 84, 93, 97, 102]. Finally, also molecular (RT-PCR) and biochemical analyses (western blot, ELISA assay) on tissues and/or culture medium confirmed the maintenance of gene expression profile (p-AKT, p-S6RP, p-mTOR, p-S6K1 and p-4EBP1 of PI3K/AKT/TOR pathway; GLI1, NEO1, NTN1 and RGMA of SHH/GLI pathway; AR target genes PSA, TMPRSS2, FKBP5; hypoxia markers CAIX, HIF-1α; KIT, AP2γ, FIG, ROS1, p-ALK, p-Met, survivin, Akt, Mcl-1, KRT19) and protein levels (VEGF, EGFR, PLGF, s-FLT1, KIT, AP2γ, FIG, ROS1, p-ALK, p-Met, survivin, Akt, Mcl-1, NEO1, NTN1, RGMA, pS6, α-SMA, THBS1, MET, EGFR, PDGFRα) of primary tumor [58, 60, 63, 74, 78, 79, 84, 86, 87, 92, 93, 97, 101].
New treatment evaluation
The ability of these systems to strictly recapitulate the real complexity of the tumor physiological microenvironment, allowed to use these models also as preclinical tool to evaluate the response to novel drugs, alternative chemotherapeutics and small molecule inhibitors [40, 52, 54, 57, 58, 60, 61, 63, 67, 71, 74, 76, 78,79,80, 97, 103, 104]. In fact, in many studies the ability to culture and preserve tumor tissue for long periods of times (up to 3–7 days) consented a more adequate exposure and response to chemotherapeutic agents and/or targeted therapies [40, 53, 54, 60, 61, 63, 74, 76, 92, 103]. For instance, Affolter et al. [98] showed that the treatment with a MEK inhibitor associated to irradiation lead to an extensive DNA damage in head and neck squamous cell carcinoma (tissues derived from the oral and nasal cavity), with decrease of p53 phosphorylation and with a strong γH2AX staining, indicative of a DNA repair response and of a decrease of proliferative activity [98]. Several authors in 3D prostate cancer tissue cultures demonstrated an anti-proliferative and pro-apoptotic responses, determined by low levels of Ki67 and high levels of cleaved caspase-3, to novel heat shock protein 90 (Hsp90) inhibitors [65, 73], PARP-1 inhibitors [67] and Jack2 inhibitors [68, 70, 72, 75]. Always using prostate cancer 3D culture it was demonstrated that the co-treatment with focal adhesion kinase (FAK) inhibitor (a cytoplasmic tyrosine kinase able to regulate a plethora of downstream signaling pathways involved in cell migration, proliferation and death) and docetaxel (an anti-mitotic chemotherapy drug), and treatment with piperlongumine decreased tumor cell viability and induced apoptosis [69, 71]. Using breast cancer tissue, it was also demonstrated that the nitroimidazole-based sulfonamide, carbonic anhydrase IX (CAIX) inhibitors, salinomycin and its modified derivative and paclitaxel (synthetic compound) decrease cancer cells proliferation, inhibit invasion and increase cell death and apoptosis [49, 61, 62]. In addition, paclitaxel revealed an antineoplastic synergistic effect in combination to natural bioactive compounds as caffeic acid, ursolic acid and rosmarinic acid [61]. Combination treatments with crizotinib and temozolomide drugs induced ~ 80% of cell death with an increase in reactive oxygen species (ROS) production [78] and the arrest of G2 phase cell cycle, in presence of histone deacetylase (HDAC) inhibitors [81], in glioblastoma cultures. Finally, several chemotherapeutic agents as cisplatin, 5-FU (5-fluorouracil), docetaxel, FAC (fluorouracil, adriamycin, cytoxan), carboplatin and gemcitabine, were also used and evaluated in 3D tumor tissues cultures [40, 54, 69, 74, 76, 82, 95, 100, 103]. In detail, gemcitabine and docetaxel induced apoptosis and decreased cell proliferation in bladder and prostate cancer tissue cultures, respectively [69, 76]. Carboplatin increased echinoderm microtubule-associated protein-like 4 (EML4) expressions [82], while cisplatin decreased proliferation and induced cell death with apoptosis and DNA damage in non-small cell lung cancer tissues [40]. Using breast cancer tissue culture, it was also demonstrated that FAC decreased proliferation rate and induced cell death [54]. Finally, Koerferet al. [103] showed that the combination cisplatin and 5-FU (5-fluorouracil) decrease tumor cellularity and increase the apoptotic processes in gastric and esophagogastric tumor tissues [103]. Some studies demonstrated that these models are also suitable for the validation of alternative anti-cancer approaches such as oncolytic viral infection or gene therapy [53, 64, 89, 91]. Using a pancreatic adenocarcinoma 3D tissues culture van Geer et al. [91] compared different viral vectors, i.e., lentiviral vectors, adenovirus (Ad) and adeno associated virus (AAV) expressing the reporter genes green fluorescent protein (GFP), to study their transduction efficiency. Reporter genes expression indicated that the pancreatic tissues was infected and transduced efficiently by Ad and AAV, whereas transduction with lentiviral vectors was limited [91]. Results obtained from Ad delivery of the firefly luciferase (FLuc) reporter gene indicated that colon tumor tissue was more amenable to Ad transduction than other tumor histologic types examined (i.e., breast and ovary). Ad transduction levels were significantly higher than a range of standard gene delivery viral and non-viral methods examined in colon tissue [89]. Also, breast cancer tissue was used to evaluated gene delivery for several vectors (Ad, AVV, lipofection, ultrasound, electroporation and naked DNA), confirming that Ad was the most efficient gene delivery vector with transduction efficiency > 50%, while ultrasound proved the optimal non-viral gene delivery method in tumor tissues [53]. Concerning oncolytic therapy, two genetically distinct viruses, vesicular stomatitis virus (VSV) and vaccinia virus (VV), were combined and used to infect tumor tissues of rectum, colon, brain and endometrium, showing that VV synergistically enhanced VSV antitumor activity, dependent in large part on the activity of the VV B18R gene product. A recombinant version of VSV expressing the fusion-associated small-transmembrane (p14FAST) protein also further enhanced the ability of VV to spread through an infected monolayer, resulting in an oncolytic effect, where in each virus enhanced the ability of the other to replicate and/or spread in tumor cells [64].
Discussion and future direction
In contrast with more simplistic models, the humanized 3D tumor tissue culture represents an attractive physiological approach able to better mimic the real in vivo tumor complexity. The great advantage of this model is that the tumor environment is the same of the clinical scenario. Furthermore, obtaining tumor tissue from patients is fast, simple, ethically correct (after Ethical Committee approval and patients’ consent), economically sound and does not require any enzymatic treatments that can alter the ‘normal’ physiological conditions of the tumor. The use of this model also allows to obtain many tissue samples from the same patient permitting an accurate control of all culture factors, which consent to have reproducible results. In addition, it is important to emphasize that the use of tumor tissue models follow the 3R (Replacement, Reduction, Refinement) principles related to a more ethical use of animals for scientific purposes. Many stimulating ideas about the use and improvement of these models emerged from this review (specific culture media, oxygen intake, dynamic conditions, support/inserts/scaffolds). In fact, it was observed that tissue culture provides the opportunity to study the tumor in the context of a natural and intact microenvironment, including all cell types as well as the native extracellular matrix, thus maintaining the naturally occurring interactions between tumor and stroma [14]. In addition, given the influence of the tumor environment and other aspects of tumor biology on drug sensitivity, the 3D tumor tissue cultures evaluated in this review appear also suitable to study and evaluate novel therapeutic strategies, also considering patient individual characteristics and specific tumors subtypes. This aspect is of fundamental importance, as it would allow to evaluate drug response for tumors and/or metastases considering a personalized approach. However, comparing the ex vivo 3D tumor tissue culture data on therapeutic strategies to what is known from clinical trials, it was observed that only few clinical data are currently available. To date, few clinical trials on small-molecule inhibitors that block the conversion of ERK to its activated form via inhibition of MEK1/2, alone or in combination with therapeutic agents, are present and showed preliminary antitumor activity in patients with different solid tumors type, i.e., ovarian cancer (NCT01663857), biliary tract cancers (NCT01943864), metastatic melanoma (NCT01584648; NCT01037127; NCT01597908; NCT01072175; NCT01271803; NCT01689519; NCT01245062) and non-small-cell lung cancer (NCT01362296). However, in two clinical trials the combination of these inhibitors with therapeutic agents did not show significant clinical activity (NCT01941927; NCT01231581) [107] and there are no clinical trials on the effects of the association of irradiation and MEK inhibitors in solid tumors. Moreover, Hsp90 inhibitors have been tested in metastatic castration-resistant prostate cancer and lung cancer showing partial responses due to their toxicities without meet primary endpoint (NCT01259089) [65]. Phase I and II trials are present and also evaluated the effectiveness, safety, tolerability, pharmacokinetics, and clinical anti-cancer activity of FAK, HDAC and CAIX inhibitors, alone or in combination with cytotoxic agents, showing a good tolerability and acceptable security profiles in patients with advanced solid tumors (NCT02915523; NCT00926640; NCT00741234; NCT02909452; NCT02032810; NCT00496444; NCT00098891; NCT02780804; NCT02805660; NCT01023737) [108,109,110]. However, at present, several clinical trials on these inhibitors alone or combined with immunotherapeutic agents are still ongoing, others have been completed and two of them did not improve the clinical outcomes in patients with thyroid cancer (NCT00134043; NCT00437957) [111]. Despite numerous clinical trials being carried out, at present, it is not possible to draw any definitive conclusions about the clinical benefit of these new and advanced therapeutic strategies evaluated through ex vivo 3D tumor tissue culture. This is principally due to the fact that the majority of clinical trials are still ongoing or have only recently completed and have yet to post results (NCT03543969; NCT02216669). Thus, currently it is not yet possible to determine the real predictive value of this ex vivo 3D tumor tissue cultures. In addition, several methodological challenges and limitations still remain including difficulty in maintenance of culture for a long period of time and currently limited developments for the translational research. The realization and production of these tissue culture models are dependent on the starting tissues availability and amount received following surgery, and on the risk of possible contamination of the tissues (even when antibiotics and antimycotics are used). Moreover, tumor tissue samples are often collected from patients with advanced disease or patients that are already been subjected to different therapeutic intervention, and this could interfere with the response of the therapy to be tested by the 3D model. Another important issue is that it is always necessary to consider that during surgery and 3D culture set-up, the vascular system of the tumor is disconnected from systemic blood flow, and this aspect may interfere with drug penetration. Hypoxia, which is present in all solid tumors, if not considered, could also represent a potential problem for the cultures. Another key point is that none of the examined studies developed and evaluated humanized 3D cultures from metastatic tissue. This is probably due to the difficulty of managing such a heterogeneous tissue, where numerous cells type and factors, i.e., cytokines, chemokines and growth factors, play different roles. In addition, in many cases, it is difficult to obtain metastatic tissues, since in specific circumstance, it is not possible to perform surgery because of the advanced disease, extensive or localized tumor in complex position, where the surgical procedure could damage critical adjacent organs and tissues. The set-up of 3D metastasis models will be of fundamental importance, as they could reproduce a realistic and controllable microenvironment that better clarifies the molecular mechanisms that support metastasis growth and colonization, and for identifying strategies able to minimize their development.
In conclusion, the use of humanized 3D tumor tissue culture provides an interesting tool that could bridge the gap between results based on monolayer culture of cancer cell lines and the reality in human solid tumors. This model reproduces overall tumor tissue viability as well as maintenance of structural integrity, both at the microscopic and ultra-structural level allowing to investigate therapy responses. Importantly, using these models, the tumor cells retain proliferative activity and morphological phenotypes. However, since each tumor has different characteristics, the set-up of tumor culture and the choice of the culture conditions should be carefully evaluated.
What remains to be proven over the longer term is whether this approach can indeed predict the correct clinical response to specific therapeutic strategies, allowing also a better quantification of drug responses or resistance in patients, thus providing high-throughput analysis and leading to new more effective tumor treatments. Future clinical studies, comparing pre- and post-treatment tissues to include parallel ex vivo cultures of pre-treatment tumor tissues, will validate the model using clinically relevant end points, correlating tissue culture parameters with patient clinical outcomes [60]. This will provide an opportunity to investigate novel mechanisms of treatment resistance and identify biomarkers of treatment response in the preclinical phase that can then be integrated into clinical studies, and are essential for the realization of personalized cancer medicine, reducing damage and increasing patients benefits [60, 112]. In addition, it would also be important to set-up a tissue bank to collect and store patient tumor tissue samples for advancing translational biomedical research to allow study of genes, RNA and proteins, and to explore the biological mechanisms that support tumors etiology and biology, and the development of novel treatments, thus to facilitate a personalized approach for tumors and metastases [113].
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Padma VV (2015) An overview of targeted cancer therapy. Biomedicine (Taipei) 5(4):19
Li XY, Hu SQ, Xiao L (2015) The cancer-associated fibroblasts and drug resistance. Eur Rev Med Pharmacol Sci 19(11):2112–2119
Helmy KY, Patel SA, Nahas GR, Rameshwar P (2013) Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv 4(10):1307–1320
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev 25(1):16–27
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5(5):402–418
Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M, Anagnostopoulos I, Weichert W, Wittschieber D, Stenzinger A (2015) The landscape of metastatic progression patterns across major human cancers. Oncotarget 6(1):570–583
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4):274–284
Nyga A, Cheema U, Loizidou M (2011) 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal 5(3):239–248
Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H (2015) Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep 33(4):1837–1843
Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, Santo VE, Barbier M, Blom S, Arundkar SC, Selvam I, Osswald A, Stein Y, Gruenewald S, Brito C, van Weerden W, Rotter V, Boghaert E, Oren M, Sommergruber W, Chong Y, de Hoogt R, Graeser R (2016) Capturing tumor complexity in vitro: comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep 6:28951
Das V, Bruzzese F, Konečný P, Iannelli F, Budillon A, Hajdúch M (2015) Pathophysiologically relevant in vitro tumor models for drug screening. Drug Discov Today 20(7):848–855
Pagani S, Fini M, Giavaresi G, Salamanna F, Borsari V (2015) The active role of osteoporosis in the interaction between osteoblasts and bone metastases. Bone 79:176–182
Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, van der Kuip H, IMI PREDECT Consortium (2014) Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J 9(9):1115–1128
Fong EL, Harrington DA, Farach-Carson MC, Yu H (2016) Heralding a new paradigm in 3D tumor modeling. Biomaterials 108:197–213
Kim JB (2005) Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15(5):365–377
Salamanna F, Contartese D, Maglio M, Fini M (2016) A systematic review on in vitro 3D bone metastases models: a new horizon to recapitulate the native clinical scenario? Oncotarget 7(28):44803–44820
Salamanna F, Borsari V, Brogini S, Giavaresi G, Parrilli A, Cepollaro S, Cadossi M, Martini L, Mazzotti A, Fini M (2016) An in vitro 3D bone metastasis model by using a human bone tissue culture and human sex-related cancer cells. Oncotarget 7(47):76966–76983
Ruggeri BA, Camp F, Miknyoczki S (2014) Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol 87(1):150–161
Basel MT, Narayanan S, Ganta C, Shreshta TB, Marquez A, Pyle M, Hill J, Bossmann SH, Troyer DL (2018) Developing a xenograft human tumor model in immunocompetent mice. Cancer Lett 412:256–263
Lee NP, Chan CM, Tung LN, Wang HK, Law S (2018) Tumor xenograft animal models for esophageal squamous cell carcinoma. J Biomed Sci 25(1):66
Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, Perkins SE, Al Hilli M, Butler KA, McKinstry S, Fink S, Jenkins RB, Hou X, Kalli KR, Goodman KM, Sarkaria JN, Karlan BY, Kumar A, Kaufmann SH, Hartmann LC, Haluska P (2014) Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res 20(5):1288–1297
Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A (2014) Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4(9):998–1013
Park D, Wang D, Chen G, Deng X (2016) Establishment of patient-derived xenografts in mice. Bio Protocol 6(22):e2008
Izumchenko E, Meir J, Bedi A, Wysocki PT, Hoque MO, Sidransky D (2016) Patient-derived xenografts as tools in pharmaceutical development. Clin Pharmacol Ther 99(6):612–621
Liu ET, Bult CJ, Shultz LD (2016) Patient-derived tumor xenografts: why now? JAMA Oncol 2(5):567–568
Xu X, Farach-Carson MC, Jia X (2014) Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv 32(7):1256–1268
Weeber F, Ooft SN, Dijkstra KK, Voest EE (2017) Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem Biol 24(9):1092–1100
Nath S, Devi GR (2016) Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol Ther 163:94–108
Salamanna F, Borsari V, Brogini S, Torricelli P, Cepollaro S, Cadossi M, Fini M (2017) A human 3D in vitro model to assess the relationship between osteoporosis and dissemination to bone of breast cancer tumor cells. J Cell Physiol 232(7):1826–1834
Albritton JL, Miller JS (2017) 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis Models Mech 10(1):3–14
Benien P, Swami A (2014) 3D tumor models: history, advances and future perspectives. Future Oncol 10(7):1311–1327
Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K (2017) Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci 108(3):283–289
Burdett E, Kasper FK, Mikos AG, Ludwig JA (2010) Engineering tumors: a tissue engineering perspective in cancer biology. Tissue Eng Part B Rev 16(3):351–359
Weiswald LB, Bellet D, Dangles-Marie V (2015) Spherical cancer models in tumor biology. Neoplasia 17(1):1–15
Huang BW, Gao JQ (2018) Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J Control Release 270:246–259
Holliday DL, Moss MA, Pollock S, Lane S, Shaaban AM, Millican-Slater R, Nash C, Hanby AM, Speirs V (2013) The practicalities of using tissue slices as preclinical organotypic breast cancer models. J Clin Pathol 66(3):253–255
Estes JM, Oliver PG, Straughn JM Jr, Zhou T, WangW GWE, Alvarez RD, Stockard CR, LoBuglio AF, Buchsbaum DJ (2007) Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8) against primary human ovarian carcinoma using a novel ex vivo tissue slice model. Gynecol Oncol 105(2):291–298
Dong M, Philippi C, Loretz B, Nafee N, Schaefer UF, Friedel G, Ammon-Treiber S, Griese EU, Lehr CM, Klotz U, Mürdter TE (2011) Tissue slice model of human lung cancer to investigate telomerase inhibition by nanoparticle delivery of antisense 2'-O-methyl-RNA. Int J Pharm 419(1–2):33–42
Karekla E, Liao WJ, Sharp B, Pugh J, Reid H, Quesne JL, Moore D, Pritchard C, MacFarlane M, Pringle JH (2017) Ex vivo explant cultures of non-small cell lung carcinoma enable evaluation of primary tumor responses to anticancer therapy. Can Res 77(8):2029–2039
Meijer TG, Naipal KA, Jager A, van Gent DC (2017) Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci OA 3(2):FSO190
Merz F, Gaunitz F, Dehghani F, Renner C, Meixensberger J, Gutenberg A, Giese A, Schopow K, Hellwig C, Schäfer M, Bauer M, Stöcker H, Taucher-Scholz G, Durante M, Bechmann I (2013) Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol 15(6):670–681
Milani C, Welsh J, Katayama ML, Lyra EC, Maciel MS, Brentani MM, Folgueira MA (2010) Human breast tumor slices: a model for identification of vitamin D regulated genes in the tumor microenvironment. J Steroid Biochem Mol Biol 121(1–2):151–155
van der Kuip H, Mürdter TE, Sonnenberg M, McClellan M, Gutzeit S, Gerteis A, SimonW FritzP, Aulitzky WE (2006) Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment. BMC Cancer 6:86
Gerlach MM, Merz F, Wichmann G, Kubick C, Wittekind C, Lordick F, Dietz A, Bechmann I (2014) Slice cultures from head and neck squamous cell carcinoma: a novel test system for drug susceptibility and mechanisms of resistance. Br J Cancer 110(2):479–488
Kendrick JE, Straughn JM Jr, Oliver PG, Wang W, Nan L, Grizzle WE, Stockard CR, Alvarez RD, Buchsbaum DJ (2008) Anti-tumor activity of the TRA-8 anti-DR5 antibody in combination with cisplatin in an ex vivo human cervical cancer model. Gynecol Oncol 108(3):591–597
Kern MA, Haugg AM, Eiteneuer E, Konze E, Drebber U, Dienes HP, Breuhahn K, Schirmacher P, Kasper HU (2006) Ex vivo analysis of antineoplastic agents in precision-cut tissue slices of human origin: effects of cyclooxygenase-2 inhibition in hepatocellular carcinoma. Liver Int 26(5):604–612
Louandre C, Donnadieu J, Lachaier E, Page C, Chauffert B, Galmiche A (2016) Personalization of the medical treatment of solid tumours using patient-derived tumour explants (review). Int J Oncol 48(3):895–899
Ward C, Meehan J, Mullen P, Supuran C, Dixon JM, Thomas JS, Winum JY, Lambin P, Dubois L, Pavathaneni NK, Jarman EJ, Renshaw L, Um IH, Kay C, Harrison DJ, Kunkler IH, Langdon SP (2015) Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget 6(28):24856–24870
Scaling AL, Prossnitz ER, Hathaway HJ (2014) GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast. Horm Cancer 5(3):146–160
Savage A, Katz E, Eberst A, Falconer RE, Houston A, Harrison DJ, Bown J (2013) Characterising the tumour morphological response to therapeutic intervention: an ex vivo model. Dis Models Mech 6(1):252–260
Rojas PA, May M, Sequeira GR, Elia A, Alvarez M, Martínez P, Gonzalez P, Hewitt S, He X, Perou CM, Molinolo A, Gibbons L, Abba MC, Gass H, Lanari C (2017) Progesterone receptor isoform ratio: a breast cancer prognostic and predictive factor for antiprogestin responsiveness. J Natl Cancer Inst 109(7):317
Rajendran S, O'Hanlon D, Morrissey D, O'Donovan T, O'Sullivan GC, Tangney M (2011) Preclinical evaluation of gene delivery methods for the treatment of loco-regional disease in breast cancer. Exp Biol Med (Maywood) 236(4):423–434
Naipal KA, Verkaik NS, Sánchez H, van Deurzen CH, den Bakker MA, Hoeijmakers JH, Kanaar R, Vreeswijk MP, Jager A, van Gent DC (2016) Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer 16:78
Muthuswamy R, Okada NJ, Jenkins FJ, McGuire K, McAuliffe PF, Zeh HJ, Bartlett DL, Wallace C, Watkins S, Henning JD, Bovbjerg DH, Kalinski P (2017) Epinephrine promotes COX-2-dependent immune suppression in myeloid cells and cancer tissues. Brain Behav Immun 62:78–86
Knutson TP, Truong TH, Ma S, Brady NJ, Sullivan ME, Raj G, Schwertfeger KL, Lange CA (2017) Post translationally modified progesterone receptors direct ligand-specific expression of breast cancer stem cell-associated gene programs. J Hematol Oncol 10(1):89
Katz E, Sims AH, Sproul D, Caldwell H, Dixon MJ, Meehan RR, Harrison DJ (2012) Targeting of Rac GTPases blocks the spread of intact human breast cancer. Oncotarget 3(6):608–619
Grosso SH, Katayama ML, Roela RA, Nonogaki S, Soares FA, Brentani H, Lima L, Folgueira MA, Waitzberg AF, Pasini FS, Góes JC, Brentani MM (2013) Breast cancer tissue slices as a model for evaluation of response to rapamycin. Cell Tissue Res 352(3):671–684
Faversani A, Vaira V, Moro GP, Tosi D, Lopergolo A, Schultz DC, Rivadeneira D, Altieri DC, Bosari S (2014) Survivin family proteins as novel molecular determinants of doxorubicin resistance in organotypic human breast tumors. Breast Cancer Res 16(3):R55
Centenera MM, Hickey TE, Jindal S, Ryan NK, Ravindranathan P, Mohammed H, Robinson JL, Schiewer MJ, Ma S, Kapur P, Sutherland PD, Hoffmann CE, Roehrborn CG, Gomella LG, Carroll JS, Birrell SN, Knudsen KE, Raj GV, Butler LM, Tilley WD (2018) A patient-derived explant (PDE) model of hormone-dependent cancer. Mol Oncol 12(9):1608–1622
Carranza-Torres IE, Guzmán-Delgado NE, Coronado-Martínez C, Bañuelos-García JI, Viveros-Valdez E, Morán-Martínez J, Carranza-Rosales P (2015) Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. Biomed Res Int 2015:618021
Antoszczak M, Urbaniak A, Delgado M, Maj E, Borgström B, Wietrzyk J, Huczyński A, Yuan Y, Chambers TC, Strand D (2018) Biological activity of doubly modified salinomycin analogs—evaluation in vitro and ex vivo. Eur J Med Chem 156:510–523
Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, Snyder E, Faversani A, Coggi G, Flavin R, Bosari S, Loda M (2010) Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci 107(18):8352–8356
Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, Kanji F, Auer R, Brown CW, Lichty BD, Parato K, Atkins H, Kirn D, Bell JC (2010) Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther 18(5):888–895
Centenera MM, Gillis JL, HansonAR JS, Taylor RA, Risbridger GP, Sutherland PD, Scher HI, Raj GV, Knudsen KE, Yeadon T, Resource APCB, Tilley WD, Butler LM (2012) Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin Cancer Res 18(13):3562–3570
Handle F, Puhr M, Schaefer G, Lorito N, Hoefer J, Gruber M, Guggenberger F, Santer FR, Marques RB, van Weerden WM, Claessens F, Erb HHH, Culig Z (2018) The STAT3 inhibitor galiellalactone reduces IL6-mediated AR activity in benign and malignant prostate models. Mol Cancer Ther 17(12):2722–2731
Köcher S, Beyer B, Lange T, Nordquist L, Volquardsen J, Burdak-Rothkamm S, Schlomm T, Petersen C, Rothkamm K, Mansour WY (2019) A functional ex vivo assay to detect PARP1-EJ repair and radiosensitization by PARP-inhibitor in prostate cancer. Int J Cancer 144(7):1685–1696
Gu L, Liao Z, Hoang DT, Dagvadorj A, Gupta S, Blackmon S, Ellsworth E, Talati P, Leiby B, Zinda M, Lallas CD, Trabulsi EJ, McCue P, Gomella L, Huszar D, Nevalainen MT (2013) Pharmacologic inhibition of Jak2-Stat5 signaling By Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin Cancer Res 19(20):5658–5674
Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, Kench JG, Stricker PD, Haynes AM, Centenera MM, Butler LM, Shreeve SM, Horvath LG, Daly RJ (2018) Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate 78(4):308–317
Liao Z, Gu L, Vergalli J, Mariani SA, De Dominici M, Lokareddy RK, Dagvadorj A, Purushottamachar P, McCue PA, Trabulsi E, Lallas CD, Gupta S, Ellsworth E, Blackmon S, Ertel A, Fortina P, Leiby B, Xia G, Rui H, Hoang DT, Gomella LG, Cingolani G, Njar V, Pattabiraman N, Calabretta B, Nevalainen MT (2015) Structure-based screen identifies a potent small molecule inhibitor of Stat5a/b with therapeutic potential for prostate cancer and chronic myeloid leukemia. Mol Cancer Ther 14(8):1777–1793
Maund SL, Nolley R, Peehl DM (2014) Optimization and comprehensive characterization of a faithful tissue culture model of the benign and malignant human prostate. Lab Invest 94(2):208–221
Maranto C, Udhane V, Hoang DT, Gu L, Alexeev V, Malas K, Cardenas K, Brody JR, Rodeck U, Bergom C, Iczkowski KA, Jacobsohn K, See W, Schmitt SM, Nevalainen MT (2018) STAT5A/B blockade sensitizes prostate cancer to radiation through inhibition of RAD51 and DNA repair. Clin Cancer Res 24(8):1917–1931
Nguyen EV, Centenera MM, Moldovan M, Das R, Irani S, Vincent AD, Chan H, Horvath LG, Lynn DJ, Daly RJ, Butler LM (2018) Identification of novel response and predictive biomarkers to Hsp90 inhibitors through proteomic profiling of patient-derived prostate tumor explants. Mol Cell Proteom 17(8):1470–1486
Shafi AA, Schiewer MJ, de Leeuw R, Dylgjeri E, McCue PA, Shah N, Gomella LG, Lallas CD, Trabulsi EJ, Centenera MM, Hickey TE, Butler LM, Raj G, Tilley WD, Cukierman E, Knudsen KE (2018) Patient-derived models reveal impact of the tumor microenvironment on therapeutic response. Eur Urol Oncol 1(4):325–337
Talati PG, Gu L, Ellsworth EM, Girondo MA, Trerotola M, Hoang DT, Leiby B, Dagvadorj A, McCue PA, Lallas CD, Trabulsi EJ, Gomella L, Aplin AE, Languino L, Fatatis A, Rui H, Nevalainen MT (2015) Jak2-Stat5a/b signaling induces epithelial-to-mesenchymal transition and stem-like cell properties in prostate cancer. Am J Pathol 185(9):2505–2522
van de Merbel AF, van der Horst G, van der Mark MH, van Uhm JIM, van Gennep EJ, Kloen P, Beimers L, Pelger RCM, van der Pluijm G (2018) An ex vivo tissue culture model for the assessment of individualized drug responses in prostate and bladder cancer. Front Oncol 8:400
Das A, Henderson F Jr, Lowe S, Wallace GC 4th, Vandergrift WA 3rd, Lindhorst SM, Varma AK, Infinger LK, Giglio P, Banik NL, Patel SJ, Cachia D (2018) Single agent efficacy of the HDAC inhibitor DATS in preclinical models of glioblastoma. Cancer Chemother Pharmacol 82(6):945–952
Das A, Cheng RR, Hilbert ML, Dixon-Moh YN, Decandio M, Vandergrift WA 3rd, Banik NL, Lindhorst SM, Cachia D, Varma AK, Patel SJ, Giglio P (2015) Synergistic effects of crizotinib and temozolomide in experimental FIG-ROS1 fusion-positive glioblastoma. Cancer Growth Metastasis 8:51–60
Parker JJ, Lizarraga M, Waziri A, Foshay KM (2017) A human glioblastoma organotypic slice culture model for study of tumor cell migration and patient-specific effects of anti-invasive drugs. J Vis Exp (125)
Parker JJ, Dionne KR, Massarwa R, Klaassen M, Foreman NK, Niswander L, Canoll P, Kleinschmidt-Demasters BK, Waziri A (2013) Gefitinib selectively inhibits tumor cell migration in EGFR-amplified human glioblastoma. Neuro Oncol 15(8):1048–1057
Xu J, Sampath D, Lang FF, Prabhu S, Rao G, Fuller GN, Liu Y, Puduvalli VK (2011) Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J Neuro Oncol 105(2):241–251
Radtke J, Rezaie SG, Kugler Ch, Zabel P, Schultz H, Vollmer E, Goldmann T, Lang DS (2010) Expression analysis of EML4 in normal lung tissue and non-small cell lung cancer (NSCLC) in the absence and presence of chemotherapeutics. Rom J Morphol Embryol 51(4):647–653
Sirchia SM, Faversani A, Rovina D, Russo MV, Paganini L, Savi F, Augello C, Rosso L, Del Gobbo A, Tabano S, Bosari S, Miozzo M (2016) Epigenetic effects of chromatin remodeling agents on organotypic cultures. Epigenomics 8(3):341–358
Leithner K, Wohlkoenig C, Stacher E, Lindenmann J, Hofmann NA, Gallé B, Guelly C, Quehenberger F, Stiegler P, Smolle-Jüttner FM, Philipsen S, PopperHH HA, Olschewski A, Olschewski H (2014) Hypoxia increases membrane metallo-endopeptidase expression in a novel lung cancer ex vivo model—role of tumor stroma cells. BMC Cancer 14:40
Hattar K, Savai R, Subtil FS, Wilhelm J, Schmall A, Lang DS, Goldmann T, Eul B, Dahlem G, Fink L, Schermuly RT, Banat GA, Sibelius U, Grimminger F, Vollmer E, Seeger W, Grandel U (2013) Endotoxin induces proliferation of NSCLC in vitro and in vivo: role of COX-2 and EGFR activation. Cancer Immunol Immunother 62(2):309–320
Cohen G, Lecht S, Oron-Herman M, Momic T, Nissan A, Lazarovici P (2013) Near infrared optical visualization of epidermal growth factor receptors levels in COLO205 colorectal cell line, orthotopic tumor in mice and human biopsies. Int J Mol Sci 14(7):14669–14688
Cohen G, Lecht S, Arien-Zakay H, Ettinger K, Amsalem O, Oron-Herman M, Yavin E, Prus D, Benita S, Nissan A, Lazarovici P (2012) Bio-imaging of colorectal cancer models using near infrared labeled epidermal growth factor. PLoS ONE 7(11):e48803
Ounpuu L, Truu L, Shevchuk I, Chekulayev V, Klepinin A, Koit A, Tepp K, Puurand M, Rebane-Klemm E, Käämbre T (2018) Comparative analysis of the bioenergetics of human adenocarcinoma Caco-2 cell line and postoperative tissue samples from colorectal cancer patients. Biochem Cell Biol 30:1–10
Rajendran S, O'Sullivan GC, O'Hanlon D, Tangney M (2013) Adenovirus-mediated transcriptional targeting of colorectal cancer and effects on treatment-resistant hypoxic cells. Clin Colorectal Cancer 12(3):152–162.e1
Scherr AL, Gdynia G, Salou M, Radhakrishnan P, Duglova K, Heller A, Keim S, Kautz N, Jassowicz A, Elssner C, He YW, Jaeger D, Heikenwalder M, Schneider M, Weber A, Roth W, Schulze-Bergkamen H, Koehler BC (2016) Bcl-xL is an oncogenic driver in colorectal cancer. Cell Death Dis 7(8):e2342
van Geer MA, Kuhlmann KF, Bakker CT, ten Kate FJ, Oude Elferink RP, Bosma PJ (2009) Ex-vivo evaluation of gene therapy vectors in human pancreatic (cancer) tissue slices. World J Gastroenterol 15(11):1359–1366
Misra S, Moro CF, Del Chiaro M, Pouso S, Sebestyén A, Löhr M, Björnstedt M, Verbeke CS (2019) Ex vivo organotypic culture system of precision-cut slices of human pancreatic ductal adenocarcinoma. Sci Rep 9(1):2133
Bazou D, Maimon N, Gruionu G, Grahovac J, Seano G, Liu H, Evans CL, Munn LL (2018) Vascular beds maintain pancreatic tumour explants for ex vivo drug screening. J Tissue Eng Regen Med 12(1):e318–e322
ElNaggar AC, Saini U, Naidu S, Wanner R, Sudhakar M, Fowler J, Nagane M, Kuppusamy P, Cohn DE, Selvendiran K (2016) Anticancer potential of diarylidenyl piperidone derivatives, HO-4200 and H-4318, in cisplatin resistant primary ovarian cancer. Cancer Biol Ther 17(10):1107–1115
Saini U, Naidu S, ElNaggar AC, Bid HK, Wallbillich JJ, Bixel K, Bolyard C, Suarez AA, Kaur B, Kuppusamy P, Hays J, Goodfellow PJ, Cohn DE, Selvendiran K (2017) Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: a potential therapeutic target. Oncogene 36(2):168–181
Theodoraki MN, Yerneni S, Sarkar SN, Orr B, Muthuswamy R, Voyten J, Modugno F, Jiang W, Grimm M, Basse PH, Bartlett DL, Edwards RP, Kalinski P (2018) Helicase-driven activation of NFκB-COX2 pathway mediates the immunosuppressive component of dsRNA-driven inflammation in the human tumor microenvironment. Cancer Res 78(15):4292–4302
Jørgensen A, Young J, Nielsen JE, Joensen UN, Toft BG, Rajpert-De Meyts E, Loveland KL (2014) Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. Br J Cancer 110(10):2604–2614
Affolter A, Muller MF, Sommer K, Stenzinger A, Zaoui K, Lorenz K, Wolf T, Sharma S, Wolf J, Perner S, Weber KJ, Freier K, Plinkert PK, Hess J, Weichert W (2016) Targeting irradiation-induced mitogen-activated protein kinase activation in vitro and in an ex vivo model for human head and neck cancer. Head Neck 38(Suppl 1):E2049–E2061
Bhattacharyya S, Sekar V, Majumder B, Mehrotra DG, Banerjee S, Bhowmick AK, Alam N, Mandal GK, Biswas J, Majumder PK, Murmu N (2017) CDKN2A-p53 mediated antitumor effect of lupeol in head and neck cancer. Cell Oncol (Dordr) 40(2):145–155
Huang P, Zhuang B, Zhang H, Yan H, Xiao Z, Li W, Zhang J, Tang Q, Hu K, Koeffler HP, Wang J, Yin D (2015) Hepatitis B virus X protein (HBx) is responsible for resistance to targeted therapies in hepatocellular carcinoma: ex vivo culture evidence. Clin Cancer Res 21(19):4420–4430
Casas BS, Adolphe C, Lois P, Navarrete N, Solís N, Bustamante E, Gac P, Cabané P, Gallegos I, Wainwright BJ, Palma V (2017) Downregulation of the Sonic Hedgehog/Gli pathway transcriptional target Neogenin-1 is associated with basal cell carcinoma aggressiveness. Oncotarget 8(48):84006–84018
Fiebitz A, Fritsch M, Reichelt U, Ruester C, Chiantera V, Vercellino GF, Darwish A, Schneider A, Mechsner S (2012) Optimized culture conditions for tissue explants of uterine leiomyoma. Clin Lab 58(11–12):1153–1164
Koerfer J, Kallendrusch S, Merz F, Wittekind C, Kubick C, Kassahun WT, Schumacher G, Moebius C, Gaßler N, Schopow N, Geister D, Wiechmann V, Weimann A, Eckmann C, Aigner A, Bechmann I, Lordick F (2016) Organotypic slice cultures of human gastric and esophagogastric junction cancer. Cancer Med 5(7):1444–1453
Bolenz C, Ikinger EM, Ströbel P, Trojan L, Steidler A, Fernández MI, Honeck P, Gabriel U, Weiss C, Grobholz R, Alken P, Michel MS (2009) Topical chemotherapy in human urothelial carcinoma explants: a novel translational tool for preclinical evaluation of experimental intravesical therapies. Eur Urol 56(3):504–511
Weissinger D, Tagscherer KE, Macher-Göppinger S, Haferkamp A, Wagener N, Roth W (2013) The soluble decoy receptor 3 is regulated by a PI3K-dependent mechanism and promotes migration and invasion in renal cell carcinoma. Mol Cancer 12(1):120
Bastos VC, Pereira NB, Diniz MG, Andrade LO, Castro WH, Kitten GT, Gomez RS, Gomes CC (2019) Bringing benign ectomesenchymal odontogenic tumours to the lab: an in vitro study using an organotypic culture model. J Oral Pathol Med 48(2):174–179
LoRusso PM, Krishnamurthi SS, Rinehart JJ, Nabell LM, Malburg L, Chapman PB, DePrimo SE, Bentivegna S, Wilner KD, Tan W, Ricart AD (2010) Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res 16(6):1924–1937
Mak G, Soria JC, Blagden SP, Plummer R, Fleming RA, Nebot N, Zhang J, Mazumdar J, Rogan D, Gazzah A, Rizzuto I, Greystoke A, Yan L, Tolson J, Auger KR, Arkenau HT (2019) A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br J Cancer 120(10):975–981
Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M, Nakagawa K (2016) A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 77(5):997–1003
Brown NF, Williams M, Arkenau HT, Fleming RA, Tolson J, Yan L, Zhang J, Swartz L, Singh R, Auger KR, Lenox L, Cox D, Lewis Y, Plisson C, Searle G, Saleem A, Blagden S, Mulholland P (2018) A study of the focal adhesion kinase inhibitor GSK2256098 in patients with recurrent glioblastoma with evaluation of tumor penetration of [11C]GSK2256098. Neuro Oncol 20(12):1634–1642
Wood A, George S, Adra N, Chintala S, Damayanti N, Pili R (2019) Phase I study of the mTOR inhibitor everolimus in combination with the histone deacetylase inhibitor panobinostat in patients with advanced clear cell renal cell carcinoma. Invest New Drugs. https://doi.org/10.1007/s10637-019-00864-7 (Epub ahead of print)
Ferraldeschi R, Attard G, de Bono JS (2013) Novel strategies to test biological hypotheses in early drug development for advanced prostate cancer. Clin Chem 59:75–84
Bryant J, Sanson-Fisher R, Fradgley E, Regan T, Hobden B, Ackland SP (2015) Oncology patients overwhelmingly support tissue banking. BMC Cancer 15:413
Acknowledgements
This work was supported by IRCCS Istituto Ortopedico Rizzoli (Ricerca Corrente) and by the Project “Oncologia di Precisione e Nuove Terapie Antitumorali (ONCOPENTA). Sviluppo di modelli preclinici avanzati per il trattamento locale di tumori primitivi e metastatici”.
Author information
Authors and Affiliations
Contributions
DC and MF designed the review. DC and FS performed the literature search. DC, FS and FV analyzed the obtained articles. DC, FS and MF wrote the paper. DC, FS and FV collected and assembled the data. DC, FS, FV and MF revised manuscript critically. All authors read and approved the submitted manuscript version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Contartese, D., Salamanna, F., Veronesi, F. et al. Relevance of humanized three-dimensional tumor tissue models: a descriptive systematic literature review. Cell. Mol. Life Sci. 77, 3913–3944 (2020). https://doi.org/10.1007/s00018-020-03513-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03513-y