Abstract
Oxygen deprivation affects human health by modulating system as well as cellular physiology. Hypoxia generates reactive oxygen species (ROS), causes oxidative stress and affects female reproductive health by altering ovarian as well as oocyte physiology in mammals. Hypoxic conditions lead to several degenerative changes by inducing various cell death pathways like autophagy, apoptosis and necrosis in the follicle of mammalian ovary. The encircling somatic cell death interrupts supply of nutrients to the oocyte and nutrient deprivation may result in the generation of ROS. Increased level of ROS could induce granulosa cells as well as oocyte autophagy. Although autophagy removes damaged proteins and subcellular organelles to maintain the cell survival, irreparable damages could induce cell death within intra-follicular microenvironment. Hypoxia-induced autophagy is operated through 5′ AMP activated protein kinase–mammalian target of rapamycin, endoplasmic reticulum stress/unfolded protein response and protein kinase C delta–c-junN terminal kinase 1 pathways in a wide variety of somatic cell types. Similar to somatic cells, we propose that hypoxia may induce granulosa cell as well as oocyte autophagy and it could be responsible at least in part for germ cell elimination from mammalian ovary. Hypoxia-mediated germ cell depletion may cause several reproductive impairments including early menopause in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reduced level of pO2 generates hypoxic condition, which is characterized by insufficient supply of oxygen to meet the physiological requirement of tissue/cells in the body. The increase of harmful gases and particulate matters in air due to rapid industrialisation, deforestation and motor vehicles affects oxygen delivery through blood and utilization capacity of a cell causing cellular/physiological hypoxia [1,2,3,4]. Further, the presence of these pollutants in air may generate inflammation in pulmonary and vascular systems [4, 4] thereby decreasing blood oxygen saturation level [4, 5, 6]. For instance, hypoxia has been observed in the placenta of women who used firewood/kerosene for cooking purpose [7].
Hypoxia affects various aspects of cell functions including metabolism, growth, cell division and cell death [8, 9]. The hypoxia-mediated changes in cellular physiology modulate cardiovascular, neuronal and reproductive physiology [10,11,12,13]. Ovary is a primary female reproductive organ responsible for generation of competent oocyte required for successful fertilization and early embryonic development (Fig. 1a). Mammalian ovary contains almost 5–6 million germ cells during 20th week of embryonic development. Majority of these germ cells are eliminated by follicular atresia, while only 1 million germ cells remain available for selective recruitment during entire life span after birth [14]. These germ cells form oogonia and migrate to gonadal ridges, enter into 1st meiotic division and remains arrested at diplotene stage of prophase I for a long period of time [15]. Those primordial follicles in response to pituitary gonadotropins may enter into process of folliculogenesis to form graffian follicle just prior to ovulation of competent oocyte [16]. The primary oocytes in mammalian ovary possess zona pellucida (ZP) encircled by layers of two major somatic cell types namely theca and granulosa cells. Theca cells are steriodogenic in nature and responsible for synthesis of estradiol-17β required for follicular growth and development. Theca cells are further differentiated into theca interna and theca externa; while granulosa cells are differentiated into mural and cumulus granulosa cells. The cumulus granulosa cells are the immediate somatic cells encircling primary oocyte that is morphologically characterized by germinal vesicle and nucleolus in center.
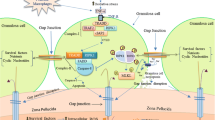
a Schematic diagram of mammalian ovary showing follicles at different stages of development. b, c Magnified schematic diagram showing proposed mechanism of hypoxia-induced autophagy in follicular cells of mammalian ovary. b In Granulosa cells, reduction of pO2 level causes cellular hypoxia. Hypoxia may induce ER stress, ROS generation, PKDδ activation and decreases PHD activity as well as ATP production in a cell. Increase of ROS level decreases PHD activity that stabilizes HIF-1α and its accumulation. HIF-1α is then translocated to nucleus, where it forms a heterodimer with HIF-1β and binds to HRE inducing transcription of BNIP3 gene. Bnip3 protein binds with Bcl-2 and disrupts its interaction with Beclin 1 making Beclin 1 free. The Beclin 1–Bcl-2 interaction can also be disrupted through phosphorylation of Bcl-2 by Jnk-1 activated in response to PKCδ. Free Beclin 1 increases catalytic efficiency of Vps-34 which then coverts PI3 into PIP3 required for phagophore formation. ATP depletion in response to hypoxia triggers phosphorylation of AMPK. The phosphorylated AMPK activates TSC2 which binds with TSC1 leading to its phosphorylation by Rheb-GTP. The phosphorylated TSC2-TSC1 inhibits mTOR and induces autophagy. ER stress induces UPR which is sensed by three different UPR sensors, EIF2AK3/PERK, ERN1/IRE1 and ATF 6. ATF 6 enters into nucleus, promotes transcription of chaperone CHOP and XBP1 and induces autophagy induction. CHOP is also upregulated in response to EIF2AK3/PERK that activates Atg 4 to cleave LCB and generates LCB-I. LC3B-I is then activated by binding of Atg7 in a ATP-dependent manner and then transferred to Atg3. Atg3 promotes conjugation of PE to LC3B-I to generate processed LC3B-II. Activated LCB-II is recruited to growing phagophore and plays important role in fusion of its edges and cargo selection in a cell. c In follicular oocyte, hypoxia may trigger all four major pathways to induce autophagy as described in b. In addition, granulosa cell death may deprive oocyte from nutrients, growth factors and survival factors that result in the activation of starvation-induced AMPK–mTOR-mediated pathway and promote phagophore formation. Once the phagophore is formed, all these pathways promote its elongation and autophagolysosome formation, which finally engulfs most of the cytoplasmic machinery that probably results in autophagic cell death
Growing body of evidences suggests the adverse impact of hypoxia on ovarian function in several ways. For instance, it triggers the depletion of follicular reserve in spiny mouse [17], reduces luteal growth in sheep ovary [13], decreases follicular development in hamster [18], promotes reactive oxygen species (ROS) generation and follicular aging in human granulosa cells [19, 20]. Although hypoxic cell employs survival strategy during mild and initial phase, sustained hypoxia may trigger various cell death pathways including autophagy, apoptosis, necrosis and necroptosis depending on duration and severity of hypoxia [21,22,23].
Autophagy is a highly regulated process of self-degradation that eliminates damaged, unwanted or surplus subcellular proteins and organelles with the help of lysosomal activity [24]. Various factors including starvation, hormones, stress and other pathological conditions may induce autophagy to maintain homeostasis and longevity of a cell [25] through the turnover of damaged proteins and organelles [24, 26]. Mammalian target of rapamycin (mTOR) is a central stress sensor and master regulator of the autophagy [27]. However, hypoxia could also trigger autophagy through various mTOR-independent pathways including protein kinase C delta–c-jun-N terminal kinase 1 (PKCδ–JNK-1) [28], endoplasmic reticulum (ER) stress or unfolded proteins response (UPR) [29] and generation of ROS [30]. Although autophagy is a protective mechanism to maintain cellular homeostasis [31, 32], excessive accumulation of indigestible materials due to autophagic degradation of damaged proteins and organelles like mitochondria, ER and ribosome could lead to autophagic cell death [33, 34]. Recent studies suggest the involvement of autophagy in the regulation of follicular development, granulosa cell as well as oocyte death leading to follicular atresia [35, 36], corpus luteum regression [37] and oocyte aging [38] and pathogenesis of metabolic disorder like Polycystic ovarian syndrome (PCOS) [39, 40].
Although hypoxia-mediated cell death has been studied in greater detail in various cell types, hypoxia-mediated autophagy remains poorly understood in the follicular cells of mammalian ovary. This review article updates the information on the involvement of autophagy in granulosa cell as well as oocyte and proposes the possible mechanism of hypoxia-mediated autophagy in the follicular cells of mammalian ovary.
Hypoxia-mediated physiological changes in ovary
The presence of certain harmful gases in air may compromise the oxygen delivery capacity of blood or alter the ability of a cell to utilize the available oxygen [1, 2]. Several pathological conditions of pulmonary as well as cardio-vascular systems may also cause reduced pO2 level in blood [41, 42]. The specialized chemoreceptor cells in arterial circulation and neuroepithelial bodies present in the airway sense hypoxic conditions and accordingly modulate pulmonary ventilation as well as perfusion to optimize the supply of O2 to the metabolizing cells/tissue. Under hypoxic conditions, peripheral blood vessels are dilated, whereas pulmonary vasculatures are constricted to shunt the blood away from poorly ventilated region for optimizing the oxygen supply to tissues [43, 44]. Most of the nucleated cells sense changes in O2 concentration and respond quickly through the activation of pre-existing proteins and in long term through the regulation of gene transcription [45]. One of the most important transcription factors induced in response to hypoxia is hypoxia-inducible factor (HIF) [46]. It regulates gene transcription to maintain oxygen homeostasis for adaption to low oxygen tension [47].
The HIF is a heterodimeric protein consisting of an oxygen-dependent α-subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed aryl hydrocarbon receptor nuclear translocator/β (ARNT/β) subunit located in the nucleus. On the other hand, HIF-1α mRNA level does not alter in normoxia as well as hypoxia [46]. However, protein is polyubiquitinylated and rapidly degraded in normoxia but gets accumulated in hypoxia [46]. In normoxia, prolyl hydroxylases (PHD1-3) hydroxylate two proline residue of HIF-1α [48,49,50]. The hydroxylated HIF-1α is then recognized by von Hippel–Lindau (VHL) protein that ubiquitinates HIF-1α and helps in proteasomal degradation [51]. In hypoxic condition, PHD activity decreases and HIF-1α proline residues are not hydroxylated, resulting in the accumulation of stabilized protein [52]. Once stabilized, HIF-1α enters into the nucleus, joins with HIF-1β to generate heterodimer transcription factor and by binding to hypoxia response elements (HRE) present in their regulatory region promotes the expression of target genes like luteinizing hormone receptor, inhibin-α VEGF, Endothelin 2, BNIP3, PDE4D, NRF2F2, disintegrin and metalloproteinase with thrombospondin-like motifs-1, etc. [53,54,55,56,57,58,59]. HIF-1α target genes affect almost all aspect of the cellular functions including metabolism [8], growth, proliferation, secretion of cytokines as well as mitogen, [60] and cell death [9]. Hypoxia-mediated changes in cellular functions affect cardio-vascular, central nervous system and reproductive physiology [10,11,12,13].
Mammalian ovary is a metabolically active organ and generates ROS at an extraordinary scale. Due to large size of follicle, follicular oocyte is more susceptible toward hypoxia [61, 62]. The chronic hypoxia may result in ovarian dysfunction and altered hormonal profile [63, 64]. Development of ovarian follicle is a dynamic process that involves proliferation, differentiation and death of somatic cells encircling oocyte [65, 66]. Follicles are recruited and selected dominant follicle ruptures to release meiotically competent oocyte, while non-selected follicle undergoes atresia [67]. Within the follicle, a bidirectional talk is important for survival and several functions of both encircling granulosa cells as well as oocyte [68]. The encircling cumulus granulosa cells nourish the oocyte by transferring nutrients, growth factors and survival factors [69]. In turn, oocyte modulates cumulus cell functions by secreting paracrine factors that include growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) [70]. The GDF9 and BMP15 modulate cell proliferation [71], metabolism [72], expansion [73], luteinisation [74] and apoptosis [75] of encircling granulosa cells within the follicular microenvironment.
The follicular oocyte is encircled by several layers of granulosa cells and thecal cells, and between both lies a tight barrier of basement membrane that blocks the infiltration of blood vessels to region of granulosa cells and oocyte separating them from blood supply. The O2 as well as nutrients have to pass through these layers of somatic cell before it becomes available to oocyte; hence, pO2 is compromised within the follicular microenvironment in mammals as the size of follicle grows larger [76]. Hypoxia induces polycystic ovaries, estradiol biosynthesis, alters estrous cycle and decreases fertility in female rat suggesting its negative impact on folliculogenesis in ovary [64]. A brief exposure of hypoxia (7–8 min) resulted in decreased number as well as diameter of primordial and primary follicle, reduced follicular reserve and ovarian volume in spiny mouse fetuses [17]. Hypoxia caused at high altitude changes the morphology and function of antral follicle and corpora lutea in sheep [13]. Further, it also increases HIF-1α and vascular endothelial growth factor (VEGF) expression level in luteal cells of sheep. These sheep also had reduced number of pre-ovulatory follicles as well as growth of corpora lutea [13]. Reduced blood flow causes hypoxic condition in follicular microenvironment, induces generation of ROS and activation of HIF-1α [77]. The active HIF-1α binds to hypoxia response elements region of VEGF gene promoter in ovarian cells and induces upregulation of VEGF [56, 57]. Increased level of ROS due to pathological conditions or drug treatment induce granulosa cell death [57, 78,79,80,81,82] inhibit follicular growth, development and induces meiotic arrest [83, 84] as well as apoptosis in rat oocytes [78, 83]. The elevated level of ROS has been reported in patients of Primary ovarian insufficiency (POI) [85], and could be used as a promising indicator for risk of POI [86]. The increased level of ROS may also be attributed to increase mutation in ATPase6 gene [85] and mitochondrial cytochrome c oxidase 1 gene in POI patients [87]. Further, high level of ROS is associated with pathogenesis of polycystic ovarian syndrome (PCOS) [88, 89]. However, the exact role of ROS in pathogenesis of PCOS is ill-understood.
The hypoxia-specific genes are upregulated in granulosa cells of aged women suggesting hypoxia as main mechanism underlying ovarian senescence and deterioration of oocyte quality [58]. Further, hypoxia induces HIF-1α and its downstream targets like phosphodiesterase 4D (PDE4D), neuron-derived orphan receptor-1 (NOR-1 or NR4A3), nuclear receptor subfamily-1 (NR2F2), neovascularization by VEGF and ATP synthesis through glycolysis [58].
The presence of air pollutant 7, 12-dimethylbenz (a) anthracene (DMBA), a polycyclic aromatic hydrocarbon affects follicular growth and development and deteriorates oocyte quality [90]. It destroys follicles, reduces ovarian volume and alters mRNA expression of number of genes involved in the cell survival, proliferation and primordial follicle activation in mouse as well as rat ovary [90, 91] resulting in the decrease of ovarian volume [92, 93]. Studies suggest the involvement of phosphatidylinositol-3 kinase (PI3K) pathway that converts phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)-trisphosphate (PIP3), leading to phosphorylation of protein kinase B (PKB/Akt) [94]. Increased Akt phosphorylation with a decrease of forkhead box O3 A (FOXO3A) phosphorylation and activation of mTOR have been observed in DMBA-treated primordial follicle as well as in oocyte of mice [95] suggesting the role of PI3K signaling and PI3K/Akt/mTOR-mediated autophagy in ovary.
Hypoxia-induced autophagy
Low pO2 is one of the major causes for the induction of autophagy [96, 97]. Depending upon the degree of severity and duration of oxygen deprivation, hypoxia triggers different pathways of autophagy. For instance, chronic and moderate hypoxia triggers HIF-1α [98] as well as PKCδ–JNK1-mediated pathways to induce autophagy [28]. On the other hand, a rapid and severe oxygen fluctuation induce autophagy via HIF-1α independent as mTOR-mediated pathway [99] and UPR [29]. Autophagy may also promote survival by removing damaged mitochondria and hypoxia-mediated ROS production [26].
HIF-1α-dependent autophagy
The BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) gene is a specific target of HIF-1α that gets fully expressed during moderate hypoxia [100]. BNIP3 and its homologue BNIP3L are prosurvival proteins, [101] that are involved in hypoxia-induced autophagy. In moderate hypoxia, HIF induces BNIP3 and disrupts the interaction between Beclin 1 and Bcl-2 [59]. The free Beclin1 induces autophagy [96] and mitophagy [59] instead of apoptosis [102] (Fig. 1b).
During hypoxia, cell is capable of supporting oxidative production of ATP through tricarboxylic acid (TCA) cycle and electron transport chain (ETC) up to some extent [103]. Leakage of electron from ETC generates ROS. On the other hand, reoxygenation following hypoxia leads to uncontrolled superoxide generation that causes increased oxidative stress [103]. The reduced pO2 as well as nitric oxide levels result in the generation of ROS and decreased PHD activity [104, 105]. The decreased PHD activity stabilizes HIF-1α and induces autophagy through BNIP/BNIP3L-mediated disruption of Beclin1 and Bcl-2 interaction [48]. Studies suggest that stabilization and/or synthesis of HIF-1α under hypoxia is dependent on the PI3K/Akt pathway [106]. In cases of severe hypoxia or anoxia, additional pathways such as platelet-derived growth factor receptor (PDGFR), which is HIF-1α dependent [107], and protein deglycase or Parkinson disease protein 7 (DJ-1/PARK7) may also regulate autophagy [29].
HIF-1α-independent autophagy
The serine/threonine kinase (mTOR) is a principal inhibitory regulator of autophagy [108, 109]. It induces autophagy during severe hypoxia (Fig. 1b). The long-term hypoxia and ATP depletion could result in the phosphorylation of 5′ AMP activated protein kinase (AMPK) that activates Tuberous sclerosis complex 2 (TSC2) proteins [110]. The activated TSC2 forms a complex with TSC1 through a combination of GTP-binding protein Rheb and inhibits mTOR function [111]. The inhibition of mTOR activity also occurs through two independent pathways, the DNA damage response 1 (REDD1) protein [111, 112] and activation of stress sensor protein, ataxia telangiectasia mutated (ATM) [113].
Under severe hypoxic conditions, autophagy is induced through UPR pathway [114]. It has been reported that UPR activates stress sensors [115] and these sensors could activate autophagy [29, 116,117,118]. During initial stage of hypoxia, PKCδ activates autophagy by promoting JNK1-mediated Bcl-2 phosphorylation that dissociates Beclin 1 from Bcl-2 proteins [119]. As the hypoxia prolongs, PKCδ and Beclin 1 proteins are cleaved by caspase-3 protein, which is associated with the apoptosis [120, 121]. On the other hand, carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]—fluoromethylketone (Z-VAD-fmk), a caspase inhibitor, induces autophagy [122]. Indeed, PKCδ–JNK1 signaling plays an important role to protect cells from hypoxic stress by inducing autophagy.
Players and pathways involved in hypoxia-mediated autophagy have been well studied in a wide variety of somatic cell types [28, 101, 114]; however, involvement of hypoxia-mediated autophagy in mammalian ovary remains poorly understood. Few studies suggest that hypoxia induces HIF-1α and VEGF expression in luteal cells, reduces number of antral follicle and the growth of corpora lutea in sheep ovary [13]. HIF-1α-mediated mTOR signaling pathway has been reported to induce mouse granulosa cells autophagy in response to follicle-stimulating hormone (FSH) [123]. The increased expression of HIF-1α is associated with mouse granulosa cells autophagy [124]. The Cobalt chloride (CoCl2)-induced hypoxia increases expression of autophagy-related genes like LC3, Atg5, Beclin 1, Atg7 and BNIP3 in mouse granulosa cells [123, 124]. Based on these studies, we propose that granulosa cell proliferation may compromise the pO2 in follicular microenvironment that may trigger HIF-1α-mediated autophagy. Autophagy has also been reported in follicle loss from the ovarian of rat and murine exposed to cigarette smoke [125, 126] probably by inducing hypoxia.
Autophagy in mammalian ovary
Involvement of autophagy has been reported in mouse [127], rat [35, 128, 129], porcine [130] goose and quail ovary [131, 132]. Autophagy plays an important role in the maintenance and regulation of ovarian primordial follicle reserve; knock-out of autophagy-related genes result in the decrease of primordial follicle pool leading to the POI [133, 134]. Germ cell-specific knock-out of ATG7 gene leads to POI with the decrease of follicle as well as oocyte number in the ovary [134]. In addition, the presence of loss of function variant of ATG7 and ATG9A genes results in the impairment of autophagy which suggests the important role of defective autophagic machinery in POI patients [135]. The POI is also associated with mutation in autophagy regulatory Tsc1 or Tsc2 genes and elevated mTOR activity that leads to premature activation and early depletion of primordial follicle pool in the ovary [136, 137]. However, rapamycin (an inhibitor of mTOR) limits the conversion of primordial follicle into developing follicle and inhibits follicular atresia, thus preventing depletion of ovarian reserve [138]. Autophagy in ovary prevents granulosa cell apoptosis in younger women, while in aged women, a decline of autophagy augments the expression of apoptotic marker, ROS and higher percentage cell death [139]. Inhibition of autophagy leads to massive accumulation of age-related catabolic waste during folliculogenesis in IL-33(−/−) mice [140]. Another protective role of autophagy can be seen in pig ovaries subjected to heat stress [141]. Heat stress increases abundance of autophagosome and expressions of beclin 1 and LC3B-II in interstitial as well as follicular cells. Abundance of BCL2L1 and phosphorylated BCL2 was also increased with no caspase 3 cleavages, suggesting the suppression of apoptotic signaling in the ovary [141]. Milk deprivation in female neonates for 12–36 h induces autophagy-mediated differentiation or the formation of primordial follicles from naked oocytes that prevents depletion of germ cells from ovarian pool. Further, starvation resulted in higher number of primordial follicle. Oocyte cytoplasm showed abundance of autophagy-related proteins and suppressed expression of apoptotic proteins such as caspase 9 and caspase 3 [142].
Autophagy has also been reported in granulosa cells of obese women due to high level of oxidized low-density lipoprotein (oxLDL) and oxidative stress [143, 144]. H2O2-induced oxidative stress also causes granulosa cell death in mouse ovary via autophagy [145, 146]. Melatonin and FSH suppress autophagy-mediated granulosa cells deaths by inhibiting JNK-mediated dissociation of BCL2/BECN1 complex [147] and activating PI3K-Akt-mTOR signaling cascade with suppressing FOXO1 transcriptional activity, respectively [146]. Autophagy, with or without apoptosis is also involved in oocyte and granulosa cell death during follicular atresia in rat [139, 143, 148]. The autophagy is mainly induced in granulosa cells during various phases of ovarian cycle in rat [35, 149], and both autophagy and apoptosis have been reported in granulosa cells of mouse ovary [150]. The primordial follicle and theca cells show weak LC3-II expression, while granulosa cells at all the stage of folliculogenesis showed high level of LC3-II expression [35]. On the other hand, LC3-II expression was not reported in follicular oocyte [35]. The granulosa cells of atretic follicle also showed intense expression of caspase-3 and LC3 immunoreactivity as compared to that of healthy follicle [35]. Gonadotropin treatment suppresses autophagy by activating PI3K-Akt-dependent or independent mTOR signaling in granulosa cells of rat [35, 151,152,153,154].
Studies suggest that autophagy is actively involved in the depletion of oocyte from rat ovary [37]. Follicular cells show simultaneous presence of both autophagic and apoptotic markers in same cell at the same time during all phases of estrous cycle in rat [37, 155]. A large number of oocytes are removed by a process sharing features of apoptosis and autophagy [156]. Most of the oocyte in early stage of death are simultaneously positive to active caspase-3, DNA breaks (apoptosis), increase of lamp1 and acid phosphatase a characteristic of autophagy [156]. A similar mechanism of cell death has been reported in oocytes of pre-pubertal rat cultured in vitro [156]. Thus, process of cell death in oocyte probably begins with the degradation of cytoplasmic components including mitochondria. During initial phase, caspase-3 is activated and oocyte undergoes apoptotic cell death [156]. Autophagy cell death accounts for massive depletion of germ cells from the ovary of Lim homeobox 8 (Lhx8) (a protein involved in patterning and differentiation) ablated mouse probably due to disrupted DNA repair mechanism. It leads to dramatic reduction of ovarian reserve and generation of sterile fibrotic ovaries [157]. Age-dependent changes in the type of cell death have been reported in oocyte where it uses different combinations of apoptosis and autophagy. For instance, oocytes are mostly eliminated by apoptosis, autophagy and even mixed events of both death pathways during prepubertal age [129]. However, autophagy can be observed at all the age group of rat [129]. The estrous cycle-dependent cell death events have also been reported in rat oocyte [37]. Apoptosis is predominant during estrous phase and autophagy is more common during proestrous stage. Both apoptosis and autophagy are observed during diestrous and metaestrous phase in rat ovary [37].
Autophagy plays a preventive role in post-maturation aging of mouse oocyte. The p62 protein expression decreased, while LC3-II puncta, autophagosome content are increased after 12 h of oocyte aging [38]. Induction of autophagy either by rapamycin or LiCl corrected the aging parameters by decreasing cytoplasmic calcium, ROS, caspase level and cytoplasmic fragmentation along with reduction in proportion of oocyte with barrel shaped spindle and congressed Autophagy was induced as natural stress response during vitrification-warming of mouse oocyte [158], but enhancing autophagy by rapamycin had negative effects on fertilization and development of oocyte [159] and inhibition activated apoptosis via caspase-9 and 12 activation [160]. Inhibition of autophagy during in vitro maturation of porcine oocyte also induced DNA damage, apoptosis and disrupted mitochondrial membrane potential exerting detrimental effects on polar body extrusion and oocyte competency [161]. These studies suggest that the increase of autophagy prevents caspases activation and apoptosis. Autophagy is reported during luteal cell death in rat [128], marmoset monkey [162] and human [163, 164]. Studies suggest that the autophagy promotes luteal cell death by regulating apoptotic cell death in non-primates species [128, 165] chromosome [38]. On the other hand, decreased autophagy accelerates the aging in mouse [38].
Recent studies suggest the role of autophagy in pathogenesis of metabolic disorder like PCOS. Autophagy-related ultra-structural changes, consistent with increased expression of LC3B and decreased SQSTM1/p62 are observed in cortex of PCOS ovary in rat [40]. Increased autophagy was also evidenced in ovarian tissue of PCOS patients along with differential expression of autophagy-related genes [40]. The granulosa cells of PCOS patients show high level of LC3-II proteins and mRNA expression with increased autophagosome formation. The increased expression of SUMO-specific protease (SENP3) induces GC autophagy in the ovary of PCOS patients [166]. Further, the elevated level of mTOR and P-mTOR has been observed in of DHEA induced PCOS mice ovary [167]. In addition, autophagy-inducing gene and transcription factor FOXO1 are reduced in endometrial tissue of PCOS patients [39]. These studies suggest the involvement of autophagy in pathogenesis of PCOS.
Conclusion
The presence of apoptosis and autophagy markers in same cells of ovary suggests the onset of autophagy and apoptosis from the beginning and only one of these processes may induce final disposal of oocyte in rat [129]. Autophagy may also be initiated when the process of apoptosis cannot be achieved [168]. Another possibility is that both processes of cell death are activated at the same time from the beginning itself and both actively participate in the disposal of oocyte. Since volume of the oocytes is significantly larger than somatic cells, it is possible that the combined degradation process may be sufficient in the elimination of a large cytoplasmic content of only one cell [129]. The role of hypoxia in modulating ovarian physiology and the role of autophagy in various physiological processes have separately been studied. However hypoxia-mediated autophagy and its impact on physiological/pathological changes in mammalian ovary remains ill-understood. From existing literature, we propose that hypoxia could be involved in the induction of autophagy within the follicular microenvironment of ovary. However, further studies are required to find out the pathways governing hypoxia-induced autophagy and the possible role of autophagy during hypoxic stress in mammalian ovary. Once the players and pathways of hypoxia-mediated autophagy are known, the therapeutic strategies could be developed to prevent the hypoxia-mediated loss of germ cell from the ovary. Studies on hypoxia-mediated autophagy in mammalian ovary could also be helpful in the management of problems like PCOS and POI in patients staying in hypoxic conditions.
References
Wald NJ, Idle M, Boreham J, Bailey A (1981) Carbon monoxide in breath in relation to smoking and carboxyhaemoglobin levels. Thorax 36:366–369
Milroy CM (2018) Deaths from environmental hypoxia and raised carbon dioxide. Acad Forensic Pathol 8:2–7
West JB (2012) Respiratory system under stress. In West JB (ed) Respiratory physiology, the essentials. Lippincott Williams & Wilkins, Philadelphia
Eltzzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Eng J Med 364(7):656–665
Gibson HL, Sarnat SE, Suh HH, Coull BA, Schwarts J, Zanobetti A, Gold DR (2014) Short-term effects of air pollution on oxygen saturation in a cohort of senior adults in steubnville, OH. J Occup Environ Med 56:149–154
DeMeo DL, Zanobetti A, Litonjua AA, Coull BA, Schwartz J, Gold DR (2004) Ambient air pollution and oxygen saturation. Am J Respir Crit Care Med 170:383–387
Dutta A, Khramtsova G, Brito K et al (2018) Household air pollution and chronic hypoxia in the placenta of pregnant Nigerian women: a randomized controlled ethanol cookstove intervention. Sci Total Environ 619(620):212–220
Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ (1994) Oxygen regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci 91:6496–6500
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci 97:9082–9087
Till A, Acker H (2004) Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol 207:3171–3188
Wiesener MS, Maxwell PH (2003) HIF and oxygen sensing; as important to life as the air we breathe? Ann Med 35:183–190
Semenza GL (2006) Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91:803–806
Parraguez VH, Urquieta B, Pérez L et al (2013) Fertility in a high-altitude environment is compromised by luteal dysfunction: the relative roles of hypoxia and oxidative stress. Reprod Biol Endocrinol 11:24
Oktem O, Urman B (2010) Understanding follicle growth in vivo. Hum Reprod 25:2944–2954
Baerwald AR, Adams GP, Pierson RA (2012) Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 18:73–91
Zeleznik AJ (2004) The physiology of follicle selection. Reprod Biol Endocrinol 2:31. https://doi.org/10.1186/1477-7827-2-31
Temple-Smith P, Pereleshina E, LaRosa D, Ellery S, Snow R, Walker D, Catt S, Dickinson H (2013) Can birth hypoxia affect ovarian follicular reserve. Hum Reprod 28:i35
Printz RH (1972) The effects of high altitude on the reproductive cycle and pregnancy in the hamster. Anat Rec 173:157–171
Tatone C, Carbone MC, Falone S et al (2006) Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod 12:655–660
Tatone C, Amicarelli F, Carbone MC et al (2008) Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update 14:31–42
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. Biol Chem 281:29776–29787
Degenhardt K, Mathew R, Beaudoin B et al (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10:51–64
Brunelle JK, Chandel NS (2002) Oxygen deprivation induced cell death: an update. Apoptosis 7:475–482
Yoshimori T (2004) Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 313:453–458
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Jin S (2006) Autophagy, mitochondrial quality control and oncogenesis. Autophagy 2:80–84
Gonzalez A, Hall MN (2017) Nutrient sensing and TOR signaling in yeast and mammals. EMBO J 36:397–408
Mazure NM, Pouyssegur J (2010) Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol 22(2):177–180
Rouschop KM, Van den Beucken T, Dubois L et al (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and A TG5. J Clin Investig 120(1):127–141
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760
Ohsumi Y (2001) Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2:211e216
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 51:1069–1075
Cuervo AM (2003) Autophagy and aging—when “all you can eat” is yourself. Sci Aging Knowl Environ 36:2
Cuervo AM (2004) Autophagy: in sickness and in health. Trends Cell Biol 14(2):70–77
Choi JY, Jo MW, Lee EY, Yoon BK, Choi DS (2010) The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil Steril 93:2532–2537
Choi J, Jo M, Lee E, Choi D (2011) Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil Steril 95:1482–1486
Escobar ML, Echeverría OM, Vázquez-Nin GH (2012) Immunohistochemical and ultrastructural visualization of different routes of oocyte elimination in adult rats. Eur J Histochem 56:e17
Lin FH, Zhang WL, Li H et al (2018) Role of autophagy in modulating postmaturation aging of mouse oocytes. Cell Death Dis 9:308
Sumarac-Dumanovic M, Apostolovic M, Janjetovic K, Jeremic D (2017) Downregulation of autophagy gene expression in endometria from women with polycystic ovary syndrome. Mol Cell Endocrinol 440:116–124
Li D, You Y, Bi FF et al (2018) Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 155:85–92
Tuder RM, Yun JH, Bhunia A, Fijalkowska I (2007) Hypoxia and chronic lung disease. J Mol Med 85:1124–1317
Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X (2003) Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 21:347–360
Costa KM, Mendonça DA, Moraes DJA, Machado BH (2014) Evolution and physiology of neural oxygen sensing. Front Physiol 5:302
Yuan XJ, Tod ML, Rubin LJ, Blaustein MP (1990) Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol 259:H281–H289
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J16:1151–1162
Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15:621–627
Brocato JY, Chervona M Costa (2014) Molecular responses to hypoxia-inducible factor 1a and beyond. Mol Pharmacol 85:651–657
Bruick RK, McKnight S (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Ivan M, Haberberger T, Gervasi DC et al (2002) Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci 99:13459–13464
Kaelin WG, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402
Yu F, White SB, Zhao Q, Lee FS (2001) HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci 98:9630–9635
Ivan M, Kondo K, Yang H et al (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:448–464
Kim J, Bagchi IC, Bagchi MK (2009) Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 150:3392–3400
Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci 90:4304–4308
Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 306:re12
Neeman M, Abramovitch R, Schiffenbauer YS, Tempel C (1997) Regulation of angiogenesis by hypoxic stress: from solid tumours to the ovarian follicle. Int J Exp Pathol 78:57–70
Kaipia A, Hsueh AJ (1997) Regulation of ovarian follicle atresia. Ann Rev Physiol 59:349–363
Molinari E, Bar H, Pyle AM, Patrizio P (2016) Transcriptome analysis of human cumulus cells reveals hypoxia as the main determinant of follicular senescence. Mol Hum Reprod 22:566–576
Zhang H, Bosch-Marce M, Shimoda LA et al (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283:10892–10903
Blagosklonn VM (2013) Hypoxia, MTOR and autophagy converging on senescence or quiescence. Autophagy 9:260–262
Blerkom J Van (2004) Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction 128:269–280
Clark AR, Stokes YM (2011) Follicle structure influences the availability of oxygen to the oocyte in antral follicles. Comput Math Methods Med 2011:287186
Macome JC, Costa LE, Martin IH, TaquiniA C (1977) Steroid biosynthesis by gonads of rats submitted to chronic hypobaric hypoxia. Acta Physiol Lat Am 27:249–257
Martin IH, Costa LE (1992) Reproductive function in female rats submitted to chronic hypobaric hypoxia. Arch Int Physiol Biochim Biophys 100:327–330
Matsuda F, Inoue N, Manabe N, Ohkura S (2012) Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 58:44–50
Edson MA, Nagaraja AK, Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30:624–712
Oktem O, Oktay K (2008) The ovary: anatomy and function throughout human life. Ann N Y Acad Sci 1127:1–9
Wigglesworth K, Lee KB, O’Brien MJ, Peng J, Matzuk MM, Eppig JJ (2013) Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci 110:E3723–E3729
Sugiura K, Eppig J (2005) Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 17:667–674
Su QY, Wu X, O’Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ (2004) Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence foran oocyte-granulosa cell regulatory loop. Dev Biol 276:64–73
McNatty KP, Lawrence S, Groome NP et al (2006) Oocyte signalling molecules and their effects on reproduction in ruminants. Reprod Fertil Dev 18:403
Sugiura K, Su YQ, Diaz FJ et al (2008) Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 135:786
Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ (1990) FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specificfactor(s) secreted by the oocyte. Dev Biol 138:16–25
Eppig J, Wigglesworth K, Pendola F, Hirao Y (1997) Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 56:976–984
Hussein TS (2005) Oocytes prevent cumulus cell apoptosis by maintaining amorphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 118:5257–5268
Bianco F, Basini G, Santini S, Grasselli F (2005) Angiogenic activity of swine granulosa cells: effects of hypoxia and the role of VEGF. Vet Res Commun 29(suppl 2):157–159
Sugino N, Nakamura Y, Okuno N, Ishimatsu M, Teyama T, Kato H (1993) Effects of ovarian ischemia–reperfusion on luteal function in pregnant rats. Biol Reprod 49:354–358
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Hydrogen peroxide modulates meiotic cell cycle and induces morphological feature characteristics of apoptosis in rat oocytes cultured in vitro. Apoptosis 10:863–874
Chaube SK, Shrivastav TG, Prasad S, Tiwari M, Tripathi A, Pandey AN, Premkumar KV (2014) Clomiphene citrate induces ROS-mediated apoptosis in mammalian oocytes. Open J Apoptosis 3:52–58
Tripathi A, Shrivastava TG, Chaube SK (2012) Aqueous extract of Azadiracta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocyte. J Assist Reprod Genet 29:15–23
Tripathi A, Shrivastava TG, Chaube SK (2013) An increase in granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Intl J Appl Basic Med Res 3:27–36
Tiwari M, Tripathi A, Chaube SK (2017) Presence of encircling granulosa cells protects against oxidative stress-induced apoptosis in rat eggs cultured in vitro. Apoptosis 22:98–107
Tiwari M, Prasad S, Tripathi A et al (2015) Apoptosis in mammalian oocytes: a review. Apoptosis 20:1019–1025
Tiwari M, Prasad S, Tripathi A, Pandey AN, Singh AK, Shrivastava TG, Chaube SK (2016) Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React Oxyg Species 1:110–116
Venkatesh S, Kumar M, Sharma A et al (2010) Oxidative stress and ATPase6 mutation is associated with primary ovarian insufficiency. Arch Gynecol Obstet 282:313–318
Tokmak A, Yıldırım G, Sarıkaya E et al (2015) Increased oxidative stress markers may be a promising indicator of risk for primary ovarian insufficiency: a cross-sectional case control study. Rev Bras Ginecol Obstet 37(9):411–416
Zhen X, Wu B, Wang J, Lu C, Gao H, Qiao J (2015) Increased incidence of mitochondrial cytochrome c oxidase 1 gene mutations in patients with primary ovarian insufficiency. PLoS One 10(7):e0132610
Lee JY, Baw CK, Gupta S, Aziz N, Agarwal A (2010) Role of oxidative stress in polycystic ovary syndrome. Curr Womens Health Rev 6:96–107
Zuo T, Zhu M, Xu W (2016) Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev 2016:8589318
Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB (2009) Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol Appl Pharmacol 234:361–369
Hoyer PB (2001) Reproductive toxicology: current and future directions. Biochem Pharmacol 41:1557–1564
Mattison DR, Schulman JD (1980) How xenobiotic chemicals can destroy oocytes. Contemp Obstet Gynecol 15:157
Weitzman M, Gortmaker S, Sobol A (1992) Maternal smoking and behavior problems of children. Pediatrics 90:342–349
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–562
Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA (2011) Understanding the Villain: DMBA induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci 123:563–575
Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM (2009) Hypoxia-induced autophagy is mediated through HIF-induction of BNIP3 and BNIP3L via their BH3-domains. Mol Cell Biol 29:2570–2581
Schaaf MB, Cojocari D, Keulers TG et al (2013) The autophagy associated gene, ULK1, promotes tolerance to chronic and acute hypoxia. Radiother Oncol 108:529–534
Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14:1983–1991
Papandreou I, Lim AL, Laderoute K, Denko NC (2008) Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ 15:1572–1581
Guo K, Searfoss G, Krolikowski D et al (2001) Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell 8:367–376
Mazure NM, Pouyssegur J (2009) Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia. Autophagy 5:868–869
Liu XW, Su Y, Zhu H et al (2010) IF-1a-dependent autophagy protects HeLa cells fromfenretinide (4-HPR)-induced apoptosis in hypoxia. Pharmacol Res 62:416–425
Wang W, Fang H, Groom L et al (2008) Superoxide flashes in single mitochondria. Cell 134:279–290
Berchner-Pfannschmidt U, Tug S, Trinidad B et al (2008) Nuclear oxygen sensing: induction of endogenous prolyl-hydroxylase 2 activity by hypoxia and nitric oxide. J Biol Chem 283:31745–31753
Tug S, Delos Reyes B, Fandrey J, Berchner-Pfannschmidt U (2009) Nonhypoxic activation of the negative regulatory feedback loop of prolylhydroxylase oxygen sensors. Biochem Biophys Res Commun 384:519–523
Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M (2003) Regulation of hypoxia-inducible factor-1 protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/ glycogen synthase kinase 3-pathway in HepG2 cells. J Biol Chem 278:31277–31285
Wilkinson S, O’Prey J, Fricker M, Ryan KM (2009) Hypoxia-selective macroautophagy and cell survival signaled by autocrine PDGFR activity. Genes Dev 23:1283–1288
Sciarretta S, Volpe M, Sadoshima J (2014) Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114:549–564
Takei N, Nawa H (2014) MTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci 7:28
Cam H, Easton JB, High A, Houghton PJ (2010) MTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1a. Mol Cell 40:509–520
Wolff NC, Vega-Rubin-de-Celis S, Xie XJ, Castrillon DH, Kabbani W, Brugarolas J (2011) Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol 31:1870–1884
DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW (2008) Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22:239–251
Cam H, Houghton PJ (2011) Regulation of mammalian target of rapamycin complex 1 (mTORC1) by hypoxia: causes and consequences. Target Oncol 6:95–102
Fang Y, Tan J, Zhang Q (2015) Signalling pathways and mechanism of hypoxia induced autophagy in animal cells. Cell Biol Int 9999:1–8
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–522
Ogata M, Hino S, Saito A et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Tam AB, Mercado EL, Hoffmann A, Niwa M (2012) ER stress activates NF-kB by integrating functions of basal IKK activity IRE1 and PERK. PLoS One 7:e45078
Gade P, Ramachandran G, Maachani UB et al (2012) An IFN-g-stimulated ATF6-C/EBP-b signaling pathway critical for the expression of death associated protein kinase 1 and induction of autophagy. Proc Natl Acad Sci 109:10316–10321
Chen JL, Lin HH, Kim KJ, Lin A, Forman HJ, Ann DK (2008) Novel roles for protein kinase Cδ-dependent signaling pathways in acute hypoxic stress-induced autophagy. J Biol Chem 283:34432–34444
Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC (2009) Caspase-mediated cleavage of Atg6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett 274:95–100
Clavijo C, Chen JL, Kim KJ, Reyland ME, Ann DK (2007) Protein kinase C delta-dependent and -independent signaling in genotoxic response to treatment of desferroxamine, a hypoxia-mimetic agent. Am J Physiol Cell Physiol 292:C2150–C2160
Colell A, Ricci JE, Tait S et al (2007) GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129:983–997
Zhou J, Yao W, Li C, Wu W, Li Q, Liu H (2017) Administration of follicle-stimulating hormone induces autophagy via upregulation of HIF-1α in mouse granulosa cells. Cell Death Dis 8:e3001
Zhou J, Li C, Yao W, Alsiddig MC, Huo L, Liu H, Miao Y (2018) Hypoxia-inducible factor-1α-dependent autophagy plays a role in glycolysis switch in mouse granulosa cells. Biol Reprod 99:308–318
Gannon AM, Stampfli MR, Foster WG (2012) Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci 125:274–284
Gannon AM, Stampfli MR, Foster WG (2013) Cigarette smoke exposure elicits increased autophagy and dysregulation of mitochondrial dynamics in murine granulosa cells. Biol Reprod 88:1–11
Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N (2008) Autophagy is essential for preimplantation development of mouse embryos. Science 321:117–120
Choi J, Jo M, Lee E, Choi D (2011) The role of autophagy in corpus luteum regression in the rat. Biol Reprod 85:465–472
Escobar ML, Echeverrıa OM, Sanchez-Sanchez L, Mendez C, Pedernera E, Vázquez-Nin GH (2010) Analysis of different cell death processes of prepubertal rat oocytes in vitro. Apoptosis 15:511–526
Lee S, Hiradate Y, Hoshino Y, Tanemura K, Sato E (2014) Quantitative analysis in LC3-II protein in vitro maturation of porcine oocyte. Zygote 22:404–410
D’Herde K, De Prest B, Roels F (1996) Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail (Coturnix coturnix japonica). Reprod Nutr Dev 36:175–189
Kovacs J, Forgo V, Peczely P (1992) The fine structure of the follicular cells of the domestic goose. Cell Tissue Res 267:561–569
Gawriluk TR, Hale AN, Flaws JA, Dillon CP, Green DR, Rucker EB III (2011) Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 141:759–765
Song ZH, Yu HY, Wang P et al (2015) Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis 6:e1589
Delcour C, Amazit L, Patino LC et al (2018) ATG7 and ATG9A loss-of-function variants trigger autophagy impairment and ovarian failure. Genet Med. https://doi.org/10.1038/s41436-018-0287-y
Adhikari D, Flohr G, Gorre N et al (2009) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 15:765–770
Adhikari D, Zheng W, Shen Y et al (2010) Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 19:397–410
Zhang XM, Li L, Xu JJ et al (2013) Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 523:82–87
Vilser C, Hueller H, Nowicki M, Hmeidan FA, Blumenauer V, Spanel-Borowski K (2010) The variable expression of lectin-like oxidized low-densitylipoprotein receptor (LOX-1) and signs of autophagy and apoptosis in freshlyharvested human granulosa cells depend on gonadotropin dose, age, and body weight. Fertil Steril 93:2706–2715
Wu J, Carlock C, Zhou C, Nakae S, Hicks J, Adams HP, Lou Y (2015) IL-33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol 194:2140–2147
Hale BJ, Hager CL, Seibert JT, Selsby JT (2017) Heat stress induces autophagy in pig ovaries during follicular development. Biol Reprod 97(3):426–437
Watanabe R, Kimura N (2018) Non-suckling starvation of neonatal mice promotes primordial follicle. J Reprod Dev 64:89–94
Duerrschmidt N, Zabirnyk O, Nowicki M et al (2006) Lectin-like oxidized low-densitylipoprotein receptor-1-mediated autophagy in human granulosa cells as analternative of programmed cell death. Endocrinology 147:3851–3860
Serke H, Vilser C, Nowicki M et al (2009) Granulosa cell subtypes respond byautophagy or cell death to oxLDL-dependent activation of the oxidizedlipoprotein receptor 1 and toll-like 4 receptor. Autophagy 5:991–1003
Shen M, Jiang Y, Guan Z, Cao Y, Sun SC, Liu H (2016) FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep 6:380–390
Shen M, Jiang Y, Zhiqiang G, Yan C et al (2017) Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 13(8):1364–1385
Cao Y, Shen M, Jiang Y, Sun SC, Liu H (2018) Melatonin reduces oxidative damage in mouse granulosa 1 cells via restraining JNK-dependent autophagy. Reproduction 155(3):307–319
Escobar ML, Echeverría OM, Casasa AS, García G, Aguilar SJ, Vázquez-Nin GH (2013) Involvement of pro-apoptotic and pro-autophagic proteins in granulosa cell death. Cell Biol 1:9–17
Gioacchini G, Dalla Valle L, Benato F et al (2013) Interplay between autophagy and apoptosis in the development of Danio rerio follicles and the effects of a probiotic. Reprod Fertil Dev 25:1115–1125
Zhou J, Yao W, Liu K, Wen Q, Wu W, Liu H, Li Q (2016) MicroRNA let-7g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor. Int J Biochem Cell Biol 78:130–140
Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M (2004) Follicle stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follic. J Biol Chem 279:19431–19440
Hunzicker-Dunn ME, Lopez-Biladeau B, Law NC, Fiedler SE, Carr DW, Maizels ET (2012) PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc Natl Acad Sci 109:E2979–E2988
Kayampilly KM, Menon PP (2007) Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology 148:3950–3957
Choi JY, Jo MW, Lee EY, Choi DS (2014) AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 147:73–80
Ortiz R, Echeverrıa OM, Salgado R, Escobar ML, Vazquez-Nin GH (2006) Fine structural and cytochemical analysis of the processes of cell death of oocytes in atretic follicles in new born and prepubertal rats. Apoptosis 11:25–37
Escobar ML, Echeverrıa OM, Ortız R, Vazquez-Nin GH (2008) Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis 13:1253–1266
D’Ignazio L, Michel M, Beyer M et al (2018) Lhx8 ablation leads to massive autophagy of mouse oocytes associated with DNA damage. Biol Reprod 98(4):532–542
Bang S, Shin H, Song H, Suh CS, Lim HJ (2014) Autophagic activation in vitrified-warmed mouse oocytes. Reproduction 148(1):11–19
Lee GK, Shin H, Lim HJ (2016) Rapamycin influences the efficiency of in vitro fertilization and development in the mouse: a role for autophagic activation. Asian Australas J Anim Sci 29(8):1102–1110
Gao HH, Li JT, Liu JJ, Yang QA, Zhang ZM (2017) Autophagy inhibition of immature oocytes during vitrification-warming and in vitro mature activates apoptosis via caspase-9 and -12 pathway. Eur J Obstet Gynecol Reprod Biol 217:89–93
Shen XS, Jin YX, Liang S et al (2018) Autophagy is required for proper meiosis of porcine oocytes maturing in vitro. Sci Rep 8:12581
Fraser HM, Lunn SF, Cowen GM, Illingworth PJ (1995) Induced luteal regression in the primate: evidence for apoptosis and changes in c-myc protein. J Endocrinol 147:131–137
Canto FD, Sierralta W, Kohen P, Munoz A, Strauss JF, Devoto L (2007) Features of natural and gonadotropin-releasing hormone antagonist induced corpus luteum regression and effects of in vivo human chorionic gonadotropin. J Clin Endocrinol Metab 92:4436–4443
Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE, Gaytan F (2000) Different patterns of structural luteolysis in the human corpus luteum of menstruation. Hum Reprod 15:2119–2128
Davis JR, Rueda BR (2000) The corpus luteum: an intraovarian structure with maternal instincts and suicidal tendencies. Front Biosci 7:949–978
Zeng X, Chai W (2018) Role of senp3 in the autophagy of granulosa. Fertil Steril 110(4):e119
Yaba A, Demir N (2012) The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J Ovarian Res 5(1):38
Yousefi S, Perozzo R, Schimid I et al (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124–1132
Acknowledgements
The laboratory is supported by Centre of Advanced Study, Department of Zoology, Banaras Hindu University, Varanasi-221005, UP, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosured.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yadav, A.K., Yadav, P.K., Chaudhary, G.R. et al. Autophagy in hypoxic ovary. Cell. Mol. Life Sci. 76, 3311–3322 (2019). https://doi.org/10.1007/s00018-019-03122-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03122-4