Abstract
Acute lymphoblastic leukemia (ALL) is the commonest childhood malignancy, accounting for approximately 80 % of leukemia in the pediatric group, and its etiology is unknown. This neoplasia is characterized by male predominance, high-risk features and poor outcome, mainly in recurrence patients and adults. In recent years, advances in the success of childhood ALL treatment were verified, and the rate of cure is over 80 % of individuals. However, there is a considerable scope for improving therapeutic outcome in this neoplasia. Improvements in ALL therapy might readily be achieved by developing additional biomarkers that can predict and refine prognosis in patients with ALL. In normal hematopoietic cells, cytokines provide the stimulus for proliferation, survival, self-renewal, differentiation and functional activation. Abnormalities of cytokines are characteristic in all forms of leukemia, including ALL. The stromal cell-derived factor-1 (SDF-1 or CXCL12) is a member of the CXC chemokine family that binds to CXC chemokine receptor 4 (CXCR4). The CXCL12/CXCR4 axis appears to play a role in dissemination of solid tumors and hematopoietic diseases. Understanding the mechanisms by which ALL cells are disseminated will provide additional information to expand therapeutic approach. Therefore, this review summarizes information relating to ALL cell biology, focusing specifically in a cytokine receptor important axis, CXCL12/CXCR4, that may have implications for novel treatment strategies to improve life expectancy of patients with this neoplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia (ALL) is characterized by the monoclonal and/or oligoclonal proliferation of hematopoietic precursor cells of the lymphoid series within the bone marrow (BM) [61]. It occurs in approximately 6,000 individuals per year and results in approximately 1,400 deaths annually in the United States [73]. In Brazil, according to National Cancer Institute (INCA), leukemia represents between 25 and 35 % of all cancer types, and ALL is the most frequent in children aged from 0 to 14 years. Furthermore, INCA 2012 annual report estimates 5.050 new cases of leukemia in men and 4.320 in women [32].
Following lymphocyte profile, two subtypes of ALL malignant cells may be involved, T cell (T-ALL) and B cell (B-ALL) [57]. T-ALL accounts for 15 % of ALL, and it is identified by male predominance, high-risk features including high white blood cell (WBC) count, mediastinal enlargement, generalized lymphadenopathy, central nervous system involvement, and poor outcome [58, 62].
In T-ALL, T cell transformation is a multi-step process in which different genetic alterations cooperate to alter the normal mechanisms that control cell growth, proliferation, survival, and differentiation during thymocyte development. Particularly, deletions of the CDKN2A locus in chromosome 9p21, which encompasses the p16/INK4A and p14/ARF suppressor genes, are present in more than 70 % of all T-ALL cases [22, 30]. Moreover, constitutive activation of NOTCH1 signaling comprises the core of the oncogenic program in the pathogenesis of T-ALL [87], cooperating with loss of p16/INK4A and p14/ARF in T cell transformation [82].
T-ALL is more aggressive than B-ALL, and limited therapeutic options are available for patients with primary resistant or relapsed disease, highlighting the urgency for treatment stratification protocols and identification of more effective antileukemic drugs [63]. This imperative was further supported by studies of the long-term effects of intensified chemotherapy in T-ALL survivors, which showed that improvements of leukemia-free survival have been achieved in parallel with significant increases in rates of acute and chronic life-threatening and debilitating toxicities [3].
B-ALL, especially B-cell precursor (BCP)-ALL, is the major form of the disease, accounting for approximately 85 % of all pediatric ALL [31]. Perturbation of B-cell differentiation in the BM must lead to B-ALL development, considering that its microenvironment provides a variety of cytokines, chemokines, growth factors and adhesion molecules that coordinately regulate B-cell development [35].
Most childhood cases of B-ALL may be subclassified by the presence of either gross or submicroscopic genetic alterations, such as aneuploidy or recurring gross chromosomal rearrangement, which are frequent in approximately 75 % of B-ALL cases [27, 63]. These rearrangements commonly perturb genes encoding regulators of hematopoiesis, tumor suppressors, oncogenes, or tyrosine kinases, but commonly it requires additional genetic hits to establish the full leukemic phenotype.
A number of chromosomal rearrangements are common in B-ALL and are critical events in leukemogenesis. Hyperdiploidy is one of the most frequent alterations in childhood ALL and is associated with favorable outcome [28]. At least five extra chromosomes are presently associated; however, the biologic basis of the acquisition of multiple chromosomal gains is poorly understood. Conversely, hypodiploidy, with fewer than 44 chromosomes, is associated with dismal prognosis [52].
Studies have identified new subtypes of ALL, and uncovered recurring submicroscopic genetic alterations in known ALL subtypes. These include loss-of-function mutations involving genes regulating lymphoid development that contribute to the arrest in maturation characteristic of B-ALL, mutations that inactivate tumor suppressor and cell cycle regulatory proteins, and mutations that drive cytokine receptor and/or kinase signaling. Concomitant lesions disrupting hematopoietic development and tumor suppression as well as driving signaling and proliferation are hallmarks of many ALL subtypes.
Importantly, several of these alterations are associated with specific subtypes of ALL defined by chromosomal alterations and different treatment outcome [51]. The translocations t(9; 22), expressing chimeric protein BCR-ABL, and t(4; 11), codifying MLL-AF4 protein, are related to poor prognosis. Patients with chromosomal alteration t(1; 19), related to E2A-PBX1 fusion protein, and t(12; 21), characterizing TEL-AML1, have a good treatment outcome [72, 75]. Contrariwise, T-ALL are derived from precursor T cells in the thymus, and infrequent but recurrent translocations lead to the overexpression of the transcription factors LYL1, HOX11, HOX11L2, and TAL1 [75]. Using gene expression profiling, Yeoh et al. [89] identified molecular markers to distinguish T-ALL subtypes with increased risk of relapse. In addition, they indicated that contemporary risk stratification fails to identify many patients who are at high risk of drug-induced toxicities or marrow relapse.
In the last years, advances of childhood ALL treatment have been achieved, with over 80 % of individuals cured [63]. This rate is supported by the accurate assignment of individual leukemia subtypes, in which genetic alterations figure primarily in most classification schemes [89]. However, a poor prognosis is still expected for a group of patients with various risk factors, such as central nervous system involvement, and those with ALL relapses. Einsiedel et al. [21] have demonstrated that more than one-third of patients may be cured from recurrent ALL with second complete remissions lasting more than 10 years. They also concluded that immunophenotype and time point of relapse are important prognostic factors that allow adapting more precisely treatment intensity to individual prognosis.
Despite the success in cure and survival rates, there is still scope for improvements, since ALL treatment is more likely to cause short- and long-term side effects, and some patients may experience relapse. Furthermore, studies about leukemic cells and niche correlation highlight the importance of therapeutically targeting the BM microenvironment [33].
In normal hematopoietic cells, cytokines provide the stimulus for proliferation, survival, self-renewal, differentiation and functional activation. Abnormalities of cytokine and growth factor signaling pathways are characteristic of all forms of leukemia: lymphoid and myeloid, acute and chronic. These pathways are usurped to sub serve critical parts of the malignant program in leukemic cells [81].
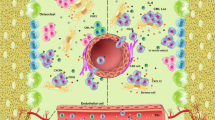
The stromal cell-derived factor-1 (SDF-1 or CXCL12) is a member of the CXC chemokine family that counteracts with its cognate receptors CXC chemokine receptor 4 (CXCR4), widely expressed in numerous tissues, including immature osteoblasts and endothelial cells within BM, epithelial cells in many organs, central nervous system and hematopoietic cells, to stimulate physiological processes [13]. CXCL12/CXCR4 signaling is essential in maintaining the progenitor hematopoietic cell pool, and also regulates hematopoietic stem cells attachment within the bone marrow niche [80].
Ayala et al. [1] outlined that a high expression of CXCR4 by leukemic blasts and activation of the CXCL12/CXCR4 axis is involved in leukemia progression and disruption of normal hematopoiesis. Moreover, in this particular, leukemia-associated bone microenvironment markers could be used as prognostic or predictive indicators of ALL progression and/or treatment outcome.
Since chemokines and their receptors have been implicated in the pathogenesis of many diseases, including cancer risk and disease progression, this work reviewed the CXCL12/CXCR4 axis in the pathogenesis of ALL and its role as a possible therapeutic target.
Chemokine CXCL12 and its receptor CXCR4
CXCL12 monomer proteins are expressed in all human cells, except in blood cells. To date, at least six CXCL12 splicing variants were described named α, β, γ, δ, ε and ϕ, and the former is the most abundant and smallest, consisting of three exons instead of four, as others do. However, CXCL12β is twice as potent in the blood, exhibiting very similar activity to CXCL12α [34].
The proteolytical degradation process of both ends regulates CXCL12 constitutive expression [15]. Degradation of N-terminus occurs in the blood and the tissues, abolishing chemokine activity and reducing its affinity to the receptor. It is splicing variant-dependent and occurs slowly. Contrariwise, C-terminus proteolysis is rapid, splicing variant-dependent, and does not cease CXCL12, but reduces its activity, occurring specifically in the blood [34].
CXCL12 plays an important role in migration of progenitor and leukemic cells to the BM [66]. Its expression by endothelial cells along with endosteum regions of BM mediates not only homing and retention of progenitor cells, but is important for their trans-endothelium migration through the expression of E-selectin [54].
ALL arises from malignant transformation of lymphocytes, undoubtedly in a single BM site; however, the spread to essentially all BM cavities, resulting in extensive disease, may have occurred by the time of diagnosis. In addition, ALL cells also infiltrate the liver, spleen, lymph nodes, and central nervous system [14]. Chemokines and theirs receptors, in which CXCL12/CXCR4 axis is supposedly involved, tightly regulate this migration process. Indeed Tokoyoda et al. [79] demonstrated the B lymphocyte location and movement between specific niches within BM during development is maintained by CXCL12 interactions in that niche.
CXCL12 may contribute to leukemic marrow infiltration by increased CXCR4 expression and migratory response in BM-derived blasts compared with circulating cells [48]. In fact, CXCR4 is one of several chemokine receptors defined by their ability to induce cell migration toward a chemotactic cytokine gradient. This receptor has been investigated in breast cancer pathogenesis [20, 56], and several reports have addressed the expression and biological role of CXCR4 at different stages of B-cell development in normal and malignant hematopoiesis.
In immature B cells, CXCL12 stimulus induces activation of small GTP-binding protein (GTPases) such as Ras-related C3 botulinum toxin substrate 1 (Rac1) [59], leading to co-location of CXCR4 and small GTPase Rac1 into membrane lipid rafts, which is necessary for cell migration in response to a CXCL12 gradient [88]. Freret et al. [23] demonstrated that inactivation of Rac1 can interfere with the mechanisms involved in receptor internalization modulating the chemotactic response to CXCL12 by regulating internalization of CXCR4, and thus, it might play a role in B-ALL cell dissemination.
Shen et al. [70] and Spiegel et al. [74] have demonstrated that down regulation of CXCR4 following exposure to high doses of CXCL12 results in significant inhibition of ALL cell homing to the BM. However, stromal cells also secrete fibronectin, a component of the extracellular matrix that enhances CXCL12-induced migration of ALL cells without influencing CXCR4 expression [67].
The role for CXCL12/CXCR4 axis in the infiltration of extramedullary sites, which commonly expresses significant levels of CXCL12 [50] is supported by the association between high expression of CXCR4 by ALL cells and extramedullary organ invasiveness [14], and inhibition of extramedullary disease by treatment with CXCR4 antagonists [37]. So, binding of CXCL12/CXCR4 is one of the key interactions between human ALL cells and BM stroma, and high expression of the chemokine receptor CXCR4 is of predictive value for early relapse in ALL childhood [68].
Pediatric patients who had B-ALL and high CXCR4 expression had significantly more prominent liver or spleen infiltration compared with patients who had low CXCR4 expression [14]. Kato et al. [39] verified that hepatomegaly in ALL patients are not only due to random infiltration but rather, the result of CXCL12/CXCR4 axis-dependent migration and expansion of leukemic cells in the hepatic niche. These data indicate that this axis stimulates not only migration but in addition, proliferation of ALL leukemic cells in vivo and in vitro, further, targeting the extramedullar microenvironment components to prevent recurrence from minimal residual disease.
Besides its crucial role in migration, there are reports indicating that CXCL12 may play a role in the pathogenesis of malignant tumors [17, 19], including leukemia [18, 55]. In this context, the primary role of CXCL12 seems to be facilitating metastasis or mobilizing tumor cell, and perhaps the establishment of cancer stem-like cell within the tumor microenvironment, where high levels of CXCL12 recruit a highly tumorigenic population of tumor cells, promoting cell survival, proliferation, angiogenesis, and metastasis.
Our research group have evaluated polymorphic mutations and gene expression of CXCL12 and CXCR4, aiming to elucidate their roles in the pathogenesis of cancer, with a focus on hematological diseases. de Oliveira et al. [18] verified that CXCL12 polymorphic alleles have implications in CML pathogenesis. de Oliveira et al. [19] studied the same SNP (rs1801157) in CXCL12 gene, although in Hodgkin’s (HL) and non-Hodgkin’s lymphoma (NHL), suggesting that this genetic variant may have important implications in this neoplasia subtype. de Oliveira Cavassin et al. [17] compared the same allelic variant between patients with lymphoid leukemias and lymphomas and indicated that Brazilian lymphoma patients are more likely to carry the polymorphic allele for CXCL12 gene, indicating a differential role for this gene in subgroups of hematological diseases. Recently, de Lourdes Perim et al. [16] verified the positive association for CXCL12 (rs1801157) and susceptibility to childhood ALL.
CXCL12/CXCR4 axis in ALL signaling
The molecular mechanism underlying CXCL12/CXCR4 signaling has been investigated extensively and revealed that multiple molecules are activated upon CXCL12 stimulation [74]. Firstly, the activation pathway of CXCL12/CXCR4 initiates after ligand CXCL12 sensitizing CXCR4, inducing receptor internalization and promoting an increase of cytoplasmic calcium store and mobilization levels.
The interaction with CXCR4 occurs between its 8 first amino acids residues in the N-terminus: the first two take part in receptor activation while further six are involved in the binding of the chemokine to the receptor. On the cell surface, CXCL12 binding to CXCR4 must be stabilized through the interaction with glycoseaminoglycans (GAGs), such as heparin sulfate, and this is responsible for leukocyte accumulation and prevention of CXCL12 proteolytic degradation [34]. Furthermore, association to GAGs can induce CXCL12 oligomerization, which in turn, promotes CXCR4 oligomerization, enhancing its activation function [10].
CXCR4 is a G-protein coupled receptor, which is composed by an intracellular heterotrimer of Gα, Gβ and Gγ subunits, bound to a guanine nucleotide GDP, in its basal state. CXCL12 ligand binding activates the receptor, and GDP is replaced by GTP, which in turn dissociates the βγ dimer. The Gα monomeric subunit can relay different GPCR signal, depending on the type of α monomer present and activated: Gαi, Gαs, Gαq and Gα12 [77].
Chemokine receptors are typically coupled Gαi proteins which act inhibiting adenyl cyclase, whereas Gαs stimulates adenyl cyclase [25]. Gαi also stimulates kinase activity of the Src family tyrosine-protein kinase c-Src, binds to the catalytic domain, and changes the conformation of c-Src. In turn, c-Src phosphorylates the adaptor Shc, recruiting GRB2 and activating the H-Ras/c-Raf-1/MEK1-2/ERK1-2 pathway. This activated pathway increases the transactivation ability of transcription factor Elk1 and repressed STAT3 transcription factor [12, 43].
Contrariwise, activated CXCR4 enables the recruitment of STAT3 by the phosphorylation of Janus quinase 2 (JAK2), activating the downstream pathway of Stats, mitogen-activated protein kinase (MAPK) and phosphatidilynositol 3-kinase (PI3K)-Akt pathway [44]. In addition, its signal induces the activation of protein kinase C and phosphorylation of dual threonine and tyrosine recognition kinase (MEK), extracellular signal-regulated kinase (ERK) and components of focal adhesion complexes in many cell types, including B-cell precursors [6, 24, 85].
Moreover, calcium flux has been used to determine chemokine activity in cells. However, Gαi does not promote this flux, but Gαq, suggesting that CXCR4 might hold other Gα proteins [65]. In addition, Gβγ subunit can trigger phospholipase C (PLC) activation and formation of diacylglycerol (DAG) and phosphatidilynositol 3 (IP3), resulting in Ca2+ mobilization from intracellular stores [46].
Chemokines and their receptors are involved in cell trafficking. Indeed, CXCL12-CXCR4 axis can mediate chemotaxis of multiple cell types, including lymphocytes, hematopoietic stem cells, endothelial and epithelial cells, and cancer cells [2, 76]. This process is mediated by the activation of PI3 kinase (PI3K) by both Gα and Gβγ subunits, leading to phosphorylation of considerable adhesion molecules, such as paxilin, focal adhesion kinase (FAK), proline-rich kinase-2 (Pyk-2), Crk substrate p130Cas, Crk, and Crk-L, Nck [85, 90].
Differences in the signaling mechanisms employed by ALL cells and normal hematopoietic stem cells (HSC) heightened the possibility of differential regulating traffic of ALL cells and thereby providing novel therapeutic applications. While both normal HSC and B cell progenitors shared a dependence on PI3K signaling [41, 90], B-ALL leukemic cells demonstrated only a minor involvement of this pathway, with dominant signaling through mitogen-activated protein kinases (p38MAPK) [6, 38].
Zhang et al. [90] demonstrated that cytoplasmic tyrosine kinase, JAK2, is involved in CXCR4 receptor-mediated signaling through PI3K and seems to be required for CXCL12-induced migration of hematopoietic progenitor cells. These results suggest that JAK2 is required for the tyrosine phosphorylation of multiple focal adhesion proteins, and for cell migration in hematopoietic progenitor cells.
The expression of CXCL12 imposes a survival potential for hematopoietic cells due to activation of PI3K-AKT-NFκB and MAPK pathways [5, 85]. In addition, it has also been shown that signal transducer and activators (STATs) are activated upon binding of CXCL12 to CXCR4 [40, 83].
Signalling through PI3K is likely necessary for CXCL12-induced activation of very late antigen 4 (VLA-4) and increased adhesion of cells to vascular cell adhesion molecule 1 (VCAM-1) and fibronectin [70]. Moreover, it has been shown that VLA-4 function is essential for BM homing of B-ALL leukemic cells [70, 74].
The participation of MAPK pathway, through PKC or Gαi, signaling to Erk1/2, Ras-activated signaling pathway, Src-related kinases (Src, Lyn, Fyn and Lck), T-cell activation molecule ZAP-70, and small GTPases have also been implicated in lymphocyte migration [6, 46, 77], suggesting that multiple signaling molecules might be accessed to support CXCL12/CXCR4 activation. However, the evidence of which of them are most important or which pathway is essential for inducing homing or migration in different ALL subtypes remains an unresolved issue.
Apparently, CXCL12/CXCR4 axis may not be directly involved in T-ALL leukemic cells signaling. Nonetheless, the analysis of the intracellular signaling profile of T-ALL patients has revealed that activation targets of CXCL12/CXCR4 signaling pathway, such as PI3K-Akt, MAPK and JAK-STAT, are implicated in oncogenic processes [11]. Thus, it is reasonable that some ALL subsets would benefit from strategic therapy concerning CXCL12/CXCR4 pathway and its derivatives.
The CXCL12/CXCR4 axis as a potential therapeutic target
The treatment of ALL is based on multidrug therapy with adjustment for risk of disease recurrence. The administered drugs include corticosteroids, metastasis inhibitors, asparaginase, antraciclics, alkylating agents, antimetabolites, and purine antagonist [4]. The remission induction therapy for ALL patients should include a glucocorticoid, vincristine, and asparaginase, not only because they are not myelosuppressive, but also because their antileukemic effects are different, and their mechanisms may act synergistically. Prednisone has been the most frequently used glucocorticoid treatment at this stage. However, dexamethasone has better results in patients with T-ALL, and appears to allow better control of the central nervous system invasion [64].
Lack of efficacy in the current treatment can be partly attributed to the fact that leukemia cells are protected by their microenvironment. Leukemic cells residing in BM niches are provided with favorable conditions for their growth and survival [8, 53] and thereby escape from chemotherapy-induced death [47]. In this context, several studies suggested that chemokine analogues or antagonists could be used in parallel with conventional therapies to improve ALL treatment. For example, Buonamici et al. [9] demonstrated that targeting the CCR7 receptor in T-ALL could block their CNS dissemination.
Additionally to the evidence that BM stromal niche can protect ALL cells against the cytotoxicity of chemotherapeutic agents, it is also a possible source of relapse. Since CXCL12/CXCR4 axis is a major determinant in the crosstalk between leukemic cells and BM stroma, the development of new drugs and approaches for the treatment of relapse remain an important goal to improve cure rates [60]. Kato et al. [39] showed that functions of the niche are maintained by CXCL12/CXCR4 axis, proposing a novel therapeutic approach targeting by inhibition of these molecules. It was demonstrated that liver dissemination of leukemia is not due to nonselective infiltration, but rather systematic invasion and proliferation of leukemic cells in hepatic niche. These findings formed the basis for therapeutic approaches that target extramedullary niche by inhibiting CXCL12/CXCR4 axis.
Mowafi et al. [49] demonstrated that the addition of recombinant CXCL12 increases proliferation of B-ALL cells in culture and induces a decreased internalization of CXCR4 receptor on the surface. However, this process does not interfere in cell proliferation. They believed that CXCL12 in childhood ALL deserves further study to clarify both the role of this chemokine in the pathogenesis of ALL and the possibility of modulating signaling directed by CXCL12.
The CXCR4 could be a potential therapeutic target, since it has been shown that this receptor neutralization enhances apoptosis and decreases proliferation in an experimental model of human non-Hodgkin’s lymphoma (NHL) [7]. Konoplev et al. [42] concluded that the activated form of CXCR4 [26, 71] is directly related to metastasis progression and provides independent prognostic information in adult patients with ALL, independently of other prognostic parameters. This observation is potentially important in both clinically and therapy as anti-CXCR4 has currently been evaluated and can be added into chemotherapy protocols designed for ALL patients. Hatse et al. [29] showed that a small-molecule CXCR4 antagonist, bicyclam or AMD3100, inhibited CXCR4 internalization, the calcium influx and chemotaxis of ALL cells. Kato et al. [39] developed a therapeutic model where AMD3100 prevented repopulation of extramedullary ALL cells after chemotherapy and dramatically improved overall survival in mice treated with AMD3100. They found that without AMD3100 administration, some leukemia cells remain in the portal region of liver after chemotherapy, contributing to leukemia relapse.
CXCR4 antagonists have been used in combination with chemotherapy in preclinical and clinical studies, which have demonstrated that blocking CXCR4 may be a novel promising approach. CXCR4 antagonists can theoretically be more effective in remission patients, as part of maintenance therapy, to destroy the residual leukemia stem cells. However, the biology of the residual leukemia stem cells after chemotherapy is different, and the targeting agents may be ineffective. Further studies that combine CXCR4 antagonists with chemotherapy in patients in complete remission are needed [77].
Some authors have proposed that CXCR4 could be a potential therapeutic target (Table 1). In this context Juarez et al. [36] demonstrated that polyphemusin II peptide analogues T140, T134 and TC14012, and AMD 3100 are potent inhibitors of CXCL12-mediated chemotaxis and BM stromal-dependent proliferation of precursor B-ALL cells. In other study, they examined the ability of CXCR4 antagonists to disrupt the interaction between precursor B-ALL cells and their supportive niche in vivo, and found that blocking CXCL12/CXCR4 interactions resulted in rapid mobilization of leukemic cells into the peripheral blood and in significant expansion reduction of precursor B-ALL, in a mice model [37].
Although higher levels of CXCR4 expression have been shown to correlate with poor patient survival, effective drugs affecting cell surface CXCR4 expression are still unknown. Matsumoto et al. [45] examined the effects of a synthetic retinoid Am80 on CXCR4 expression of cultured T-ALL cells. They observed that it inhibited surface CXCR4 expression and CXCL12-induced chemotaxis by the acceleration of CXCR4 internalization. Therefore, Am80 may be an effective drug to inhibit the extramedullary infiltration of T-ALL cells.
Disruption of ALL cell microenvironmental interaction could be used to enhance the effectiveness of chemotherapeutic agents due to loss of protection by the stroma. The treatment with AMD3100 causes maintenance of leukemic cells in peripheral blood for a longer time than normal hematopoietic progenitors, prolonging exposure to chemotherapeutic agents. Finally, AMD3100 increases the proportion of cells in the circulation that are actively cycling, a factor that is likely to increase sensitivity to cell cycle dependent agents commonly used for ALL treatment, such as vincristine [86].
Among other cytokines, IL-8 is highly expressed in T-ALL cells refractory to chemotherapy. The involvement of transcription factor NFκB is of particular interest as a key molecule in the establishment of T-ALL and, consequently, inhibiting agents are considered attractive candidates to T-ALL treatment. The IL-8 could be one NFκB target gene involved in the progression of T-ALL and the characterization of molecular mechanisms leading to IL-8 upregulation could be relevant to elucidate the development of T-ALL and design new therapeutic strategies [69]. It was demonstrated that NFκB and AP-1 transcription factors activity are central to induced IL-8 expression. [84].
Parameswaran et al. [60] demonstrated that the survival of mice bearing human and murine ALL cell lines could be extended by the combination of a CXCR4 antagonist AMD11070 and chemotherapy. It could represent an additional target to conventional chemotherapy treatments, without, however, replacing them. Within this context, CXCR4 has emerged as a promising therapeutic target, although further studies and consideration are required. In some way, it is plausible that inhibiting CXCR4 would result in mobilization of leukemic cells within circulation, which could cooperate to extramedullary invasion.
Understanding the mechanisms by which ALL cells disseminate may provide information to benefit developing therapeutic strategies based on targeting the ALL cell trafficking. Nevertheless, blocking CXCL12/CXCR4 axis could represent an important mechanism on managing therapeutic approaches in ALL.
References
Ayala F, Dewar R, Kieran M, Kalluri R (2009) Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 23(12):2233–2241
Baggiolini M (1998) Chemokines and leukocyte traffic. Nature 392(6676):565–568
Barrett AJ, Horowitz MM, Pollock BH, Zhang MJ, Bortin MM, Buchanan GR, Camitta BM, Ochs J, Graham-Pole J, Rowlings PA et al (1994) Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med 331(19):1253–1258
Bartram CR, Schrauder A, Kohler R, Schrappe M (2012) Acute lymphoblastic leukemia in children: treatment planning via minimal residual disease assessment. Deutsches Arzteblatt int 109(40):652–658. doi:10.3238/arztebl.2012.0652
Bendall L (2005) Chemokines and their receptors in disease. Histol Histopathol 20(3):907–926
Bendall LJ, Baraz R, Juarez J, Shen W, Bradstock KF (2005) Defective p38 mitogen-activated protein kinase signaling impairs chemotaxic but not proliferative responses to stromal-derived factor-1alpha in acute lymphoblastic leukemia. Cancer Res 65(8):3290–3298
Bertolini F, Dell’Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, Gobbi A, Pruneri G, Martinelli G (2002) CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin’s lymphoma. Cancer Res 62(11):3106–3112
Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ (2000) Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia 14(5):882–888
Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F, Gehrie E, Li M, Newcomb E, Zavadil J, Meruelo D, Lipp M, Ibrahim S, Efstratiadis A, Zagzag D, Bromberg JS, Dustin ML, Aifantis I (2009) CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459(7249):1000–1004
Busillo JM, Benovic JL (2007) Regulation of CXCR4 signaling. Biochim Biophys Acta 1768(4):952–963
Cardoso BA, Girio A, Henriques C, Martins LR, Santos C, Silva A, Barata JT (2008) Aberrant signaling in T-cell acute lymphoblastic leukemia: biological and therapeutic implications. Braz J Med Biol Res 41(5):344–350
Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA (2003) Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int J Oncol 22(3):469–480
Chotinantakul K, Leeanansaksiri W (2012) Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res 2012:270425
Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Mohle R, Meister B (2001) High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol 115(3):545–553
De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HH, Fales H, Tosato G (2004) Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood 103(7):2452–2459
de Lourdes Perim A, Guembarovski RL, Oda JM, Lopes LF, Ariza CB, Amarante MK, Fungaro MH, de Oliveira KB, Watanabe MA (2013) CXCL12 and TP53 genetic polymorphisms as markers of susceptibility in a Brazilian children population with acute lymphoblastic leukemia (ALL). Mol Biol Rep 40(7):4591–4596
de Oliveira Cavassin GG, De Lucca FL, Delgado Andre N, Covas DT, Pelegrinelli Fungaro MH, Voltarelli JC, Watanabe MA (2004) Molecular investigation of the stromal cell-derived factor-1 chemokine in lymphoid leukemia and lymphoma patients from Brazil. Blood Cells Mol Dis 33(1):90–93
de Oliveira CE, Cavassin GG, Perim Ade L, Nasser TF, de Oliveira KB, Fungaro MH, Carneiro JL, Watanabe MA (2007) Stromal cell-derived factor-1 chemokine gene variant in blood donors and chronic myelogenous leukemia patients. J Clin Lab Anal 21(1):49–54
de Oliveira KB, Oda JM, Voltarelli JC, Nasser TF, Ono MA, Fujita TC, Matsuo T, Watanabe MA (2009) CXCL12 rs1801157 polymorphism in patients with breast cancer, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma. J Clin Lab Anal 23(6):387–393
do Val Carneiro JL, Nixdorf SL, Mantovani MS, da Silva do Amaral Herrera AC, Aoki MN, Amarante MK, Fabris BA, Pelegrinelli Fungaro MH, Ehara Watanabe MA (2009). Plasma malondialdehyde levels and CXCR4 expression in peripheral blood cells of breast cancer patients. J Cancer Res Clin Oncol 135(8):997–1004
Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann G, Hahlen K, Gobel U, Klingebiel T, Ludwig WD, Henze G (2005) Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol 23(31):7942–7950
Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland DG, Lander ES, Golub TR, Look AT (2002) Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1(1):75–87
Freret M, Gouel F, Buquet C, Legrand E, Vannier JP, Vasse M, Dubus I (2011) Rac-1 GTPase controls the capacity of human leukaemic lymphoblasts to migrate on fibronectin in response to SDF-1alpha (CXCL12). Leuk Res 35(7):971–973
Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE (1998) The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273(36):23169–23175
Goldsmith ZG, Dhanasekaran DN (2007) G protein regulation of MAPK networks. Oncogene 26(22):3122–3142
Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R (1997) Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem 272(45):28726–28731
Harrison CJ (2009) Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br J Haematol 144(2):147–156
Harrison CJ, Foroni L (2002) Cytogenetics and molecular genetics of acute lymphoblastic leukemia. Rev Clin Exp Hematol 6(2):91–113 (discussion 200–112)
Hatse S, Princen K, Bridger G, De Clercq E, Schols D (2002) Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 527(1–3):255–262
Hebert J, Cayuela JM, Berkeley J, Sigaux F (1994) Candidate tumor-suppressor genes MTS1 (p16INK4A) and MTS2 (p15INK4B) display frequent homozygous deletions in primary cells from T- but not from B-cell lineage acute lymphoblastic leukemias. Blood 84(12):4038–4044
Hosking FJ, Papaemmanuil E, Sheridan E, Kinsey SE, Lightfoot T, Roman E, Irving JA, Allan JM, Taylor M, Tomlinson IP, Greaves M, Houlston RS (2010) Genome-wide homozygosity signatures and childhood acute lymphoblastic leukemia risk. Blood 115(22):4472–4477
INCA (2012). Instituto Nacional de Câncer (INCA) (Brasil). Câncer no Brasil: estimativa 2012: incidência de câncer no Brasil
Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D (2007) Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest 117(4):1049–1057
Janowski M (2009) Functional diversity of SDF-1 splicing variants. Cell Adhes Migr 3(3):243–249
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249
Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ (2003) Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia 17(7):1294–1300
Juarez J, Dela Pena A, Baraz R, Hewson J, Khoo M, Cisterne A, Fricker S, Fujii N, Bradstock KF, Bendall LJ (2007) CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia 21(6):1249–1257
Juarez JG, Thien M, Dela Pena A, Baraz R, Bradstock KF, Bendall LJ (2009) CXCR4 mediates the homing of B cell progenitor acute lymphoblastic leukaemia cells to the bone marrow via activation of p38MAPK. Br J Haematol 145(4):491–499
Kato I, Niwa A, Heike T, Fujino H, Saito MK, Umeda K, Hiramatsu H, Ito M, Morita M, Nishinaka Y, Adachi S, Ishikawa F, Nakahata T (2011) Identification of hepatic niche harboring human acute lymphoblastic leukemic cells via the SDF-1/CXCR4 axis. PLoS One 6(11):e27042
Kato Y, Iwama A, Tadokoro Y, Shimoda K, Minoguchi M, Akira S, Tanaka M, Miyajima A, Kitamura T, Nakauchi H (2005) Selective activation of STAT5 unveils its role in stem cell self-renewal in normal and leukemic hematopoiesis. J Exp Med 202(1):169–179
Kim CH, Hangoc G, Cooper S, Helgason CD, Yew S, Humphries RK, Krystal G, Broxmeyer HE (1999) Altered responsiveness to chemokines due to targeted disruption of SHIP. J Clin Invest 104(12):1751–1759
Konoplev S, Jorgensen JL, Thomas DA, Lin E, Burger J, Kantarjian HM, Andreeff M, Medeiros LJ, Konopleva M (2011) Phosphorylated CXCR4 is associated with poor survival in adults with B-acute lymphoblastic leukemia. Cancer 117(20):4689–4695
Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ (2004) CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 35(3):233–245
Levine RL, Pardanani A, Tefferi A, Gilliland DG (2007) Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 7(9):673–683
Matsumoto T, Jimi S, Hara S, Takamatsu Y, Suzumiya J, Tamura K (2010) Am 80 inhibits stromal cell-derived factor-1-induced chemotaxis in T-cell acute lymphoblastic leukemia cells. Leuk Lymphoma 51(3):507–514
Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC (2001) Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol 19:397–421
Mishra S, Zhang B, Cunnick JM, Heisterkamp N, Groffen J (2006) Resistance to imatinib of bcr/abl p190 lymphoblastic leukemia cells. Cancer Res 66(10):5387–5393
Mohle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H, Brugger W, Kanz L (2000) Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol 110(3):563–572
Mowafi F, Cagigi A, Matskova L, Bjork O, Chiodi F, Nilsson A (2008) Chemokine CXCL12 enhances proliferation in pre-B-ALL via STAT5 activation. Pediatr Blood Cancer 50(4):812–817
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56
Mullighan CG (2012) Molecular genetics of B-precursor acute lymphoblastic leukemia. J Clin Invest 122(10):3407–3415
Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, Dastugue N, Schrappe M, Pui CH, Basso G, Silverman LB, Janka-Schaub GE (2007) Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 110(4):1112–1115
Nagasawa T (2006) Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol 6(2):107–116
Naiyer AJ, Jo DY, Ahn J, Mohle R, Peichev M, Lam G, Silverstein RL, Moore MA, Rafii S (1999) Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood 94(12):4011–4019
Nishii K, Katayama N, Miwa H, Shikami M, Masuya M, Shiku H, Kita K (1999) Survival of human leukaemic B-cell precursors is supported by stromal cells and cytokines: association with the expression of bcl-2 protein. Br J Haematol 105(3):701–710
Oda JM, de Oliveira KB, Guembarovski RL, de Lima KW, da Silva do Amaral Herrera AC, Guembarovski AL, Sobrinho WJ, Derossi DR, Watanabe MA (2012). TGF-beta polymorphism and its expression correlated with CXCR4 expression in human breast cancer. Mol Biol Rep 39(12):10131–10137
Onciu M (2009) Acute lymphoblastic leukemia. Hematol Oncol Clin N Am 23(4):655–674
Oyekunle AA, Castagnetti F, Gugliotta G, Soverini S, Baccarani M, Rosti G (2011) F317L BCR-ABL1 kinase domain mutation associated with a sustained major molecular response in a CML patient on dasatinib. Leuk Res 35(7):e118–e120
Palmesino E, Moepps B, Gierschik P, Thelen M (2006) Differences in CXCR4-mediated signaling in B cells. Immunobiology 211(5):377–389
Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N (2011) Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia 25(8):1314–1323
Pui CH, Evans WE (2006) Treatment of acute lymphoblastic leukemia. N Engl J Med 354(2):166–178
Pui CH, Relling MV, Downing JR (2004) Acute lymphoblastic leukemia. N Engl J Med 350(15):1535–1548
Pui CH, Robison LL, Look AT (2008) Acute lymphoblastic leukaemia. Lancet 371(9617):1030–1043
Pui CH, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, Furman WL, Ribeiro RC, Spunt SL, Rubnitz JE, Jeha S, Hudson MM, Kun LE, Merchant TE, Kocak M, Broniscer A, Metzger ML, Downing JR, Leung W, Evans WE, Gajjar A (2012) Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children's Research Hospital, 1992 through 2007. J Clin Oncol 30(16):2005–2012. doi:10.1200/JCO.2011.40.8617
Rubin JB (2009) Chemokine signaling in cancer: one hump or two? Semin Cancer Biol 19(2):116–122
Sahin AO, Buitenhuis M (2012) Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adhes Migr 6(1):39–48
Sbaa-Ketata E, Vasse M, Lenormand B, Schneider P, Soria C, Vannier JP (2001) Fibronectin increases the migration induced by stromal cell-derived factor-1 alpha (SDF-1 alpha) in pre-B acute lymphoblastic leukemia cells. Eur Cytokine Netw 12(2):223–230
Schneider P, Vasse M, Al Bayati A, Lenormand B, Vannier JP (2002) Is high expression of the chemokine receptor CXCR-4 of predictive value for early relapse in childhood acute lymphoblastic leukaemia? Br J Haematol 119(2):579–580
Scupoli MT, Donadelli M, Cioffi F, Rossi M, Perbellini O, Malpeli G, Corbioli S, Vinante F, Krampera M, Palmieri M, Scarpa A, Ariola C, Foa R, Pizzolo G (2008) Bone marrow stromal cells and the upregulation of interleukin-8 production in human T-cell acute lymphoblastic leukemia through the CXCL12/CXCR4 axis and the NF-kappaB and JNK/AP-1 pathways. Haematologica 93(4):524–532
Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF (2001) The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Exp Hematol 29(12):1439–1447
Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, Luther MA, Wells TN, Hoxie JA, Marsh M (1997) Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol 139(3):651–664
Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Arkin S, Declerck L, Cohen HJ, Sallan SE (2001) Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 97(5):1211–1218
Society AC (2012) American Cancer Society: Cancer Facts and Figures 2012. American Cancer Society, Atlanta
Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, Rechavi G, Vormoor J, Lapidot T (2004) Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood 103(8):2900–2907
Staudt LM (2002) It’s ALL in the diagnosis. Cancer Cell 1(2):109–110
Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J (2010) CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 29(4):709–722
Tavor S, Petit I (2010) Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Seminars in cancer biology 20(3):178–185. doi:10.1016/j.semcancer.2010.07.001
Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16(11):2927–2931
Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T (2004) Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20(6):707–718
Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM (2011) Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117(2):429–439
Van Etten RA (2007) Aberrant cytokine signaling in leukemia. Oncogene 26(47):6738–6749
Van Vlierberghe P, Ferrando A (2012) The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest 122(10):3398–3406
Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M (1999) The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J 13(13):1699–1710
Vilimas T, Mascarenhas J, Palomero T, Mandal M, Buonamici S, Meng F, Thompson B, Spaulding C, Macaroun S, Alegre ML, Kee BL, Ferrando A, Miele L, Aifantis I (2007) Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med 13(1):70–77
Wang JF, Park IW, Groopman JE (2000) Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood 95(8):2505–2513
Welschinger R, Liedtke F, Basnett J, Dela Pena A, Juarez JG, Bradstock KF, Bendall LJ (2013) Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol 41(3):293–302 (e291)
Weng AP, Aster JC (2004) Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev 14(1):48–54
Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ (2005) Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood 105(1):40–48
Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, Behm FG, Raimondi SC, Relling MV, Patel A, Cheng C, Campana D, Wilkins D, Zhou X, Li J, Liu H, Pui CH, Evans WE, Naeve C, Wong L, Downing JR (2002) Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell 1(2):133–143
Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE (2001) Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood 97(11):3342–3348
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lourdes Perim, A., Amarante, M.K., Guembarovski, R.L. et al. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell. Mol. Life Sci. 72, 1715–1723 (2015). https://doi.org/10.1007/s00018-014-1830-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1830-x