Abstract
The mouse is often used as a model for understanding human placentation and offers multiple advantages, including the ability to manipulate gene expression in specific compartments and to derive trophoblast stem cells, which can be maintained or differentiated in vitro. Nevertheless, there are numerous differences between the mouse and human placentas, only the least of which are structural. This review aims to compare mouse and human placentation, with a focus on signaling pathways involved in trophoblast lineage-specific differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human placenta is a very poorly understood organ, and as a result, so is our understanding of numerous pregnancy-related conditions, including pre-eclampsia and fetal growth restriction [1]. Many of these conditions are caused by abnormalities early during human pregnancy and placentation, which are challenging to study. For these and other reasons, mouse models have been utilized extensively, giving us comprehensive knowledge regarding the role of specific genes in the formation and function of the placenta and the associated contribution to fetal growth and development. At times, however, knowledge of processes in the mouse has been extended to the human system without additional studies. This review seeks to compare mouse and human placentation based on studies done using relevant models, including in vitro (trophoblast stem-cell based) and in vivo experiments in the mouse, and in vitro experiments using validated human trophoblast cell culture systems. The uniqueness of this review is its particular focus on specific trophoblast subtypes in placentas of both species and comparison of the signaling pathways required for the maintenance and/or differentiation of each lineage subtype.
Placental structure and terminology
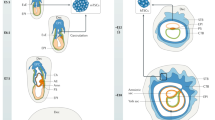
Both mouse and human have a hemochorial placenta, where maternal blood comes in direct contact with fetal-derived trophoblast. However, certain anatomical differences exist. In the mouse labyrinth, three layers of trophoblast separate maternal and fetal blood, while in the chorionic villi of the human placenta, there are at first two, and, later in gestation, functionally one layer of trophoblast separating maternal and fetal blood (Fig. 1). Similarly, the trophoblast cells anchoring the placenta to the uterine wall in the mouse (parietal giant cells and glycogen trophoblasts) are not nearly as invasive as the equivalent cells (extravillous trophoblast/EVT) in human, where these cells invade up to one-third of the thickness of the uterine wall, including the maternal arterioles [2].
Mouse and human placenta. Although both mouse and human placentas are hemochorial, some structural differences can be observed. a Mouse placenta: the labyrinth is the functional structure where gas/nutrient exchange occurs. Maternal blood is separated from fetal blood by three layers of trophoblasts (SynT-I, SynT-II and sinusoidal giant cells). The supportive junctional zone contains spongiotrophoblast, which give rise to trophoblast giant cells (TGCs) and glycogen trophoblasts. Both TGCs and glycogen trophoblasts are only modestly invasive, compared to the human extravillous trophoblasts (EVTs). b Human placenta: the functional structure for gas/nutrient exchange is the chorionic villus. In the first trimester, maternal blood is separated from the fetal blood by two layers of trophoblast, the syncytiotrophoblast (STB) and the cytotrophoblast (CTB) stem cells. Later in gestation, the continuous CTB layer disappears and only sparse CTBs are visible at term. The EVTs arise from the trophoblast cell column, and are highly invasive, penetrating up to one-third of the thickness of the uterus. Endovascular EVTs extensively remodel maternal spiral arterioles to ensure correct blood supply to the growing embryo. c Table listing functionally and/or structurally analogous mouse and human trophoblast subtypes
At the cellular level, the two species appear more equivalent, with syncytiotrophoblast formation arising from cell fusion in the interhemal compartment (the labyrinth in the mouse and chorionic villi in human), and hyperdiploid trophoblast cells forming in the placental implantation site of both species (Fig. 1). The latter arise through a process called endoreduplication (DNA synthesis without nuclear division) in the mouse, leading to “giant” nuclei (hence the name “trophoblast giant cells”) [3], while the process leading to hyperdiploidy in human EVT is less clearly defined [4].
Early events and the trophoblast stem cell niche
Both mouse and human placentation start with the formation of trophectoderm (TE) in the pre-implantation blastocyst (Fig. 2). TE specification in the mouse is marked by expression of CDX2 and exclusion of inner cell mass (ICM)-specific OCT4 (Pou5f1) [5]. In vitro, using a combination of mouse embryonic fibroblasts (MEFs) as a feeder layer and FGF4, trophoblast stem (TS) cell lines have been established from both mouse blastocysts and extraembryonic ectoderm, which recapitulate the above gene expression profile [6]. Mouse TS cells require a combination of FGF4 and TGFβ signaling to proliferate and maintain their undifferentiated state, while withdrawal of these factors leads to terminal differentiation [6–8]. Similar attempts have so far failed to yield human TS cells, raising doubts about the existence of these cells in the human pre-implantation blastocyst [9]. In fact, unlike the mouse embryo, where the post-implantation period is characterized by expansion of proliferative TS cells in the extraembryonic ectoderm [10], the early post-implantation human embryo (days 10–12 post-coitum) is characterized by formation of highly invasive trophoblasts, which help the embryo burrow into the endometrium [2, 11]. Only after this point in time does the mononuclear cytotrophoblast begin to proliferate to form the cytotrophoblastic shell. This and other data have led to the proposal that, in human, TS cells may, in fact, not exist in the placenta until the post-implantation period (on and after day 13 post-coitum), where co-expression of the ELF5 and CDX2 in a subgroup of cytotrophoblast (CTB) may in fact point to such a niche [12]. Another suggestion of a human TS cell niche is the Ki67+ cells in the proximal trophoblast cell column. In fact, in the context of a first trimester explant, FGF4 and activin, the growth factors that contribute to mouse TS cell maintenance, have each been shown to expand the CTB layer, although activin also induces some markers of EVT [13, 14]. Finally, the chorionic mesenchyme has also been suggested as a TS cell niche in the human placenta, though whether these cells are truly multipotent trophoblast remains to be confirmed [15]. Whether these, or other compartments, constitute a stem cell niche in the placenta, remains to be elucidated. The lack of knowledge about human TS cell markers, combined with our inability to maintain primary human CTB in culture, remain major obstacles to progress in this field.
Timeline comparison of mouse and human placental development. Timeline for both species is calculated from conception, with assignment of day of observation of the copulation plug as E0.5 in the mouse, and mid-menstrual cycle (corresponding to time of ovulation followed by fertilization) as coitus in human. The formation of blastocyst defines two distinct populations of cells: the inner cell mass and the outer trophectoderm, at E3.5 in mouse and approximately day 5 post-coitum in human. Implantation occurs at E4.5 in mouse and day 7–8 post-coitum in human. In mouse, chorio-allantoic attachment starts off the formation of the labyrinth around E8.0, with branching morphogenesis complete by E10.5, the half point through gestation, although the labyrinth continues to grow in the latter half of gestation. In human, villous formation starts early, around day 13 post-coitum, and the villi are fully vascularized by the end of the first/beginning of the second trimester. However, further maturation of both the stroma and trophoblast continues through the second trimester. In mouse, the junctional zone and TGCs are also fully formed by E10.5, although glycogen cells continue to differentiate and invade the uterus along with specific TGC subtypes. In human, EVT invasion and remodeling of maternal spiral arterioles are complete by the end of the first trimester, at which point the chorionic villi become fully bathed in well-oxygenated maternal blood
Trophoblast of the interhemal region
Morphogenesis
The interhemal region of the placenta (labyrinth in the mouse and chorionic villi in human) is composed of cells whose main function is to perform nutrient and gas exchange between the maternal and fetal blood. In both species, this compartment is composed of extraembryonic ectoderm (chorion)-derived trophoblast and extraembryonic mesoderm (allantois)-derived fetal mesenchyme and blood vessels. However, the union of these two structures (referred to as chorio-allantoic attachment in rodents) is temporally distinct in the two species. In mice, chorionic morphogenesis and branching are not initiated until after embryonic day 8.5 (E8.5), when the allantoic mesoderm attaches to the basal surface of the chorion [16]; however, in the human placenta, villus formation and branching initiate early (day 13 post-coitum) and are followed by infiltration of allantoic blood vessels, which is not concluded until the end of the first trimester [2].
Gcm1: The master regulator of the interhemal compartment
In mouse, the major transcription factor to initiate labyrinthine formation is the product of the Gcm1 gene [17]. Gcm1 is involved both in branching morphogenesis and in syncytiotrophoblast formation through regulation of syncytin genes, involved in cell–cell fusion [17–19]. Gcm1 is initially expressed in the basal layer of the chorion, which comes into direct contact with the allantois [19]; following completion of branching morphogenesis, it is co-expressed with Synb and Cebpa in the syncytiotrophoblast layer II (SynT-II) of the labyrinth [19]. In vitro, Gcm1 promotes G1-to-G0 transition of mouse TS cells, in preparation for fusion and syncytiotrophoblast formation [20]. The expression pattern of GCM1 in the human placenta is more complex, with the RNA expressed in both villous CTB and cell column trophoblasts, and the protein detected in the nuclei of a subgroup of villous CTB and few cells in the distal cell column [21]; nevertheless, similar to its function in the mouse placenta, it appears to promote cell cycle exit and syncytiotrophoblast formation in chorionic villi [22], also through regulation of syncytins [23].
Progenitor trophoblasts in the interhemal compartment
Most recently, two groups have described labyrinthine trophoblast “progenitor” cells in the mouse placenta. Hughes et al. [24] have identified LY6E as a surface marker of the SYNA+ cells in the upper portion of the chorion as early as E8.5. When sorted out of mouse TS cultures, LY6E+ cells expressed higher levels of SYNA and, when plated in differentiation media, readily formed multinucleated syncytiotrophoblast [24]. Ueno et al. [25] have identified EPCAMhi BrdU+ cell clusters during labyrinthine morphogenesis between E9.5 and E14.5. During co-culture with VCAM1-expressing OP9 cells, these EPCAMhi cells efficiently formed multinucleated syncytiotrophoblast, and expressed markers of SynT-I, SynT-II and sinusoidal giant cells, suggesting they are progenitors of all three layers of the labyrinth [25]. In addition, Ueno et al. have suggested that HGF signaling through its receptor, c-Met, is a pathway through which these EPCAMhi labyrinthine progenitor cells are maintained in vivo. c-Met knockout mice showed labyrinthine hypoplasia, intra-uterine growth restriction, and embryonic lethality by E14.5; specifically, EPCAMhi cells were present in similar numbers at E9.5, but were significantly decreased from E10.5 onward [25]. Similar trophoblast progenitors have not been described in the human chorionic villi, and HGF signaling has only been implicated in promotion of trophoblast motility, signaling through phosphatidylinositol 3-kinase (PI3-kinase) (see below) [26].
Signaling pathways regulating differentiation of the interhemal trophoblasts
Activin/Nodal/TGFβ signaling
One signaling pathway identified as a regulator of labyrinth growth in the mouse is that of Activin/Nodal. In vitro, in the absence of FGF4, Activin-A inhibits differentiation of mouse TS cells into spongiotrophoblast and trophoblast giant cells (TGC), instead promoting differentiation towards a labyrinthine cell fate [27]. Interestingly, Activin is thought to originate from the giant cells, suggesting paracrine control of differentiation between the distinct trophoblast layers in vivo [27]. While both Activin and Nodal utilize similar receptors, studies on transgenic mice have described Nodal to be the primary player in vivo. In knockout mice, Nodal produced by the epiblast has been shown to be required to maintain FGF4 signaling that supports TS cells early in gestation [28]. After mid-gestation, Nodal is expressed in spongiotrophoblast and its loss or reduction leads to a smaller labyrinth, as well as alteration of the spongiotrophoblast and a thickened giant cell layer [29, 30] (Table 1).
In the context of human trophoblast differentiation, Activin has been shown to promote both proliferation and EVT differentiation in first trimester villous explants (see below) [13], and hCG production and syncytiotrophoblast differentiation in context of term CTB [31]. The source of Activin-A is also distinct in human chorionic villi, being the mesenchymal villous core [32]. Another family member, TGFβ, also plays a role in human EVT differentiation (see “Invasive Trophoblast” section below and Table 1). Conversely, Nodal has been shown to inhibit proliferation and induce apoptosis in human in vitro trophoblast models, while it inhibits cell invasion and migration in HTR8 and first trimester CTB explants [33, 34].
PPARγ signaling
Another pathway involves signaling through the nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ). Lack of PPARγ in the mouse placenta leads to embryonic lethality at E10.5, due to placental abnormalities, including arrest of labyrinth formation [35, 36]. In vitro, PPARγ-null mouse TS cells fail to form syncytiotrophoblast, and are unable to upregulate Gcm1 upon switching to differentiation media [37]. In vitro, PPARγ agonists appear to have similar effects on mouse and human trophoblast, inhibiting differentiation into trophoblast giant cells in mouse [37] and interfering with invasion of human EVT [38]. Their effect on syncytiotrophoblast differentiation is more subtle, with modest induction of Gcm1 in mouse TS cells [37] and variable effects on hCG secretion in human term CTB [39, 40].
The role of oxygen tension and hypoxia-inducible factor
Alterations in oxygen tension, and its main effector pathway through the hypoxia-inducible factor (HIF) complex, have also been identified as a modulator of syncytiotrophoblast differentiation. Specifically, culture of term human CTB under low oxygen tension reduces cell–cell fusion and hCG secretion [41]. In mouse, formation of an intact HIF complex is required for invasive trophoblast differentiation (see below); when disrupted, such as in HIF1β (or Arnt)-null mouse TS cells, the latter differentiate exclusively into multinucleated syncytiotrophoblast [42]. In wild-type mouse TS cells, culture in 20 % oxygen on a fibronectin-rich matrix mimics the Arnt-null phenotype, leading to syncytiotrophoblast formation [43]. Treatment with the chromatin modifiers, histone deacetylase inhibitors, similarly, promotes differentiation into syncytiotrophoblast [42, 44], although this particular pathway appears to be both GCM1- and PPARγ-independent [44].
Canonical Wnt signaling
In mice, Wnt signaling appears to be required for chorio-allantoic attachment and labyrinthine development [45]. Specifically, Gcm1 appears to be the main target of canonical Wnt signaling in mouse placentation as defects in this pathway are associated with decreased expression of this transcription factor [45, 46]. More recently, Lu et al. [47] have provided additional evidence for the role of canonical Wnt signaling in regulation of Gcm1 expression in TS cells. In this study, signaling through Fzd5 was shown to lead to up-regulation of Gcm1 expression and fusion of trophoblast cells [47]. A similar effect of Wnt/TCF signaling on GCM1 and syncytialization has been shown in the human trophoblast cell line, BeWo [46], although in human, this pathway is better known for its role in EVT differentiation (see below and Table 1).
Protein kinase A-based signaling
Finally, two other pathways have long been known to promote CTB differentiation in chorionic villi of the human placenta: epidermal growth factor (EGF) [48] and hCG itself [49]. Both act through their specific receptors to increase intracellular levels of the second messenger cAMP, thus activating protein kinase A (PKA), and promoting cell fusion through induction of expression of syncytins [50, 51]. In the mouse, EGF signaling has not been shown to regulate syncytiotrophoblast formation but rather implicated in mediating differentiation of spongiotrophoblast and glycogen trophoblast in the junctional zone [52].
Invasive trophoblast
Morphogenesis and subtype-specific markers in the mouse
In mice, the first invasive trophoblasts arise from the mural trophectoderm as primary TGCs [53]. Later, the polar trophectoderm gives rise to the ectoplacental cone, which further differentiates into the spongiotrophoblast layer; these regions are characterized by expression of Ascl2 (previously known as Mash2) and Tpbpa, and are the source of secondary TGCs [53, 54]. TGCs are polyploid cells, which arise through a process of endoreduplication, or DNA synthesis without nuclear division [55, 56]. They secrete multiple hormones, including placental lactogens and proliferins, required for maintenance of pregnancy [54]; they also secrete metalloproteinases, which facilitate their locally invasive potential [54]. Unlike their human counterpart (see below), mouse TGCs have a fairly limited “invasive” potential. In fact, their invasive behavior can be characterized as more “phagocytic” than migratory: as mouse TS cells differentiate into TGC in vitro, they decrease their membrane protrusive activity, and instead develop highly organized actin stress fibers, large internal focal adhesions, and stable cell–cell interactions [57]. Phosphorylation of focal adhesion kinase (FAK), required for focal adhesion turnover, also decreases with differentiation into TGC [57].
Four types of TGCs have been characterized based on expression of placental lactogens Pl1, Pl2, Plf, and Ctsq, including parietal TGC (Pl1+/Pl2+/Plf+), canal TGC (Plf+/Pl2+), spiral artery-associated TGC (Plf+), and the sinusoidal TGC (Ctsq+/Pl2+) [54]. The latter cells are considered part of the labyrinth and, unlike the other TGCs, arise exclusively from Tpbpa− cells [54]. All four subtypes require expression of the bHLH transcription factor Hand1, as differentiated Hand1−/− TS cells fail to express any of these markers [54]. Another group of “invasive” trophoblast in the mouse placenta is glycogen trophoblast cells, which arise from Tpbpa+ cells [54]. These cells are characterized by PAS+ intracellular content and express the gap junction protein connexin 31 (GJB3) [3]. Some glycogen cells migrate deep into the maternal decidua where they are thought to help enhance maternal blood flow [53]. Recently, a report by Mould et al. shed further light on the development of these trophoblast subtypes [58]. In this study, the authors showed that glycogen trophoblast as well as spiral artery and canal TGC subtypes derive from a specific subpopulation of Tpbpa+ cells. These progenitor cells express Prdm1 (also known as Blimp1) and, through lineage tracing studies, were shown to be distinct from the Tpbpa+ cells that give rise to spongiotrophoblast and parietal TGCs [58]. This work further defines the complex relationship between progenitor and terminally differentiated trophoblast cells.
Morphogenesis and subtype-specific markers in human
In the human placenta, the extravillous trophoblast or EVTs are the functional equivalent of mouse TGC and glycogen cells. While there is an initial round of differentiation into highly invasive EVT early following implantation, the majority of EVT later in gestation arises from trophoblast cell columns in anchoring villi [2]. In comparison to mouse, much less is known about EVT subtypes and markers in the human placenta. Nonetheless, we know that human EVTs are in fact highly invasive, invading up to one-third of the myometrial thickness in the uterus [2]. Functionally, these cells either invade the uterine wall (interstitial EVT) or the maternal spiral arterioles (endovascular EVT); the latter invade through the arterial wall and replace maternal endothelial lining in a process termed vascular remodeling [59]. This process involves changes in integrin expression, with progression from integrins α6β4 in villous CTB, to α5β1 in cell column trophoblast, to α1β1 in mature invasive EVT [60]. In addition, trophoblasts in cell columns also lose their epithelial phenotype, decreasing expression of E-cadherin, and gain a vascular phenotype, acquiring endothelial markers such as PECAM1 (CD31), VE-cadherin, and MCAM (CD146) [61, 62]. Interestingly, however, E-cadherin expression appears to be restored in a subset of mature EVT, particularly the endovascular subtype [63]. In addition, unlike TGCs in the mouse, FAK phosphorylation is increased upon CTB differentiation into EVT, as focal adhesion turnover is required for enhanced migration of these cells [64]. Finally, it is important to note that, in contrast to mouse, where TGCs are the primary producers of hormones such as placental lactogens, in human, syncytiotrophoblast produce similar hormones, including hCG and hPL [65, 66]. Human EVT do produce a hyperglycosylated form of hCG, which promotes invasion in an autocrine fashion [67–69].
Less is known about the transcription factors that distinguish between different EVT subtypes. The human homolog of Mash2, ASCL2, has been shown to be expressed in trophoblast cell columns and invasive EVT by in situ hybridization in early gestation human placentas [70, 71], although whether it is in fact required for EVT differentiation has yet to be explored. The role of HAND1 in human trophoblast is less clear, as its RNA has been detected in the trophectoderm of human blastocysts, but not in samples of first trimester placental tissue or immunopurified CTB [72, 73]. Other transcription factors, including members of the homeobox family and the nuclear receptor PPARγ, are not specific to EVT, as they are also expressed in villous trophoblast [74]. In fact, as discussed above, PPARγ appears to be required for syncytiotrophoblast differentiation; interestingly, induction of PPARγ has been shown to inhibit differentiation of both mouse and human invasive trophoblast: in mouse TS cells, treatment with the PPARγ agonist, rosiglitazone, reduced markers of spongiotrophoblast and TGC [37], while similar treatment of human first trimester cytotrophoblast inhibited invasion in a dose-dependent manner [75].
Signaling pathways regulating invasive trophoblast differentiation
Activin/TGFβ signaling
Numerous signaling pathways are known to modulate differentiation and function of invasive trophoblast [76] (Table 1). As mentioned above, Activin induces EVT differentiation in the human placenta [13], an effect that contrasts sharply with promotion of mouse TS proliferation and labyrinthine differentiation (see above). In context of the first trimester chorionic villi, Activin induced outgrowth of CTB into the surrounding matrix and induced EVT-associated markers, including HLA-G and production of matrix metalloproteinases, MMP-2 and -9 [13]. In contrast to Activin, TGFβ signaling has both anti-proliferative and anti-invasive activity in human CTB [77–79]; the latter are mediated through production of tissue inhibitors of metalloproteinases (TIMPs) and plasminogen activator inhibitor 1 (PAI-1), which block MMP and urokinase plasminogen activator (uPA) activities, respectively [77, 79]. Again, this contrasts with the effect of TGFβ on mouse TS cells, which, similar to Activin, is pro-proliferative [7].
Canonical Wnt signaling
Another signaling pathway, which has been shown to promote human EVT differentiation, is Wingless/β-catenin (canonical Wnt). This pathway has been nicely detailed in a recent review [80], and thus will not be expanded on in detail here. In short, Wnt3a was shown to stimulate first trimester CTB to migrate and invade through Matrigel; this is at least partly mediated through TCF3/4, whose expression is increased in invading trophoblast, and its interaction with β-catenin, leading to induction of Wnt/TCF target genes [81]. In mice, Wnt signaling is dispensable for blastocyst development, but is otherwise required for blastocyst activation and implantation [45]. Nevertheless, in contrast to human EVT, DKK1, an inhibitor of canonical Wnt pathway, has been shown to increase motility of mouse ectoplacental cone cells, the precursors to giant cells, in vitro [82]. As described above, Wnt/TCF signaling also affects development of the interhemal region in both mouse and human placenta, through its effects on GCM1 and syncytialization [46].
Notch signaling
Another pathway, which has been shown to play a role in invasive trophoblast, is Notch signaling. While numerous Notch receptors and ligands are expressed throughout the mouse placenta at different gestational ages [83], most of the work has focused on Notch2, which is expressed in all TGC subtypes [83, 84]. Conditional deletion of this receptor in the mouse reduced the size of maternal blood sinuses and placental perfusion, leading to embryonic lethality by E11.5 [84]. The role of Notch signaling in human EVT differentiation and invasion is less clear. Similar to the mouse placenta, multiple Notch receptors (including Notch2) and ligands are expressed in villous CTB, cell column trophoblast, and EVT of the human placenta [85]. However, the effect of Notch inhibition on first trimester CTB invasion appears to be different in different studies, reducing Matrigel invasion in one study [84] and promoting proliferation, EVT differentiation, and migration of these cells in another study [85]. Although the same Notch inhibitors were used for some of these experiments in both studies, one major difference was the use of different matrices for culturing and assaying primary human CTB (Matrigel in Hunkapiller et al. [84] and fibronectin or collagen I in Haider et al. [85]).
Oxygen tension and the role of hypoxia-inducible factor
Hypoxia and signaling through the hypoxia-inducible factor have also been shown to modulate invasive trophoblast differentiation. As described above, mouse TS cells unable to form an intact HIF complex fail to differentiate into TGC, instead differentiating exclusively into syncytiotrophoblast in vitro [42]. In addition, culture of mouse TS cells in 2 % oxygen on a fibronectin-rich matrix redirects differentiation into TGC [43]. In another rodent model, the pregnant rat, where endovascular trophoblasts are similarly invasive as in the human placenta, maternal exposure to 11 % oxygen during mid-gestation (E6.5–E13.5) enhanced uterine vascular remodeling by increasing invasion of endovascular, but not interstitial, trophoblast [86]. In human, there are some discrepancies in results from numerous studies. It is known that early placentation (<8 weeks) occurs under low oxygen tension (<20 mmHg, <3 % oxygen), and that oxygen levels rise (to >50 mmHg, >7.5 %) by 12 weeks gestation [87]. In accordance with these findings, expression of HIF1α, the subunit of HIF sensitive to hypoxia, drops precipitously after 8 weeks of gestation. During this period, the human placenta grows rapidly, but much differentiation into EVT and invasion are also taking place [2]. Paradoxically, when compared to 20 % oxygen, culture of primary first trimester CTB in 2 % oxygen appears to induce proliferation, inhibit integrin-α1 expression, and reduce invasion [88]. Nevertheless, in the context of the first trimester explant, the number of cells expressing HLA-G, a marker of EVT, appears to expand [89]. Similarly, expression of integrin-α5, a marker of cell column trophoblasts, which are intermediate in differentiation between CTB and mature EVT, is increased when first trimester human placental explants are cultured under 3 % oxygen [90]. Finally, treatment of these explants with HIF1α-antisense oligonucleotides under 3 % oxygen, promoted a more invasive EVT phenotype, including enhanced integrin-α1 and gelatinase B expression [90]. These data, considered in whole, appear to indicate that lower oxygen tension (generally below 3 % oxygen) promotes differentiation of CTB into an intermediate EVT phenotype, characterized by expression of HLA-G and integrin-α5, while higher oxygen tensions are required for maturation of this phenotype, including the switch to integrin-α1.
Phosphoinositide 3-kinase-based signaling
Phosphoinositide 3-kinase (PI3K) is a major signaling pathway, activated downstream of growth factor receptor-tyrosine kinases as well as G protein-coupled receptors, and, in turn, activates the serine/threonine kinase, Akt [76]. While it has not been shown to promote differentiation per se, signaling through PI3K does promote growth factor-mediated trophoblast migration, a phenotype characteristic of the EVT lineage. Specifically, HGF and EGF were found to induce EVT migration, acting through the PI3K pathway, in two human EVT cell culture models, SGHPL-5 and HTR8/SVneo cells, respectively [26, 91]. In rodents, PI3K inhibitors have been shown to interfere with expression of giant-cell-associated genes during differentiation of the rat choriocarcinoma cell line, Rcho-1 [92].
Summary, limitations, and conclusions
In summary, mouse and human placentation do share some markers and signaling pathways in both the interhemal and invasive lineages (Table 1). One major limitation, which should be kept in mind, is the systems available for assessment of various factors in human trophoblast differentiation. In this review, we have focused on data from first trimester human CTB or explants, which are thought to be bipotential [14]; clear notation has been made where data with other cell types (term CTB or human trophoblast cell lines) are described. It is clear, however, that much work remains. This will include further comparisons of signaling pathways using more optimal in vitro systems for human trophoblast differentiation; one such system may be human pluripotent stem cells, following treatment with BMP4 [93–95]. In addition, identification of additional human trophoblast lineage-specific markers is required to better understand trophoblast function and placental abnormalities associated with pregnancy complications. Finally, while the mouse continues to be invaluable as a genetic tool for the study of placental development, the importance of comparative studies using human placental tissues and isolated CTB must not be disregarded, particularly when drawing conclusions about the roles of analogous genes or pathways with regard to human placental disorders.
Abbreviations
- CTB:
-
Cytotrophoblast
- EGF:
-
Epidermal growth factor
- EPC:
-
Ectoplacental cone
- EVT:
-
Extravillous trophoblast
- FAK:
-
Focal adhesion kinase
- FGF:
-
Fibroblast growth factor
- HGF:
-
Hepatocyte growth factor
- HIF:
-
Hypoxia-inducible factor
- ICM:
-
Inner cell mass
- MEF:
-
Mouse embryonic fibroblast
- PI3K:
-
Phosphoinositide 3-kinase
- PKA:
-
Protein kinase A
- STB:
-
Syncytiotrophoblast
- TE:
-
Trophectoderm
- TGC:
-
Trophoblast giant cell
- TS:
-
Trophoblast stem (cells)
References
Guttmacher AE, Maddox YT, Spong CY (2014) The Human Placenta Project: placental structure, development, and function in real time. Placenta 35(5):303–304
Benirschke K, Kaufmann P (2000) Pathology of the human placenta. Springer, New York
Hu D, Cross JC (2010) Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol 54(2–3):341–354
Weier JF, Weier HG, Jung CJ, Gormley M, Zhou Y, Chu LW, Genbacev O, Wright AA, Fisher SJ (2005) Human cytotrophoblasts acquire aneuploidies as they differentiate to an invasive phenotype. Dev Biol 279(2):420–432
Strumpf D, Mao C, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J (2005) Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132(9):2093–2102
Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science 282(5396):2072–2075
Erlebacher A, Price KA, Glimcher LH (2004) Maintenance of mouse trophoblast stem cell proliferation by TGF-β/activin. Dev Biol 275(1):158–169
Kubaczka C, Senner C, Araúzo-Bravo M, Sharma N, Kuckenberg P, Becker A, Zimmer A, Brüstle O, Peitz M, Hemberger M, Schorle H (2014) Derivation and Maintenance of Murine Trophoblast Stem Cells under Defined Conditions. Stem Cell Rep 2(2):232–242
Niakan KK, Eggan K (2013) Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol 375(1):54–64
Roberts RM, Fisher SJ (2011) Trophoblast Stem Cells. Biol Reprod 84(3):412–421
James JL, Carter AM, Chamley LW (2012) Human placentation from nidation to 5 weeks of gestation. Part I: what do we know about formative placental development following implantation? Placenta 33(5):327–334
Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ (2010) ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet 19(12):2456–2467
Caniggia I, Lye SJ, Cross JC (1997) Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 138(9):3976–3986
Baczyk D, Dunk C, Huppertz B, Maxwell C, Reister F, Giannoulias D, Kingdom JC (2006) Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta 27(4–5):367–374
Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, Larocque N, Goldfien G, Zdravkovic T, McMaster MT, Fisher SJ (2011) Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells 29(9):1427–1436
Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC (2003) Genes, development and evolution of the placenta. Placenta 24(2–3):123–130
Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 25(3):311–314
Schubert SW, Lamoureux N, Kilian K, Klein-Hitpass L, Hashemolhosseini S (2008) Identification of integrin-alpha4, Rb1, and syncytin a as murine placental target genes of the transcription factor GCMa/Gcm1. J Biol Chem 283(9):5460–5465
Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC (2008) Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development 135(12):2083–2091
Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC (2004) The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol 271(1):26–37
Baczyk D, Satkunaratnam A, Nait-Oumesmar B, Huppertz B, Cross JC, Kingdom JC (2004) Complex patterns of GCM1 mRNA and protein in villous and extravillous trophoblast cells of the human placenta. Placenta 25(6):553–559
Baczyk D, Drewlo S, Proctor L, Dunk C, Lye S, Kingdom J (2009) Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ 16(5):719–727
Yu C, Shen K, Lin M, Chen P, Lin C, Chang G, Chen H (2002) GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem 277(51):50062–50068
Hughes M, Natale BV, Simmons DG, Natale DR (2013) Ly6e expression is restricted to syncytiotrophoblast cells of the mouse placenta. Placenta 34(9):831–835
Ueno M, Lee L, Chhabra A, Kim Y, Sasidharan R, Van HB, Wang Y, Kamata M, Kamran P, Sereti K, Ardehali R, Jiang M, Mikkola HA (2013) c-Met-dependent multipotent labyrinth trophoblast progenitors establish placental exchange interface. Dev Cell 27(4):373–386
Cartwright JE, Tse WK, Whitley GS (2002) Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res 279(2):219–226
Natale DRC, Hemberger M, Hughes M, Cross JC (2009) Activin promotes differentiation of cultured mouse trophoblast stem cells towards a labyrinth cell fate. Dev Biol 335(1):120–131
Guzman-Ayala M, Ben-Haim N, Beck S, Constam DB (2004) Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. PNAS 101(44):15656–15660
Park CB, Dufort D (2011) Elsevier Trophoblast Research Award lecture: the multifaceted role of Nodal signaling during mammalian reproduction. Placenta 32(Suppl 2):S125–S129
Ma GT, Soloveva V, Tzeng SJ, Lowe LA, Pfendler KC, Iannaccone PM, Kuehn MR, Linzer DI (2001) Nodal regulates trophoblast differentiation and placental development. Dev Biol 236(1):124–135
Song Y, Keelan J, France JT (1996) Activin-A stimulates, while transforming growth factor beta 1 inhibits, chorionic gonadotrophin production and aromatase activity in cultured human placental trophoblasts. Placenta 17(8):603–610
Gerbaud P, Pidoux G, Guibourdenche J, Pathirage N, Costa JM, Badet J, Frendo J, Murthi P, Evain-Brion D (2011) Mesenchymal activin-A overcomes defective human trisomy 21 trophoblast fusion. Endocrinology 152(12):5017–5028
Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C (2004) Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem 279(30):31277–31286
Nadeem L, Munir S, Fu G, Dunk C, Baczyk D, Caniggia I, Lye S, Peng C (2011) Nodal signals through activin receptor-like kinase 7 to inhibit trophoblast migration and invasion: implication in the pathogenesis of preeclampsia. Am J Pathol 178(3):1177–1189
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4(4):585–595
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4(4):597–609
Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS (2009) PPARγ regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One 4(11):e8055
Tarrade A, Schoonjans K, Guibourdenche J, Bidart JM, Vidaud M, Auwerx J, Rochette-Egly C, Evain-Brion D (2001) PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology 142(10):4504–4514
Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, Sadovsky Y (2000) Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocr Metab 85(10):3874–3881
Elchalal U, Humphrey RG, Smith SD, Hu C, Sadovsky Y, Nelson DM (2004) Troglitazone attenuates hypoxia-induced injury in cultured term human trophoblasts. Am J Obstet Gynecol 191(6):2154–2159
Alsat E, Wyplosz P, Malassiné A, Guibourdenche J, Porquet D, Nessmann C, Evain-Brion D (1996) Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol 168(2):346–353
Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ (2005) Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 132(15):3393–3403
Choi HJ, Sanders TA, Tormos KV, Ameri K, Tsai JD, Park AM, Gonzalez J, Rajah AM, Liu X, Quinonez DM, Rinaudo PF, Maltepe E (2013) ECM-Dependent HIF induction directs trophoblast stem cell fate via LIMK1-mediated cytoskeletal rearrangement. PLoS One 8(2):e56949
Tache V, Ciric A, Moretto-Zita M, Li Y, Peng J, Maltepe E, Milstone DS, Parast MM (2013) Hypoxia and trophoblast differentiation: a key role for PPARγ. Stem Cells Dev 22(21):2815–2824
Rinkenberger JL, Cross JC, Werb Z (1997) Molecular genetics of implantation in the mouse. Dev Genet 21(1):6–20
Matsuura K, Jigami T, Taniue K, Morishita Y, Adachi S, Senda T, Nonaka A, Aburatani H, Nakamura T, Akiyama T (2011) Identification of a link between Wnt/β-catenin signalling and the cell fusion pathway. Nat Commun 2:548
Lu J, Zhang S, Nakano H, Simmons DG, Wang S, Kong S, Wang Q, Shen L, Tu Z, Wang W, Wang B, Wang H, Wang Y, Es JH, Clevers H, Leone G, Cross JC, Wang H (2013) A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS Biol 11(4):e1001536
Morrish DW, Bhardwaj D, Dabbagh LK, Marusyk H, Siy O (1987) Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrinol Metab 65(6):1282–1290
Shi QJ, Lei ZM, Rao CV, Lin J (1993) Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132(3):1387–1395
Keryer G, Alsat E, Tasken K, Evain-Brion D (1998) Cyclic AMP-dependent protein kinases and human trophoblast cell differentiation in vitro. J Cell Sci 111(Pt 7):995–1004
Frendo J, Olivier D, Cheynet V, Blond J, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F (2003) Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol 23(10):3566–3574
Dackor J, Caron KM, Threadgill DW (2009) Placental and embryonic growth restriction in mice with reduced function epidermal growth factor receptor alleles. Genetics 183(1):207–218
John R, Hemberger M (2012) A placenta for life. Reprod Biomed Online 25(1):5–11
Simmons DG, Fortier AL, Cross JC (2007) Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 304(2):567–578
Gardner RL, Davies TJ (1993) Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. J Exp Zool 265(1):54–60
MacAuley A, Cross JC, Werb Z (1998) Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol Biol Cell 9(4):795–807
Parast MM, Aeder S, Sutherland AE (2001) Trophoblast giant-cell differentiation involves changes in cytoskeleton and cell motility. Dev Biol 230(1):43–60
Mould A, Morgan MA, Li L, Bikoff EK, Robertson EJ (2012) Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev 26(18):2063–2074
Fisher SJ, Damsky CH (1993) Human cytotrophoblast invasion. Semin Cell Biol 4(3):183–188
Damsky CH, Fitzgerald ML, Fisher SJ (1992) Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 89(1):210–222
Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH (1997) Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 99(9):2139–2151
Lee Y, Kim K, McKeon F, Yang A, Boyd TK, Crum CP, Parast MM (2007) A unifying concept of trophoblastic differentiation and malignancy defined by biomarker expression. Hum Pathol 38(7):1003–1013
Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B (2000) Localization of E-cadherin in villous, extravillous and vascular trophoblasts during intrauterine, ectopic and molar pregnancy. Mol Human Reprod 6(10):943–950
Ilić D, Genbacev O, Jin F, Caceres E, Almeida EA, Bellingard-Dubouchaud V, Schaefer EM, Damsky CH, Fisher SJ (2001) Plasma membrane-associated pY397FAK is a marker of cytotrophoblast invasion in vivo and in vitro. Am J Pathol 159(1):93–108
Fujimoto S, Hamasaki K, Ueda H, Kagawa H (1986) Immunoelectron microscope observations on secretion of human placental lactogen (hPL) in the human chorionic villi. Anat Rec 216(1):68–72
Cole LA (2012) hCG, five independent molecules. Clin Chim Acta 413(1–2):48–65
Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T (2007) Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology 148(10):5011–5019
Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, Brion DE, Fournier T (2010) Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocr Metab 95(10):E240–E244
Cole LA (2010) Hyperglycosylated hCG, a review. Placenta 31(8):653–664
Miyamoto T, Hasuike S, Jinno Y, Soejima H, Yun K, Miura K, Ishikawa M, Niikawa N (2002) The human ASCL2 gene escaping genomic imprinting and its expression pattern. J Assist Reprod Genet 19(5):240–244
Meinhardt G, Husslein P, Knöfler M (2005) Tissue-specific and ubiquitous basic helix-loop-helix transcription factors in human placental trophoblasts. Placenta 26(7):527–539
Knöfler M, Meinhardt G, Vasicek R, Husslein P, Egarter C (1998) Molecular cloning of the human Hand1 gene/cDNA and its tissue-restricted expression in cytotrophoblastic cells and heart. Gene 224(1–2):77–86
Knöfler M, Meinhardt G, Bauer S, Loregger T, Vasicek R, Bloor DJ, Kimber SJ, Husslein P (2002) Human Hand1 basic helix-loop-helix (bHLH) protein: extra-embryonic expression pattern, interaction partners and identification of its transcriptional repressor domains. Biochem J 361(Pt 3):641–651
Murthi P, Kalionis B, Cocquebert M, Rajaraman G, Chui A, Keogh RJ, Evain-Brion D, Fournier T (2013) Homeobox genes and down-stream transcription factor PPARγ in normal and pathological human placental development. Placenta 34(4):299–309
Tarrade A, Kuen RL, Malassiné A, Tricottet V, Blain P, Vidaud M, Evain-Brion D (2001) Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest 81(9):1199–1211
Knöfler M (2005) Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta 26(Suppl A):S21–S30
Graham CH, Lala PK (1991) Mechanism of control of trophoblast invasion in situ. J Cell Physiol 148(2):228–234
Graham CH, Lysiak JJ, McCrae KR, Lala PK (1992) Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod 46(4):561–572
Graham CH (1997) Effect of transforming growth factor-beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta 18(2–3):137–143
Knöfler M, Pollheimer J (2013) Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Front Genet 4:190
Meinhardt G, Haider S, Haslinger P, Proestling K, Fiala C, Pollheimer J, Knöfler M (2014) Wnt-dependent T-cell factor-4 controls human etravillous trophoblast motility. Endocrinology 155(5):1908–1920
Peng S, Li J, Miao C, Jia L, Hu Z, Zhao P, Li J, Zhang Y, Chen Q, Duan E (2008) Dickkopf-1 secreted by decidual cells promotes trophoblast cell invasion during murine placentation. Reproduction 135(3):367–375
Gasperowicz M, Rai A, Cross JC (2013) Spatiotemporal expression of Notch receptors and ligands in developing mouse placenta. Gene Expr Patterns 13(7):249–254
Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ (2011) A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development 138(14):2987–2998
Haider S, Meinhardt G, Velicky P, Otti GR, Whitley G, Fiala C, Pollheimer J, Knöfler M (2014) Notch signaling plays a critical role in motility and differentiation of human first-trimester cytotrophoblasts. Endocrinology 155(1):263–274
Rosario GX, Konno T, Soares MJ (2008) Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 314(2):362–375
Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157(6):2111–2122
Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ (1996) Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97(2):540–550
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ (1997) Regulation of human placental development by oxygen tension. Science 277(5332):1669–1672
Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 105(5):577–587
Qiu Q, Yang M, Tsang BK, Gruslin A (2004) Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod 10(9):677–684
Kent LN, Kent LN, Konno T, Soares MJ, Soares MJ (2010) Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol 10(1):97
Amita M, Adachi K, Alexenko AP, Sinha S, Schust DJ, Schulz LC, Roberts RM, Ezashi T (2013) Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. PNAS 110(13):E1212–E1221
Li Y, Moretto-Zita M, Soncin F, Wakeland A, Wolfe L, Leon-Garcia S, Pandian R, Pizzo D, Cui L, Nazor K, Loring JF, Crum CP, Laurent LC, Parast MM (2013) BMP4-directed trophoblast differentiation of human embryonic stem cells is mediated through a ΔNp63 + cytotrophoblast stem cell state. Development 140(19):3965–3976
Li Y, Parast MM (2014) BMP4 regulation of human trophoblast development. Int J Dev Biol 58(2–4):239–246
Acknowledgments
This work was supported by funds from a California Institute for Regenerative Medicine New Faculty Award (RN2-00931-1) and the National Institutes of Health (R01HD071100) to M.M.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soncin, F., Natale, D. & Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: a comparative review. Cell. Mol. Life Sci. 72, 1291–1302 (2015). https://doi.org/10.1007/s00018-014-1794-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1794-x